Abstract
Cytomegalovirus (CMV) is a significant cause of morbidity and mortality in immunocompromised hosts, many of whom undergo significant periods of lymphopenia. However, the impact of lymphopenia and subsequent immune reconstitution on T cell responses and pulmonary pathology are poorly understood. Using a model of primary murine CMV infection in mice treated with cyclophosphamide (CY), the relationship of CD8+ T cell reconstitution to pneumonitis pathology was studied. Female BALB/c mice were infected with murine CMV (MCMV) with/without CY on day 1 post-infection. Lung pathology and viral specific T cell responses were assessed on days 7–28. T cell lymphocyte subsets, effector responses, and MCMV specificity were assessed at baseline and after in vitro stimulation of cells with immediate–early peptide pp89. CY treatment of MCMV-infected mice resulted in interstitial pneumonitis not seen with MCMV alone. Compared to MCMV alone, on day 14, MCMV/CY mice had greater number of CD8+ T cells, a fourfold increase in absolute number of pp89 tetramer-specific CD8+ cells, and an eightfold increase in MCMV specific T cell effector responses (IFN-γ; p<0.001). This expansion was preceded by transient lymphopenia, increased viral titers, and, most strikingly, a 10-fold increased proliferative capacity in MCMV/CY mice. In the setting of CY-associated lymphopenia, concurrent MCMV infection alters immune reconstitution toward a hyperexpanded MCMV-specific CD8+ effector T cell pool that correlates with significant lung immunopathology.
Introduction
Viral pneumonitis is an important cause of morbidity and mortality in immunocompromised hosts (39,41). The pathophysiology of virus-associated respiratory disease may depend on the specific virus and host environments, and depending on this, secondary infections, prolonged viral replication, and inflammatory immunopathology have all been shown to play a role (2,17,24,50). In particular, cytomegalovirus (CMV) infections remain a major cause of morbidity and mortality in immunocompromised hosts, particularly those undergoing lymphocyte depleting therapies, such as with allogeneic hematopoietic cell transplant (HCT) or with cytoreduction induction therapies for autoimmune disease and solid organ transplant, despite the availability of antiviral therapies post-transplant (3,8,16). In this setting, cytoreductive immunosuppression may not only promote viral replication but also alter mechanisms of immune reconstitution due to the creation of a lymphopenic space (20,40). Although the presence of CMV-specific CD8+ T cells has been shown to be critical for protection from lethal infection, the pathogenesis of CMV pneumonitis remains incompletely understood (25,35,38).
In experimental models of murine CMV (MCMV), immune responses vary based on the strain-specific differences in natural killer (NK) and CD8+ T cell responses (11,18,19). For example, in susceptible BALB/c mice, which lack the Ly49h NK receptor, host defense is primarily CD8+ T cell dependent, whereas both cell populations play a role in viral immunity in the more resistant C57BL/6 mouse strain (7,19). Early work demonstrated that MCMV-infected BALB/c mice treated with single pulse high-dose cyclophosphamide (CY) developed severe pneumonitis not seen in control MCMV or CY alone mice (44,45). Although these studies found that MCMV pneumonitis was characterized by lung mononuclear infiltration and concluded an important role for immunopathology in its pathogenesis, the identification, antigen-specificity, and functionality of infiltrating cells was not determined. Subsequent studies of MCMV infection in irradiated BALB/c syngeneic bone-marrow transplant recipients established the lung as an important site of primary viral infection and latency, and demonstrated that CD8+ T cells were necessary and sufficient for control of active viral replication (1,34,37,46). Subsequently, several MHC class I-restricted immediate–early epitopes were identified as immunodominant in the BALB/c MCMV CD8+ T cell response (12,36). However, these studies did not assess potential differences between immunocompromised versus immunocompetent hosts with regards to pathology, viral load, and antiviral CD8+ T cell responses.
Cytotoxic agents, including high-dose CY, that induce transient lymphopenia are increasingly being used as induction agents in a variety of conditions, including allogeneic solid organ transplant, and autoimmune and hematologic malignancies(5,13,31,47) These agents have been shown to increase the risk of CMV reactivation, including CMV pneumonitis(5,13,31,47). The present study evaluated the effects of single-pulse high-dose CY on the development of MCMV-specific CD8+ T cell responses associated with MCMV pneumonitis pathology in BALB/c mice. Surprisingly, it is reported that immunosuppression with high-dose CY at 24 h following primary MCMV intranasal infection results in a delayed but markedly hyperexpanded and highly functional MCMV-specific CD8+ T cell response associated with the development of pneumonitis pathology.
Materials and Methods
Mice
Female BALB/c (I-ad, H-2d) mice, 5–8 weeks old, were purchased from the National Cancer Institute. Animal protocols were approved by the Johns Hopkins Animal Care and Use Committee.
MCMV infection
BALB/c mice were infected intranasally with 2×105 plaque forming units (PFU) of MCMV with or without CY 200 mg/kg administered intraperitoneally (i.p.) 24 h after infection.
MCMV virus and peptides
MCMV strain MW97.01 and H2d-restricted immunodominant nonapeptide pp89 (YPHFMPTNL) were a gift from Dr. Ann Hill (Oregon Health and Science University, Portland, OR). MCMV was grown and purified on mouse embryo fibroblasts and stored at –80°C (11). Viral plaque assay for quantification using BALB/3T3 cells (American Type Culture Collection) was performed by thawing viral-infected tissue, and centrifuging at 1,500 g for 5 min. Serial log10 dilutions of viral supernatant were plated on BALB/3T3 cells for 2 h, overlayed with complete media supplemented with carboxymethylcellulose, and incubated at 37°C for 6 days. Subsequently, wells were fixed, stained with formalin/crystal violet, and plaques counted. The titer of the final stock was quantified at 1.88×107 PFU/mL.
Cell preparations, stimulation, and cytokine detection
The lungs and spleen were harvested from the mice on days 7, 14, 21, and 28, and mononuclear cells were isolated via enzyme digestion and Percoll density gradient centrifugation as per previously described methods, and maintained in complete cell culture medium (42). Fluorophore-conjugated antibodies (Abs) to the following murine markers were purchased from BD Biosciences or Ebioscience: anti-CD4, anti-CD8, anti-H2Dd, anti-CD44, anti-IFN-γ, Foxp3, anti-TNF-α, anti-granzyme B (grB), Ki-67, and respective isotype Abs. Single-cell suspensions of the lung and spleen were washed and incubated with anti-CD16 to block Fc receptors. Intracellular cytokine staining was performed after restimulating cells for 6 h with or without pp89 (2 μg/mL) in the presence of Brefeldin A (42). Cells were fixed, permeabilized, and stained with surface and intracellular Abs as previously described or per eBioscience FoxP3 buffer system for Ki-67 and Foxp3 (42). Flow cytometry analysis was performed using a FACSCalibur or FACS Aria CellQuest (Becton Dickinson), and Flowjo software (Tree Star).
Tetramer staining
Tetramer complexes of mouse H-2Ld incorporating the nonapeptide YPHFMPTNL (pp89) were produced by the National Institute of Allergy and Infectious Disease Tetramer Facility, and directly conjugated to the APC fluorochrome.
Bromodeoxyuridine cell proliferation assays
Mice were injected with 1.0 mg of 5 bromodeoxyuridine (BrdU; i.p.) on day 0 and were administered 0.8 mg/mL BrdU in drinking water for 7 days before sacrifice. BrdU incorporation was assayed with a BrdU FITC Flow Kit (BD Pharmingen) per the manufacturer's protocol.
Histopathology
The lungs were fixed in 10% formalin, embedded in paraffin, sectioned, and stained using hematoxylin and eosin. Pneumonitis scores were calculated by two independent blinded reviewers using a 4-point scale, which assigned one point each for the following parameters: perivascular inflammation, peribronchial inflammation, interstitial infiltrate or alveolar septal thickening, and viral cytopathic effect.
Statistical analysis
Ordinal and continuous integral variables were compared by t-test for paired comparisons or Kruskal–Wallis test for multigroup comparisons using SPSS software and values reported as mean±standard error of the mean (SEM). A p-value of <0.05 was considered statistically significant.
Results
Compared to MCMV or CY alone, MCMV/CY-treated mice develop significant pneumonitis pathology on day 14 following a transient increase in viral load
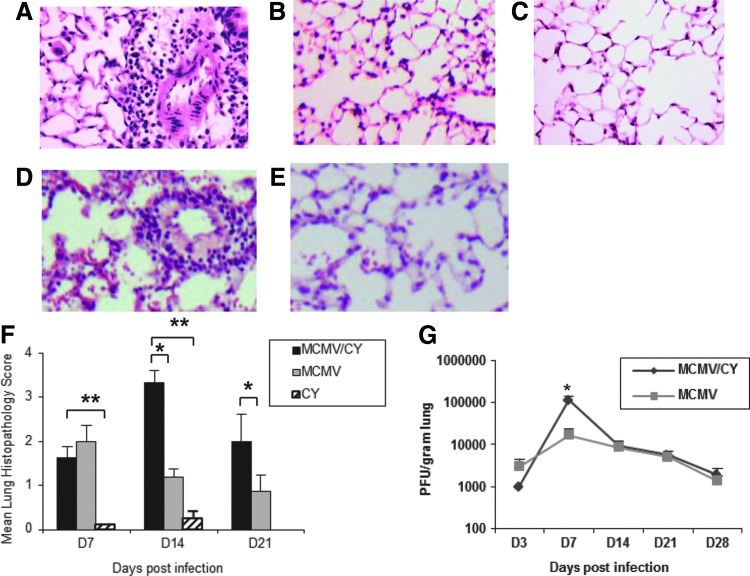
To establish the pathology previously reported with combined MCMV infection and CY treatment, lung histology in BALB/c mice was compared with MCMV infection alone, CY treatment alone, or MCMV/CY over three time points on days 7, 14, and 21. Using a standardized scoring system (see Materials and Methods), it was found that both MCMV/CY- and MCMV-treated mice had modestly increased pneumonitis scores compared to CY alone on day 7 as shown in Figure 1A–D (p<0.003). However, MCMV/CY-treated mice continued to develop severe lung pathology on day 14 compared to CY and MCMV alone (p<0.001), with only partial resolution of pneumonitis by day 21 compared with either MCMV or CY alone (p<0.05).
FIG. 1.
Murine cytomegalovirus/cyclophosphamide (MCMV/CY) mice develop significant pneumonitis preceded by a transient increase in viral loads compared to MCMV alone. Wild type recipient mice were infected with intranasal MCMV with or without concomitant CY administration. (A)–(E) Representative day 14 histopathology from MCMV/CY (A: 10×; D: 40×), MCMV alone (B: 10×; b: 40×), and CY-treated mice (C); lung sections were fixed, stained with hematoxylin and eosin as per Materials and Methods. (F) Mean lung histopathology scores with bars representing mean values±standard error of the mean (SEM) for treatment group, with p-values determined by t-test. (G) Viral load counts as quantified by plaque forming units on days 3, 7, 14, 21, and 28. Bars represent mean values±SEM of viral loads for treatment group, with p-values determined by t-test for paired samples and Kruskal–Wallis test for multiple groups. Results represent pathology and counts from individual mice with a minimum of six to eight mice per group. *p<0.05; **p<0.01. Color images available online at www.liebertpub.com/vim
Next, to determine whether pneumonitis was associated with increased MCMV replication, MCMV lung viral loads were quantified on days 3, 7, 14, 21, and 28 in MCMV and MCMV/CY mice via the plaque assay. Although viral loads peaked on day 7 in both groups, viral loads in the MCMV/CY-treated mice were approximately sixfold higher (p<0.05). However, at the peak of pneumonitis pathology on day 14 and later time points, MCMV viral loads were comparable between the MCMV alone and MCMV/CY-treated groups and continued to decline at a similar rate (Fig. 1E).
Together, these data indicate that while increased MCMV viral titers precede the peak of MCMV pneumonitis in MCMV/CY-treated mice, the peak of pneumonitis pathology is associated with declining viral loads. Furthermore, the persistence of MCMV-CY pneumonitis on day 21, despite a similar decline in viral loads, suggests additional pathogenic mechanisms beyond total viral burden.
The kinetics of pneumonitis development in MCMV/CY-treated mice correlates with a massive influx of CD8+ T lymphocytes in the lung
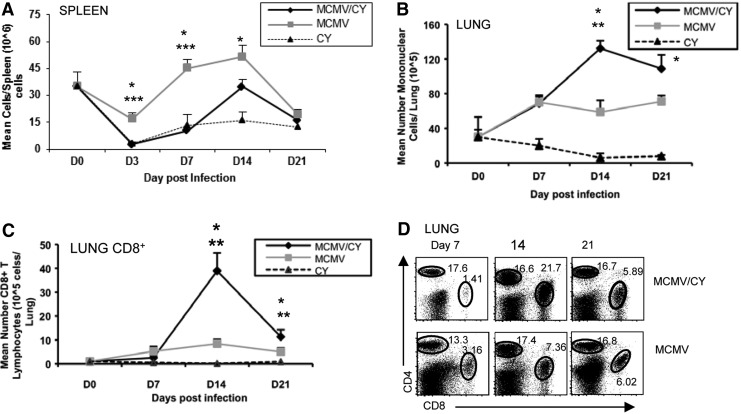
Given similar lung viral loads between MCMV- and MCMV/CY-treated mice at the peak of pneumonitis pathology, next the inflammatory response in both cohorts were examined. First, the kinetics of the inflammatory response were characterized by quantifying the total number of mononuclear cells isolated from the lung (LMNCs) on days 3, 7, 14, and 21, and this was compared to a systemic response as represented by mononuclear cells from spleen. In the spleen, an initial reduction in splenocyte counts was found on day 3 in MCMV/CY-treated mice compared to MCMV alone on day 3 (p<0.002) followed by a gradual increase in splenocytes (Fig. 2A). In the lung, by contrast, a reduction in LMNCs was not observed on day 3 in MCMV/CY-treated mice. However, these mice had fewer LMNCs compared to the MCMV alone group (Fig. 2B; p<0.008). Notably, MCMV/CY-treated mice demonstrated a delayed and markedly augmented peak influx of LMNCs on day 14 compared to the MCMV alone group (Fig. 2B; p<0.002). These findings demonstrate a transient cytopenia followed by a delayed and augmented pulmonary inflammatory response in MCMV/CY-treated mice that temporally correlates with pneumonitis pathology.
FIG. 2.
MCMV/CY mice develop transient lymphopenia followed by augmented influx of CD8+ T cells in the lungs at the time of pneumonitis pathology. (A) Spleens from BALB/c mice infected with MCMV alone, MCMV/CY treatment, or CY alone were harvested at prior to treatment day 0, and on days 3, 7, 14 and 21, and splenocytes isolated as per Materials and Methods. Data points represent mean cell counts±SEM, with p-values determined by t-test from individual mice with six to eight mice per group. (B) Lung mononuclear cells from mice infected with MCMV alone, MCMV/CY treatment, or CY alone were isolated prior to treatment on day 0, and on days 3, 7, 14, and 21. Data points represent mean cell counts±SEM, with p-values determined by rank sum test from individual mice with six to eight mice per group. (C) CD8+ T cells were isolated and purified from the lung of MCMV-, MCMV/CY-, and CY-treated mice on days 0, 7, 14, and 21. For (A), (B), and (C), data points represent mean cell counts per lung±SEM, with p-values determined by t-test from individual mice with six to eight mice per group. (D) Representative flow cytometry for CD4, and CD8 surface expression on days 7, 14, and 21 MCMV- and MCMV/CY-treated mice in CD8+ T cells. Quadrant values represent detected frequencies of each population, gating on lung mononuclear cells. *p<0.05; **p<0.01; ***p<0.005.
Previous studies have reported >80% lymphocyte predominant cells in the LMNC compartment, with a relative paucity of macrophages. To characterize the lymphocyte subsets further, B- and T cell subsets in LMNCs were measured by quantifying the relative frequencies and absolute numbers of B220, CD8, and CD4. While no significant difference was found in B- and CD4+ T lymphocyte frequencies between MCMV and MCMV/CY mice at any time point, as shown in a representative experiment in Figure 2C, a twofold increase was observed in CD8+ T lymphocyte frequencies (26.0±2.31% vs. 12.2%±1.77%; p<0.001), and a fourfold increase was observed in absolute CD8+ T cell numbers in the lungs of MCMV/CY-treated mice on day 14 compared to MCMV alone (Fig. 2D; p<0.001). This differential expansion of CD8+ T cells in MCMV/CY mice was specific to the lung compartment and not observed in the splenic compartment (p=0.048; data not shown). By day 21, there was a partial contraction in lung CD8+ T lymphocytes in MCMV/CY mice, but not to the baseline observed in MCMV alone. Taken together, these data indicate that MCMV pneumonitis is characterized by delayed but significantly increased numbers of CD8+ T lymphocytes in the lung compartment in MCMV/CY-treated mice, in contrast to mice with MCMV alone.
Pneumonitis in MCMV/CY-treated mice is characterized by increased frequency and total numbers of viral specific CD8+ T lymphocytes
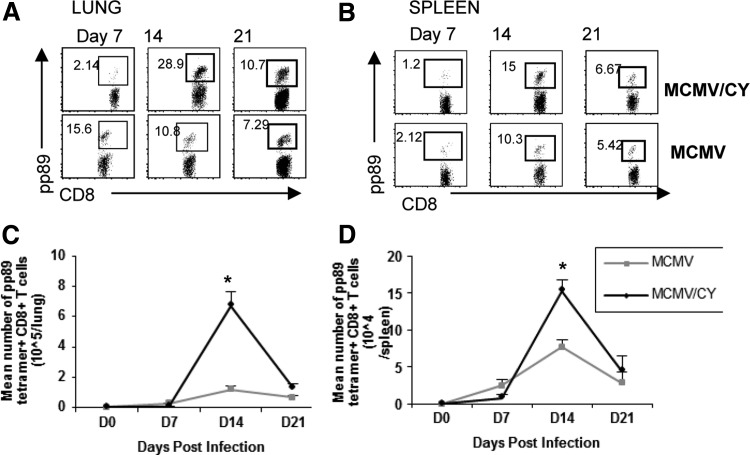
Because MCMV/CY-treated mice had an early increase in viral load followed by increased CD8+ T cells in the lung, next this study considered whether this augmented immune response was directed against the virus. To assess this, CD8+ T cells from the lung and spleen of MCMV- and MCMV/CY-treated mice were tetramer stained for the immune-dominant MCMV antigen pp89 on days 7, 14, and 21 post-infection. As shown in a representative experiment in Figure 3A, the frequency of lung CD8+tetramer+ T cells markedly increased from days 7 to 14 in MCMV/CY mice. In contrast, in the MCMV-alone group, the frequencies of pp89-specific CD8 T cells peaked earlier on day 7, but with a smaller peak frequency. Combined, the increased quantity of CD8+ T cells in the lungs of MCMV/CY mice along with a larger percentage of pp89-specific CD8+ T cells resulted in a six-fold increase in the total number of pp89+CD8+ T cells on day 14 compared to MCMV mice (Fig. 3C; p<0.001).
FIG. 3.
MCMV/CY pneumonitis is characterized by a massive expansion of MCMV-specific CD8+ T lymphocytes in the lung. (A) and (B) Representative flow cytometry for CD8, and pp89 tetramer surface expression on days 7, 14, and 21 MCMV- and MCMV/CY-treated mice. Quadrant values represent detected frequencies of each population, gating on lung mononuclear cells, and splenocytes, respectively. (C) and (D) Data points represent mean cell counts per lung±SEM, with p-values determined by t-test from individual mice with six to eight mice per group. *p<0.05.
Systemically, in the spleen, there were higher frequencies of pp89-tetramer+ T cells on day 14 in MCMV/CY mice compared to MCMV alone, resulting in two-fold increase in absolute numbers of splenic pp89-tetramer+ on day 14 in MCMV/CY mice compared to MCMV mice (Fig. 3D; p<0.01). The absolute number of pp89-tetramer+CD8+ T cells sharply contracted in both the lung and spleen to similar levels in both groups by day 21, coincident with the previously shown decline in MCMV viral load. Together, these data demonstrate that pneumonitis pathology is associated with a skewing of the CD8+ T cell repertoire, leading to significantly increased numbers of MCMV-specific CD8+ T cells in both the lung and spleen compartments.
CD8+ T lymphocytes in MCMV/CY mice demonstrate increased MCMV-specific effector function at the time of pneumonitis pathology
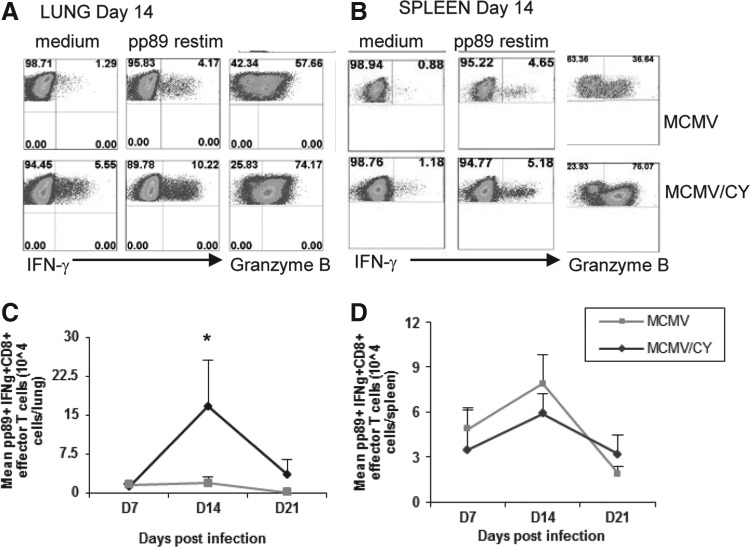
Given the strikingly increased numbers of pp89-tetramer+ CD8+ T cells in the lungs of MCMV/CY mice on day 14, next, effector function was examined. To assess this, mononuclear cells were isolated from the lung and spleen on days 7, 14, and 21, and cultured for 5 h in vitro in the presence or absence of pp89 peptide antigen, followed by staining for CD8, effector cytokine IFN-γ, and the cytolytic molecule grB. As shown in in Figure 4A, on day 7, similar total numbers of pp89-specific CD8+IFN-γ+ T cells were observed in MCMV/CY mice as compared to MCMV control mice. However, by day 14, the mean frequencies of lung pp89-specific CD8+IFN-γ+ T cells from MCMV/CY mice were significantly increased compared to MCMV controls, resulting in a 10-fold higher total absolute number of pp89-specific CD8+IFN-γ+ T cells in MCMV/CY mice (Fig. 4B; p<0.012). By day 21, pp89-specific CD8+IFN-γ+ T cells contracted but remained significantly higher in MCMV/CY mice compared to MCMV control mice, despite similar viral loads (p<0.04). These CD8+ T cells also demonstrated a similar pattern in their expression of grB, with similar levels on day 7 in both MCMV/CY and MCMV alone, but higher frequencies by day 14 in MCMV/CY mice as compared to MCMV alone (p<0.01).
FIG. 4.
CD8+ T cell isolated from the lungs of MCMV/CY mice have increased MCMV-specific effector function during pneumonitis, and higher absolute numbers of intralung effector CD8+ T lymphocytes. (A) and (B) Representative ICCS for IFN-γ, and granzyme B in MCMV and MCMV/CY mice on day 14 CD8+ T cells with medium or ex vivo pp89 restimulation. Quadrant values represent detected frequencies of each population, gating on CD8+ T cells from the lung or spleen, respectively. (C) and (D) Mean numbers of CD8+ cells secreting IFN-γ by ICS upon ex vivo pp89 restimulation on days 7, 14, and 21 from the lung or spleen of MCMV/CY and MCMV mice. Data points represent mean values±SEM from each treatment group, with p-values determined by t-test. Results represent minimum three separate experiments with individual responses from 8–10 mice per group. *p<0.05.
As a systemic control, pp89-specific CD8+IFN-γ+ T cells in the spleen were assessed, and were found to be similar in both frequency and total number on days 7, 14, and 21 between MCMV/CY and MCMV-alone groups (Fig. 3D). These data indicate that the intrapulmonary expansion of CD8+ T cells during MCMV/CY-associated pneumonitis is highly skewed toward an MCMV-specific, effector/cytolytic phenotype compared to MCMV alone.
Increased numbers of MCMV-specific CD8+ T cells in MCMV/CY mice are not associated with a relative deficiency in the frequency of regulatory T cells
Given the magnitude of the CD8+ T lymphocyte influx at the peak of pneumonitis, this study questioned whether this was due to impaired regulation of the immune response. To determine this, CD4+ regulatory T cells were measured by quantifying CD4+CD25+Foxp3+ T cells in both MCMV/CY and MCMV mice on days 7 and 14 using flow cytometry analysis. As shown in a representative experiment in Figure 5A and cumulative lung data in Figure 5B, there was no significant difference in the frequencies of CD4+CD25+Foxp3+ T cells in the lungs of MCMV/CY versus MCMV mice at either day 7 (p=0.46) or 14 (p=0.34). These similar lung and splenic frequencies of regulatory T cells as a percent of total lymphocytes in MCMV/CY mice suggest that the hyperexpansion of MCMV-specific CD8+ T cells was not likely attributable to a relative deficiency in regulatory T cells in MCMV/CY mice.
FIG. 5.
MCMV/CY mice do not demonstrate increased frequency of regulatory T lymphocytes compared to MCMV alone. (A) Representative flow cytometry and ICS for surface CD25 and Foxp3 intracellular expression on days 7 and 14 from lung mononuclear cells (LMNCs) and splenic lymphocytes in MCMV/CY and MCMV control mice. Quadrant values represent detected frequencies of each population, gating on CD4+ T cells. (B) Mean lung frequencies of CD4+CD25+Foxp3+ cells isolated from the lung on days 7 and 14 in MCMV/CY and MCMV control recipients. Bars represent mean values±SEM from each treatment group, with p-values determined by t-test. Results represent minimum four separate experiments with individual responses from six mice per group.
MCMV/CY mice demonstrate a delayed but massive proliferative expansion of MCMV-specific CD8+ T cells prior to pneumonitis pathology
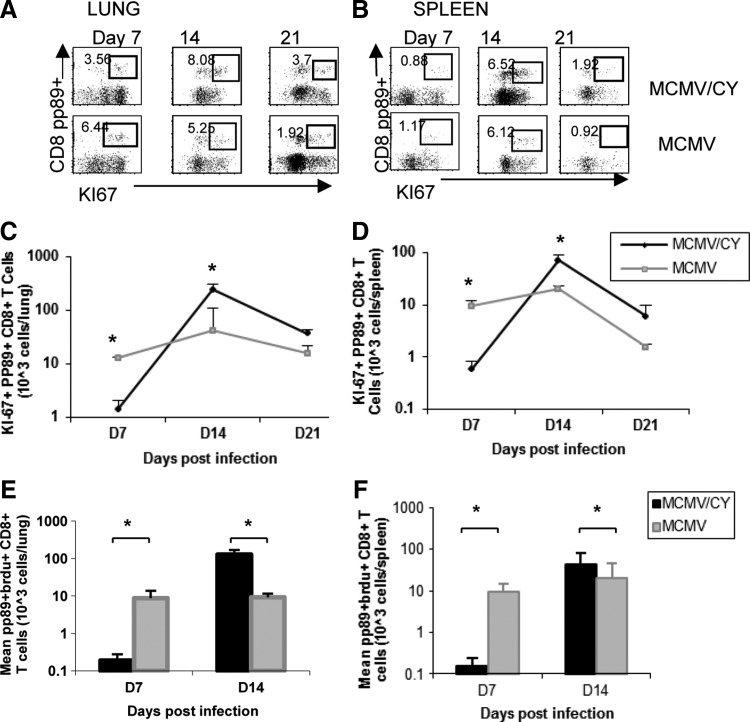
Next, this study asked whether MCMV-specific CD8+ T cells in MCMV/CY mice demonstrated increased proliferative expansion to account for the markedly increased numbers detected in the lung. To do this, Ki-67 expression was measured in pp89-tetramer+ lung and splenic CD8+ T cells on days 7, 14, and 21. On day 7, the frequencies and absolute numbers of pp89-tetramer+Ki67+CD8+ T cells in MCMV/CY mice were significantly reduced compared to MCMV alone (Fig. 6A and B; p<0.001). However, by day 14, while relatively similar frequencies were observed, there was a significant increase in absolute numbers of lung pp89-tetramer+Ki67+CD8+ T cells in MCMV/CY mice in contrast to MCMV mice, followed by a contraction in proliferating cells in both groups by day 21. Similar findings were observed in the splenic compartment, where MCMV/CY mice demonstrated delayed kinetics of pp89-tetramer+Ki67+CD8+ T cells compared to MCMV controls (p<0.004), followed by a significant increase in proliferating cells in MCMV/CY mice by day 14 (p<0.001), as shown in Figure 6C and D. As in the lung, a marked contraction of splenic pp89+Ki67+CD8+ T cells occurred by day 21.
FIG. 6.
MCMV/CY mice demonstrate massive proliferation between days 7 and 14 compared to MCMV alone. (A) and (B) Representative flow cytometry and ICS for Ki-67, pp89, and CD8 expression on days 7, 14, and 21 from LMNC and splenic lymphocytes in MCMV/CY and MCMV control mice. Quadrant values represent detected frequencies of each population, gating on CD8+ T cells. Mean absolute numbers of CD8+pp89-tetramer+Ki-67+ cells isolated from the lung and spleen (C) and (D) respectively on days 7 and 14 in MCMV/CY and MCMV control recipients. Bars represent mean values±SEM from each treatment group, with p-values determined by t-test. Results represent a minimum of four separate experiments with individual responses from four to six mice per group. (E) and (F) In separate experiments, recipient mice were administered BrdU from days 0–7 and 6–14 (see Materials and Methods). Lung mononuclear cells and splenocytes were isolated and immediately surface stained for CD8 and BrdU incorporation followed by flow cytometry analysis. Bar graphs represent mean absolute numbers of pp89+ BRDU+CD8+ T cells±SEM on days 7 and 14 graft CD8+ cells isolated from LMNCs and the spleen of MCMV/CY versus MCMV mice, with p-values determined by t-test. Results are for individual mice from three separate experiments with four to six mice per group. *p<0.05.
To confirm a delayed hyperexpansion of MCMV-specific CD8+ T cells in MCMV/CY mice further, incorporation of the synthetic thymidine analog, bromodeoxyuridine (BrdU), was evaluated in both groups to determine the period of maximal proliferation. As shown in Figure 6E, the number of pp89-tetramer+BrdU+CD8+ T cells in MCMV/CY mice increased most demonstrably in the lungs between days 7 and 14 (p=0.01), in marked contrast to BrdU incorporation in MCMV mice. Similar findings were also observed in the spleen (p=0.001) as shown in Figure 6F. Collectively, these data show an initially delayed kinetics in MCMV-specific CD8+ T cell proliferation, followed by a striking hyperexpansion of these cells in MCMV/CY mice, in association with pneumonitis pathology.
Discussion
Our studies demonstrate that the pulmonary pathology seen in lymphopenia-associated MCMV/CY pneumonitis is characterized by a massive expansion of MCMV-specific CD8+ T cell responses that is many times higher than that seen in MCMV infection of a normal wild type host. These studies are consistent with previous studies in a stem-cell transplant model in which investigators demonstrated a striking CD8+ T cell infiltration in response of MCMV infection in mice that underwent stem-cell transplant (33). However, those studies did not compare immune responses to MCMV in otherwise immunocompetent hosts. Moreover, the present data show that the increased number and prolonged presence of CD8+ T cells in MCMV/CY mice does not appear to be a result of a regulatory T cell deficiency, but rather a function of massively increased proliferation.
In the present model, expansion of CD8+ T cells and peak pneumonitis pathology in MCMV/CY mice was preceded by a transient increase in viral loads, which decreased to similar levels between both groups by day 14. As these findings are consistent with those reported in the index study in this model by Shanley et al., this raises the question of whether a quantitative early difference in viral antigen load is the driving force for the inflammatory response and pneumonitis of MCMV/CY mice (45).
Our studies suggest that the presence of viral antigen is necessary for the massive proliferative expansion and development of pneumonitis, as uninfected CY-treated mice did not demonstrate pathology. The observation of viral cytopathic effect on histopathology supports this role of active viral replication in pneumonitis pathology. It is also acknowledged that it is possible that increased early viral replication in MCMV-CY mice plays a role in the increased T cell responses on day 14, and may mediate cytolysis triggered by persisting viral products, even when the in vitro viral loads were similar to the control group. However, viral burden alone does not appear to be the only factor contributing to pathology.
Earlier studies of this model by Shanley et al. demonstrated that although that the lung viral load in MCMV/CY-infected mice was directly proportional to the inoculated viral dose, inoculation of MCMV/CY mice with up to a 3 log lower virus dose or treatment with antiviral therapy to reduce viral loads by greater than 1 log did not prevent the development of pneumonitis (44). Our studies support this observation, in the persistence of pneumonitis only in MCMV-CY mice from day 14–21, despite very similar declining viral loads between MCMV and MCMV-CY mice. Furthermore, no studies have described the development of pneumonitis pathology, even when lethal doses of MCMV were administered in otherwise immunocompetent BALB/c mice (1,43). Thus, it is concluded that the effects of CY therapy in MCMV-infected mice extend beyond early increased viral replication from transient immunosuppression. These observations are also consistent with human models of primary CMV infection, such as our studies in lung transplant where it was demonstrated that the magnitude of expansion of CMV-specific T cells in the lung does not necessarily correlate with BAL viral load levels (32).
The present findings support the creation of a transient lymphopenic “space” prior to the massive expansion and influx of lymphocyte cells to the lung that characterize pneumonitis. Although a lymphopenic environment in RAG-1–/– mice has been shown to induce an IL-7 mediated “homeostatic” proliferation that restores total T cell numbers, the tempo of homeostatic proliferation in the absence of foreign antigen is typically much slower (with three or four rounds of division over 1 week) than that seen with antigen-driven T cell proliferation in a normal host (6–8 h for cell division) (15,49). However, in our studies, the combination of both a lymphopenic “space” and the presence of foreign antigen in the MCMV/CY group resulted in a massive hyperexpansion of MCMV-specific lymphocytes.
Although several studies in experimental murine models have shown that both CD8+ and CD4+ T cell proliferation in lymphopenic hosts is accelerated and skewed in its repertoire in the presence of foreign antigen(10,15,23), this study is unique in that it directly compared antigen-driven proliferation in a lymphopenic host versus a normal host, and demonstrated that the magnitude of the proliferation exceeds that seen with antigen-driven proliferation in normal hosts. An “intense” form of proliferation has been attributed to IL-2/IL-15 mediated pathways in prior models of homeostatic proliferation, potentially driven by self or external ligands (6). These cytokines would be candidates for future studies to support the contribution of the lymphopenic environment in driving proliferation, beyond its role in increasing overall antigenic burden.
In addition to a massive increase in absolute numbers of CD8+ T cells, our studies demonstrated a significant increase in the frequency of pp89-tetramer+MCMV-specific CD8+ T cells in MCMV/CY mice compared to MCMV alone, suggesting that the immune reconstitution was skewed toward a MCMV-specific repertoire. This is consistent with recent studies of homeostatic proliferation in several disease phenotypes, including human multiple sclerosis, CMV, and allogeneic kidney transplant, in which lymphopenia depletion induces expansion of a clonally-restricted T cell repertoire that leads to autoimmunity or acute allograft rejection, respectively (14,26,48).
Importantly, the present model, consistent with studies above, demonstrates that this massive and skewed expansion leads to a population of cells that is associated with clinically significant tissue injury, that is, pneumonitis pathology. These findings may have important implications for potential complications of lymphocyte-depleting immunosuppressive regimens in a variety of clinical settings, such as solid and hematopoietic organ transplant and treatment of autoimmune disease. For example, a closer examination of human CMV following the use of contemporary lymphocyte depleting protocols suggests that there may be a higher burden of CMV-associated organ injury in these recipients. Upon engraftment with a CMV seropositive donor, hematopoietic stem-cell transplant recipients can undergo a massive expansion of CMV-specific T cells which appear to confer protection from CMV infection (4,30). However, in a study of partial T cell depleting myeloablative regimens in an allogeneic stem-cell transplant, there was a significantly higher incidence of CMV infection in recipients with lung injury, and increased mortality in patients with lung injury, raising the question of the role of viral-associated immunopathology in these subjects. In another study by Kim et al., the use of T lymphocyte-depleting therapy, alemtuzumab, for HSCT conditioning was not associated with increased CMV viremia, but a strikingly higher incidence of CMV disease was found compared to alternate conditioning regimens (28). Further human studies are needed to examine the kinetics and quality of the CMV-specific immune response in these specific settings.
In our studies, CD8+ T cells from MCMV/CY mice demonstrated higher peak frequencies of effector function compared to MCMV alone, and were associated with clearance of virus. The findings are consistent with those seen in a bone-marrow transplant model of MCMV pneumonia, in which mice developed a similarly massive lung infiltration of effector CD8+ T cell prior to resolution of infection (12,33). In addition, Holtappels et al. found that the recovered cytotoxic lymphocytes in their model displayed high cytolytic activity but only approximately 20% specific to pp89-specific lysis (12). This effector phenotype appears not only to reflect antigen-driven T cell activation, but also the unique characteristics of T cell expansion under homeostatic proliferation following creation of a lymphopenic space. Under these settings, various models have shown the expansion of previously naive T cells that subsequently display an effector/memory phenotype (26,29). Although our studies primarily examined the acute immune response and contraction to primary CMV viremia, CMV is a virus that establishes latency. This is characterized by memory CD8+ T cell inflation to a restricted number of CMV epitopes, including IE1 and IE3 peptides. Future studies to examine the impact of CY treatment on inflationary responses during latency may be warranted.
Studies of peripheral expansion following lymphopenia in tumor and vaccine models have shown enhanced vaccine or tumor-specific (22,49) T cell immunity was not just dependent on IL-7 and IL-2/IL-15 mediated pathways but was also influenced by regulatory T cells (9,27). Because studies in other models have seen an inhibition of regulatory T cell numbers and function after CY-induced lymphopenia (21), this subset was examined to determine if a relative deficiency of regulatory T cells contributed to the massive CD8 expansion. However, in the present model, similar frequencies of day 7 and 14 regulatory T cells were found in both lung and spleen compartments between MCMV- and MCMV/CY-treated mice, suggesting that in our population, the augmented and persistent expansion of CD8+ T cells was not attributable to a quantitative deficiency in regulatory T cells. Thus, it appears that the contribution of regulatory T cells in the regulation of lymphopenia-induced CD8+ T cell hyperexpansion is variable and may depend on the relative contribution of a driving antigen and other factors.
In conclusion, the present studies find that MCMV/CY pneumonitis is characterized by a massive infiltration of MCMV-specific functional CD8+ effector T cells upon immune reconstitution. This immune response was associated with augmented proliferative capacity and functional viral-specific CD8+ T cells following a transient lymphopenic environment and effective viral clearance. Although this response may be protective from lethal viral infection, the magnitude of the immune response is such as to result in demonstrable immunopathology. Given the increasing use of cytoreductive therapies in hematopoietic transplant and other disorders, further studies are needed to determine the mechanisms that regulate this response.
Acknowledgments
Funding source: AI079175 (J.F.M.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Balthesen M, Messerle M, and Reddehase MJ. Lungs are a major organ site of cytomegalovirus latency and recurrence. J Virol 1993;67:5360–5366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brincks EL, Roberts AD, Cookenham T, et al. Antigen-specific memory regulatory CD4+Foxp3+ T cells control memory responses to influenza virus infection. J Immunol 2013;190:3438–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broers AE, van Der Holt R, van Esser JW, et al. Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood 2000;95:2240–2245 [PubMed] [Google Scholar]
- 4.Chalandon Y, Degermann S, Villard J, et al. Pretransplantation CMV-specific T cells protect recipients of T-cell-depleted grafts against CMV-related complications. Blood 2006;107:389–396 [DOI] [PubMed] [Google Scholar]
- 5.Charlier C, Henegar C, Launay O, et al. Risk factors for major infections in Wegener granulomatosis: analysis of 113 patients. Ann Rheum Dis 2009;68:658–663 [DOI] [PubMed] [Google Scholar]
- 6.Cho JH, Boyman O, Kim HO, et al. An intense form of homeostatic proliferation of naive CD8+ cells driven by IL-2. J Exp Med 2007;204:1787–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniels KA, Devora G, Lai WC, et al. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J Exp Med 2001;194:29–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi MK, and Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis 2004;4:725–738 [DOI] [PubMed] [Google Scholar]
- 9.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 2005;202:907–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge Q, Rao VP, Cho BK, et al. Dependence of lymphopenia-induced T cell proliferation on the abundance of peptide/MHC epitopes and strength of their interaction with T cell receptors. Proc Natl Acad Sci U S A 2001;98:1728–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold MC, Munks MW, Wagner M, et al. Murine cytomegalovirus interference with antigen presentation has little effect on the size or the effector memory phenotype of the CD8 T cell response. J Immunol 2004;172:6944–6953 [DOI] [PubMed] [Google Scholar]
- 12.Holtappels R, Podlech J, Geginat G, et al. Control of murine cytomegalovirus in the lungs: relative but not absolute immunodominance of the immediate-early 1 nonapeptide during the antiviral cytolytic T-lymphocyte response in pulmonary infiltrates. J Virol 1998;72:7201–7212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huisman C, van der Straaten HM, Canninga-van Dijk MR, et al. Pulmonary complications after T-cell-depleted allogeneic stem cell transplantation: low incidence and strong association with acute graft-versus-host disease. Bone Marrow Transplant 2006;38:561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones JL, Thompson SA, Loh P, et al. Human autoimmunity after lymphocyte depletion is caused by homeostatic T-cell proliferation. Proc Natl Acad Sci U S A 2013;110:20200–20205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kieper WC, Troy A, Burghardt JT, et al. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J Immunol 2005;174:3158–3163 [DOI] [PubMed] [Google Scholar]
- 16.Kotloff RM, Ahya VN, and Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2004;170:22–48 [DOI] [PubMed] [Google Scholar]
- 17.La Gruta NL, Kedzierska K, Stambas J, et al. A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol 2007;85:85–92 [DOI] [PubMed] [Google Scholar]
- 18.Lathbury LJ, Allan JE, Shellam GR, et al. Effect of host genotype in determining the relative roles of natural killer cells and T cells in mediating protection against murine cytomegalovirus infection. J Gen Virol 1996;77:2605–2613 [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Zafer A, de Repentigny Y, et al. Transgenic expression of the activating natural killer receptor Ly49H confers resistance to cytomegalovirus in genetically susceptible mice. J Exp Med 2003;197:515–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lugthart G, van Ostaijen-Ten Dam MM, Jol-van der Zijde CM, et al. Early cytomegalovirus reactivation leaves a specific and dynamic imprint on the reconstituting T cell compartment long-term after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014;20:655–661 [DOI] [PubMed] [Google Scholar]
- 21.Lutsiak ME, Semnani RT, De Pascalis R, et al. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 2005;105:2862–2868 [DOI] [PubMed] [Google Scholar]
- 22.Ma J, Urba WJ, Si L, et al. Anti-tumor T cell response and protective immunity in mice that received sublethal irradiation and immune reconstitution. Eur J Immunol 2003;33:2123–2132 [DOI] [PubMed] [Google Scholar]
- 23.Mackall CL, Bare CV, Granger LA, et al. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol 1996;156:4609–4616 [PubMed] [Google Scholar]
- 24.Madhi SA, and Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med 2004;10:811–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitsani D, Nguyen MH, Girnita DM, et al. A polymorphism linked to elevated levels of interferon-gamma is associated with an increased risk of cytomegalovirus disease among Caucasian lung transplant recipients at a single center. J Heart Lung Transplant 2011;30:523–529 [DOI] [PubMed] [Google Scholar]
- 26.Moxham VF, Karegli J, Phillips RE, et al. Homeostatic proliferation of lymphocytes results in augmented memory-like function and accelerated allograft rejection. J Immunol 2008;180:3910–3918 [DOI] [PubMed] [Google Scholar]
- 27.Oh S, Berzofsky JA, Burke DS, et al. Coadministration of hiv vaccine vectors with vaccinia viruses expressing il-15 but not il-2 induces long-lasting cellular immunity. Proc Natl Acad Sci U S A 2003;100:3392–3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SH, Choi SM, Lee DG, et al. Infectious complications associated with alemtuzumab use for allogeneic hematopoietic stem cell transplantation: comparison with anti-thymocyte globulin. Transpl Infect Dis 2009;11:413–423 [DOI] [PubMed] [Google Scholar]
- 29.Pearl JP, Parris J, Hale DA, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant 2005;5:465–474 [DOI] [PubMed] [Google Scholar]
- 30.Peggs KS, Verfuerth S, Pizzey A, et al. Reconstitution of T-cell repertoire after autologous stem cell transplantation: influence of CD34 selection and cytomegalovirus infection. Biol Blood Marrow Transplant 2003;9:198–205 [DOI] [PubMed] [Google Scholar]
- 31.Peleg AY, Husain S, Kwak EJ, et al. Opportunistic infections in 547 organ transplant recipients receiving alemtuzumab, a humanized monoclonal cd-52 antibody. Clinical Infect Dis 2007;44:204–212 [DOI] [PubMed] [Google Scholar]
- 32.Pipeling MR, West EE, Osborne CM, et al. Differential CMV-specific CD8+ effector T cell responses in the lung allograft predominate over the blood during human primary infection. J Immunol 2008;181:546–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Podlech J, Holtappels R, Pahl-Seibert MF, et al. Murine model of interstitial cytomegalovirus pneumonia in syngeneic bone marrow transplantation: persistence of protective pulmonary CD8-T-cell infiltrates after clearance of acute infection. J Virol 2000;74:7496–7507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Podlech J, Holtappels R, Wirtz N, et al. Reconstitution of CD8 T cells is essential for the prevention of multiple-organ cytomegalovirus histopathology after bone marrow transplantation. J Gen Virol 1998;79:2099–2104 [DOI] [PubMed] [Google Scholar]
- 35.Quinnan GV, Jr, Kirmani N, Rook AH, et al. Cytotoxic T cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med 1982;307:7–13 [DOI] [PubMed] [Google Scholar]
- 36.Reddehase MJ, Mutter W, Munch K, et al. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol 1987;61:3102–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddehase MJ, Weiland F, Munch K, et al. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol 1985;55:264–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reusser P, Riddell SR, Meyers JD, et al. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood 1991;78:1373–1380 [PubMed] [Google Scholar]
- 39.Ruuskanen O, Lahti E, Jennings LC, et al. Viral pneumonia. Lancet 2011;377:1264–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt-Hieber M, Schwarck S, Stroux A, et al. Immune reconstitution and cytomegalovirus infection after allogeneic stem cell transplantation: the important impact of in vivo T cell depletion. Int J Hematol 2010;91:877–885 [DOI] [PubMed] [Google Scholar]
- 41.Shah PD, and McDyer JF. Viral infections in lung transplant recipients. Semin Respir Crit Care Med 2010;31:243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah PD, West EE, Whitlock AB, et al. CD154 deficiency uncouples allograft CD8+ T-cell effector function from proliferation and inhibits murine airway obliteration. Am J Transplant 2009;9:2697–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shanley JD, Biczak L, and Forman SJ. Acute murine cytomegalovirus infection induces lethal hepatitis. J Infect Dis 1993;167:264–269 [DOI] [PubMed] [Google Scholar]
- 44.Shanley JD, and Pesanti EL. The relation of viral replication to interstitial pneumonitis in murine cytomegalovirus lung infection. J Infect Dis 1985;151:454–458 [DOI] [PubMed] [Google Scholar]
- 45.Shanley JD, Pesanti EL, and Nugent KM. The pathogenesis of pneumonitis due to murine cytomegalovirus. J Infect Dis 1982;146:388–396 [DOI] [PubMed] [Google Scholar]
- 46.Steffens HP, Kurz S, Holtappels R, et al. Preemptive CD8 T-cell immunotherapy of acute cytomegalovirus infection prevents lethal disease, limits the burden of latent viral genomes, and reduces the risk of virus recurrence. J Virol 1998;72:1797–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takizawa Y, Inokuma S, Tanaka Y, et al. Clinical characteristics of cytomegalovirus infection in rheumatic diseases: multicentre survey in a large patient population. Rheumatology (Oxford) 2008;47:1373–1378 [DOI] [PubMed] [Google Scholar]
- 48.Verfuerth S, Peggs K, Vyas P, et al. Longitudinal monitoring of immune reconstitution by CDR3 size spectratyping after T-cell-depleted allogeneic bone marrow transplant and the effect of donor lymphocyte infusions on T-cell repertoire. Blood 2000;95:3990–3995 [PubMed] [Google Scholar]
- 49.Williams KM, Hakim FT, and Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol 2007;19:318–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoo JK, Kim TS, Hufford MM, et al. Viral infection of the lung: host response and sequelae. J Allergy Clin Immunol 2013;132:1263–1276; quiz 1277. [DOI] [PMC free article] [PubMed] [Google Scholar]