Abstract
Although studies have established that immune mechanisms are important in controlling tick-borne encephalitis virus (TBEV) infection, the interactions of different TBEV strains with cells of innate and adaptive immunity are not well understood. In this study, the ability of two Far Eastern subtype TBEV strains (Dal'negorsk and Primorye-183) with various degrees of pathogenicity for humans to modulate the expression of membrane molecules differently on human immune cells were investigated using a whole-blood flow cytometry-based assay. The whole-blood samples (from 10 healthy donors) were infected with TBEV strains and analyzed for the virus binding to the blood cells, as well as expression of adhesion (CD11b and ICAM-1) and activation (CD69, CD25, CD95) molecules on the surfaces of monocytes, granulocytes, natural killer (NK) cells, and T-lymphocytes (CD4+, CD8+) at selected times (3, 6, and 24 h post-infection). It was found that the highly pathogenic Dal'negorsk strain penetrated rapidly and was actively replicated in the blood cells, inducing downregulation of CD11b, ICAM-1, and CD69 on monocytes and a significant decrease of NK cells expressing CD69, CD25, CD95, and CD8 T-lymphocytes expressing CD69 compared with the mock-infected cells. The nonpathogenic Primorye-183 strain penetrated slowly and was replicated in the blood cells, but caused a significant increase in the adhesion and activation of molecule expression to trigger innate defense mechanisms and enable the rapid elimination of the virus from the organism. Thus, TBEV-induced activation or suppression of adhesion and activation receptors expression form an essential part of fundamental virus properties, that is, virulence and pathogenicity.
Introduction
Tick-borne encephalitis (TBE), known in many Eurasian countries, is one of the most dangerous neuroviral infections, which has consistently attracted the attention of researchers. According to the international classification, tick-borne encephalitis virus (TBEV) belongs to the genus Flavivirus in the family Flaviviridae, and has three subtypes: Far Eastern, Siberian, and European (19). In the endemic areas, where TBEV strains of the Far Eastern subtype circulate, severe forms of disease with high mortality rates that can exceed 20% (23) are detected more often than in the regions dominated by virus strains of Siberian and European subtypes. Besides, each TBEV subtype includes genetically different lines or clusters that indicate heterogeneity of a virus population that can cause various forms of disease (6,13,25). A number of authors, who have described clinical polymorphism of TBE, link it to genotypic and phenotypic variability of the TBEV population (38,65).
Along with that, it has been established that the ability of all flaviviruses, including TBEV, to modulate the host immune response can have a pivotal effect on the course and outcome of the disease (32,41,43,53,62). The key to understanding how these viruses are able to evade the host's immune response and make their way from the site of infection to the central nervous system and the brain is studying viral–host cell interactions. Mutational analyses of the flaviviral envelope E protein, which is critical for cellular infectivity, have demonstrated a striking ability of flaviviruses to adapt to different cells and receptors (2,11,31). In this study, the interaction between TBEV and human blood leukocytes was examined, since it is known that these cells could differently contribute to the protective and pathogenic manifestations of flavivirus infections (4,28,32,37). In addition, TBEV, as has been previously established, can be isolated from human blood leukocytes during the first days after the tick bite, indicating that the virus is replicating in blood cells (23,24). However, the molecular mechanisms that allow TBEV to manipulate host cellular functions for successful infection are still unclear. Here, the ability of two TBEV strains with various degrees of pathogenicity for humans to modulate the expression of membrane molecules on different subpopulations of human blood leukocytes were investigated.
Materials and Methods
TBEV strains
TBEV strains of the Far Eastern subtype—Dal'negorsk (Dal) and Primorye-183 (P-183)—were used. The Dal strain, isolated in 1973 from the brain of a dead patient who had the focal form of TBE, is highly pathogenic in humans (Gene Bank Whole Genome Sequence Number: FJ402886). The P-183 strain, isolated in 1991 from the blood leukocytes of a person with an inapparent form of TBE, is nonpathogenic in humans (Gene Bank Whole Genome Sequence Number: JQ825153). Isolation of these strains and the study of their molecular genetic characteristics and biological properties was performed as previously described (6,25,26). In this study, the brains of infected suckling mice (Dal strain: 9 passage; P-183 strain: 5 passage) stored at −80°C were taken. Ten percent of brain suspensions were prepared, and their titers were determined on pig embryo kidney cell (PK cells) monolayers as previously described (25,26). The titer of the Dal strain was 1010 TCID50/mL; the titer of the P-183 strain was 107 TCID50/mL.
Ex vivo modeling of TBE infection
Whole-blood samples (15 mL) were collected using heparin as an anticoagulant from healthy donors (n=10) without a history of TBE and unvaccinated against TBE. The average age of the donors was 32 years (range 28–37 years). All donors gave informed written consent according to the local ethics committee. Each of the whole-blood samples (approximately 5×106 cells/mL) was divided into three portions: one portion of the blood (6 mL) was infected with the Dal strain at a multiplicity of infection (MOI) of 0.01 TCID50/cell; another portion (6 mL) was infected with the P-183 strain of TBEV at 0.01 TCID50/cell; and the third portion (3 mL) was mock infected (phosphate-buffered saline [PBS]). Immediately, each portion of infected and mock-infected blood was divided into aliquots of 1 mL and incubated at 37°C for 24 h. At 3, 6, 12, and 24 h post-infection (p.i.), respectively, virus binding to blood cells and expression of adhesion and activation molecules on the surface of blood cells was determined.
Virology methods
Infected and mock-infected blood samples (1 mL aliquots) were taken at the given time points, centrifuged at 400 g for 5 min, and then TBEV strains binding to the blood cells were assessed by the number of virus-infected cells and amount of virus remaining in the plasma.
The number of blood cells infected with TBEV strains was determined by indirect immunofluorescence. Infected and mock-infected pelleted cells were plated on microscope slides that were fixed with chilled acetone within 20 min. Then, the cells were applied to human serum containing antibodies to TBEV (1:40 dilution). The slides were incubated in a wet chamber at 37°C for 1 h, washed with PBS (pH 7.2) for 10 min, and air-dried. Then the cells were applied to antihuman FITC-conjugated immunoglobulins (1:64 dilution; Medgamal). They were then incubated in a wet chamber at 37°C for 1 h again, washed with PBS (pH 7.2), and visualized in a luminescent microscope (Micros 200A). To calculate the percentage of the blood cells that was TBEV-infected at each time point, both total cells and immunofluorescence-positive cells in each sample of blood cells in at least five microscopy fields (100–120 cells per field) were counted. The number of virus-infected cells staining positive in relation to the total number of cells was expressed as the percentage of virus-positive cells.
The amount of unbound virus in the plasma samples was determined by titration of samples on PK cells. Infected and mock-infected plasma samples were serially diluted (10–1 to 10–6), and 100 μL of each dilution was added to an overnight monolayer of PK cells grown on 24-well plates. After 1 h of incubation at 37°C, the inoculated monolayer was washed with culture medium 199 followed by the addition of cell maintenance medium 199 with gentamicin and 1% fetal bovine serum (FBS). It was then, incubated at 37°C in a CO2-incubator for 7 days. The virus titer was expressed as log10 TCID50/mL.
Flow cytometry analysis
Expression levels of adhesion (CD11b and CD54) and activation (CD69, CD25, and CD95) molecules on the human whole-blood cell surface were determined in aliquots (1 mL) infected with TBEV strains and mock-infected blood samples, taken at 3 and 24 h p.i., using monoclonal antibodies (MAb) against the related antigens (49).
For this study, the MAb panels (Beckman Coulter) anti-CD45-FITC/CD14-PE, anti-CD3-FITC, anti-CD4-FITC, anti-CD8-FITC, anti-CD16-FITC, anti-CD56-FITC, anti-CD11b-PE, anti-CD54-PE, anti-CD69-PE, anti-CD25-PE, anti-CD95-PE, anti-CD3-PC5, as well as related isotype controls, were used.
The expression level of membrane molecules was assessed by a standard method of two- or three-color immunofluorescent staining of whole blood. Heparinized whole blood (100 μL) was incubated in the presence of 5 μL of antihuman cell surface molecule MAbs for 30 min in the dark and at room temperature. Then, erythrocytes lysis was performed using 2 mL of FACS Lysing Solution (Becton Dickinson), followed by incubation for 10 min at room temperature. The leukocyte suspension was further washed with 2 mL of PBS (Cell Wash, Becton Dickinson). Data collection was performed using a flow cytometer FACSCalibur (Becton Dickinson). The CELLQUEST™ software provided by the manufacturer was used for data acquisition and analysis. At least 10,000 cells were examined in each sample.
The gating of granulocyte, monocyte, and lymphocyte subpopulations was performed using a light scatter (forward and side scatter), and natural killer (NK) cell gating was carried out using CD16 or CD56 fluorescence in the CD3-negative lymphocyte area. Data were analyzed with CellQuest Pro (Becton Dickinson) software.
The results of expression analysis for CD11b, CD54, and CD69 on monocytes, granulocytes, and NK cells are indicated as mean intensity fluorescence (MFI). Results of expression analysis for CD69, CD25, and CD95 within lymphocyte subpopulations (NK cells, CD4+ and CD8+ T-lymphocytes) are shown as the mean percentage of the cells that express markers.
Statistical analysis
Statistical analysis was performed using the Mann–Whitney U-test. Statistical significance was considered at the level of p≤0.05.
Results
The ex vivo interaction of different pathogenic TBEV strains (Dal and P-183) with the immune cells of healthy donors' whole blood was examined. It is of note that the use of whole blood does not require the extraction of cells or their preparation for cultivation, which eliminates nonspecific cell activation at separation stages, while retaining the existing in vivo balance of various types of blood cells. Therefore, assessment of adhesion and activation markers expression on whole-blood cells most closely reflects the processes that take place in vivo.
Dynamics of infection of donors' blood cells by TBEV strains
The early stages of infection by different TBEV strains in donors' whole-blood samples infected with these strains within 24 h p.i were examined.
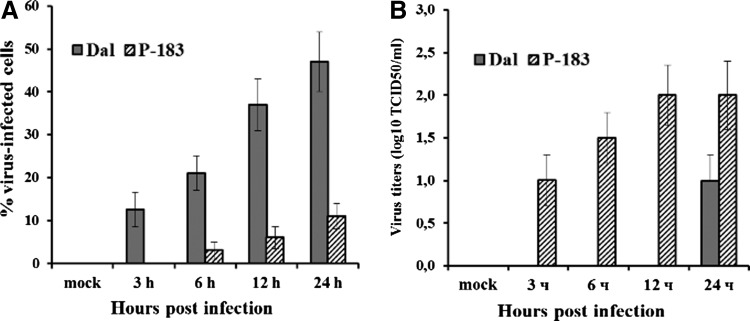
In the blood samples infected with the P-183 strain (MOI 0.01), which caused an inapparent form of TBE, virus binding to blood cells was only detected at 24 h p.i. (11±3% virus-infected cells; Fig. 1A), whereas unbound virus was revealed in plasma samples during the whole experiment: virus titers 1–24 h p.i. were from 1.0±0.3 to 2.0±0.4 log10 TCID50/mL (Fig. 1B).
FIG. 1.
Dynamics of infection of donors' blood cells by tick-borne encephalitis virus (TBEV) strains: (A) in blood cells and (B) in plasma. The whole-blood samples were mock-infected and infected with the Dal strain at a multiplicity of infection (MOI) of 0.01 TCID50/cell (gray columns) and with the P-183 strain at a MOI of 0.01 TCID50/cell (columns with hatching). Virus binding to the blood cells was analyzed at 3, 6, and 24 h post-infection (p.i.). (A) The number of blood cells infected with TBEV strains was determined by indirect immunofluorescence and expressed as the percentage of virus-positive cells. (B) The amount of unbound virus in the plasma samples was determined by titration of samples on pig embryo kidney (PK) cells and expressed as log10 TCID50/mL. The results shown were obtained from 10 independent blood samples (each data point represents the mean±SD).
Infection of donors' blood cells with the Dal strain, which caused a focal form of TBE, had the opposite dynamics. In the blood samples infected with this strain (MOI 0.01), the percentage of virus-infected cells, starting from 3 h p.i. (12±4%), increased within the whole observation period to reach a mean value at 47±7% (Fig. 1A). In plasma samples, unbound virus was only found at 24 h p.i. (Fig. 1B), and this probably indicates that by this time the reproduced viral particles from the blood cells were beginning to enter the plasma.
These results confirm the results of the authors' previous studies (20, 26) as well as other studies that showed replication of highly virulent strains of TBEV starting from 12 h pi by using different cell cultures (31,35,45,52).
Influence of TBEV strains on expression of adhesion and activation molecules by innate immune system cells
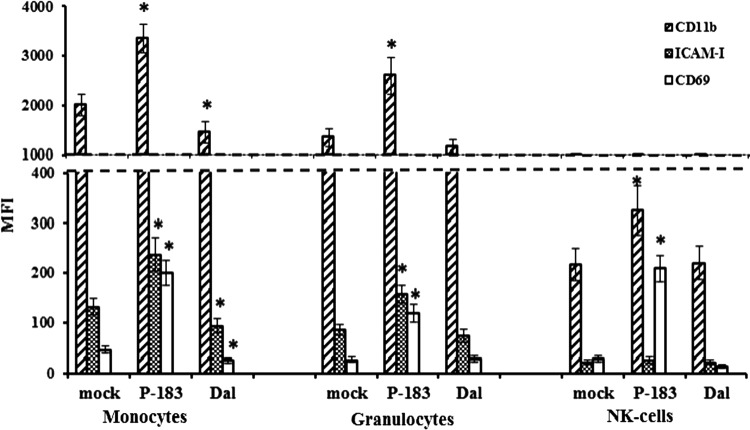
The expression of molecules CD11b was studied, belonging to the family of β2-integrins, ICAM-1 (CD54), relating to the immunoglobulin superfamily, and early activation antigen CD69 on monocytes, granulocytes, and NK cells in blood samples infected with TBEV strains and mock-infected at 3 and 24 h p.i. At 3 h p.i., a change in fluorescence intensity of adhesion and activation molecules by action of TBEV strains was observed only for molecules CD11b that are known (8,57) to be constitutively expressed on these cells. By this time, the level of CD11b expression on monocytes, granulocytes, and NK cells in the blood samples infected with the P-183 strain increased by 1.3–1.4 times, and in samples infected with the Dal strain, it did not change compared to mock-infected samples (data not shown).
Changes in the level of CD11b, ICAM-1, and CD69 expression on monocytes, granulocytes, and NK cells induced by TBEV strains at 24 h p.i. are shown in Figure 2. It was found that by this time, in blood samples infected with the P-183 strain, the fluorescence intensity of the CD11b molecules on cells of innate immunity was 1.6–1.7 times higher than in samples infected with the Dal strain (p≤0.05) and mock-infected samples (p≤0.05). At 24 h p.i. in blood samples infected with the Dal strain, the level of CD11b expression on monocytes (p=0.030) decreased, while that on granulocytes and NK cells did not differ from that on mock-infected cells (p>0.05).
FIG. 2.
CD11b, ICAM-1, and CD69 expression on innate immune cells after infection of blood cells by TBEV strains. The whole-blood samples were mock-infected and infected with TBEV strains (P-183 and Dal) at a MOI of 0.01 TCID50/cell and analyzed at 24 h p.i. by flow cytometry for expression of CD11b, ICAM-1, and CD69 on the surface of monocytes, granulocytes, and NK cells. The results shown were obtained from 10 independent blood samples and are presented as mean fluorescence intensity (MFI; each data point represents the mean±SD). Asterisks (*) indicate the differences between parameters of TBEV-infected and mock-infected cells that were statistically significant (p≤0.05).
Intercellular adhesion molecules ICAM-1, which belong to the immunoglobulin superfamily, are constitutively expressed on endothelial cells, while expression on peripheral blood leukocytes requires their activation by virus or cytokines, that is, IL-1, IFNγ, or TNFα (44). It was shown that the maximum level of ICAM-1 expression on blood cells was registered in monocytic and granulocytic cell populations after 24 h p.i. (Fig. 2). In blood samples infected with the P-183 strain, the level of ICAM-1 expression on monocytes and granulocytes increased 1.8 times compared with that in mock-infected samples (p≤0.05). During blood-cell infection by the Dal strain, fluorescence intensity of ICAM-1 on the surface of the monocytes decreased (p=0.032), while expression of these molecules on granulocytes and NK cells was within variability of values in mock-infected samples (Fig. 2).
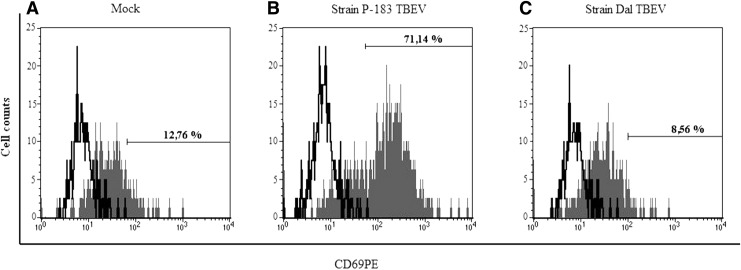
Molecule CD69 is the earliest activated antigen that is expressed on the surface of leukocytes, monocytes, and NK cells within 1–2 h of cell activation (3,9,27,29). At the same time, Craston et al. showed that the dynamics of CD69 expression differ with respect to the cell type and the method of stimulation. Here, it was found that at 24 h p.i. in blood samples infected with the P-183 strain, the level of CD69 expression on monocytes (Figs. 2 and 3) and granulocytes increased by 4.5 times, and NK cells by 7.5 times, compared with those in mock-infected samples (p≤0.05; Fig. 2). Blood-cell infection by the Dal strain over the same period revealed a significant decrease in fluorescence intensity of CD69 on the surface of monocytes and NK cells compared with that on mock-infected cells (p≤0.05). The distribution of activation molecules CD69 on monocytes in blood samples infected with TBEV at 24 h p.i. is shown in Figure 3.
FIG. 3.
CD69 expression on the monocytes after infection of blood cells by TBEV strains. The whole-blood samples were mock-infected (A) and infected with the P-183 strain at a MOI of 0.01 TCID50/cell (B) and the Dal strain at a MOI of 0.01 TCID50/cell (C), and analyzed at 24 h p.i. by flow cytometry for expression of CD69 on the surface of the monocytes. Isotype control: unpainted peaks; the fluorescence CD69+ monocytes: painted gray peaks. The histogram shows the number of CD69+ monocytes after 24 h p.i.: (A) 12.76%, (B) 71.14%, and (C) 8.56%. The data shown are from 1 of 10 experiments.
Influence of TBEV strains on expression of activation molecules by donors' lymphocytes
Transmission of the activation signal that triggers lymphocyte activation is a development of the cascade reaction, which is accomplished by the expression of various genes and their receptors on the cell surface, that is, activation antigens of lymphocytes. Several dozens of such activation antigens that are expressed on the lymphocyte membrane have been studied so far (18,39,61). Activation markers that are most often studied are CD69, CD25, and CD95, with their expression being indicative of passing certain stages of the cell cycle.
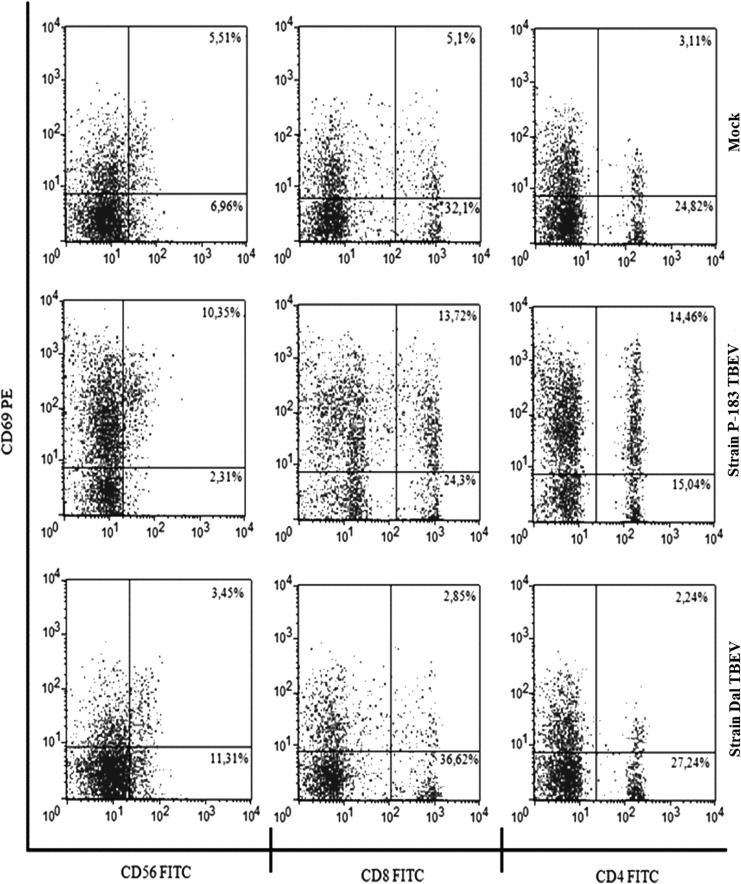
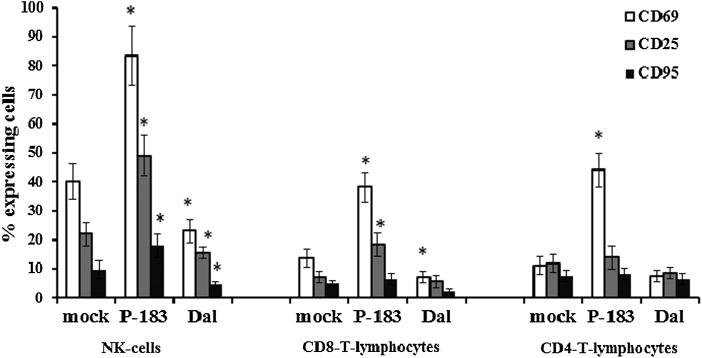
It has been found that at 3 h p.i. in blood samples infected with TBEV strains, the number of lymphocytes that expressed CD69 did not differ from those in mock-infected samples (p>0.05). At 24 h p.i. in blood samples infected with the P-183 strain, a significant increase of expression of this activation marker was detected on NK cells, CD8+ T-lymphocytes, and CD4+ T-lymphocytes, whereas infection with the Dal strain showed a reduction in the expression of CD69 compared to mock-infected cells (p≤0.05; Figs.4 and 5). By this time, the greatest changes in the level of CD69 expression were detected on NK cells: the number of NK cells expressing CD69 in mock-infected samples was 40.2% (34.3–45.9%), while in blood samples infected with the P-183 and Dal strains the number of cells were 83.4% (71.2–93.8%) and 23.1% (19.5–27.2%), respectively (Fig. 5).
FIG. 4.
CD69 expression on the lymphocyte subpopulations after infection of blood cells by TBEV strains. The whole-blood samples were mock-infected (top panels) and infected with the P-183 strain at a MOI of 0.01 TCID50/cell (middle panels) and the Dal strain at a MOI of 0.01 TCID50/cell (bottom panels), and analyzed at 24 h p.i. by flow cytometry for the expression of CD69 on the surface of NK cells (CD3–CD56+), T-lymphocytes (CD3+CD8+ and CD3+CD4+). The data shown are from 1 of 10 experiments.
FIG. 5.
CD69, CD25, and CD95 expression on the lymphocyte subpopulations after infection of blood cells by TBEV strains. The whole-blood samples were mock-infected and infected with TBEV strains (P-183 and Dal) at a MOI of 0.01 TCID50/cell and analyzed at 24 h p.i. by flow cytometry for expression of CD69, CD25, and CD95 within lymphocyte subpopulations (NK cells, CD4+, and CD8+ T-lymphocytes). The results obtained from 10 independent blood samples and presented as mean percentage of cells expressing markers (each data point represents the mean±SD of results) are shown. Uncoated (white) columns, % of cells expressing CD69; gray, % of cells expressing CD25; black, % of cells expressing CD95. *Significance of the differences between the parameters of TBEV-infected and mock-infected cells (p≤0.05).
In addition, at 24 h p.i. in blood samples infected with TBEV strains, changes in the number of NK cells and CD8+ T-lymphocytes expressing the activation antigen CD25 were registered. The percentage of NK cells expressing these molecules in mock-infected samples was 22.0% (18.9–26.1%)%, while in blood samples infected with the P-183 and Dal strains, the percentages were 49.0% (42.7–56.0%) and 15.4% (12.1–17.0%), respectively (Fig. 5). The content of CD8+ T-lymphocytes expressing CD25 in blood samples infected with the P-183 strain increased to 18.3% (14.5–22.1%) compared with 7.2% (5.1–9.5%) of those of mock-infected samples and 5.5% (3.0–7.8%) of samples infected by the Dal strain.
Studies of the expression of the activation marker CD95 on lymphocytes has shown that its level at 24 h p.i. only increases on NK cells during infection with the P-183 strain (Fig. 5). This strain of TBEV induced 18.0% (13.8–22.1%) of cells that express CD95 (9.6% [6.5–12.0%] in mock-infected samples); when infected by Dal strain, the percentage of NK cells expressing CD95 was 4.5% (3.0–5.6%).
Discussion
This study continued the authors' experimental research of the biological and molecular genetic features of TBEV strains, isolated from people with various forms of disease in the Russian Far East (6,24–26,40). This research in animal models, mostly mice, has shown that pathogenic potential (virulence) of the strains isolated from patients with inapparent infection was significantly lower compared to the strains causing severe focal TBE (6,25). In cell culture models (PK cells and culture of human peripheral blood mononuclear cells), it has been found that TBEV strains differ in their replicative activity: nonpathogenic strains have lower rates of penetration and replication of viral particles in cells than pathogenic strains (20,26).
In the present study, the focus was on the initial stage of infection, which largely predetermines the course and outcome of the disease. Within 24 h p.i. in an ex vivo model, the interaction of different pathogenic TBEV strains (Dal and P-183) with the cells of healthy donors' whole blood was examined.
It was found that during the infection of blood cells by these strains, their dynamics of binding to cells differ significantly in that period of observation (Fig. 1). So, the nonpathogenic P-183 strain of TBEV showed slow penetration and a low level of virus binding to blood cells, as well as its constant presence in plasma samples throughout the entire monitoring period. However, the Dal strain in contrast is highly pathogenic for humans, and so it rapidly penetrated and actively reproduced in blood cells. New viral particles of this strain from the cells appear in the extracellular space (in the plasma) after only 24 h p.i. The same slow phase of viral particles moving out was previously described for flavivirus Kunjin (58) and the highly virulent Hypr strain of TBEV (30,35). Overby et al. and Miorin et al. explained this 24 h delay as an escape strategy of TBEV from the immune system, according to which TBEV rearranged intracellular cytoplasmic compartments and reproduced in them, making them unavailable for detection by pattern-recognition receptors. This causes a time delay in the activation of these cells, the belated synthesis of biologically active substances, including IFN, which, in the end, allows the virus to spread in an organism rapidly and smoothly, before an efficient antiviral response is launched (35).
To determine the features of strategies to escape from immune control that are used by TBEV strains, the ability of these strains to modulate the expression of membrane molecules on different subpopulations of human blood leukocytes (granulocytes, monocytes, NK cells, and T-lymphocytes) was investigated. Granulocytes and monocytes/macrophages are known to be innate immune system cells, which, on the one hand, can have an antiviral effect by absorption, neutralization, and elimination of flaviviruses and cells infected by them, and, on the other hand, can serve as a reservoir for the replication and dissemination of these viruses in peripheral organs (1,4,7,37,42). A crucial component of the innate defense against flaviviruses are NK cells, which control infection either through the lysis of infected cells, or by the release of inflammatory cytokines. The early activation of NK cells in humans has been associated with mild clinical diseases (12,28,56,63). T-lymphocytes, as cells of adaptive immunity, also play an important role in viral clearance and the prevention of virus dissemination (59,64), although in animal models of flavivirus infection pathogenic effects of T-cell responses have also been demonstrated (47,48,55).
Here, it is shown that TBEV strains of different pathogenicity for humans changed the expression of adhesive (CD11b and ICAM-1) molecules and the activation antigen CD69 on membranes of innate immune system cells (i.e., granulocytes, monocytes, and NK cells) in different ways (Figs. 2 and 3). It is known that molecules CD11b (α-chain of β2-integrin, Mac-1α, CR3α), which are expressed on the leukocyte surfaces and mediate the identification of various ligands (including molecules ICAM-1, iC3b fragments of C3 component of complement, γ-chain of fibrinogen), regulate certain stages of inflammatory response, the most important of which is participation in leukocyte adhesion to the endothelium (5,8,16,17). Intercellular adhesion molecules ICAM-1 are expressed on blood leukocytes during activation, and participate in the contact interaction of cells in immune reactions: T-lymphocytes with monocytes, and cytotoxic T-lymphocytes with target cells (44,50). The earliest inducible activation marker CD69, which is expressed on lymphocytes, NK cells, monocytes, and granulocytes, acts as a transduction signal that causes the synthesis of key response mediators to viral infections, such as IFNα/β or pro-inflammatory cytokines (9,15,29).
Increased expression of adhesion molecules on monocytes and granulocytes, induced by the P-183 strain of TBEV, shows that, on the one hand, the processes of intercellular interaction facilitate dissemination of the virus replicating in these target cells, while, on the other hand, improvement of activation and adhesion abilities of monocytes, granulocytes, and NK cells is associated with the activation of such cells, as this strengthens their phagocytic and killing activity, as well as the antigen-induced activation of T-lymphocytes.
Decline in the adhesion and activation abilities of antigen-presenting cells (i.e., monocytes) and NK cells, caused by the Dal strain of TBEV that actively replicates in blood cells, can be one of the mechanisms of virus-induced suppression of the innate immune system. The data obtained confirm the clinical research of Pirogova et al., which showed that patients with inapparent TBE forms have increased phagocytic and receptor activity of monocytes and neutrophils, contrary to those who have manifest TBE forms. These authors link this to differences in the biological properties of TBEV that are able to infect humans.
The presentation of a viral peptide in complex with molecules MHC-I and MHC-II by antigen-presenting cells (i.e., monocytes, dendritic cells) to T-lymphocytes acts as a triggering mechanism of the adaptive immune response to pathogens. Antigen identification by T-cells triggers their activation and the expression of numerous activation antigens (46,48,55,59). Contrary to T-lymphocytes, NK cells do not require a cascade reaction of antigen presentation, and act independently of MHC presence on infected cell membranes (14,28,33,34,60). Analysis of the number of lymphocytes that have activation marker expression resulted in the following order being identified: CD69→CD25→HLA-DR→CD95. These are responsible for the consecutive passage of certain stages of lymphocyte differentiation to activation apoptosis, allowing the activation processes that take place in immune system to be estimated (27,39,61). The present study describes the influence of TBEV strains on the activation profile of lymphocyte subpopulations, that is, T-lymphocytes (CD4+, CD8+) and NK cells (Figs. 4 and 5). At 24 h p.i. in blood samples infected with the P-183 strain, the most activated cells turned out to be those that had cytotoxic potential, that is, NK cells and CD8+ lymphocytes, which was confirmed by the high level of CD69 and CD25 expression on these cells. By that time, the P-183 strain had induced expression of only the earliest activation marker, that is, CD69, on CD4+ lymphocytes. In contrast, the Dal strain of TBEV suppressed the activation of antigen-presenting cells (i.e., monocytes), and caused the inhibition of activation of CD8+ lymphocytes and NK cells.
The NS3 protein region was defined as a primary source of flaviviral peptide determinants for CD4+ T-cells (21, 22), which was confirmed by the presence of incomplete NS3-specific CD4+ T-cellular reactions in patients who had severe encephalitis (21). This allowed the identification of flaviviral peptide determinants that identify CD8+ T-cells: they include peptides of E (51), NS4B (54), and NS3 proteins (21). As previously reported, TBEV strains, studied by the authors, have differences in these regions of viral proteins (6) that may cause various strain influences on T-lymphocyte activation.
Thus, processes of virus binding to blood cells and modulation of the expression of intercellular adhesion molecules and activation markers on monocytes, granulocytes, NK cells, and T-lymphocyte surfaces were different for the studied TBEV strains.
Apparently, the mechanisms used by the highly pathogenic Dal strain to escape the host's immune response are similar to those employed by other virulent strains of TBEV. So, virulent TBEV strains were unavailable for cell recognition and delayed cell activation that gave the virus a replicative advantage within the cells during the first 24 h of infection (30,35). In contrast, the P-183 strain is rapidly recognized by immune cells and induces their activation, which then triggers innate antiviral defense mechanisms. It can be anticipated that such strains, which are nonpathogenic to humans, do not have the above-described strategy of “escape” from immune control, and are rapidly eliminated from the organism, in contrast to the highly virulent TBEV strains. Thus, mechanisms of virus-induced up- or downregulation of the expression of adhesion and activation receptors are associated with molecular genetic features of virus strains, and constitute an essential part of fundamental virus properties such as virulence and pathogenicity.
Acknowledgments
The authors are grateful to Prof. S.I. Belikov, and also to O.S. Maistrovskaya for his valuable advice when discussing and preparing the paper.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ahantarig A, Růzek D, Vancová M, et al. Tick-borne encephalitis virus infection of cultured mouse macrophages. Intervirology 2009;52:283–290 [DOI] [PubMed] [Google Scholar]
- 2.Anderson R. Manipulation of cell surface macromolecules by flaviviruses. Adv Virus Res 2003;59:229–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atzeni F, Schena M, Ongari AM, et al. Induction of CD69 activation molecule on human neutrophils by GM-CSF, IFN-γ and IFN-α. Cell Immunol 2002;220:20–29 [DOI] [PubMed] [Google Scholar]
- 4.Bai F, Kong K-F, Dai J, et al. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J Infect Dis 2010;202:1804–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becchetti A, and Arcangeli A. Integrins and ion channels in cell migration: implications for neuronal development, wound healing and metastatic spread. Adv Exp Med Biol 2010;674:107–123 [DOI] [PubMed] [Google Scholar]
- 6.Belikov SI, Kondratov IG, Potapova UV, et al. The relationship between the structure of the tick-borne encephalitis virus strains and their pathogenic properties. PLoS One 2014;9:e94946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaturvedi UC, Nagar R, and Shrivastava R. Macrophage and dengue virus: friend or foe? Indian J Med Res 2006;124:23–40 [PubMed] [Google Scholar]
- 8.Cifarelli V, Libman IM, DeLuca A, et al. Increased expression of monocyte CD11b (Mac-1) in overweight recent-onset type 1 diabetic children. Rev Diabet Stud 2007;4:113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clausen J, Vergeiner B, Enk M, et al. Functional significance of the activation-associated receptor CD25 and CD69 on human NK-cells and NK-like T-cells. Immunobiology 2003;207:85–93 [DOI] [PubMed] [Google Scholar]
- 10.Craston R, Koh M, McDermott A, et al. Temporal dynamics of CD69 expression on lymphoid cells. J Immunol Methods 1997;10:37–45 [DOI] [PubMed] [Google Scholar]
- 11.Diamond MS. Evasion of innate and adaptive immunity by flaviviruses. Immunol Cell Biol 2003;81:196–206 [DOI] [PubMed] [Google Scholar]
- 12.Diamond MS, Shrestha B, Mehlhop E, et al. Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral Immunol 2003;16:259–278 [DOI] [PubMed] [Google Scholar]
- 13.Ecker M, Allison S, Meixner T, et al. Sequence analysis and genetic classification of tick-borne encephalitis viruses from Europe and Asia. J Gen Virol 1999;80:179–185 [DOI] [PubMed] [Google Scholar]
- 14.Farag SS, and Caligiuri MA. Human natural killer cell development and biology. Blood Rev 2006;20:123–137 [DOI] [PubMed] [Google Scholar]
- 15.Hayasaka D, Nagata N, Fujii Y, et al. Mortality following peripheral infection with tick-borne encephalitis virus results from a combination of central nervous system pathology, systemic inflammatory and stress responses. Virology 2009;390:139–150 [DOI] [PubMed] [Google Scholar]
- 16.Humphries MJ. Integrin structure. Biochem Soc Trans 2000;28:311–339 [PubMed] [Google Scholar]
- 17.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002;110:673–687 [DOI] [PubMed] [Google Scholar]
- 18.Khaitov RM, and Ilina NI. Allergy and Immunology: National Leadership. Moscow: GEOTAR—Media, 2009. [in Russian] [Google Scholar]
- 19.King AMQ, Adams MJ, Carstens EB, et al. Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses. San Diego, CA: Elsevier Academic Press, 2012 [Google Scholar]
- 20.Krylova NV, Leonova GN, and Maistrovskaya OS. Cytokine production by blood leukocytes infected with Far Eastern subtype TBEV strains. J Microbiol Epidemiol Immunobiol 2006;Suppl 3:95–99. [in Russian] [Google Scholar]
- 21.Kumar P, Sulochana P, Nirmala G, et al. Impaired T helper 1 function of nonstructural protein3-specific T cells in Japanese patients with encephalitis with neurological sequelae. J Infect Dis 2004;189:880–891 [DOI] [PubMed] [Google Scholar]
- 22.Larena M, and Lobigs M. Immunobiology of Japanese encephalitis virus. In: Ruzek D, ed. Flavivirus Encephalitis. Rijeka, Croatia: In Tech, 2011:317–338 [Google Scholar]
- 23.Leonova GN. Tick-Borne Encephalitis in Primorsky Krai. Vladivostok: Dalnauka, 1997. [in Russian] [Google Scholar]
- 24.Leonova GN, Borisevich VG, and Gagach GF. A way of express diagnostics of tick-borne encephalitis. Patent No. 2217758 C2, Russian Federation, 2003
- 25.Leonova GN, Belikov SI, Kondratov IG, et al. Comprehensive assessment of the genetics and virulence of tick-borne encephalitis virus strains isolated from patients with inapparent and clinical forms of the infection in the Russian Far East. Virology 2013;443:89–98 [DOI] [PubMed] [Google Scholar]
- 26.Leonova GN, Maystrovskaya OS, Kondratov IG, et al. The nature of replication of tick-borne encephalitis virus strains isolated from residents of the Russian Far East with inapparent and clinical forms of infection. Virus Res 2014;189:34–42 [DOI] [PubMed] [Google Scholar]
- 27.Lindsey W, Lowdell M, Marti G, et al. CD69 expression as an index of T-cell function: assay standardization, validation and use in monitoring immune recovery. Cytotherapy 2007;9:123–132 [DOI] [PubMed] [Google Scholar]
- 28.Lisnić VJ, Krmpotić A, and Jonjić S. Modulation of natural killer cell activity by viruses. Curr Opin Microbiol 2010;13:530–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marzio R, Mauel J, and Betz-Corradin S. CD69 and regulation of the immune function. Immunopharmacol Immunotoxicol 1999;21:565–582 [DOI] [PubMed] [Google Scholar]
- 30.Miorin L, Albornoz A, Baba MM, et al. Formation of membrane-defined compartments by tick-borne encephalitis virus contributes to the early delay in interferon signaling. Virus Res 2012;163:660–666 [DOI] [PubMed] [Google Scholar]
- 31.Mitzel DN, Best SM, Masnick MF, et al. Identification of genetic determinants of a tick-borne flavivirus associated with host-specific adaptation and pathogenicity. Virology 2008;381:268–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasyrova RF, Ryazantseva NV, Zhoukova NG, et al. Molecular and cellular basis of pathogenesis of tick-borne encephalitis. Bull Siberian Med 2006;Suppl 1:42–51. [in Russian] [Google Scholar]
- 33.Orange JS, Fassett MS, Koopman LA, et al. Viral evasion of natural killer cells. Nature Immunol 2002;3:1006–1012 [DOI] [PubMed] [Google Scholar]
- 34.Orange JS, and Ballas ZK. Natural killer cells in human health and disease. Clin Immunol 2006;118:1–10 [DOI] [PubMed] [Google Scholar]
- 35.Overby AK, Popov VL, Niedrig M, et al. Tick-borne encephalitis virus delays interferon induction and hides its double-stranded RNA in intracellular membrane vesicles. J Virology 2010;84:8470–8483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pirogova NP, Mikhailova OV, and Karpova MR. Features of the phagocytic activity of peripheral blood leukocytes in patients with tick-borne encephalitis. Bull Exp Biol Med 2002;Suppl 1:82–85. [in Russian] [Google Scholar]
- 37.Plekhova NG, Somova LM, Lyapun IN, et al. The cells of innate systems in tick-borne encephalitis. In: Ruzek D, ed. Flavivirus Encephalitis. Rijeka, Croatia: In Tech, 2011:167–194 [Google Scholar]
- 38.Pogodina VV, Karan LS, Koliasnikova NM, et al. Evolution of tick-borne encephalitis and a problem of evolution of its causative agent. Vopr Virusol 2007;52:16–21. [in Russian] [PubMed] [Google Scholar]
- 39.Poryadin GV, Orshanko AM, Salmasi JM, et al. Activation processes in lymphocytes of patients with latent sensitization. Path Physiol Exp Therapy 2009;1:23–25. [in Russian] [PubMed] [Google Scholar]
- 40.Potapova UV, Feranchuk SI, Potapov VV, et al. NS2B/NS3 protease: allosteric effect of mutations associated with the pathogenicity of tick-borne encephalitis virus. J Biomol Struct Dyn 2012;30:638–651 [DOI] [PubMed] [Google Scholar]
- 41.Ratnikova L, Ter-Bagdasarian L, and Mironov IL. Current views on the pathogenesis of tick-borne encephalitis. Epid Infect Dis 2002;5:41–45. [in Russian] [Google Scholar]
- 42.Rios M, Zhang MJ, Grinev A, et al. Monocytes-macrophages are a potential target in human infection with West Nile virus through blood transfusion. Transfusion 2006;46:659–662 [DOI] [PubMed] [Google Scholar]
- 43.Robertson SJ, Mitzel DN, Taylor RT, et al. Tick-borne flaviviruses: dissecting host immune responses and virus countermeasures. Immunol Res 2009;43:172–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roebuck KA, and Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol 1999;66:876–888 [DOI] [PubMed] [Google Scholar]
- 45.Rouha H, Hoenninger VM, Thurner C, et al. Mutational analysis of three predicted 5′-proximal stem-loop structures in the genome of tick-borne encephalitis virus indicates different roles in RNA replication and translation. Virology 2011;417:79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Růžek D, Salát J, Palus M, et al. CD8+ T-cells mediate immunopathology in tick-borne encephalitis. Virology 2009;384:1–6 [DOI] [PubMed] [Google Scholar]
- 47.Růžek D, Salát J, Singh SK, et al. Breakdown of the blood-brain barrier during tick-borne encephalitis in mice is not dependent on CD8+ T-cells. PLoS One 2011;6:e20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samuel MA, and Diamond MS. Pathogenesis of West Nile virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J Virology 2006;80:9349–9360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shapiro JM. Practical Flow Cytometry. 4th ed. New York: John Wiley, 2003 [Google Scholar]
- 50.Shen J.T-To SS, Schrieber L, et al. Early E-selectin, VCAM-1, ICAM-1, and late major histocompatibility complex antigen induction on human endothelial cells by flavivirus and comodulation of adhesion molecule expression by immune cytokines. J Virology 1997;71:9323–9332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takada K, Masaki H, Konishi E, et al. Definition of an epitope on Japanese encephalitis virus (JEV) envelope protein recognized by JEV specific murine CD8+ cytotoxic T lymphocytes. Arch Virol 2000;145:523–534 [DOI] [PubMed] [Google Scholar]
- 52.Ternovoi VA, Protopopova EV, Chausov EV, et al. Novel variant of tick-borne encephalitis virus, Russia. Emerging Infect Dis 2007;13:1574–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tigabu B, Juelich T, Bertrand J, et al. Clinical evaluation of highly pathogenic tick-borne flavivirus infection in the mouse model. J Med Virol 2009;81:1261–1269 [DOI] [PubMed] [Google Scholar]
- 54.Trobaugh DW, Yang L, Ennis FA, et al. Altered effector functions of virus-specific and virus cross-reactive CD8+ T cells in mice immunized with related flaviviruses. Eur J Immunol 2010;40:1315–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turtle L, Griffiths MJ, and Solomon T. Encephalitis caused by flaviviruses. Q J Med 2012;105:219–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vargin VV, and Semenov BF. Changes of natural killer cell activity in different mouse lines by acute and asymptomatic flavivirus infections. Acta Virol 1986;30:303–308 [PubMed] [Google Scholar]
- 57.Walzog B, and Gaehtgens P. Adhesion molecules. The path to a new understanding of acute inflammation. News Physiol Sci 2000;15:107–113 [DOI] [PubMed] [Google Scholar]
- 58.Westaway EG. Proteins specified by group B togaviruses in mammalian cells during productive infections. Virology 1973;51:454–465 [DOI] [PubMed] [Google Scholar]
- 59.Whitmire JK. Induction and function of virus-specific CD4+ -T cell responses. Virology 2011;411:216–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilk E, Kalippke K, Buyny S, et al. New aspects of NK cell subset identification and inference of NK cells' regulatory capacity by assessing functional and genomic profiles. Immunobiology 2008;213:271–283 [DOI] [PubMed] [Google Scholar]
- 61.Yarilin AA. Immunology. Moscow: GEOTAR-Media, 2010. [in Russian] [Google Scholar]
- 62.Ye J, Zhu B, Fu ZF, et al. Immune evasion strategies of flaviviruses. Vaccine 2013;31:461–471 [DOI] [PubMed] [Google Scholar]
- 63.Zhang M, Daniel S, Huang Y, et al. Anti-West Nile virus activity of in vitro expanded human primary natural killer cells. BMC Immunol 2010;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang N, and Bevan MJ. CD8+ T cells: foot soldiers of the immune system. Immunity 2011;35:161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhoukova OB, Ryazantseva NV, and Novitsky VV. Viral persistence: immunological and molecular genetic aspects of pathogenesis. Bull Siberian Med 2003;4:113–120. [in Russian] [Google Scholar]