Abstract
Tamoxifen, an antineoplastic agent, is active in vitro and in vivo against the parasitic protozoa Leishmania. As part of our efforts to unravel this drug's mechanisms of action against the parasite and understand how resistance could arise, we tried to select tamoxifen-resistant Leishmania amazonensis. Three different strategies to generate tamoxifen resistant mutants were used: stepwise increase in drug concentration applied to promastigote cultures, chemical mutagenesis followed by drug selection and treatment of infected mice followed by selection of amastigotes. For amastigote selection, we employed a method with direct plating of parasites recovered from lesions into semi-solid media. Tamoxifen resistant parasites were not rescued by any of these methods. Miltefosine was used as a control in selection experiments and both stepwise selection and chemical mutagenesis allowed successful isolation of miltefosine resistant mutants. These findings are consistent with a multi-target mode of action to explain tamoxifen's leishmanicidal properties. Considering that drug resistance is a major concern in anti-parasitic chemotherapy, these findings support the proposition of using tamoxifen as a partner in drug combination schemes for the treatment of leishmaniasis.
Keywords: Leishmania amazonensis, Chemotherapy, Drug resistance, Tamoxifen
Abbreviations: TM, tamoxifen; MNNG, N-methyl-N-nitroso-N′-nitroguanidine; MT, miltefosine transporter; PBS, phosphate buffered solution; NBD-PC, 2-(6-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)hexanoyl-1-hexadecanoyl-sn-glycero-3-phosphocholine
Graphical abstract
Highlights
-
•
Tamoxifen is effective in the treatment of cutaneous and visceral leishmaniasis.
-
•
Resistance to tamoxifen was not found in promastigotes upon mutagenesis/selection.
-
•
Resistance to tamoxifen was not detected in amastigotes after in vivo selection.
-
•
Tamoxifen may be a good partner in drug combination schemes for leishmaniasis.
1. Introduction
Leishmaniasis is a complex of vector-borne infectious diseases transmitted by sand flies and caused by protozoan parasites of the genus Leishmania. The disease is widespread in the world in tropical and subtropical regions (Alvar et al., 2012). Recent data indicate that approximately 0.2–0.4 million and 0.7 to 1.2 million cases of visceral and cutaneous leishmaniasis respectively occur each year (Alvar et al., 2012).
The limited drug arsenal available for the treatment of leishmaniasis presents numerous shortcomings, such as toxicity, the need for prolonged and mostly parenteral medication and price. Furthermore, failure has been reported for most drugs used in the treatment of the disease (Bryceson, 2001; Croft, 2001) and, in some settings, this failure has been attributed to drug resistance. Widespread resistance to antimonials is found in the Northeast of India (Sundar, 2001; Sundar et al., 2001) and there is great concern on the selection of parasites resistant to miltefosine (Cojean et al., 2012; Rijal et al., 2013), the first oral drug used in the treatment of leishmaniasis. For all these reasons, there is an urgent need to discover and develop new antileishmanial drugs as well as strategies to protect current and future therapies from the threat of resistance.
Drug resistance is related to the ability of pathogens to circumvent the effects of drugs and is due to the genetic adaptability that enables the selection of appropriate strategies against these drugs (Vanaerschot et al., 2013). Selection of drug resistant parasites has been used for the identification of drug resistance genes in parasitic protozoa and has also contributed to understanding the mechanisms of action of some of these therapeutic agents (Ouellette et al., 2004; Muller and Hemphill, 2011; Alsford et al., 2013). In Leishmania, this strategy has been applied in vitro using stepwise increase in the concentration of the drug of interest, followed by the use of different molecular techniques for the analysis of differentially expressed genes/proteins and for identification of point mutations in drug resistant parasites compared to wild-type lines (Berg et al., 2013; Vanaerschot et al., 2013). The dihydrofolate reductase/thymidilate synthase was the first gene identified in a methotrexate selected resistant line of Leishmania major using this strategy (Beverley et al., 1984, 1986). Stepwise increase in drug concentration has also been applied to the selection of miltefosine resistant mutants and this phenotype has been linked to point mutations on a P-type ATPase, known as miltefosine transporter (MT), responsible for the translocation of phospholipids across the plasma membrane (Perez-Victoria et al., 2003; Coelho et al., 2012, 2014).
We have previously reported that tamoxifen, a selective oestrogen receptor modulator, is active in vitro and in vivo against Leishmania species (Miguel et al., 2007, 2008, 2009). We have demonstrated that tamoxifen's antileishmanial activity is not dependent on the interaction with oestrogen receptors (Bonano et al., 2014) but the precise antileishmanial mechanism of action is still uncertain. The aim of this work was to apply drug selection experimental protocols to Leishmania amazonensis with the objective of developing parasite lines that would be suitable to the characterization of tamoxifen's mechanism of action.
2. Material and methods
2.1. Drugs
Tamoxifen, tamoxifen citrate, miltefosine and N-methyl-N-nitroso-N′-nitroguanidine (MNNG) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Stock solutions of tamoxifen and miltefosine (10 mM) were prepared in ethanol or sterile water, respectively. MNNG was diluted in DMSO at a concentration of 10 mg/mL. Tamoxifen citrate was used for the treatment of BALB/c mice infected with L. amazonensis, prepared daily with saline.
2.2. Parasites and cells
L. amazonensis (MHOM/BR/1973/M2269) promastigotes were grown in medium 199 (Sigma–Aldrich) supplemented with 10% heat-inactivated fetal calf serum, 0.25% hemin, 12 mM NaHCO3, 50 U/mL penicillin and 50 μg/mL streptomycin at 25 °C. Amastigotes were obtained from infected mice, as described (Arruda et al., 2008). In brief, female BALB/c mice (4–5 week-old) were infected with 106 stationary-phase parasites injected subcutaneously in the right hind footpad. After 8–12 weeks, lesions were removed and homogenized in phosphate-buffered saline (PBS); the suspension was cleared of cell debris by centrifugation at 50 g for 8 min. The supernatant containing amastigotes was recovered and washed three times in PBS. Amastigotes were counted in a Neubauer hemocytometer.
MCF-7 breast cancer cell line was cultured in DMEM supplemented with l-glutamine, glucose and 10% fetal bovine serum (Life Technologies). This line was maintained in exponential growth phase by sub-culturing twice weekly in 25-cm2 flasks at 37 °C and 5% CO2. For sub-culturing, media was removed from the flasks, cells were washed with PBS and then detached by incubation with 2 mL of Trypsin/EDTA solution (Vitrocell Embriolife, Campinas, Brazil) for 5–10 min followed by inactivation with DMEM. Cells were counted and resuspended in growth media at 105 cells/mL.
2.3. Drug selection in promastigotes
Selection of resistant parasites was initiated using different concentrations of tamoxifen (2, 4, 6, 8 and 12 μM) and 10 μM of miltefosine. For miltefosine, the drug was increased using a stepwise selection until they were resistant to 150 μM. Selection was performed with at least three successive passages for each dose (Coelho et al., 2014). For tamoxifen, parasites were kept for at least 40 passages in the presence of 12 μM tamoxifen (around 250 days of treatment) after previous treatment with 8 μM of tamoxifen for 10 passages.
2.4. Mutagenesis in vitro
Mutagenesis was applied to L. amazonensis wild-type promastigotes (5 × 106 parasites/mL in M199 medium) that were initially treated with 3 μg/mL of MNNG (Sigma–Aldrich) for a period of 4 or 24 h at 25 °C in a total of at least 250 mL of liquid medium, as described (Iovannisci and Ullman, 1984). Mutagenized parasites were then washed three times with PBS and resuspended in fresh medium at a concentration of 5 × 106 parasites/mL. Viability post-treatment was evaluated by cell counting and once the cell cultures started to grow, mutagenized parasites were submitted to selection in the presence of 20 μM tamoxifen or 70–75 μM miltefosine. Selection was performed in liquid or semi-solid 199 medium. Parasites were seeded in 150 mL of M199 at a density of 2 × 106 parasites/mL or plated in semi-solid medium at concentration of 4 × 107 parasites/plate in a total of at least 20 plates.
2.5. Drug selection in amastigotes
Mice were infected as described above. Five weeks after infection, treatment with tamoxifen was initiated. Infected animals received intraperitoneal injections of 30.4 mg tamoxifen citrate/kg/day (equivalent to 20 mg/kg/day tamoxifen) for 15 days. Sixty days after the end of treatment, mice were euthanized and amastigotes were purified from lesions. Amastigotes recovered from infected mice treated or not with tamoxifen were counted and then plated in medium 199 containing 1% of agar (Invitrogen Corporation, NY, USA) and 0.6 μg/mL of biopterin (Sigma–Aldrich). A total of 5 × 106 amastigotes were directly plated in triplicate, in plates containing 20, 30 or 50 μM of tamoxifen or in the absence of drug.
2.6. Parasite viability
Susceptibility to tamoxifen, miltefosine or MNNG was evaluated by a [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] (MTT, Sigma–Aldrich) viability test assay as previously described (Zauli-Nascimento et al., 2010). Briefly, promastigotes (2 × 106 per well) in M199 were incubated in the presence of increasing concentrations of drug (tamoxifen, 2–128 μM; miltefosine, 3–200 μM or MNNG, 1.5–96 μg/mL assayed at a 2-fold dilution) for 24 h. MTT cleavage was measured in a microplate reader (POLARstar Omega, BMG Labtech, Ortenberg, Germany) with a test wavelength of 595 nm and a reference wavelength of 690 nm. Assays were performed in triplicate and results are expressed as the mean and standard deviation (SD) of at least three independent experiments.
2.7. Uptake of fluorescent phosphocholine
Log-phase promastigotes were labelled with NBD-PC (Molecular Probes) as described (Coelho et al., 2014). Briefly, parasites were labelled with 10 μM NBD-PC for 30 min. After washing, parasites were resuspended in PBS for flow cytometry analysis. Labelled parasites were analysed at room temperature using Guava EasyCyte Mini Flow Cytometer System (Millipore). Data from 5000 cells, defined by gating at data acquisition, was collected and analysed using CytoSoft version 4.2.1 software (Guava Technologies) and FlowJo version 9.4.9 software (Tree Star, Ashland, Oregon).
2.8. DNA manipulation
For nucleotide sequencing of the MT gene, total genomic DNA of L. amazonensis parasites was purified using DNAzol (Invitrogen). The MT gene of the miltefosine resistant clones was amplified by PCR in two overlapping fragments of approximately 2.8 and 1.3 kb using two pairs of primers (F and R3, 5′-CGCTCTAGACACCACCACCACTCCTGCCT-3′ and 5′- CGGCATGTGCACCTTCCAGC – 3′, and F3 and R, 5′-GCGCAACGACTTCATCGACC – 3′ and 5′- CGCAAGCTTTCTGCTCACGTTCCGCCCTC, respectively). After electrophoresis, PCR amplified products were purified from 0.8% agarose gels using QIAquick PCR purification kit (Qiagen, Valencia, USA) and cloned in pGEM-T easy (Promega Corporation, Madison, WI, USA). All nucleotide sequences were determined automatically with the Big Dye Terminator v3.1 Cycle Sequencing kit (Life Technologies) using pUC/M13 primers and internal primers of MT gene of L. amazonensis previously described (Coelho et al., 2014). Nucleotide sequence analyses were performed using Lasergene Software (DNASTAR) and Clone Manager 9.0 Software.
2.9. Selection of tamoxifen resistant human breast cancer cell lines and cytotoxicity studies
MCF-7 tamoxifen resistant line was selected by exposing cell culture to 1 μM tamoxifen during 30 days as described (Coser et al., 2009; Mo et al., 2013). For cytotoxicity studies, 105 cells/well were seeded on a 24-well plate. Cells were allowed to attach and grow overnight and then treated with 1–50 μM tamoxifen. Cells were incubated for 3 days at 37 °C with 5% CO2 followed by detaching with Trypsin/EDTA solution and cell counting using a Neubauer hemocytometer. The resistant and sensitive lines were tested in duplicate for each tamoxifen concentration in three independent experiments. The EC50s were determined as described above.
2.10. Statistical analysis
The half-maximal effective concentrations (EC50) were determined from sigmoidal regression of the concentration–response curves. Growth curves were analysed after calculating the area under the curve (AUC) for the means of three independent experiments performed with duplicates and compared through one-way ANOVA followed by Tukey's post-tests. Unpaired two-tailed Student's t tests were used to compare EC50 determined in MCF-7 cells. Statistical analysis were performed using GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA USA).
2.11. Ethics statement
Animal experiments were approved by the Ethics Committee for Animal Experimentation (Protocol: 178/138/02) in agreement with the guidelines of the Sociedade Brasileira de Ciência de Animais de Laboratório (SBCAL) and of the Conselho Nacional de Controle da Experimentação Animal (CONCEA).
3. Results
The first strategy used with the purpose of selecting tamoxifen-resistant Leishmania was to submit promastigote cultures to sub-EC50 concentrations of drug. Since the EC50 against L. amazonensis promastigotes is 12.66 ± 1.75 μM (Table 1), parasites were cultivated in the presence of 2, 4, 6 and 8 μM tamoxifen as the initial selection condition. While no change in growth was observed at 2 and 4 μM of tamoxifen compared to untreated parasites, a delay in reaching the exponential phase was observed when parasites were exposed to 6 and 8 μM of the drug (data not shown). After 5 passages, tamoxifen's EC50 was determined for these lines and no differences were observed in comparison with the EC50 prior to selection (Table 1). Two independent lines initially selected at 8 μM (TM8.1 and TM8.2) were kept for 10 passages in the presence of this drug concentration. At the end of this period, there was no significant change in tamoxifen's EC50 (Table 1).
Table 1.
Tamoxifen (TM) susceptibility in wild-type and selected lines.
| Parasite linesa | EC50 ± S.D. (μM)b | Fold resistancec |
|---|---|---|
| L. amazonensis 2269 (wild-type) | 12.66 ± 1.75 | 1 |
| TM2 | 13.4 ± 3 | 1.06 |
| TM4 | 13.2 ± 1.6 | 1.04 |
| TM6 | 11.1 ± 0.1 | 0.88 |
| TM8.1 | 9.99 ± 1.64 | 0.8 |
| TM8.2 | 11.36 ± 2.07 | 0.9 |
| TM12.1 | 13.71 ± 2.86 | 1.08 |
| TM12.2 | 12.42 ± 2.34 | 0.98 |
L. amazonensis promastigotes of the wild-type strain and lines selected in the presence of 2, 4, 6, 8 or 12 μM tamoxifen.
EC50 ± standard deviation (in μM). Results are the average of at least three independent experiments.
Fold resistance is the ratio between the EC50 of selected lines over the EC50 for the wild-type strain.
Drug concentration on TM8.1 and TM8.2 cultures was then raised to 12 μM for 40 passages (TM12.1 and TM12.2). Wild-type parasites were unable to grow in the presence of 12 μM tamoxifen (Fig. 1). TM12.1 and TM12.2 promastigotes survived at this drug concentration but their growth curves showed significantly delayed exponential phases and lower densities at the stationary phase as compared with control wild-type curves (Fig. 1). This delayed growth pattern was reversible upon removal of the drug and, in the absence of tamoxifen, the growth curves for TM12.1 and TM12.2 promastigotes were not significantly different from the control wild-type cells (Fig. 1).
Fig. 1.
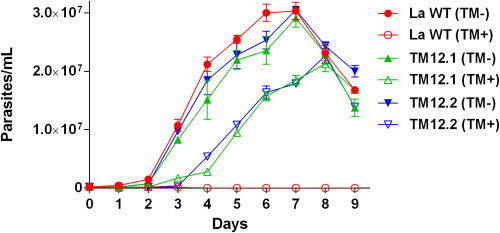
Growth curves for TM12.1 and TM12.2 lines and wild-type L. amazonensis promastigotes in the presence or absence of tamoxifen. Promastigotes were cultivated in M199 medium at 25 °C in the absence (TM-) or presence of 12 μM tamoxifen (TM+). Cultures were seeded at 2 × 105 cells/mL and parasites were counted using a Neubauer hemocytometer. Data is the mean and standard error from three independent experiments. The AUC was significantly different between WT (TM-) and TM12.1(TM+) or TM12.2 (TM+) (ANOVA with Tukey's post-test, P < 0.0001).
In spite of TM12.1 and TM12.2 ability to survive in the presence of 12 μM tamoxifen, there was no change in tamoxifen's EC50 after 24 h treatment as compared to the EC50 against parasites not exposed to the drug (Table 1). Several attempts were made to increase drug pressure in TM12.1 and TM12.2 cultures. However, we were never able to recover living parasites when tamoxifen concentrations were raised to 20 μM or higher.
As a positive control for the drug selection protocol, miltefosine was used in increasing concentrations against L. amazonensis promastigotes. Stepwise selection with miltefosine generated highly resistant mutants with 8-fold increase in the EC50 as compared with wild-type parasites, after about 100 days in culture (data not shown and (Coelho et al., 2014)).
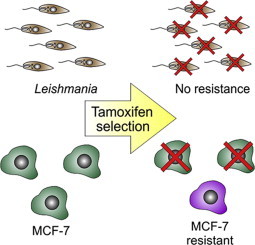
Having shown that we could select for drug resistant Leishmania, it remained to ascertain whether we could select for tamoxifen resistance. That was tested using a human breast cancer cell line, MCF-7, for which it had been previously shown that exposure to tamoxifen led to selection of resistant lines (Coser et al., 2009; Mo et al., 2013). MCF-7 cells were cultivated with 1 μM tamoxifen for 30 days and then evaluated for their susceptibility to tamoxifen. Tamoxifen's EC50 against the parental MCF-7 cells was determined as 14.7 ± 3.6 μM (Fig. S1). MCF-7 cells exposed to tamoxifen for 30 days had their susceptibility significantly decreased with and EC50 calculated as 34.1 ± 4.9 μM (Fig. S1). Therefore, the lack of resistance to tamoxifen in Leishmania was not due to any faults in the protocol.
An alternative to increasing the frequency of drug resistant parasites is the use of mutagenic agents. This strategy has already been applied successfully in the selection of drug resistant parasitic protozoa, as Toxoplasma gondii and Leishmania (Iovannisci and Ullman, 1984; Kink and Chang, 1987; McFadden et al., 2000; Perez-Victoria et al., 2003). MNNG was chosen to generate mutagenized promastigotes. The toxicity of MNNG against L. amazonensis promastigotes was evaluated through viability tests allowing the determination of an EC50 of 3.3 μg/mL ± 0.55 (Fig. 2A). Based on these findings, a concentration of 3 μg/mL MNNG was used to treat parasites for 4 or 24 h followed by a drug-free medium replacement for growth recovery. Parasites treated with MNNG for 24 h took 8 days to start growing again (Fig. 2B), while parasites treated for 4 h recovered after 3 days (Fig. 2C). When culture growth was restored, parasites were selected in the presence of tamoxifen or miltefosine, in liquid culture and in plates. We were unable to recover living parasites when 20 μM of tamoxifen was added to the culture media. On the other hand, MNNG treatment followed by miltefosine selection allowed the recovery of several colonies. For cells treated with MNNG for 24 h followed by selection at 70 μM miltefosine, we observed a 10−8 recovery efficiency while for cells treated with MNNG for 4 h the efficiency was 3.75 × 10−7.
Fig. 2.
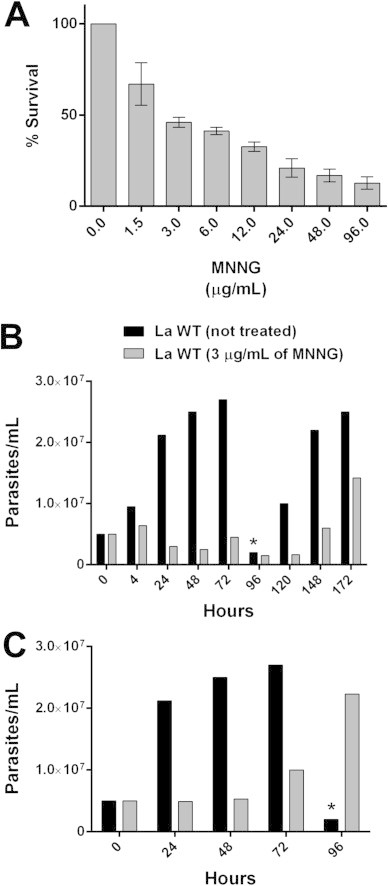
Activity of MNNG against L. amazonensis. (A) Promastigotes were cultivated in M199 medium at 25 °C for 24 h with increasing concentrations of MNNG and viability was determined by MTT. (B and C) L. amazonensis promatigotes were treated with 3 μg/mL of MNNG for 24 h (B) or 4 h (C), washed three times with PBS and seeded at 5 × 106 cells/mL. Cell density was evaluated daily by counting using a Neubauer hemocytometer. (*) Wild-type parasites were sub-cultured when a density of 2.7 × 107 cells/mL was reached.
Ten resistant clones selected in semisolid medium containing miltefosine from populations treated for 4 h with MNNG were transferred to liquid medium containing 80 μM miltefosine. Susceptibility tests showed that these clones were highly resistant to the drug with up to 8-fold increases in the EC50 as compared with the control parasites (Table 2). These resistant lines were also compared to MF150.3–1, a previously selected miltefosine resistant L. amazonensis line (Coelho et al., 2014) which was found to be defective in the accumulation of NBD-phosphocholine (NBD-PC) due to mutations in the MT gene (Coelho et al., 2014). In all ten clones selected for miltefosine resistance, we observed reduced accumulation of phosphocholine (Table 2 and Fig. 3). Nucleotide sequence analysis of the MT alleles in one of these clones (clone 10) identified the following mutations: G410E, L512H, T932A, E951K, T955T and W1005STOP, confirming previous observations correlating resistance to miltefosine in Leishmania to mutations in the MT gene (Perez-Victoria et al., 2003; Coelho et al., 2012, 2014).
Table 2.
Miltefosine susceptibility in wild-type and miltefosine resistant clones generated after treatment with MNNG.
| Parasite lines | EC50 ± S.D. (μM)a | NBD-PC reduced accumulationb |
|---|---|---|
| L. amazonensis 2269 (wild-type) | 21.9 ± 0.45 | No |
| MF150.3–1c | 167.3 ± 16.8 | Yes |
| MNNG-MF clone 2 | 108.2 ± 4.27 | Yes |
| MNNG-MF clone 3 | 120.3 ± 14.4 | Yes |
| MNNG-MF clone 5 | 121 ± 8.2 | Yes |
| MNNG-MF clone 6 | 104 ± 9.8 | Yes |
| MNNG-MF clone 8 | 101.1 ± 12.1 | Yes |
| MNNG-MF clone 10 | 145 ± 34.1 | Yes |
| MNNG-MF clone 11 | 117.3 ± 19.1 | Yes |
| MNNG-MF clone 12 | 76.2 ± 18.21 | Yes |
| MNNG-MF clone 13 | 128.9 ± 1.8 | Yes |
| MNNG-MF clone 15 | 110.3 ± 7 | Yes |
EC50 ± standard deviation values in μM are indicated. Results are the average of three independent experiments.
NBD-PC accumulation was determined by FACS as indicated in Fig. 3.
The miltefosine resistant line MF150.3–1 is an L. amazonensis M2269 strain selected by stepwise selection (Coelho et al., 2014).
Fig. 3.
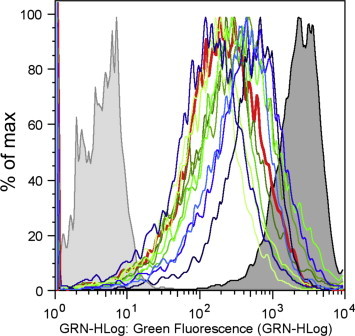
NBD-phosphocholine accumulation in L. amazonensis clones resistant to miltefosine. Wild-type, MF150.3–1 miltefosine resistant line selected in vitro (Coelho et al., 2014) and miltefosine resistant clones were incubated with the fluorescent analogue NBD-PC for 30 min at 25 °C, washed and evaluated by flow cytometry. Non-labelled and labelled wild-type parasites are shown in light and dark gray respectively. MF150.3–1 trace is shown in red and MNNG-MF resistant clones 2, 3, 5, 6, 8, 10, 11, 12, 13 and 15 appear in different shades of blue and green (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.).
We then made use of in vivo selection to try to identify tamoxifen resistant parasites. We had previously shown that mice treated with tamoxifen exhibited a marked decrease in the size of lesions and parasite burden but there was no sterile cure (Miguel et al., 2008). We investigated whether parasites remaining at the lesion site after tamoxifen treatment displayed altered susceptibility to the drug. For this evaluation, amastigotes recovered from lesions were directly plated in M199-agar containing 0, 20, 30 or 50 μM tamoxifen and incubated at 25 °C. Parasites from treated and untreated animals differentiated and grew as promastigotes forming colonies in the absence of tamoxifen. However, no growth was observed when parasites were plated in the presence of drug (data not shown). Taken together, these data indicate that treatment with tamoxifen does not induce detectable selection of resistant parasites in vivo.
4. Discussion
Tamoxifen is a nonsteroidal anti-oestrogen compound capable of binding the oestrogen receptor (Jordan, 1992, 2002). The drug has a low cost and is widely used in the treatment of oestrogen receptor positive breast cancer and as a preventive therapy in women with a high risk of developing the disease (Clarke et al., 2003). However, tamoxifen resistance in tumour cells does exist and may be categorized into intrinsic or acquired. Several factors have been associated with breast cancer resistance to tamoxifen including silencing of the gene encoding oestrogen receptor α, mutations in the oestrogen receptor, altered expression of various growth factor receptors or signalling molecules (reviewed in (Clarke et al., 2003; Riggins et al., 2007; Musgrove and Sutherland, 2009)). The lack or disturbance of the oestrogen receptor activation or transduction ultimately leads to the generation of survival signals that counteract apoptotic death programs. In all instances, clinical or laboratorial resistance to tamoxifen in tumour cells seems to be related directly or indirectly to receptor activation.
Previous analyses in our laboratory did not identify an oestrogen receptor encoding homologue in the genome of Leishmania. Furthermore, structure–activity relationship studies have clearly shown that the antileishmanial activity of SERM-like molecules was present even when groups required for oestrogen receptor binding were absent (Bonano et al., 2014). In addition, oestrogen did not compete out the antileishmanial activity of tamoxifen (Miguel et al., 2007). All these data indicated that the drug activity against the parasite is unrelated to the receptor or to activation of oestrogen receptor-signalling pathways.
In this study, we applied various tools aimed at selecting tamoxifen resistant L. amazonensis mutants in an attempt to understand resistance mechanisms and the anti-parasite mode of action. The protocol employing stepwise increase in drug concentration has been used successfully in the selection of several lines of drug-resistant Leishmania. For instance, the method was used to select for resistance against methotrexate (Coderre et al., 1983), antimony (Callahan and Beverley, 1991; do Monte-Neto et al., 2011), pentamidine (Mukherjee et al., 2006; Coelho et al., 2008) and miltefosine (Perez-Victoria et al., 2003; Coelho et al., 2012, 2014). Nevertheless, we were unsuccessful in selecting tamoxifen resistant mutants, even after long and continuous drug treatment (around 250 days) with concentrations equivalent to the EC50 in lines TM12.1 and TM12.2 (Table 1). These lines showed an increased tolerance to the drug (Fig. 1 and Table 1), but did not exhibit decreased susceptibility.
Three independent investigators, in a timeframe spanning two years, used this in vitro selection protocol with small variations, always with the same results. In parallel, miltefosine resistant parasites as well as MCF-7 cells resistant to tamoxifen were selected.
Mutagenesis was also employed as a tool to increase the frequency of mutations that could lead to tamoxifen resistance. The use of MNNG to generate Leishmania resistant to tubercidin and tunicamycin had been previously described (Iovannisci et al., 1984; Iovannisci and Ullman, 1984; Kink and Chang, 1987). In our hands, the mutagenic agent did increase the recovery of miltefosine resistant mutants but not of tamoxifen resistant parasites. On the other hand, these data helped confirm that miltefosine resistance can be easily acquired. Furthermore, previous findings correlating miltefosine resistance to drastic reductions of drug accumulation due to specific point mutations in the MT gene (Perez-Victoria et al., 2003; Coelho et al., 2012, 2014) were also confirmed here.
Recently, an alternative method to select for resistance in Leishmania using macrophage cultures infected with amastigotes was described (Hendrickx et al., 2014). We employed a different strategy, using in vivo infections, to verify whether tamoxifen resistant amastigotes could be selected. After treating L. amazonensis infected BALB/c mice with tamoxifen, we recovered the lesion parasites and submitted those to selection by plating in the presence of tamoxifen. We had previously reported that parasites recovered from mice after treatment with tamoxifen were as susceptible to the drug as the parasites used to initiate the infection. However, in those circumstances, parasites differentiated from amastigotes recovered from the site of infection were grown as promastigotes without drug pressure for a posterior determination of tamoxifen's EC50. This method could potentially miss eventual resistant parasites that, due to a growth disadvantage would be selected out of the population in the initial rounds of culture. On the other hand, using the protocol described here, better conditions were used to allow the identification of a possible mutant. To the best of our knowledge, this is the first description of recovering amastigotes from lesions and selecting those by plating in semi-solid agar, without any further manipulation. In our hands, the plating efficiency was comparable when seeding the plates with promastigotes or lesion-derived amastigotes. This method also indicated the absence of resistant parasites.
Taken together, we consider these data to be an indication that tamoxifen must have multiple targets in the parasite in agreement with observations in tumour cells where, besides being able to bind and modulate oestrogen receptor activity, tamoxifen has been shown to exert other effects such as interference in membranes, ceramide metabolism, calcium-calmodulin modulation and protein kinase C activity (Lam, 1984; Custodio et al., 1994; Cabot, 1996; Li et al., 2012).
Failure to select drug resistant organisms may be considered a positive trait in drug development. Yet, it is not often described, partly due to the difficulty in knowing how far you have to go to make sure resistance is unattainable. However, while we cannot completely dismiss the possibility of finding tamoxifen resistant Leishmania, we have gathered enough evidence to rule it out as a common occurrence and to strengthen the proposition of using tamoxifen in combination with other drugs in the chemotherapy of the disease.
Acknowledgments
We thank Prof. Carlos Menck for the kind donation of MNNG and Prof. Glaucia M. Machado-Santelli for providing the human breast cancer cell line MCF-7. We also thank Prof. Carlos Renato Machado, Prof. Alejandro Katzin and Dr. Juliana Reimão for helpful suggestions and discussions. This work was supported by research grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, 2011/20484-7) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 473343/2012-6), Brazil. SRBU is the recipient of a senior researcher scholarship from CNPq. ACC and CTT are fellows supported by FAPESP (2012/14629-5 and 2011/18858-6 respectively) and JKUYY is supported by CNPq (473343/2012-6).
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Alsford S., Kelly J.M., Baker N., Horn D. Genetic dissection of drug resistance in trypanosomes. Parasitology. 2013;140:1478–1491. doi: 10.1017/S003118201300022X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvar J., Velez I.D., Bern C., Herrero M., Desjeux P., Cano J., Jannin J., den Boer M. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda D.C., D'Alexandri F.L., Katzin A.M., Uliana S.R. Leishmania amazonensis: biosynthesis of polyprenols of 9 isoprene units by amastigotes. Exp. Parasitol. 2008;118:624–628. doi: 10.1016/j.exppara.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Berg M., Mannaert A., Vanaerschot M., Van Der Auwera G., Dujardin J.C. Genomic approaches to tackle drug resistance in Leishmania. Parasitology. 2013;140:1492–1505. doi: 10.1017/S0031182013000140. [DOI] [PubMed] [Google Scholar]
- Beverley S.M., Coderre J.A., Santi D.V., Schimke R.T. Unstable DNA amplifications in methotrexate-resistant Leishmania consist of extrachromosomal circles which relocalize during stabilization. Cell. 1984;38:431–439. doi: 10.1016/0092-8674(84)90498-7. [DOI] [PubMed] [Google Scholar]
- Beverley S.M., Ellenberger T.E., Cordingley J.S. Primary structure of the gene encoding the bifunctional dihydrofolate reductase-thymidylate synthase of Leishmania major. Proc. Natl. Acad. Sci. U. S. A. 1986;83:2584–2588. doi: 10.1073/pnas.83.8.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano V.I., Yokoyama-Yasunaka J.K., Miguel D.C., Jones S.A., Dodge J.A., Uliana S.R. Discovery of synthetic Leishmania inhibitors by screening of a 2-arylbenzothiophene library. Chem. Biol. Drug Des. 2014;83:289–296. doi: 10.1111/cbdd.12239. [DOI] [PubMed] [Google Scholar]
- Bryceson A. A policy for leishmaniasis with respect to the prevention and control of drug resistance. Trop. Med. Int. Health. 2001;6:928–934. doi: 10.1046/j.1365-3156.2001.00795.x. [DOI] [PubMed] [Google Scholar]
- Cabot M.C., Giuliano A.E., Volner A., Han T.Y. Tamoxifen retards glycosphingolipid metabolism in human cancer cells. FEBS Lett. 1996;394:129–131. doi: 10.1016/0014-5793(96)00942-8. [DOI] [PubMed] [Google Scholar]
- Callahan H.L., Beverley S.M. Heavy metal resistance: a new role for P-glycoproteins in Leishmania. J. Biol. Chem. 1991;266:18427–18430. [PubMed] [Google Scholar]
- Clarke R., Liu M.C., Bouker K.B., Gu Z., Lee R.Y., Zhu Y., Skaar T.C., Gomez B., O'Brien K., Wang Y., Hilakivi-Clarke L.A. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene. 2003;22:7316–7339. doi: 10.1038/sj.onc.1206937. [DOI] [PubMed] [Google Scholar]
- Coderre J.A., Beverley S.M., Schimke R.T., Santi D.V. Overproduction of a bifunctional thymidylate synthetase-dihydrofolate reductase and DNA amplification in methotrexate-resistant Leishmania tropica. Proc. Natl. Acad. Sci. U. S. A. 1983;80:2132–2136. doi: 10.1073/pnas.80.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho A.C., Gentil L.G., da Silveira J.F., Cotrim P.C. Characterization of Leishmania (Leishmania) amazonensis promastigotes resistant to pentamidine. Exp Parasitol. 2008;120:98–102. doi: 10.1016/j.exppara.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Coelho A.C., Boisvert S., Mukherjee A., Leprohon P., Corbeil J., Ouellette M. Multiple mutations in heterogeneous miltefosine-resistant Leishmania major population as determined by whole genome sequencing. PLoS Negl. Trop. Dis. 2012;6:e1512. doi: 10.1371/journal.pntd.0001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho A.C., Trinconi C.T., Costa C.H., Uliana S.R. In vitro and in vivo miltefosine susceptibility of a Leishmania amazonensis isolate from a patient with diffuse cutaneous leishmaniasis. PLoS Negl. Trop. Dis. 2014;8:e2999. doi: 10.1371/journal.pntd.0002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cojean S., Houze S., Haouchine D., Huteau F., Lariven S., Hubert V., Michard F., Bories C., Pratlong F., Le Bras J., Loiseau P.M., Matheron S. Leishmania resistance to miltefosine associated with genetic marker. Emerg. Infect. Dis. 2012;18:704–706. doi: 10.3201/eid1804.110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coser K.R., Wittner B.S., Rosenthal N.F., Collins S.C., Melas A., Smith S.L., Mahoney C.J., Shioda K., Isselbacher K.J., Ramaswamy S., Shioda T. Antiestrogen-resistant subclones of MCF-7 human breast cancer cells are derived from a common monoclonal drug-resistant progenitor. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14536–14541. doi: 10.1073/pnas.0907560106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft S.L. Monitoring drug resistance in leishmaniasis. Trop. Med. Int. Health. 2001;6:899–905. doi: 10.1046/j.1365-3156.2001.00754.x. [DOI] [PubMed] [Google Scholar]
- Custodio J.B., Dinis T.C., Almeida L.M., Madeira V.M. Tamoxifen and hydroxytamoxifen as intramembraneous inhibitors of lipid peroxidation. Evidence for peroxyl radical scavenging activity. Biochem. Pharmacol. 1994;47:1989–1998. doi: 10.1016/0006-2952(94)90073-6. [DOI] [PubMed] [Google Scholar]
- do Monte-Neto R.L., Coelho A.C., Raymond F., Legare D., Corbeil J., Melo M.N., Frezard F., Ouellette M. Gene expression profiling and molecular characterization of antimony resistance in Leishmania amazonensis. PLoS Negl. Trop. Dis. 2011;5:e1167. doi: 10.1371/journal.pntd.0001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx S., Boulet G., Mondelaers A., Dujardin J.C., Rijal S., Lachaud L., Cos P., Delputte P., Maes L. Experimental selection of paromomycin and miltefosine resistance in intracellular amastigotes of Leishmania donovani and L. infantum. Parasitol. Res. 2014;113:1875–1881. doi: 10.1007/s00436-014-3835-7. [DOI] [PubMed] [Google Scholar]
- Iovannisci D.M., Ullman B. Characterization of a mutant Leishmania donovani deficient in adenosine kinase activity. Mol. Biochem. Parasitol. 1984;12:139–151. doi: 10.1016/0166-6851(84)90131-2. [DOI] [PubMed] [Google Scholar]
- Jordan C. Historical perspective on hormonal therapy of advanced breast cancer. Clin. Ther. 2002;24(Suppl. A):A3–A16. doi: 10.1016/s0149-2918(02)85031-7. [DOI] [PubMed] [Google Scholar]
- Jordan V.C. The role of tamoxifen in the treatment and prevention of breast cancer. Curr. Probl. Cancer. 1992;16:129–176. doi: 10.1016/0147-0272(92)90002-6. [DOI] [PubMed] [Google Scholar]
- Kink J.A., Chang K.P. Tunicamycin-resistant Leishmania mexicana amazonensis: expression of virulence associated with an increased activity of N-acetylglucosaminyltransferase and amplification of its presumptive gene. Proc. Natl. Acad. Sci. U. S. A. 1987;84:1253–1257. doi: 10.1073/pnas.84.5.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam H.Y. Tamoxifen is a calmodulin antagonist in the activation of cAMP phosphodiesterase. Biochem.. Biophys. Res. Commun. 1984;118:27–32. doi: 10.1016/0006-291x(84)91062-3. [DOI] [PubMed] [Google Scholar]
- Li Z., Wang N., Fang J., Huang J., Tian F., Li C., Xie F. Role of PKC-ERK signaling in tamoxifen-induced apoptosis and tamoxifen resistance in human breast cancer cells. Oncol. Rep. 2012;27:1879–1886. doi: 10.3892/or.2012.1728. [DOI] [PubMed] [Google Scholar]
- McFadden D.C., Tomavo S., Berry E.A., Boothroyd J.C. Characterization of cytochrome b from Toxoplasma gondii and Q(o) domain mutations as a mechanism of atovaquone-resistance. Mol. Biochem. Parasitol. 2000;108:1–12. doi: 10.1016/s0166-6851(00)00184-5. [DOI] [PubMed] [Google Scholar]
- Miguel D.C., Yokoyama-Yasunaka J.K., Andreoli W.K., Mortara R.A., Uliana S.R. Tamoxifen is effective against Leishmania and induces a rapid alkalinization of parasitophorous vacuoles harbouring Leishmania (Leishmania) amazonensis amastigotes. J. Antimicrob. Chemother. 2007;60:526–534. doi: 10.1093/jac/dkm219. [DOI] [PubMed] [Google Scholar]
- Miguel D.C., Yokoyama-Yasunaka J.K., Uliana S.R. Tamoxifen is effective in the treatment of Leishmania amazonensis infections in mice. PLoS Negl. Trop. Dis. 2008;2:e249. doi: 10.1371/journal.pntd.0000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel D.C., Zauli-Nascimento R.C., Yokoyama-Yasunaka J.K., Katz S., Barbieri C.L., Uliana S.R. Tamoxifen as a potential antileishmanial agent: efficacy in the treatment of Leishmania braziliensis and Leishmania chagasi infections. J. Antimicrob. Chemother. 2009;63:365–368. doi: 10.1093/jac/dkn509. [DOI] [PubMed] [Google Scholar]
- Mo Z., Liu M., Yang F., Luo H., Li Z., Tu G., Yang G. GPR30 as an initiator of tamoxifen resistance in hormone-dependent breast cancer. Breast Cancer Res. 2013;15:R114. doi: 10.1186/bcr3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A., Padmanabhan P.K., Sahani M.H., Barrett M.P., Madhubala R. Roles for mitochondria in pentamidine susceptibility and resistance in Leishmania donovani. Mol. Biochem. Parasitol. 2006;145:1–10. doi: 10.1016/j.molbiopara.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Muller J., Hemphill A. Drug target identification in intracellular and extracellular protozoan parasites. Curr. Top. Med. Chem. 2011;11:2029–2038. doi: 10.2174/156802611796575876. [DOI] [PubMed] [Google Scholar]
- Musgrove E.A., Sutherland R.L. Biological determinants of endocrine resistance in breast cancer. Nat. Rev. Cancer. 2009;9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- Ouellette M., Drummelsmith J., Papadopoulou B. Leishmaniasis: drugs in the clinic, resistance and new developments. Drug Resist. Updat. 2004;7:257–266. doi: 10.1016/j.drup.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Perez-Victoria F.J., Gamarro F., Ouellette M., Castanys S. Functional cloning of the miltefosine transporter. A novel P-type phospholipid translocase from Leishmania involved in drug resistance. J. Biol. Chem. 2003;278:49965–49971. doi: 10.1074/jbc.M308352200. [DOI] [PubMed] [Google Scholar]
- Riggins R.B., Schrecengost R.S., Guerrero M.S., Bouton A.H. Pathways to tamoxifen resistance. Cancer Lett. 2007;256:1–24. doi: 10.1016/j.canlet.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijal S., Ostyn B., Uranw S., Rai K., Bhattarai N.R., Dorlo T.P., Beijnen J.H., Vanaerschot M., Decuypere S., Dhakal S.S., Das M.L., Karki P., Singh R., Boelaert M., Dujardin J.C. Increasing failure of miltefosine in the treatment of Kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin. Infect. Dis. 2013;56:1530–1538. doi: 10.1093/cid/cit102. [DOI] [PubMed] [Google Scholar]
- Sundar S. Drug resistance in Indian visceral leishmaniasis. Trop. Med. Int. Health. 2001;6:849–854. doi: 10.1046/j.1365-3156.2001.00778.x. [DOI] [PubMed] [Google Scholar]
- Sundar S., Pai K., Kumar R., Pathak-Tripathi K., Gam A.A., Ray M., Kenney R.T. Resistance to treatment in Kala-azar: speciation of isolates from northeast India. Am. J. Trop. Med. Hyg. 2001;65:193–196. doi: 10.4269/ajtmh.2001.65.193. [DOI] [PubMed] [Google Scholar]
- Vanaerschot M., Huijben S., Van den Broeck F., Dujardin J.C. Drug resistance in vectorborne parasites: multiple actors and scenarios for an evolutionary arms race. FEMS Microbiol. Ver. 2013;38:41–55. doi: 10.1111/1574-6976.12032. [DOI] [PubMed] [Google Scholar]
- Zauli-Nascimento R.C., Miguel D.C., Yokoyama-Yasunaka J.K., Pereira L.I., Pelli de Oliveira M.A., Ribeiro-Dias F., Dorta M.L., Uliana S.R. In vitro sensitivity of Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis Brazilian isolates to meglumine antimoniate and amphotericin B. Trop. Med. Int. Health. 2010;15:68–76. doi: 10.1111/j.1365-3156.2009.02414.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


