Abstract
Insects and their products are included in the traditional pharmacopoeia of various ethnic groups worldwide. In the Brazilian semiarid region can be highlighted the use of the termite Nasutitermes corniger for the treatment of various diseases. This study evaluated the ethanol extract of N. corniger and its nest as an antimicrobial agent and as a modulator of bacterial resistance against multidrug strains. The Minimum Inhibitory Concentration (MIC) of the extract on Staphylococcus aureus and Escherichia coli by microdilution was determined, as well as MIC of antibiotics in the presence and absence of extract. Despite having no significant antimicrobial activity (MIC ⩾ 1000 μg mL−1), the extract showed additive activity to the antibiotic efficacy, significantly reducing its MIC. These results suggest that N. corniger and its nest are promising natural products for use in antimicrobial therapy.
Keywords: Modification of resistance, Synergistic effect, Antimicrobial activity, Insect, Zootherapy, Ethnopharmacology
1. Introduction
Escherichia coli is the most prevalent Gram-negative rod-shaped bacterium in human fecal flora optional, usually inhabiting the colon as a harmless commensal (Johnson, 1991); however, pathogenic variables have the ability to cause diarrhea and extra intestinal diseases. Staphylococcus aureus is an important pathogen that is part of the normal flora of the human skin and mucous membranes. Infection with this microorganism often occurs through a lesion on the skin which can lead to the appearance of wounds, but also S. aureus can infect any tissue of the body, causing potentially fatal diseases such as osteomyelitis, endocarditis, pneumonia and septicemia (O’Riordan and Lee, 2004; Fournier and Philpott, 2005).
The prevalence of resistant bacterial strains has increased in several countries, where it was found that a range of antibiotics including penicillins, cephalosporins and aminoglycosides no longer had efficacy on certain pathogens (Subramanian et al., 2009; Croxen et al., 2013). Such resistance increases the bacterial pathogenic potential and presents a serious challenge to human health, increasing morbidity and mortality, as well as the costs for the treatment of infections (Andersson and Hughes, 2010).
The use of natural products derived from animals, plants and microorganisms has been seen as an alternative to antibiotics. Apart from substances with direct antimicrobial activity, researchers have sought substances that can act as adjuvants by modifying the effectiveness of antimicrobial agents (Coutinho et al., 2010a; Veras et al., 2012).
The insect class is the largest and most widely distributed taxonomic group. It has about one million described species, comprising approximately three-quarters of organisms on Earth, which represents a colossal biomass in nature (Gullan and Cranston 2005; Chakravorty et al., 2011). These characteristics have made insects an important resource for many civilizations over the centuries, which have been using these animals for several purposes, including the use in healing rituals (Morris, 2004).
The use of insects and their products in traditional medicine has been documented by several authors in different parts of the world (Pemberton, 1999; Costa-Neto, 2002; Lehman et al., 2007). Extracts of insects have been considered important and studied by modern science, revealing important therapeutic functions, which consider these animals as an important tool for the discovery of new drugs (Feng et al., 2009).
In Brazil, among the species most commonly used for therapeutic purposes are the termites (Isoptera) (Alves and Rosa 2006, 2007), including Nasutitermes corniger (Motschulsky, 1855), whose natural products are listed in the traditional pharmacopoeia of the semiarid region for the treatment of asthma, cough, flu and sore throat (Ferreira et al., 2012).
This study aimed at evaluating, through experimental models in vitro, the extract of N. corniger and its nest as an antibacterial agent and as a modifier of resistance against pathogenic microorganisms.
2. Materials and methods
2.1. Zoological material
The collection of N. corniger along with the nest was performed on the farm Farinha, municipality of Pocinhos, semiarid region of the Paraíba state, (7°07′S, 36°07′W). The species was identified by Prof. Alexandre Vasconcellos from the Department of Systematics and Ecology at the Universidade Federal da Paraíba (UFPB). A sample was deposited to the Isoptera collection at the Center of Exact and Natural Sciences UFPB under the number CICB 69.
2.2. Extract preparation
Twenty grams of termites with nest were subjected to extraction by maceration with 100 mL of absolute ethanol for 5 days at room temperature (25 ± 3 °C) with occasional stirring. After filtration, the extracts were concentrated on a rotary evaporator at 40 °C.
2.3. Determination of total polyphenols
The total polyphenol content of plant extracts was measured using spectrophotometry in the visible region by the method of Folin–Ciocalteu described by Chandra and Mejia (2004) with minor modifications. The extracts (25 mg) were dissolved in distilled water to obtain a final concentration of 200 μg mL−1. From each solution, a 1 mL aliquot was added to 1 mL of 1 mol/L Folin–Ciocalteu reagent. This mixture remained undisturbed for 2 min before the addition of 2 mL of 20% (w/v) Na2CO3 solution and left undisturbed for 10 min. Thereafter the reading was performed using Spectrophotometer Shimadzu, model UV-mini 1240, at 757 nm. The calibration curve was obtained with a stock solution of gallic acid (1000 μg mL−1), from which dilutions were made at concentrations between 1 and 40 μg mL−1.
2.4. Determination of total flavonoids
The total flavonoids were determined by the method described by Meda et al. (2005). The extracts were diluted with methanol at 1000 μg mL−1. To 5 mL of each test solution was added the same volume of 2% (w/v) AlCl3 solution in methanol. This mixture remained undisturbed for 10 min before the UV spectrophotometric reading at 415 nm wavelength. The total flavonoids were determined by the calibration curve using quercetin (Sigma–Aldrich) as standard at concentrations between 2 and 30 μg mL−1.
2.5. Determination of condensed tannins
The content of condensed tannins was verified through the method of vanillin–HCl described by Makkar and Becker (1993), where 0.25 mL of the sample was added to 1.5 mL of vanillin solution in methanol (4% w/v) and subsequently, 0.75 mL of concentrated HCl (37%). After HCl addition, the tube content was shaken in a water bath at 30 °C for 3–4 s before reading on a spectrophotometer at 500 nm wavelength. Catechin was used as a standard at concentrations between 10 and 100 μg mL−1.
2.6. Determination of saponins
The quantification of total saponins followed the method described by Makkar et al. (2007). 250 μL of 8% vanillin solution in ethanol was added to 250 μL extract solution in 80% methanol; then 2.5 mL of 72% sulfuric acid was added. The tubes were incubated at 60 °C in a water bath for 10 min and transferred to an ice bath, staying for 4 min. The absorbance reading at 544 nm was performed against a blank consisting of the vanillin solution, 80% methanol and sulfuric acid. The calibration curve was obtained from a diosgenin solution at concentrations between 100 and 500 μg mL−1.
2.7. Microbial strains
We used the clinical isolates of Staphylococcus aureus resistant to ampicillin, ciprofloxacin, cephalexin, erythromycin, penicillin and amoxicillin and Escherichia coli resistant to amoxicillin, cephalothin, levofloxacin, chloramphenicol, tetracycline and gentamicin. The tested bacterial strains were incubated at 37 °C for 24 h in Mueller–Hinton agar and maintained on agar slants in tubes on the same medium.
2.8. Drugs
All the drugs tested were obtained from Sigma Chemical Corp., St. Louis, MO, USA, and dissolved in sterile water before use.
2.9. Minimal Inhibitory Concentration (MIC) Determination and Modulation activity
The minimum inhibitory concentration (MIC) was determined by the microdilution method in 96-well plates (CLSI, 2012) using Mueller–Hinton broth. Colonies of microorganisms were suspended in 0.9% saline solution and the suspension was adjusted by the spectrophotometric method at 625 nm to a final concentration of 5 × 106 CFU mL−1. We performed serial dilutions of the extract solubilized in 10% DMSO in the range of 1000–3.9 μg mL−1 and antibiotics in the range of 2500–2.4 μg mL−1. DMSO 10% was included as negative control. The plates were incubated at 37 ± 1 °C for 24 h. Bacterial growth was indicated by the addition of 20 μL of 0,01% resazurin aqueous (Sigma–Aldrich) with incubation at 37 ± 1 °C for 2 h, and MIC values were identified as the lowest concentration in which no bacterial growth is visible. Evaluation of extracts as modulators of antibiotic resistance was performed according to Coutinho et al. (2010a). The MIC of the antibiotic was determined in the presence and absence of sub-inhibitory concentrations (125 μg mL−1). Plates were incubated as described above and each assay was performed in triplicate.
2.10. Statistical analysis of microbiological tests
The test results were expressed as geometric means. The two-way analysis of variance followed by Bonferroni post-test was applied using the GraphPad Prism 5.0 software (Matias et al., 2013).
3. Results and discussion
Results of chemical tests are described in Table 1. The termite N. corniger and its nest have plant secondary metabolites, probably from their host plant. This is because this species of termite is xylophage and compounds present in the plant consumed may still be in the digestive system of the animals or the excrement used for making the nest (Noirot and Darlington, 2000; Thorne and Haverty, 2000).
Table 1.
Concentration of secondary metabolites (mg g−1) determined for the extract of N. corniger.
| Metabolites | Total polyphenols | Flavonoids | Condensed tannins | Total saponins |
|---|---|---|---|---|
| Concentration | 17.56 ± 0.23 | 6.75 ± 0.05 | 10.5 ± 0.09 | 38.22 ± 13.21 |
With respect to antimicrobial activity, the extract showed no clinically significant activity against resistant strains of S. aureus and E. coli, with the Minimum Inhibitory Concentration (MIC) > 1000 μg mL−1. Ríos and Recio (2005) suggest that in the study on medicinal plants,the extract should be considered as possessing antimicrobial activity if the MIC is <1000 μg mL−1. Despite the extracts tested in this study not being obtained from plant material, we take this value as a reference.
Moreover, by adding the extract of N. corniger and its nest in sub-inhibitory concentration (125 μg mL−1) to the culture medium, there was a significant reduction (P < 0.001) in the MIC of all antibiotics tested, except for cephalothin on E. coli (Figs. 1 and 2). For S. aureus, the best modulation effects were observed in association with erythromycin extract, which provided a more marked reduction of MIC, 2500–625 μg mL−1, while for E. coli there was a MIC decrease from 156.25 to 39.06 μg mL−1 when combining the extract and gentamicin.
Figure 1.
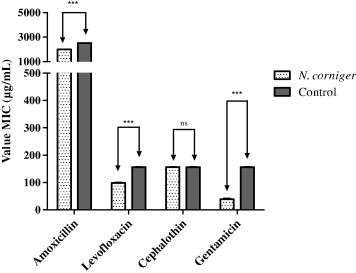
Graph demonstrating the modulatory activity of the ethanol extract of N. corniger on resistance of E. coli to antibiotics. ∗∗∗ – statistically significant with P value < 0.001; ns – not statistically significant value with P > 0.05.
Figure 2.
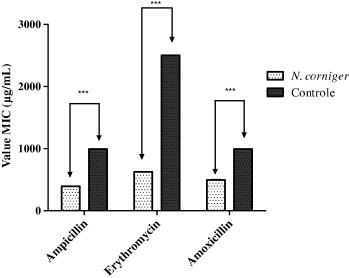
Graph demonstrating the modulatory activity of the ethanol extract of N. corniger on resistance of S. aureus to antibiotics. ∗∗∗ – statistically significant with P value < 0.001.
Similar results were obtained by Coutinho et al. (2010a,b) who evaluated the synergism between decoctions of N. corniger and aminoglycosides on resistant strains of E. coli. Although decoctions have not shown significant antimicrobial activity (MIC > 1024 μg mL−1) when combined with antibiotics, they promoted significantly reduced MIC.
The biological activity exhibited both in this work as in those above should probably be related to the presence of the host plant secondary metabolites, peptides produced by the animals as a defense mechanism, or even substances produced by mound’s microorganisms.
Regarding the substances of plant origin, tannins can act on the metabolism in microorganisms through inhibition of enzymes, oxidative phosphorylation and the electron transport system, inactivation of microbial adhesins and proteins of the cell envelope (Scalbert 1991; Cowan, 1999). Flavonoids can disrupt lipid bilayers by directly penetrating and disrupting the barrier function, but may also cause membrane fusion, resulting in leakage of intramembranous materials (Ikigai et al., 1993; Cushnie and Lamb, 2005). Saponins cause disturbance in the lipid component forming pores in membranes (Francis et al., 2002; Sung and Lee, 2008).
Termites have developed the ability to deal with a rich microbial community and are often infectious inhabiting their nests and feeding sites, developing, among other defense mechanisms, the synthesis of antimicrobial peptides (Traniello et al., 2002; Schmid-Hempel, 2005). Two peptides with antimicrobial properties have been isolated from Pseudacanthotermes spiniger by Lamberty et al. (2001), “termicin”, which showed essentially antifungal properties and “spinigerin”, which showed antifungal and antibacterial activities. Cysteine-rich peptides and α-helical peptides, such as termicin and spinigerin respectively, have the ability to permeabilize microbial cytoplasmic membranes (Dimarcq et al., 1998), which was confirmed by Lee et al. (2003) when studying the antimicrobial properties of Spinigerin. Bulmer and Crozier (2004, 2006) in studies on the molecular biology of Nasutitermes termites identified genes responsible for the synthesis of antimicrobial peptides.
The nests of social insects, for its characteristics of temperature and humidity, have a rich associated microbiota, where some species live in symbiosis with insects, protecting them from infections by bacteria and fungi. Madden et al. (2013) reported the antimicrobial activity of actinobacteria isolated from the nest of Polistes dominulus (Christ). Visser et al. (2012) isolated actinobacteria from nests of the termites Macrotermes natalensis, Microtermes sp. and Odontotermes spp. and also confirmed their antimicrobial activity. Bonfim (2010) confirmed the antimicrobial activity of actinobacteria and Bacillus isolated from nests of Nasutitermes mainly on Gram-positive bacteria. Extracts, such as the one used in the present study, represent a complex mixture of substances, affecting and interfering in several mechanisms related to synergistic or additive effects when combined with antibiotics or other drugs (Imming et al., 2006; Schmidt et al., 2008; Wagner and Ulrich-Merzenich, 2009).
4. Conclusion
The results of this study revealed N. corniger and its nest as having modulating activity of multidrug bacterial resistance to commercial antibiotics and can be used as adjuvants in antimicrobial therapy.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgment
The authors thank Mr. José Leonardo Clementino de Araújo for sample collection.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alves R.R.N., Rosa I.M.L. From cnidarians to mammals: the use of animals as remedies in fishing communities in NE Brazil. J. Ethnopharmacol. 2006;107:259–276. doi: 10.1016/j.jep.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Alves R.R.N., Rosa I.M.L. Zootherapy goes to town: the use of animal-based remedies in urban areas of NE and N Brazil. J. Ethnopharmacol. 2007;113:541–555. doi: 10.1016/j.jep.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Andersson D.I., Hughes D. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat. Rev. 2010;8:260–271. doi: 10.1038/nrmicro2319. [DOI] [PubMed] [Google Scholar]
- Bonfim, G.F., 2010. Atividade antimicrobiana de microrganismos isolados de cupinzeiros da região da Mata de Cipó, Bahia. Dissertação (Mestrado em Biotecnologia). Feira de Santana: Universidade Estadual de Feira de Santana.
- Bulmer M.S., Crozier R.H. Duplication and diversifying selection among termite antifungal peptides. Mol. Bio. Evol. 2004;21:2256–2264. doi: 10.1093/molbev/msh236. [DOI] [PubMed] [Google Scholar]
- Bulmer M.S., Crozier R.H. Variation in positive selection in térmite GNBPs and Relish. Mol. Bio. Evol. 2006;23:317–326. doi: 10.1093/molbev/msj037. [DOI] [PubMed] [Google Scholar]
- Chakravorty J., Ghosh S., Meyer-Rochow V.B. Practices of entomophagy and entomotherapy by members of the Nyishi and Galo tribes, two ethnic groups of the of Arunachal Pradesh (North-East India) J. Ethnob. Ethnom. 2011;7:5. doi: 10.1186/1746-4269-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S., Mejia E.G. Polyphenolic Compounds, antioxidant capacity, and quinone reductase activity of an aqueous extract of Ardisia compressa in comparison to mate (Ilex paraguariensis) and green (Camellia sinensis) teas. J. Agric. Food Chem. 2004:3583–3589. doi: 10.1021/jf0352632. [DOI] [PubMed] [Google Scholar]
- CLSI – Clinical and Laboratory Standards Institute, 2012. Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Second Informational Supplement, ninth ed. Document M100–S22. Pensilvânia, USA: NIH.
- Costa-Neto E.M. The use of insects in folk medicine in the state of Bahia, Northeastern Brazil, with notes on insects reported elsewhere in Brazilian folk medicine. Hum. Ecol. 2002;30:245–263. [Google Scholar]
- Coutinho H.D.M., Costa J.G., Falcão-Silva V.S., Siqueira J.P., Jr, Lima E.O. Effect of Momordica charantia L. in the resistance to aminoglycosides in methicilin-resistant Staphylococcus aureus. Comp. Immunol. Microbiol. Infect. Dis. 2010;33:467–471. doi: 10.1016/j.cimid.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Coutinho H.D.M., Almeida Filho G.G., Pessoa H.L.F., Gadelha C.A., Gadelha T.S. Natural products from the termite Nasutitermes corniger lowers aminoglycoside MIC. Pharmacogn. Mag. 2010;6:1–4. doi: 10.4103/0973-1296.59958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxen M.A., Law R.J., Scholz R., Keeney K.M., Wlodarska M., Finlay B.B. Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013;26:822–880. doi: 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushnie T.P.T., Lamb A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimarcq J.L., Bulet P., Hetru C., Hoffmann J. Cysteine-rich antimicrobial peptides in invertebrates. Pept. Sci. 1998;47:465–477. doi: 10.1002/(SICI)1097-0282(1998)47:6<465::AID-BIP5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Feng Y., Zhao M., He Z., Chen Z., Sun L. Research and utilization of medicinal insects in China. Entomol. Res. 2009;39:313–316. [Google Scholar]
- Ferreira F.S., Albuquerque U.P., Coutinho H.D.M., Almeida W.O., Alves R.R.N. The Trade in Medicinal Animals in Northeastern Brazil. Evid. Based Complement. Alternat. Med. 2012;2012:1–20. doi: 10.1155/2012/126938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier B., Philpott D.J. Recognition of Staphylococcus aureus by the innate immune system. Clin. Microbiol. Rev. 2005;18:521–540. doi: 10.1128/CMR.18.3.521-540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis G., Kerema Z., Makkara H.P.S., Beckera K. The biological action of saponins in animal systems: a review. Br. J. Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- Gullan P.J., Cranston P.S. Blackwell Publishing Ltd; Oxford: 2005. The Insects an Outline of Entomology. [Google Scholar]
- Ikigai H., Nakae T., Hara Y., Shimamura T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta. 1993;1147:132–136. doi: 10.1016/0005-2736(93)90323-r. [DOI] [PubMed] [Google Scholar]
- Imming P., Sinning C., Meyer A. Drugs, their targets and the nature and number of drug targets. Drug Discov. 2006;5:821–834. doi: 10.1038/nrd2132. [DOI] [PubMed] [Google Scholar]
- Johnson J.R. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberty M., Zachary D., Lanot R., Bordereau C., Robert A., Hoffmann J.A., Bulet P. Constitutive expression of a Cysteine-rich antifungal and a linear antibacterial peptide in a termite insect. J. Biol. Chem. 2001;276:4085–4092. doi: 10.1074/jbc.M002998200. [DOI] [PubMed] [Google Scholar]
- Lee K.H., Shin S.Y., Hong J.E., Yang S.T., Kim J.I., Hahm K.S., Kim Y. Solution structure of termite-derived antimicrobial peptide, spinigerin, as determined in SDS micelle by NMR spectroscopy. Biochem. Biophys. Res. 2003;309:591–597. doi: 10.1016/j.bbrc.2003.08.043. [DOI] [PubMed] [Google Scholar]
- Lehman A.D., Dunkel F.V., Klein R.A., Ouattara S., Diallo D., Gamby K.T., N’Diaye M. Insect management products from Malian traditional medicine-Establishing systematic criteria for their identification. J. Ethnopharmacol. 2007;110:235–249. doi: 10.1016/j.jep.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Madden A.A., Grassetti A., Soriano J.A.N., Starks P.T. Actinomycetes with antimicrobial activity isolated from paper wasp (Hymenoptera: Vespidae: Polistinae) nests. Environ. Entomol. 2013;42:703–710. doi: 10.1603/EN12159. [DOI] [PubMed] [Google Scholar]
- Makkar H.P.S., Becker K. Vanillin–HCl method for condensed tannins: effect of organic solvents used for extraction of tannins. J. Chem. Ecol. 1993;4:613–621. doi: 10.1007/BF00984996. [DOI] [PubMed] [Google Scholar]
- Makkar H.P.S., Siddhuraju P., Becker K. Humana Press; Totowa, NJ: 2007. Plant Secondary Metabolities. [DOI] [PubMed] [Google Scholar]
- Matias E.F.F., Alves E.F., Santos B.S., Souza C.E.S., Ferreira J.V.A., Lavor A.K.L.S., Figueredo F.G., Lima L.F., Santos F.A.V., Peixoto F.S.N., Colares A.V., Boligon A.A., Saraiva R.A., Athayde M.L., Rocha J.B.T., Menezes I.R.A., Coutinho H.D.M., Costa J.G.M. Biological activities and chemical characterization of Cordia verbenacea DC as tool to validate the ethnobiological usage. Evid. Based Complement. Alternat. Med. 2013;2013:1–7. doi: 10.1155/2013/164215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda A., Lamien C.E., Romito M., Millogo J., Nacoulma O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. [Google Scholar]
- Morris B. Oxford International Publishers; Oxford: 2004. Insects and human life. [Google Scholar]
- Noirot C., Darlington J.P.E.C. Termite nests: architecture, regulation and defense. In: Abe T., Higashi M., Bignell D.E., editors. Termites: Evolution, Sociality, Symbiosis, Ecology. Kluwer Academic Publications; Dordrecht: 2000. [Google Scholar]
- O’Riordan K., Lee J.C. Staphylococcus aureus Capsular Polysaccharides. Clin. Microbiol. Rev. 2004;17:218–234. doi: 10.1128/CMR.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton R.W. Insects and other arthropods used as drugs in Korean traditional medicine. J. Ethnopharmacol. 1999;65:207–216. doi: 10.1016/s0378-8741(98)00209-8. [DOI] [PubMed] [Google Scholar]
- Ríos J.L., Recio M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005;100:80–84. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Scalbert A. Antimicrobial properties of tannins. Phytochemistry. 1991;30:3875–3883. [Google Scholar]
- Schmid-Hempel P. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 2005;50:529–551. doi: 10.1146/annurev.ento.50.071803.130420. [DOI] [PubMed] [Google Scholar]
- Schmidt B., Ribnicky D.M., Poulev A., Logendra S., Cefalu W.T., Raskin I. A natural history of botanical therapeutics. Metabolism. 2008;57(Suppl 1):S3–S9. doi: 10.1016/j.metabol.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian K., Selvakkumar C., Vinaykumar K.S., Goswami N., Meenakshisundaram S., Balakrishnan A., Lakshmi B.S. Tackling multiple antibiotic resistance in enteropathogenic Escherichia coli (EPEC) clinical isolates: a diarylheptanoid from Alpinia officinarum shows promising antibacterial and immunomodulatory activity against EPEC and its lipopolysaccharide-induced inflammation. Int. J. Antimicrob. Agents. 2009;33:244–250. doi: 10.1016/j.ijantimicag.2008.08.032. [DOI] [PubMed] [Google Scholar]
- Sung W.S., Lee D.G. The combination effect of Korean red ginseng saponins with kanamycin and cefotaxime against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 2008;31:1614–1617. doi: 10.1248/bpb.31.1614. [DOI] [PubMed] [Google Scholar]
- Thorne B.L., Haverty M.I. Nest Growth and Survivorship in Three Species of Neotropical Nasutitermes (Isoptera: Termitidae) Environ. Ecol. 2000;29:256–264. [Google Scholar]
- Traniello J.F.A., Rosengaus R.B., Savoie K. The development of immunity in a social insect: evidence for the group facilitation of disease resistance. Proc. Natl. Acad. Sci. 2002;99:6838–6842. doi: 10.1073/pnas.102176599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veras H.N.H., Rodrigues F.F.G., Colares A.V., Menezes I.R.A., Coutinho H.D.M., Botelho M.A., Costa J.G.M. Synergistic antibiotic activity of volatile compounds from the essential oil of Lippia sidoides and thymol. Fitoterapia. 2012;83:508–512. doi: 10.1016/j.fitote.2011.12.024. [DOI] [PubMed] [Google Scholar]
- Visser A.A., Nobre T., Currie C.R., Aanen D.K., Poulsen M. Exploring the potential for actinobacteria as defensive symbionts in fungus-growing termites. Microb. Ecol. 2012;63:975–985. doi: 10.1007/s00248-011-9987-4. [DOI] [PubMed] [Google Scholar]
- Wagner H., Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16:97–110. doi: 10.1016/j.phymed.2008.12.018. [DOI] [PubMed] [Google Scholar]


