Abstract
Neural tube defects (NTDs), including spina bifida and anencephaly, are severe birth defects of the central nervous system that originate during embryonic development when the neural tube fails to close completely. Human NTDs are multifactorial, with contributions from both genetic and environmental factors. The genetic basis is not yet well understood, but several nongenetic risk factors have been identified as have possibilities for prevention by maternal folic acid supplementation. Mechanisms underlying neural tube closure and NTDs may be informed by experimental models, which have revealed numerous genes whose abnormal function causes NTDs and have provided details of critical cellular and morphological events whose regulation is essential for closure. Such models also provide an opportunity to investigate potential risk factors and to develop novel preventive therapies.
Keywords: anencephaly, spina bifida, folic acid, genetics
INTRODUCTION
Neural tube defects (NTDs) are severe birth defects of the central nervous system that originate during embryogenesis and result from failure of the morphogenetic process of neural tube closure (see sidebar). In higher vertebrates, the neural tube is generated by the processes that shape, bend, and fuse the neural plate, and fusion in the dorsal midline progressively seals the neural tube as it forms. If closure is not completed, the neuroepithelium remains exposed to the environment and consequently subject to degeneration and neuronal deficit. The type and severity of these open NTDs vary with the level of the body axis affected. Thus, failure of closure in the prospective brain and spinal cord results in anencephaly and open spina bifida (myelomeningocele), respectively.
Although the unifying feature of open NTDs is incomplete neural tube closure, evidence points to many different possible causes, both genetic and environmental. In humans, it appears that most NTDs are multifactorial, resulting from an additive contribution of several risk factors, which are each individually insufficient to disrupt neural tube closure (the multifactorial threshold model) (Harris & Juriloff 2007). The challenge of identifying the primary cause of NTDs in individual patients is highlighted by the numerous candidate genes and environmental factors indicated by epidemiologic studies and experimental models. Moreover, the potential for gene-gene and gene-environment interactions introduces further potential complexity.
UNDERSTANDING THE EMBRYONIC BASIS OF NTDs: NEURAL TUBE CLOSURE
Determining the specific causes of NTDs is best achieved in the context of an understanding of the mechanisms underlying neural tube closure (reviewed by Copp & Greene 2013, Greene & Copp 2009). Given the inaccessibility of the neurulation-stage human embryo, our knowledge of the key principles of neural tube closure comes mainly from analysis of experimental models, particularly other mammals, amphibians, and birds, in which primary neural tube closure is achieved through folding and fusion of the neuroepithelium.
Primary Neurulation: Subtypes of NTDs Relate to Stages of Closure
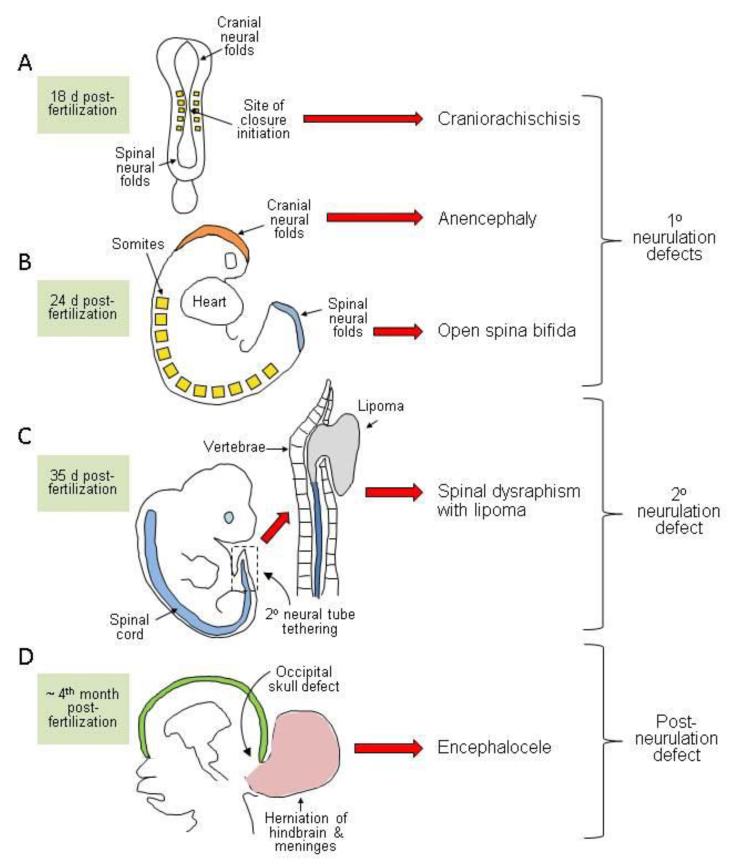
In the prospective brain and most of the spinal cord, neural tube formation essentially involves the bending of the neuroepithelium at the midline to generate neural folds that elevate, meet, and fuse in the dorsal midline (primary neurulation). Rather than simultaneously rolling up along the extent of the rostrocaudal axis, neural tube closure is discontinuous with distinct sites of initiation located at characteristic axial levels. Moreover, the morphological and molecular requirements for closure vary along the body axis, such that an individual NTD usually affects only a portion of the neural tube. NTDs can thus be attributed to failure of particular initiation events or disruption of the progression of closure between these sites (Figure 1).
Figure 1.
Diagrammatic representation of the developmental origin of malformations broadly classified as neural tube defects in humans. (a,b) Disorders of primary neurulation include craniorachischisis (a) in which the neural tube fails to initiate closure, leaving most of the brain and the entire spine open. If closure initiates successfully, then the cranial and/or spinal neural folds may fail to close (b) generating exencephaly/anencephaly and open spina bifida (myelomeningocele), respectively. (c) Disorders of secondary neurulation comprise failure of the neural tube to separate completely from adjacent tissues, resulting in tethering and diminished mobility. The spinal cord is covered by skin and often associated with fatty tissue accumulation (lipoma) through as-yet-unknown mechanisms. (d) Postneurulation defects can arise when the bony structure of the skeleton fails to develop fully. Herniation of the meninges, with or without brain tissue, through a skull defect (shown here as occipital but sometimes parietal or fronto-ethmodial) generates encephalocele, while an analogous defect in the spinal region produces meningocele.
In mice, closure is first achieved on embryonic day 8.5 at the level of the hindbrain/cervical boundary (closure 1) (Figure 1a), and failure of this event leads to craniorachischisis (Copp et al. 2003). Closure initiates at a second site on embryonic day 9, closure 2, in the caudal forebrain or forebrain/midbrain boundary. Once initial contact and fusion have been established between the tips of the neural folds, closure spreads bidirectionally from the sites of closures 1 and 2 and in a caudal direction from the rostral end of the neural tube (closure 3). The open regions of neural folds, termed neuropores, gradually shorten, leading to complete closure of the anterior neuropore (between closures 2 and 3) on embryonic day 9 and the hindbrain neuropore (between closures 1 and 2) a few hours later. Cranial NTDs (anencephaly) result from failure of closure 2, or incomplete “zippering” between closures 1 and 2, which closes the midbrain and hindbrain. If fusion does not progress from the anterior end of the neural plate (closure 3), the resultant phenotype is a split face usually accompanied by forebrain anencephaly.
Unlike the cranial region where closure proceeds bidirectionally, spinal neurulation is entirely caudally directed as the embryo continues to grow. Primary neurulation completes with final closure of the posterior neuropore on embryonic day 10. Impaired progression of closure, and consequently the presence of a persistently open posterior neuropore, results in spina bifida, and the size of the ensuing lesion relates directly to the axial level at which closure stops.
Primary Neurulation in Humans
Examination of human embryos suggests that initiation of closure is discontinuous, as in the mouse (Nakatsu et al. 2000, O’Rahilly & Müller 2002). Bending of the neural plate begins at approximately 18 days after fertilization, with an event equivalent to closure 1 at approximately 21 days and completion of closure at the posterior neuropore by 26--28 days postfertilization (Figure 1a,b). It appears that closure of the forebrain and midbrain in human embryos may be achieved by progression between the site of closure 1 and the rostral end of the neural plate without an intervening initiation site analogous to closure 2 (O’Rahilly & Müller 2002, Sulik et al. 1998).
Secondary Neurulation
In mice and humans, the neural tube caudal to the midsacral region is continuous with the caudal end of the primary neural tube but forms by a distinct process, termed secondary neurulation (Copp & Brook 1989, Schoenwolf 1984). This process involves condensation of a population of tail bud--derived cells to form an epithelial rod that undergoes canalization to form the lumen of the tube in the lower sacral and coccygeal regions. Malformations resulting from disturbance of secondary neurulation are closed (skin covered) and often involve tethering of the spinal cord, with associated ectopic lipomatous material (Figure 1c) (Lew & Kothbauer 2007).
MECHANISMS UNDERLYING NEURAL TUBE CLOSURE
Studies of neurulation-stage embryos, both normal and developing NTDs, provide insights into key molecular and cellular pathways underlying the morphological tissue movements of neural tube closure (Copp & Greene 2010). The occurrence of isolated NTDs at cranial or caudal levels in humans and different mouse models suggests the likely involvement of region-specific mechanisms, dependent on different gene products, in addition to ubiquitous requirements that are essential at all levels.
Shaping of the Neural Plate: Convergent Extension Is Required to Initiate Closure
Concomitant with the onset of neural tube closure, the neural plate undergoes narrowing in the mediolateral axis (convergence) and elongation in the rostrocaudal axis (extension), owing to intercalation of cells at the midline (Keller 2002). Convergent extension depends on activity of a noncanonical Wnt signaling pathway, homologous to the planar cell polarity (PCP) pathway first described in Drosophila as regulating cell polarity in the plane of epithelia (Goodrich & Strutt 2011). Signaling occurs via Frizzled (Fzd) membrane receptors and cytoplasmic Dishevelled (Dvl) but without stabilization of β-catenin.
PCP: planar cell polarity
Functional disruption of PCP mediators prevents convergent extension, and the neural plate remains broad in Xenopus (Wallingford & Harland 2001, 2002) and mouse embryos (Greene et al. 1998, Ybot-Gonzalez et al. 2007b). Hence, closure 1 fails, leading to craniorachischisis in mice homozygous for mutations in core PCP genes including Vangl2 and Celsr1, or double mutants for Dvl-1 and -2, or Fzd-3 and -6 (Juriloff & Harris 2012a). Craniorachischisis also results from mutation of the PCP-related genes Scrb1 (Murdoch et al. 2001) and Ptk7 (Lu et al. 2004) or genes encoding accessory proteins, such as Sec24b, which affects Vangl2 transport (Merte et al. 2010). Ultimately, failure of closure initiation in PCP-mutant embryos is thought to result from insufficient proximity of the neural folds, owing to the broadened midline.
Failure of closure 1 in most of the core PCP mutant embryos precludes analysis of a requirement for convergent extension at later stages of neurulation. However, spina bifida occurs in some loop-tail heterozygotes (Vangl2Lp/+) (Copp et al. 1994) and in compound heterozygotes of Vangl2Lp/+ with mutations of Ptk7, Sec24b, or Sdc4 (Escobedo et al. 2013, Lu et al. 2004, Merte et al. 2010). Moreover, non-canonical Wnt signaling is compromised in Lrp6 null embryos that develop spina bifida (Gray et al. 2013). These observations suggest that PCP signaling maycontinue to be required as spinal neurulation proceeds.
Despite the entirely open spinal neural tube in Vangl2Lp/Lp embryos with craniorachischisis, closure does occur in the forebrain and much of the midbrain, implying that PCP-dependent convergent extension is not required throughout the cranial region. Nonetheless, exencephaly is observed in digenic combinations of Vangl2Lp/+ with some Wnt pathway genes (e.g., Dvl3+/−, Fzd1+/−, and Fzd2+/−) (Etheridge et al. 2008, Yu et al. 2010). Exencephaly also develops in mutants for the PCP effector genes Fuz or Intu, but the role of these genes in cilium-dependent hedgehog signaling seems more likely to explain their loss-of-function effect on cranial neural tube closure than does a role in regulating convergent extension (Gray et al. 2009, Heydeck & Liu 2011, Zeng et al. 2010) (see Bending of the Neural Folds: Regulation by Shh and BMP Signaling, below). Thus, components of PCP signaling potentially affect neural tube closure via multiple cellular mechanisms.
Bending of the Neural Folds: Regulation by Shh and BMP Signaling
To achieve closure, the neuroepithelium must bend to bring the tips of the neural folds into apposition. Bending occurs in a stereotypical manner at hinge points: a median hinge point (MHP) in the midline and paired dorsolateral hinge points (DLHPs) that arise laterally (Shum & Copp 1996). The morphology varies along the body axis with differing modes in the upper (MHP only), mid-spine (MHP and DLHPs), and caudal (DLHPs only) regions of the primary spinal neural tube.
The mechanisms underlying neuroepithelial bending are not fully understood, but one notable feature of the MHP is the predominance of wedge-shaped cells (wider basally than apically) compared with nonbending regions (Schoenwolf & Smith 1990). At neural plate stages, the neuroepithelium is a pseudostratified epithelium in which nuclei move to the basal pole during S-phase, owing to interkinetic nuclear migration. Prolongation of S-phase at the MHP provides a possible means by which regulation of the cell cycle may contribute to cell wedging and hence MHP formation (Schoenwolf & Smith 1990).
Bending is regulated by signals emanating from nonneural tissues dorsal and ventral to the neural folds (reviewed by Greene & Copp 2009). The MHP is induced by signals from the notochord, located immediately ventral to the midline of the neuroepithelium (Smith & Schoenwolf 1989, Ybot-Gonzalez et al. 2002). At the molecular level, notochord-derived Shh induces the floor plate of the neural tube at the MHP (Chiang et al. 1996, Placzek & Briscoe 2005). However, this action is not essential for spinal neural tube closure, which completes in the absence of a floor plate in mouse embryos lacking Shh or Fox A2 (Ang & Rossant 1994, Chiang et al. 1996). Thus, the MHP may be functionally important in floor plate development but is not essential for neural tube closure.
In contrast to the MHP, DLHPs appear essential for the neural tube to close in the low spinal region. For example, Zic2 mutant embryos, in which DLHPs are absent, develop severe spina bifida (Ybot-Gonzalez et al. 2007a). The formation of DLHPs is actively regulated; the interplay of inhibitory and inductive signals determines their appearance at different axial levels (Copp & Greene 2013). These signals include inhibitory effects of Shh from the notochord and BMP signaling from the surface ectoderm at the dorsal tips of the neural folds. These signals are opposed by the BMP antagonist noggin, whose expression in the dorsal neural folds is sufficient to induce DLHPs (Ybot-Gonzalez et al. 2002, 2007a).
In contrast to the effects of an absence of Shh signaling, NTDs do result from mutations that enhance Shh signaling, for example, through deficient function of inhibitory or cilia-related genes such as Gli3, Rab23, Fkbp8, Tulp3, and Ift40 (Miller et al. 2013, Murdoch & Copp 2010). Mutants involving increased Shh signaling display NTDs at cranial and/or spinal levels. Although spina bifida in some of these models appears to be associated with suppression of dorsolateral bending of the neural folds (Murdoch & Copp 2010), the mechanism underlying cranial NTDs is not clear.
Cranial Neurulation: Additional Complexity and Sensitivity to Disruption
The neural folds in the cranial region bend in the midline and dorsolaterally as in the mid-spinal region, but the closure process appears morphologically more complex. The folds are initially biconvex, with the tips facing away from the midline, and then switch to a biconcave shape allowing the tips to approach in the midline. The additional complexity of cranial compared with spinal neurulation appears to be reflected in a more extensive genetic underpinning and a greater sensitivity to disruption, at least in rodents. Exencephaly occurs in approximately three times as many knockout mouse models as does spina bifida and is the NTD type most commonly induced by teratogens (Copp et al. 1990, Harris & Juriloff 2010).
Cranial neurulation may rely on specific contributory factors that are not involved in the spinal region such as expansion of the mesenchyme underlying the neural folds (Greene & Copp 2009, Zohn & Sarkar 2012). Moreover, disruption of the actin cytoskeleton prevents closure in the cranial but not the spinal region (Morriss-Kay & Tuckett 1985, Ybot-Gonzalez & Copp 1999). Similarly, exencephaly is observed, but spinal neurulation completes successfully in null mutants for several cytoskeletal components (e.g., n-cofilin, vinculin) (Gurniak et al. 2005, Xu et al. 1998). Nevertheless, apically located actin microfilaments are present throughout the neuroepithelium (Sadler et al. 1982) and functional disruption of the cytoskeleton-associated proteins MARCKS-related protein or Shroom3 causes both spinal and cranial NTDs (Hildebrand & Soriano 1999, Xu et al. 1998) suggesting that regulation of the actomyosin cytoskeleton plays a role in closure in both regions. Shroom proteins appear to play a key role: Expression of Shroom in Xenopus is sufficient to induce apical constriction of epithelial cells, whereas functional disruption inhibits neural fold bending and suppresses closure (Haigo et al. 2003).
Adhesion and Fusion of the Neural Folds
Once the neural folds meet at the dorsal midline, processes of adhesion, fusion, and remodeling give rise to two discrete epithelial layers, with the nascent neural tube overlain by an intact surface ectoderm (Pai et al. 2012). At the closure site, the neural fold tips are composed of neuroepithelium continuous with the nonneural surface ectoderm. The cell type that adheres first may differ at varying axial levels (Geelen & Langman 1979, Ray & Niswander 2012). Nevertheless, at all levels initial contact appears to involve subcellular protrusions, resembling lamellipodia and filopodia, observed by electron microscopy (Geelen & Langman 1979) and in live embryos (Pyrgaki et al. 2010). The molecular basis of adhesion is not well characterized, perhaps owing to functional redundancy among the proteins involved. However, a role for the interaction of cell surface ephrin receptors with Eph ligands is suggested by the occurrence of cranial NTDs in mice lacking ephrin-A5 or EphA7 (Holmberg et al. 2000) and by delayed spinal closure in embryos exposed to peptides that block ephrin-A/EphA interactions (Abdul-Aziz et al. 2009).
Knockout of protease-activated receptors (PAR1 and PAR2) in the surface ectoderm also causes cranial NTDs, implicating a role for signaling via these G protein--coupled receptors in closure (Camerer et al. 2010). Further evidence for the function of the nonneural ectoderm is provided by Grhl2 null mutants, which fail in closure throughout the cranial region and exhibit spina bifida (Brouns et al. 2011, Rifat et al. 2010, Werth et al. 2010). Grhl2 is expressed in the surface ectoderm overlying the neural folds and regulates expression of several components of the apical adhesion junction complex, including E-cadherin (Pyrgaki et al. 2011, Werth et al. 2010).
Regulation of Cell Proliferation and Cell Death
During neurulation the embryo grows rapidly. Cell cycle exit and neuronal differentiation begin in the neuroepithelium shortly after closure, and maintenance of adequate proliferation in the neuroepithelium appears crucial for closure, particularly in the cranial region. Thus, in mice, NTDs can be caused by exposure to antimitotic agents (Copp et al. 1990) or mutation of genes encoding proteins associated with cell-cycle progression (e.g., neurofibromin 1, nucleoporin) or prevention of neuronal differentiation (e.g., Notch pathway genes Hes1, Hes3, RBP-Jκ) (Harris & Juriloff 2007, 2010). Conversely, excessive cell proliferation is also associated with NTDs in several mouse models, such as Phactr4 mutants (Kim et al. 2007).
Characteristic patterns of apoptotic cell death occur in the neural folds and the midline of the closed neural tube (Geelen & Langman 1979, Massa et al. 2009, Yamaguchi et al. 2011). Increased cell death could inhibit closure by compromising the functional and/or mechanical integrity of the neuroepithelium. It is associated with NTDs in several teratogen-induced and genetic models, although only rarely has a direct causal link been definitively established (Copp & Greene 2013, Fukuda et al. 2011). The occurrence of exencephaly in mice lacking apoptosis-related genes such as caspase3 or Apaf1 suggests a requirement for apoptosis in closure (Harris & Juriloff 2010). However, forebrain and spinal closure occurs normally in these models and pharmacological suppression of apoptosis does not cause NTDs, suggesting that it is dispensable to complete closure (Massa et al. 2009).
CLINICAL FEATURES OF NEURAL TUBE DEFECTS
Owing to their multifactorial causation NTDs represent a group of disorders. However, after failure of neural fold closure has occurred defects, originating from various primary causes, may share similar pathogenic features.
Open NTDs and Associated Conditions
Open NTDs can result from failure of closure at a de novo initiation site or incomplete progression of closure following successful initiation (Figure 1). Where embryos are available for examination, as in experimental models, NTDs can be recognized during or immediately after neurulation stages owing to the persistently open neural folds. However, at later embryonic and fetal stages, the morphological appearance changes considerably owing to secondary changes and degeneration.
In cranial NTDs, the open neural folds undergo growth and differentiation and typically appear to bulge from the developing brain, termed exencephaly. Inability to form the skull vault over the open region causes the exposed neural tissue to degenerate, leading to the characteristic appearance of anencephaly, observed later in human or rodent pregnancy (Wood & Smith 1984, Seller 1995). Both anencephaly and craniorachischisis (~10% of NTDs) are lethal conditions at or shortly after birth.
Open neural folds in the spinal region prevent the sclerotome-derived vertebral arches from covering the neuroepithelium, the consequent opening in the vertebral column giving rise to the term spina bifida (Copp et al. 2013). The neural tissues may be contained within a meninges-covered sac that protrudes through the open vertebrae (myelomeningocele; spina bifida cystica) or exposed directly to the amniotic fluid (myelocele). Babies born with open spina bifida usually survive with appropriate medical care but suffer neurological impairment, the severity of which depends on the level of the lesion. Associated conditions include hydrocephalus, Chiari malformation type II, and vertebral abnormalities as well as genitourinary and gastrointestinal disorders.
Diagnosis, Treatment, and Maternal-Fetal Surgery
NTDs can be diagnosed prenatally by ultrasound (Cameron & Moran 2009). However, where prenatal diagnosis is not routinely available and/or therapeutic abortion is not an option, many babies with NTDs are born. Postnatal medical care for babies born with open spina bifida usually involves surgery to close and cover the lesion. Multiple subsequent surgeries are commonly required to alleviate tethering of the spinal cord, treat hydrocephalus, and/or address orthopedic and urological problems.
As open NTDs arise early during pregnancy, there is a prolonged period during which secondary neurological damage may occur owing to exposure of nervous tissue to the amniotic fluid environment. These considerations provided impetus for the development of in utero fetal surgery for spina bifida, which may improve neurological outcomes compared with postnatal repair, although with fetal and maternal risks (Adzick et al. 1998, 2011). Experimental models of spina bifida are being used to investigate the possible combination of surgical intervention with additional therapy, intended to remediate neural damage. Examples include the implantation of biodegradable scaffolds to promote neural regeneration and/or neural stem cells to populate the damaged spinal cord (Saadai et al. 2011, 2013).
Disorders of the Closed Neural Tube
This review focuses on open NTDs, characterized by failure of neural tube closure. Various other conditions are also associated with abnormalities of the closed brain or spinal cord and are often categorized as NTDs under a broader definition (Figure 1). There is also a less well-defined group of closed spinal NTDs in which the vertebral arches are malformed but covered by skin. These conditions, including spina bifida occulta and spinal dysraphisms, vary widely in clinical presentation. The more severe subtypes are associated with various abnormalities of the spinal cord, lipoma (Figure 1c), and/or anorectal abnormalities. The embryonic origin of closed spina bifida is not well defined but is hypothesized to involve abnormalities of secondary neurulation (Copp et al. 2013). Abnormal development of the skull or vertebrae may also allow herniation of the closed neural tube through the affected bony region, as in encephalocele (Figure 1d) or meningocele, respectively.
CAUSES OF NTDs
NTDs are among the most common birth defects worldwide with a prevalence that varies from 0.5 to more than 10 per 1,000 pregnancies. This variance likely reflects differing contributions from risk factors such as nutritional status, prevalence of obesity and diabetes, usage of folic acid supplementation and/or fortification, the presence of environmental toxicants, and differing genetic predisposition among ethnic groups. In most populations, there is also a striking gender bias: Anencephaly is more prevalent among females than males. Many NTD mouse strains also show a female preponderance among cranial NTDs, apparently reflecting a fundamental higher sensitivity of cranial neural tube closure to disturbance in female embryos (Juriloff & Harris 2012b). Overall, although studies have identified numerous risk factors, these may account for less than half of NTDs, suggesting that additional genetic and nongenetic factors remain to be identified (Agopian et al. 2013).
ENVIRONMENT FACTORS
Various teratogenic agents induce NTDs in rodent models (Copp et al. 1990, Copp & Greene 2010). In humans, teratogens that have been associated with NTDs include the anticonvulsant drug valproic acid (Wlodarczyk et al. 2012) and the fungal product fumonisin (Missmer et al. 2006). Other nongenetic risk factors include maternal fever and excessive use of hot tubs (Moretti et al. 2005), consistent with the induction of NTDs by hypothermia in rodent models.
Maternal obesity and diabetes are well-recognized risk factors for NTDs (Correa et al. 2003). Determining the cause of diabetes-related NTDs is hampered by the complexity of the diabetic milieu, although hyperglycemia alone is sufficient to cause NTDs in cultured rodent embryos. NTDs may result from increased oxidative stress, altered expression of genes such as Pax3, and neuroepithelial cell apoptosis (Fine et al. 1999, Reece 2012). Recent findings suggest that activation of apoptosis signal-regulating kinase 1 (ASK1) in hyperglycemic conditions leads to activation of the apoptosis mediator caspase 8 by stimulating the FoxO3a transcription factor (Yang et al. 2013).
Nutritional factors and folate
The historical link between lower socioeconomic status and higher risk of birth defects led investigators to examine the possible involvement of nutritional factors in NTDs. Lower blood levels of the B-vitamin folate were observed in mothers of NTD fetuses (Smithells et al. 1976), prompting an intervention trial of a folic acid--containing multivitamin supplement to prevent NTD recurrence (Schorah 2008, Smithells et al. 1981). A multicenter randomized controlled trial confirmed that maternal folic acid supplementation (at 4 mg per day) significantly reduces the recurrence risk (Wald et al. 1991). Additional clinical trials provided evidence for reduction of occurrence risk (Berry et al. 1999, Czeizel et al. 2011, Czeizel & Dudás 1992).
Questions remain concerning the mechanism by which folic acid prevents NTDs (Blom et al. 2006, Copp et al. 2013). Although maternal folate status is a risk factor, in most cases, maternal folate levels are within the normal range and rarely clinically deficient. Nonetheless, data have shown an inverse relationship between blood folate concentration and risk of an affected pregnancy (Daly et al. 1995). Suboptimal folate levels may contribute to NTD development in individuals who are genetically susceptible. Such a gene-environment interaction has been demonstrated in mice, where folate deficiency does not cause NTDs unless deficiency is present in combination with a mutation of a predisposing gene, such as Pax3 (Burren et al. 2008).
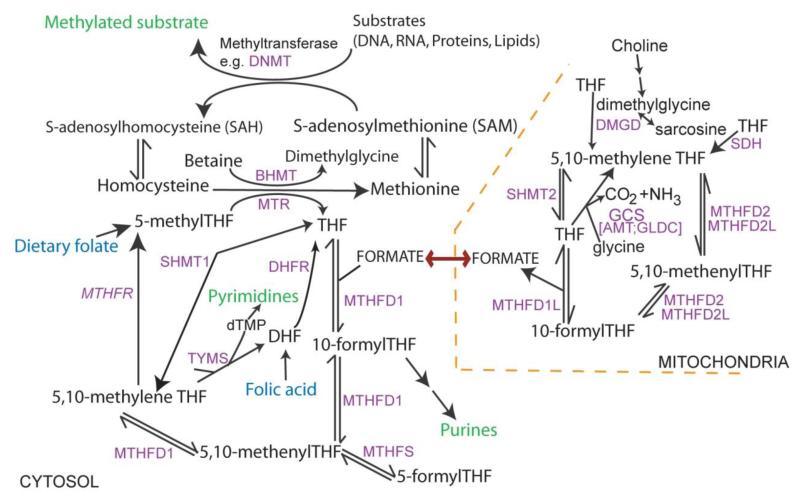
Folate one-carbon metabolism (Figure 2) comprises a complex network of interlinked reactions that mediate transfer of one-carbon groups for several biosynthetic processes (Stover 2009). Among these, attention has focused particularly on the requirement for nucleotide biosynthesis and methylation reactions in neural tube closure. Abnormal thymidylate and purine biosynthesis have been identified in mouse NTD models (Beaudin et al. 2011, Fleming & Copp 1998) and in a proportion of NTD cases (Dunlevy et al. 2007), whereas deficient methylation may also be implicated in NTDs (see Gene-Regulatory Mechanisms and NTDs, below).
Figure 2. Overview of folate one-carbon metabolism.
Folates provide a backbone for the transfer of one-carbon units. Key outputs (in green) include nucleotide biosynthesis and methylation. Among methylation cycle intermediates, homocysteine may also be converted to cystathionine in the transulfuration pathway and S-adenosylmethionine is involved in polyamine biosynthesis. FOCM is compartmentalised: one-carbon units from the mitochondria enter cytoplasmic FOCM as formate while reactions of thymidylate biosynthesis also operate in the nucleus (catalysed by SHMT1, TYMS and DHFR). In loss-of-function mouse models, NTDs arise in mutants for Mthfd1l and genes encoding the glycine cleavage system (GCS). Shmt1 and Mthfr null mice are viable to birth but may develop NTDs under folate-deficient conditions.
GENETICS OF NTDS
Most NTDs occur sporadically, with a relative scarcity ofmultigenerational families. Nevertheless, strong evidence demonstrates a genetic component in the etiology of NTDs, and the pattern of inheritance favors a multifactorial polygenic or oligogenic model, as opposed to an effect of single genes with partial penetrance (Harris & Juriloff 2007). Most studies of NTD genetics have focused on one or more candidate genes (reviewed by Boyles et al. 2005, Greene et al. 2009, Harris & Juriloff 2010). In general, candidates have been (a) human orthologs of genes whose mutation causes NTDs in mice, of which there are more than 200 examples; or (b) genes related to environmental risk factors, particularly folate metabolism.
Case-control association studies have implicated several genes, whereas mutation screening by sequencing has identified putative pathogenic mutations. However, the definitive assignment of a gene variant as causative is complicated by the apparent multigenic nature of NTDs and by the large number of possible candidate genes, modifier genes, epigenetic factors, and environmental influences. Moreover, where putative mutations have been identified in specific genes, each has been involved in only a small proportion of NTD patients, suggesting that there is considerable heterogeneity underlying the genetic basis of NTDs. Thus, although the morphological and cellular bases of neural tube closure have become increasingly well understood, the genetic basis of NTDs in individual cases remains largely unclear.
Gene-gene interactions and effect of modifier genes
Mouse studies suggest three broad mechanisms by which genetic interactions may result in NTDs. First, in some instances functional redundancy makes it necessary for two orthologous genes to be mutated [e.g., Dvl1-Dvl2 (Hamblet et al. 2002), Cdx1-Cdx2 double knockouts (Savory et al. 2011)], in order to reveal a requirement in neural tube closure. Second, additive effects of heterozygous mutations may result in NTDs that resemble those of individual homozygotes [e.g., Dvl3 with Vangl2Lp (Etheridge et al. 2008)]. Third, variation in the penetrance and expressivity of NTD phenotypes between inbred mouse strains is widely reported to reflect variants in modifier genes. For example, the rate of exencephaly resulting from Cecr1 mutation is strongly affected by strain background (Davidson et al. 2007). Whereas the identity of modifier genes for NTDs has rarely been determined, a variant in Lmnb1 is present in some mouse strains and significantly increases the frequency of NTDs in curly tail (Grhl3ct) embryos (de Castro et al. 2012).
Genes implicated through experimental models
In mice, mutation of genes encoding components of the PCP pathway causes NTDs (see Shaping of the Neural Plate, above). Sequencing of PCP genes in humans has identified putative mutations in CELSR1, VANGL1, VANGL2, FZD6, SCRIB1, and DVL2 in some patients with craniorachischisis, spina bifida, anencephaly, or closed forms of spina bifida (Chandler et al. 2012; De Marco et al. 2013; Kibar et al. 2007; Lei et al. 2010, 2013; Robinson et al. 2012; and reviewed by Juriloff & Harris 2012a). As in mice, heterozygous human PCP mutations may interact with other genetic NTD risk factors in a digenic or polygenic fashion to cause a range of NTD types. This interaction could involve summation of multiple variants in PCP genes. For example, a putative mutation in DVL2 was identified in a spina bifida patient in combination with a second, previously identified missense variant in VANGL2 (De Marco et al. 2013).
Among other genes implicated in NTDs from mouse models, association studies have not provided evidence for a major contribution to risk, and few positive results have emerged from sequencing-based mutation screens. As data begin to emerge from large-scale exome sequencing studies of NTD patients, it will become possible to evaluate the contribution of multiple genes in the same patient cohorts and the mutational load associated with individual risk.
Analysis of genes related to environmental risk factors
The identification of environmental factors such as maternal diabetes and folate status as risk factors for NTDs provides impetus for researchers to analyze related genes in affected families. Risk could be associated with maternal genotype if genetic variation alters maternal metabolism and secondarily affects the developing embryo. However, the inheritance of maternal alleles by the embryo complicates interpretation of such effects. Alternatively, a genetically determined abnormality in the embryo itself could influence risk of NTDs, potentially through interaction with a predisposing environmental factor. For example, it may be informative to analyze genetic data on folate-related genes in the context of maternal folate status (Etheredge et al. 2012).
Association with risk of spina bifida has been reported for several genes implicated in diabetes, obesity, glucose metabolism, and oxidative stress. These potential ‘risk’ genes include GLUT1, SOD1, and SOD2 (Davidson et al. 2008, Kase et al. 2012). Maternal variants in the obesity-related genes FTO, LEP, and TCF7L2 are also associated with NTDs, consistent with maternal obesity as a risk factor (Lupo et al. 2012).
Genes related to folate one-carbon metabolism have been perhaps the most intensively studied group of candidates for NTDs (reviewed by Blom et al. 2006, Greene et al. 2009, Shaw et al. 2009). The C677T polymorphism of MTHFR, which encodes an alanine-to-valine substitution, has been associated with NTDs. The TT genotype is found at higher frequency among NTD cases than in controls in some populations (e.g., Irish) but not others (e.g., Hispanics) (Botto & Yang 2000). Several studies indicate positive associations with other folate-related genes, including MTRR, although these have generally not been observed in all study populations.
In mice, mutations in folate-metabolizing enzymes (e.g., Mthfd1) are sometimes lethal before the stage of neural tube closure (e.g., Christensen et al. 2013, MacFarlane et al. 2009), whereas others do not disrupt closure (e.g., Chen et al. 2001, Di Pietro et al. 2002). Null embryos for the folate receptor, Folr1, die preneurulation but develop NTDs when supplemented with sufficient folic acid to prevent early lethality (Piedrahita et al. 1999). NTDs are also observed in Shmt1 knockouts, under folate-deficient conditions (Beaudin et al. 2011). In contrast, NTDs occur spontaneously in mice carrying loss-of-function alleles of Amt (Narisawa et al. 2012) or Mthfd1L (Momb et al. 2013), both of which encode enzymes of mitochondrial folate metabolism (see sidebar and Figure 2) (Tibbetts & Appling 2010). The homologous genes in humans have also been linked to NTDs. Missense mutations have been identified in NTD patients in AMT as well as in GLDC, which encodes its partner enzyme in the glycine cleavage system (Narisawa et al. 2012). Genetic associations with NTDs have been reported for MTHFD1L (Parle-McDermott et al. 2009) and SLC23A32 (MFTC), encoding a mitochondrial folate transporter (Pangilinan et al. 2012). Altogether, these findings suggest that NTD risk is influenced by the function of mitochondrial folate metabolism, a major source of one-carbon units to the cytoplasm.
Gene-Regulatory Mechanisms and NTDs
Identification of causative genes may be complicated, in addition to the potential multigenic nature of NTDs, by the potential involvement of aberrant gene expression, perhaps resulting from mutations in regulatory elements. For example, mutations resulting in insufficient expression of Grhl3 or excess expression of Grhl2 cause NTDs in mice in the absence of coding mutations (Brouns et al. 2011, Gustavsson et al. 2007). Further complexity may be added by the potential for regulation by epigenetic modifications such as DNA methylation, histone modification, or chromatin remodeling, each of which has been associated with NTDs in mice and in some cases in humans (reviewed by Greene et al. 2011, Harris & Juriloff 2010). For example, methylation of LINE-1 genomic elements was lower than normal in DNA of anencephalic but not spina bifida fetuses (Wang et al. 2010).
A simple model predicts a positive correlation between folate status and methylation. However, data from human pregnancy suggest that the relationship is not straightforward (Crider et al. 2012). A recent study found an inverse correlation of LINE-1 methylation with maternal and cord blood folate, whereas different imprinted genes showed positive or negative associations (Haggarty et al. 2013). Somewhat counterintuitively, use of folic acid supplements was associated with reduced LINE-1 methylation.
A requirement for DNA methylation in mouse neural tube closure is suggested by the occurrence of NTDs in knockouts of Dnmt3b, encoding a DNA methyltransferase, and in embryos cultured with 5-azacytidine (Matsuda & Yasutomi 1992, Okano et al. 1999). Similarly, inhibition of the methylation cycle reduces DNA methylation and causes NTDs in cultured mouse embryos (Burren et al. 2008, Dunlevy et al. 2006). However, Mthfr null embryos do not develop NTDs despite a significant reduction in global DNA methylation (Chen et al. 2001), nor is there an exacerbating effect of Mthfr loss-of-function on Pax3 or curly tail mutants, although both show increased rates of NTDs under folate-deficient conditions (Burren et al. 2008, de Castro et al. 2010, Pickell et al. 2009). Thus, questions remain about the relationships among folate status, DNA methylation, and risk of NTDs.
Other epigenetic mechanisms include various modifications of histone proteins, which potentially misregulate genes that influence neurulation. NTDs occur in mice carrying mutations in the histone demethylases Jarid2 (Takeuchi et al. 1999) and Fbxl10 (Fukuda et al. 2011). Similarly, histone acetylases and deacetylases, which regulate the equilibrium of histone acetylation, are implicated in NTDs. An acetylase-specific knockin mutation of Gcn5 causes cranial NTDs (Bu et al. 2007), as does loss-of-function of another histone acetylase, p300 (Yao et al. 1998). Increased acetylation is also associated with NTDs. For example, cranial NTDs occur in mice carrying mutations in histone deacetylases Sirt1 or Hdac4 (Cheng et al. 2003, Vega et al. 2004). The teratogenic effects of valproic acid and trichostin A may also be mediated through their inhibition of histone deacetylases (Finnell et al. 2002).
PRIMARY PREVENTION OF NTDs
Once the neural tube has failed to close, ensuing damage to the exposed neural tissue is irreversible, despite possible palliative benefit of in utero surgery (see Diagnosis, Treatment, and Maternal-Fetal Surgery). Therefore, primary prevention is the optimal approach for reducing the burden of NTDs.
Folic Acid Supplementation and Fortification
The reduction in risk of NTDs following maternal folic acid supplementation led to public health recommendations that women who may become pregnant should consume 0.4 mg of folic acid daily or 4 mg daily following a previous affected pregnancy (Czeizel et al. 2011). To ensure that additional folate was received, food fortification programs were introduced in many countries. This approach has raised blood folate levels and has been associated with lower NTD frequency (Crider et al. 2011). The magnitude of effect varies, with greatest reduction found where preexisting rates were highest (Blencowe et al. 2010, Rosenthal et al. 2013). Some countries have delayed a decision on fortification owing to safety concerns (e.g., possible enhancement of bowel cancer), but a recent meta-analysis found no evidence for increased cancer rates following folic acid supplementation (Vollset et al. 2013).
Folate-Resistant NTDs
Folic acid supplementation in clinical trials has not approached 100% NTD prevention, and an estimated one-third of NTDs may be folic acid resistant (Blencowe et al. 2010). A study in the United States, where folate fortification of food is mandatory, found no apparent protective effect of folic acid supplements (Mosley et al. 2009), suggesting that increased dosage would not necessarily provide additional preventive effects.
Given the multifactorial causation of NTDs it seems reasonable to suppose that optimal prevention will require a combination of multiple interventions. Possible approaches may relate to folate one-carbon metabolism. For example, as with folate, evidence shows a graded relationship between lower levels of circulating vitamin B12 and increased risk of an NTD-affected pregnancy (Molloy et al. 2009). Perhaps use of B12 supplements would further reduce NTD frequency, although this approach remains to be tested.
Another possibility is that folic acid cannot ameliorate some defects that result from abnormal folate metabolism, owing to defects in the intervening enzymes required to transfer one-carbon units to key downstream metabolites. In this case, supplementation with alternative folates, such as 5-methyl THF (Czeizel et al. 2011), or key downstream molecules may be advantageous. For example, supplementation with formate prevented NTDs in Mthfd1L null mice (Momb et al. 2013), whereas combinations of thymidine and purine precursors prevented NTDs in curly tail mice, in which folic acid is not protective (Leung et al. 2013).
In addition to low levels of folate and vitamin B12, lower maternal levels of other vitamins, including vitamin C, have been reported in NTDs (Smithells et al. 1976). Conversely, intake of several vitamins and maternal diet are associated with lower risk of NTDs, which suggests that nutrients other than folic acid may be beneficial (Chandler et al. 2012, Sotres-Alvarez et al. 2013). Experimental analysis of individual vitamins found that myo-inositol deficiency caused NTDs in cultured rodent embryos (Cockroft 1988). Inositol supplementation significantly reduced NTD frequency in curly tail mice (Greene & Copp 1997) and in rodent models of diabetes (Reece et al. 1997) and inositol is in clinical testing for prevention of NTD recurrence.
SUMMARY
Experimental models provide systems for analysis of the developmental events of neural tube closure, and fundamental cellular and morphological processes continue to be defined in more detail. In principle, NTDs may result from insufficiency of one or more of the key driving forces (e.g., cellular properties and/or morphogenetic movements) that are necessary to achieve closure, for example, through mutation of a PCP gene. Alternatively, a genetic lesion or environmental insult may disrupt the closure process even where the underlying machinery is intact, for example through induction of aberrant cellular behaviors such as excess apoptosis. Experimental models require careful analysis to disentangle these possibilities. A key challenge will be to understand how the molecular and cellular determinants of neurulation relate to the biomechanical forces required to fold the neuroepithelium to achieve closure.
Advances in exome and whole-genome sequencing may help researchers begin to understand the genetic basis of NTDs in humans. The multifactorial complexity of NTDs means that analysis of data from such studies will present a major challenge. Moreover, investigators will need to integrate genetic data with information on epigenetic and environmental factors to obtain a more complete understanding of the cause of individual NTDs.
Folic acid supplementation provides a means to reduce NTD risk and represents a major public health advance. Nevertheless, the heterogeneity of NTDs suggests that primary prevention may be achieved best by multiple interventions, and use of additional micronutrients alongside folic acid may provide additional opportunities to further reduce risk.
ACKNOWLEDGMENTS
Work in the authors’ laboratory is funded by the Medical Research Council (J003794), the Newlife Foundation (11-1206), and the Wellcome Trust (087525).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Abdul-Aziz NM, Turmaine M, Greene ND, Copp AJ. EphrinA-EphA receptor interactions in mouse spinal neurulation: implications for neural fold fusion. Int. J. Dev. Biol. 2009;53:559–68. doi: 10.1387/ijdb.082777na. [DOI] [PubMed] [Google Scholar]
- Adzick NS, Sutton LN, Crombleholme TM, Flake AW. Successful fetal surgery for spina bifida. Lancet. 1998;352:1675–76. doi: 10.1016/S0140-6736(98)00070-1. [DOI] [PubMed] [Google Scholar]
- Adzick NS, Thom EA, Spong CY, Brock JW, III, Burrows PK, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N. Engl. J. Med. 2011;364:993–1004. doi: 10.1056/NEJMoa1014379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agopian AJ, Tinker SC, Lupo PJ, Canfield MA, Mitchell LE. Proportion of neural tube defects attributable to known risk factors. Birth Defects Res. A Clin. Mol. Teratol. 2013;97(1):42–46. doi: 10.1002/bdra.23100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang S-L, Rossant J. HNF-3β is essential for node and notochord formation in mouse development. Cell. 1994;78:561–74. doi: 10.1016/0092-8674(94)90522-3. [DOI] [PubMed] [Google Scholar]
- Beaudin AE, Abarinov EV, Noden DM, Perry CA, Chu S, et al. Shmt1 and de novo thymidylate biosynthesis underlie folate-responsive neural tube defects in mice. Am. J. Clin. Nutr. 2011;93:789–98. doi: 10.3945/ajcn.110.002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry RJ, Li Z, Erickson JD, Li S, Moore CA, et al. Prevention of neural-tube defects with folic acid in China. N. Engl. J. Med. 1999;341(20):1485–90. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Modell B, Lawn J. Folic acid to reduce neonatal mortality from neural tube disorders. Int. J. Epidemiol. 2010;39(Suppl. 1):i110–21. doi: 10.1093/ije/dyq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom HJ, Shaw GM, den Heijer M, Finnell RH. Neural tube defects and folate: case far from closed. Nat. Rev. Neurosci. 2006;7(9):724–31. doi: 10.1038/nrn1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto LD, Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am. J. Epidemiol. 2000;151(9):862–77. doi: 10.1093/oxfordjournals.aje.a010290. [DOI] [PubMed] [Google Scholar]
- Boyles AL, Hammock P, Speer MC. Candidate gene analysis in human neural tube defects. Am. J. Med. Genet. C. Semin. Med. Genet. 2005;135(1):9–23. doi: 10.1002/ajmg.c.30048. [DOI] [PubMed] [Google Scholar]
- Brouns MR, De Castro SC, Terwindt-Rouwenhorst EA, Massa V, Hekking JW, et al. Over-expression of Grhl2 causes spina bifida in the Axial defects mutant mouse. Hum. Mol. Genet. 2011;20:1536–46. doi: 10.1093/hmg/ddr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu P, Evrard YA, Lozano G, Dent SY. Loss of Gcn5 acetyltransferase activity leads to neural tube closure defects and exencephaly in mouse embryos. Mol. Cell. Biol. 2007;27:3405–16. doi: 10.1128/MCB.00066-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burren KA, Savery D, Massa V, Kok RM, Scott JM, et al. Gene-environment interactions in the causation of neural tube defects: folate deficiency increases susceptibility conferred by loss of Pax3 function. Hum. Mol. Genet. 2008;17:3675–85. doi: 10.1093/hmg/ddn262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer E, Barker A, Duong DN, Ganesan R, Kataoka H, et al. Local protease signaling contributes to neural tube closure in the mouse embryo. Dev. Cell. 2010;18:25–38. doi: 10.1016/j.devcel.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron M, Moran P. Prenatal screening and diagnosis of neural tube defects. Prenat. Diag. 2009;29:402–11. doi: 10.1002/pd.2250. [DOI] [PubMed] [Google Scholar]
- Chandler AL, Hobbs CA, Mosley BS, Berry RJ, Canfield MA, et al. Neural tube defects and maternal intake of micronutrients related to one-carbon metabolism or antioxidant activity. Birth Defects Res. A Clin. Mol. Teratol. 2012;94(11):864–74. doi: 10.1002/bdra.23068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Melnyk S, et al. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum. Mol. Genet. 2001;10(5):433–43. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc. Natl. Acad. Sci. USA. 2003;100(19):10794–99. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–13. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Christensen KE, Deng L, Leung KY, Arning E, Bottiglieri T, et al. A novel mouse model for genetic variation in 10-formyltetrahydrofolate synthetase exhibits disturbed purine synthesis with impacts on pregnancy and embryonic development. Hum. Mol. Genet. 2013;22(18):3705–19. doi: 10.1093/hmg/ddt223. [DOI] [PubMed] [Google Scholar]
- Cockroft DL. Changes with gestational age in the nutritional requirements of postimplantation rat embryos in culture. Teratology. 1988;38:281–90. doi: 10.1002/tera.1420380312. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Brook FA. Does lumbosacral spina bifida arise by failure of neural folding or by defective canalisation? J. Med. Genet. 1989;26:160–66. doi: 10.1136/jmg.26.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp AJ, Brook FA, Estibeiro JP, Shum ASW, Cockroft DL. The embryonic development of mammalian neural tube defects. Prog. Neurobiol. 1990;35:363–403. doi: 10.1016/0301-0082(90)90037-h. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Checiu I, Henson JN. Developmental basis of severe neural tube defects in the loop-tail (Lp) mutant mouse: use of microsatellite DNA markers to identify embryonic genotype. Dev. Biol. 1994;165:20–29. doi: 10.1006/dbio.1994.1230. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Greene NDE. Genetics and development of neural tube defects. J. Pathol. 2010;220:217–30. doi: 10.1002/path.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp AJ, Greene NDE. Neural tube defects--disorders of neurulation and related embryonic processes. Wiley Interdiscip. Rev. Dev. Biol. 2013;2(2):213–27. doi: 10.1002/wdev.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp AJ, Greene NDE, Murdoch JN. The genetic basis of mammalian neurulation. Nat. Rev. Genet. 2003;4:784–93. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Stanier P, Greene ND. Neural tube defects: recent advances, unsolved questions, and controversies. Lancet Neurol. 2013;12(8):799–810. doi: 10.1016/S1474-4422(13)70110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa A, Botto L, Liu YC, Mulinare J, Erickson JD. Do multivitamin supplements attenuate the risk for diabetes-associated birth defects? Pediatrics. 2003;111:1146–51. [PubMed] [Google Scholar]
- Crider KS, Bailey LB, Berry RJ. Folic acid food fortification--its history, effect, concerns, and future directions. Nutrients. 2011;3(3):370–84. doi: 10.3390/nu3030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012;3(1):21–38. doi: 10.3945/an.111.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel AE, Dudás I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N. Engl. J. Med. 1992;327:1832–35. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Dudás I, Paput L, Bánhidy F. Prevention of neural-tube defects with periconceptional folic acid, methylfolate, or multivitamins? Ann. Nutr. Metab. 2011;58:263–71. doi: 10.1159/000330776. [DOI] [PubMed] [Google Scholar]
- Daly LE, Kirke PN, Molloy A, Weir DG, Scott JM. Folate levels and neural tube defects: implications for prevention. JAMA. 1995;274(21):1698–702. doi: 10.1001/jama.1995.03530210052030. [DOI] [PubMed] [Google Scholar]
- Davidson CE, Li Q, Churchill GA, Osborne LR, McDermid HE. Modifier locus for exencephaly in Cecr2 mutant mice is syntenic to the 10q25.3 region associated with neural tube defects in humans. Physiol. Genomics. 2007;31:244–51. doi: 10.1152/physiolgenomics.00062.2007. [DOI] [PubMed] [Google Scholar]
- Davidson CM, Northrup H, King TM, Fletcher JM, Townsend I, et al. Genes in glucose metabolism and association with spina bifida. Reprod. Sci. 2008;15(1):51–58. doi: 10.1177/1933719107309590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro SC, Leung KY, Savery D, Burren K, Rozen R, et al. Neural tube defects induced by folate deficiency in mutant curly tail (Grhl3) embryos are associated with alteration in folate one-carbon metabolism but are unlikely to result from diminished methylation. Birth Defects Res. A Clin Mol. Teratol. 2010;88:612–18. doi: 10.1002/bdra.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro SC, Malhas A, Leung K-Y, Gustavsson P, Vaux DJ, et al. Lamin B1 polymorphism influences morphology of the nuclear envelope, cell cycle progression, and risk of neural tube defects in mice. PLoS Genet. 2012;8(11):e1003059. doi: 10.1371/journal.pgen.1003059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco P, Merello E, Consales A, Piatelli G, Cama A, et al. Genetic analysis of Disheveled 2 and Disheveled 3 in human neural tube defects. J. Mol. Neurosci. 2013;49(3):582–88. doi: 10.1007/s12031-012-9871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro E, Sirois J, Tremblay ML, Mackenzie RE. Mitochondrial NAD-dependent methylenetetrahydrofolate dehydrogenase-methenyltetrahydrofolate cyclohydrolase is essential for embryonic development. Mol. Cell. Biol. 2002;22(12):4158–66. doi: 10.1128/MCB.22.12.4158-4166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlevy LPE, Burren KA, Mills K, Chitty LS, Copp AJ, Greene NDE. Integrity of the methylation cycle is essential for mammalian neural tube closure. Birth Defects Res. A Mol. Teratol. 2006;76:544–52. doi: 10.1002/bdra.20286. [DOI] [PubMed] [Google Scholar]
- Dunlevy LPE, Chitty LS, Burren KA, Doudney K, Stojilkovic-Mikic T, et al. Abnormal folate metabolism in foetuses affected by neural tube defects. Brain. 2007;130:1043–49. doi: 10.1093/brain/awm028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo N, Contreras O, Muñoz R, Farías M, Carrasco H, et al. Syndecan 4 interacts genetically with Vangl2 to regulate neural tube closure and planar cell polarity. Development. 2013;140(14):3008–17. doi: 10.1242/dev.091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheredge AJ, Finnell RH, Carmichael SL, Lammer EJ, Zhu H, et al. Maternal and infant gene-folate interactions and the risk of neural tube defects. Am. J. Med. Genet. A. 2012;158A(10):2439–46. doi: 10.1002/ajmg.a.35552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, et al. Murine Dishevelled 3 functions in redundant pathways with Dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine EL, Horal M, Chang TI, Fortin G, Loeken MR. Evidence that elevated glucose causes altered gene expression, apoptosis, and neural tube defects in a mouse model of diabetic pregnancy. Diabetes. 1999;48(12):2454–62. doi: 10.2337/diabetes.48.12.2454. [DOI] [PubMed] [Google Scholar]
- Finnell RH, Waes JGV, Eudy JD, Rosenquist TH. Molecular basis of environmentally induced birth defects. Annu. Rev. Pharmacol. Toxicol. 2002;42:181–208. doi: 10.1146/annurev.pharmtox.42.083001.110955. [DOI] [PubMed] [Google Scholar]
- Fleming A, Copp AJ. Embryonic folate metabolism and mouse neural tube defects. Science. 1998;280:2107–9. doi: 10.1126/science.280.5372.2107. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Tokunaga A, Sakamoto R, Yoshida N. Fbxl10/Kdm2b deficiency accelerates neural progenitor cell death and leads to exencephaly. Mol. Cell Neurosci. 2011;46:614–24. doi: 10.1016/j.mcn.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Geelen JAG, Langman J. Ultrastructural observations on closure of the neural tube in the mouse. Anat. Embryol. 1979;156:73–88. doi: 10.1007/BF00315716. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138(10):1877–92. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JD, Kholmanskikh S, Castaldo BS, Hansler A, Chung H, et al. LRP6 exerts non-canonical effects on Wnt signaling during neural tube closure. Hum. Mol. Genet. 2013;22(21):4267–81. doi: 10.1093/hmg/ddt277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RS, Abitua PB, Wlodarczyk BJ, Szabo-Rogers HL, Blanchard O, et al. The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat. Cell Biol. 2009;11:1225–32. doi: 10.1038/ncb1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene NDE, Copp AJ. Inositol prevents folate-resistant neural tube defects in the mouse. Nat. Med. 1997;3:60–66. doi: 10.1038/nm0197-60. [DOI] [PubMed] [Google Scholar]
- Greene NDE, Copp AJ. Development of the vertebrate central nervous system: formation of the neural tube. Prenat. Diag. 2009;29:303–11. doi: 10.1002/pd.2206. [DOI] [PubMed] [Google Scholar]
- Greene NDE, Gerrelli D, Van Straaten HWM, Copp AJ. Abnormalities of floor plate, notochord and somite differentiation in the loop-tail (Lp) mouse: a model of severe neural tube defects. Mech. Dev. 1998;73:59–72. doi: 10.1016/s0925-4773(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Greene NDE, Stanier P, Copp AJ. Genetics of human neural tube defects. Hum. Mol. Genet. 2009;18:R113–29. doi: 10.1093/hmg/ddp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene NDE, Stanier P, Moore GE. The emerging role of epigenetic mechanisms in the etiology of neural tube defects. Epigenetics. 2011;6:875–83. doi: 10.4161/epi.6.7.16400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurniak CB, Perlas E, Witke W. The actin depolymerizing factor n-cofilin is essential for neural tube morphogenesis and neural crest cell migration. Dev. Biol. 2005;278(1):231–41. doi: 10.1016/j.ydbio.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Gustavsson P, Greene NDE, Lad D, Pauws E, de Castro SCP, et al. Increased expression of Grainyhead-like-3 rescues spina bifida in a folate-resistant mouse model. Hum. Mol. Genet. 2007;16(21):2640–46. doi: 10.1093/hmg/ddm221. [DOI] [PubMed] [Google Scholar]
- Haggarty P, Hoad G, Campbell DM, Horgan GW, Piyathilake C, McNeill G. Folate in pregnancy and imprinted gene and repeat element methylation in the offspring. Am. J. Clin. Nutr. 2013;97(1):94–99. doi: 10.3945/ajcn.112.042572. [DOI] [PubMed] [Google Scholar]
- Haigo SL, Hildebrand JD, Harland RM, Wallingford JB. Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr. Biol. 2003;13(24):2125–37. doi: 10.1016/j.cub.2003.11.054. [DOI] [PubMed] [Google Scholar]
- Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, et al. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–38. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- Harris MJ, Juriloff DM. Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res. A Clin. Mol. Teratol. 2007;79(3):187–210. doi: 10.1002/bdra.20333. [DOI] [PubMed] [Google Scholar]
- Harris MJ, Juriloff DM. An update to the list of mouse mutants with neural tube closure defects and advances toward a complete genetic perspective of neural tube closure. Birth Defects Res. A Clin. Mol. Teratol. 2010;88:653–69. doi: 10.1002/bdra.20676. [DOI] [PubMed] [Google Scholar]
- Heydeck W, Liu A. PCP effector proteins inturned and fuzzy play nonredundant roles in the patterning but not convergent extension of mammalian neural tube. Dev. Dyn. 2011;240:1938–48. doi: 10.1002/dvdy.22696. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD, Soriano P. Shroom, a PDZ domain-containing actin-binding protein, is required for neural tube morphogenesis in mice. Cell. 1999;99(5):485–97. doi: 10.1016/s0092-8674(00)81537-8. [DOI] [PubMed] [Google Scholar]
- Holmberg J, Clarke DL, Frisén J. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature. 2000;408:203–6. doi: 10.1038/35041577. [DOI] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ. A consideration of the evidence that genetic defects in planar cell polarity contribute to the etiology of human neural tube defects. Birth Defects Res. A Clin. Mol. Teratol. 2012a;94(10):824–40. doi: 10.1002/bdra.23079. [DOI] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ. Hypothesis: the female excess in cranial neural tube defects reflects an epigenetic drag of the inactivating X chromosome on the molecular mechanisms of neural fold elevation. Birth Defects Res. A Clin. Mol. Teratol. 2012b;94(10):849–55. doi: 10.1002/bdra.23036. [DOI] [PubMed] [Google Scholar]
- Kase BA, Northrup H, Morrison AC, Davidson CM, Goiffon AM, et al. Association of copper-zinc superoxide dismutase (SOD1) and manganese superoxide dismutase (SOD2) genes with nonsyndromic myelomeningocele. Birth Defects Res. A Clin. Mol. Teratol. 2012;94(10):762–69. doi: 10.1002/bdra.23065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–54. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Kibar Z, Torban E, McDearmid JR, Reynolds A, Berghout J, et al. Mutations in VANGL1 associated with neural-tube defects. N. Engl. J. Med. 2007;356(14):1432–37. doi: 10.1056/NEJMoa060651. [DOI] [PubMed] [Google Scholar]
- Kim TH, Goodman J, Anderson KV, Niswander L. Phactr4 regulates neural tube and optic fissure closure by controlling PP1-, Rb-, and E2F1-regulated cell-cycle progression. Dev. Cell. 2007;13(1):87–102. doi: 10.1016/j.devcel.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Lei Y, Zhu H, Duhon C, Yang W, Ross ME, et al. Mutations in planar cell polarity gene SCRIB are associated with spina bifida. PLoS ONE. 2013;8(7):e69262. doi: 10.1371/journal.pone.0069262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei YP, Zhang T, Li H, Wu BL, Jin L, Wang HY. VANGL2 mutations in human cranial neural-tube defects. N. Engl. J. Med. 2010;362:2232–35. doi: 10.1056/NEJMc0910820. [DOI] [PubMed] [Google Scholar]
- Leung KY, De Castro SC, Savery D, Copp AJ, Greene ND. Nucleotide precursors prevent folic acid-resistant neural tube defects in the mouse. Brain. 2013;136(Pt. 9):2836–41. doi: 10.1093/brain/awt209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew SM, Kothbauer KF. Tethered cord syndrome: an updated review. Pediatr. Neurosurg. 2007;43(3):236–48. doi: 10.1159/000098836. [DOI] [PubMed] [Google Scholar]
- Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- Lupo PJ, Canfield MA, Chapa C, Lu W, Agopian AJ, et al. Diabetes and obesity-related genes and the risk of neural tube defects in the national birth defects prevention study. Am. J. Epidemiol. 2012;176:1101–9. doi: 10.1093/aje/kws190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane AJ, Perry CA, Girnary HH, Gao D, Allen RH, et al. Mthfd1 is an essential gene in mice and alters biomarkers of impaired one-carbon metabolism. J. Biol. Chem. 2009;284(3):1533–39. doi: 10.1074/jbc.M808281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa V, Savery D, Ybot-Gonzalez P, Ferraro E, Rongvaux A, et al. Apoptosis is not required for mammalian neural tube closure. Proc. Natl. Acad. Sci. USA. 2009;106:8233–38. doi: 10.1073/pnas.0900333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Yasutomi M. Inhibition of cephalic neural tube closure by 5-azacytidine in neurulating rat embryos in vitro. Anat. Embryol. 1992;185:217–23. doi: 10.1007/BF00211820. [DOI] [PubMed] [Google Scholar]
- Merte J, Jensen D, Wright K, Sarsfield S, Wang Y, et al. Sec24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure. Nat. Cell Biol. 2010;12:41–46. doi: 10.1038/ncb2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KA, Ah-Cann CJ, Welfare MF, Tan TY, Pope K, et al. Cauli: a mouse strain with an ift140 mutation that results in a skeletal ciliopathy modelling Jeune syndrome. PLoS. Genet. 2013;9(8):e1003746. doi: 10.1371/journal.pgen.1003746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missmer SA, Suarez L, Felkner M, Wang E, Merrill AH, Jr, et al. Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ. Health Perspect. 2006;114:237–41. doi: 10.1289/ehp.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy AM, Kirke PN, Troendle JF, Burke H, Sutton M, et al. Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic acid fortification. Pediatrics. 2009;123:917–23. doi: 10.1542/peds.2008-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momb J, Lewandowski JP, Bryant JD, Fitch R, Surman DR, et al. Deletion of Mthfd1l causes embryonic lethality and neural tube and craniofacial defects in mice. Proc. Natl. Acad. Sci. USA. 2013;110:549–54. doi: 10.1073/pnas.1211199110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti ME, Bar-Oz B, Fried S, Koren G. Maternal hyperthermia and the risk for neural tube defects in offspring: systematic review and meta-analysis. Epidemiology. 2005;16:216–19. doi: 10.1097/01.ede.0000152903.55579.15. [DOI] [PubMed] [Google Scholar]
- Morriss-Kay GM, Tuckett F. The role of microfilaments in cranial neurulation in rat embryos: effects of short-term exposure to cytochalasin D. J. Embryol. Exp. Morphol. 1985;88:333–48. [PubMed] [Google Scholar]
- Mosley BS, Cleves MA, Siega-Riz AM, Shaw GM, Canfield MA, et al. Neural tube defects and maternal folate intake among pregnancies conceived after folic acid fortification in the United States. Am. J. Epidemiol. 2009;169:9–17. doi: 10.1093/aje/kwn331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch JN, Copp AJ. The relationship between sonic Hedgehog signalling, cilia and neural tube defects. Birth Defects Res. A Clin. Mol. Teratol. 2010;88:633–52. doi: 10.1002/bdra.20686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch JN, Rachel RA, Shah S, Beermann F, Stanier P, et al. Circletail, a new mouse mutant with severe neural tube defects: chromosomal localisation and interaction with the loop-tail mutation. Genomics. 2001;78:55–63. doi: 10.1006/geno.2001.6638. [DOI] [PubMed] [Google Scholar]
- Nakatsu T, Uwabe C, Shiota K. Neural tube closure in humans initiates at multiple sites: evidence from human embryos and implications for the pathogenesis of neural tube defects. Anat. Embryol. 2000;201(6):455–66. doi: 10.1007/s004290050332. [DOI] [PubMed] [Google Scholar]
- Narisawa A, Komatsuzaki S, Kikuchi A, Niihori T, Aoki Y, et al. Mutations in genes encoding the glycine cleavage system predispose to neural tube defects in mice and humans. Hum. Mol. Genet. 2012;21:1496–503. doi: 10.1093/hmg/ddr585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rahilly R, Müller F. The two sites of fusion of the neural folds and the two neuropores in the human embryo. Teratology. 2002;65:162–70. doi: 10.1002/tera.10007. [DOI] [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99(3):247–57. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- Pai YJ, Abdullah NL, Mohd-Zin SW, Mohammed RS, Rolo A, et al. Epithelial fusion during neural tube morphogenesis. Birth Defects Res. A Clin. Mol. Teratol. 2012;94:817–23. doi: 10.1002/bdra.23072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangilinan F, Molloy AM, Mills JL, Troendle JF, Parle-McDermott A, et al. Evaluation of common genetic variants in 82 candidate genes as risk factors for neural tube defects. BMC Med. Genet. 2012;13:62. doi: 10.1186/1471-2350-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parle-McDermott A, Pangilinan F, O’Brien KK, Mills JL, Magee AM, et al. A common variant in MTHFD1L is associated with neural tube defects and mRNA splicing efficiency. Hum. Mutat. 2009;30:1650–56. doi: 10.1002/humu.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickell L, Li D, Brown K, Mikael LG, Wang XL, et al. Methylenetetrahydrofolate reductase deficiency and low dietary folate increase embryonic delay and placental abnormalities in mice. Birth Defects Res. A Clin. Mol. Teratol. 2009;85(6):531–41. doi: 10.1002/bdra.20575. [DOI] [PubMed] [Google Scholar]
- Piedrahita JA, Oetama B, Bennett GD, van Waes J, Kamen BA, et al. Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development. Nat. Genet. 1999;23(2):228–32. doi: 10.1038/13861. [DOI] [PubMed] [Google Scholar]
- Placzek M, Briscoe J. The floor plate: multiple cells, multiple signals. Nat. Rev. Neurosci. 2005;6:230–40. doi: 10.1038/nrn1628. [DOI] [PubMed] [Google Scholar]
- Pyrgaki C, Liu A, Niswander L. Grainyhead-like 2 regulates neural tube closure and adhesion molecule expression during neural fold fusion. Dev. Biol. 2011;353:38–49. doi: 10.1016/j.ydbio.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrgaki C, Trainor P, Hadjantonakis AK, Niswander L. Dynamic imaging of mammalian neural tube closure. Dev. Biol. 2010;344:941–47. doi: 10.1016/j.ydbio.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray HJ, Niswander L. Mechanisms of tissue fusion during development. Development. 2012;139(10):1701–11. doi: 10.1242/dev.068338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece EA. Diabetes-induced birth defects: what do we know? What can we do? Curr. Diab. Rep. 2012;12(1):24–32. doi: 10.1007/s11892-011-0251-6. [DOI] [PubMed] [Google Scholar]
- Reece EA, Khandelwal M, Wu YK, Borenstein M. Dietary intake of myo-inositol and neural tube defects in offspring of diabetic rats. Am. J. Obstet. Gynecol. 1997;176:536–39. doi: 10.1016/s0002-9378(97)70543-x. [DOI] [PubMed] [Google Scholar]
- Rifat Y, Parekh V, Wilanowski T, Hislop NR, Auden A, et al. Regional neural tube closure defined by the Grainy head-like transcription factors. Dev. Biol. 2010;345:237–45. doi: 10.1016/j.ydbio.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Robinson A, Escuin S, Doudney K, Vekemans M, Stevenson RE, et al. Mutations in the planar cell polarity genes CELSR1 and SCRIB are associated with the severe neural tube defect craniorachischisis. Hum. Mutat. 2012;33:440–47. doi: 10.1002/humu.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal J, Casas J, Taren D, Alverson CJ, Flores A, Frias J. Neural tube defects in Latin America and the impact of fortification: a literature review. Public Health Nutr. 2013;17:537–50. doi: 10.1017/S1368980013000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadai P, Nout YS, Encinas J, Wang A, Downing TL, et al. Prenatal repair of myelomeningocele with aligned nanofibrous scaffolds--a pilot study in sheep. J. Pediatr. Surg. 2011;46(12):2279–83. doi: 10.1016/j.jpedsurg.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Saadai P, Wang A, Nout YS, Downing TL, Lofberg K, et al. Human induced pluripotent stem cell-derived neural crest stem cells integrate into the injured spinal cord in the fetal lamb model of myelomeningocele. J. Pediatr. Surg. 2013;48(1):158–63. doi: 10.1016/j.jpedsurg.2012.10.034. [DOI] [PubMed] [Google Scholar]
- Sadler TW, Greenberg D, Coughlin P, Lessard JL. Actin distribution patterns in the mouse neural tube during neurulation. Science. 1982;215:172–74. doi: 10.1126/science.7031898. [DOI] [PubMed] [Google Scholar]
- Savory JG, Mansfield M, Rijli FM, Lohnes D. Cdx mediates neural tube closure through transcriptional regulation of the planar cell polarity gene Ptk7. Development. 2011;138:1361–70. doi: 10.1242/dev.056622. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC. Histological and ultrastructural studies of secondary neurulation in mouse embryos. Am. J. Anat. 1984;169:361–76. doi: 10.1002/aja.1001690402. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Smith JL. Epithelial cell wedging: a fundamental cell behavior contributing to hinge point formation during epithelial morphogenesis. In: Keller RE, Fristrom D, editors. Control of Morphogenesis. Vol. 1. Saunders; London: 1990. pp. 325–34. [Google Scholar]
- Schorah C. Dick Smithells, folic acid, and the prevention of neural tube defects. Birth Defects Res. A Clin. Mol. Teratol. 2008;85:254–59. doi: 10.1002/bdra.20544. [DOI] [PubMed] [Google Scholar]
- Seller MJ. Sex, neural tube defects, and multisite closure of the human neural tube. Am. J. Med. Genet. 1995;58:332–36. doi: 10.1002/ajmg.1320580406. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Lu W, Zhu H, Yang W, Briggs FB, et al. 118 SNPs of folate-related genes and risks of spina bifida and conotruncal heart defects. BMC Med. Genet. 2009;10(1):49. doi: 10.1186/1471-2350-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum ASW, Copp AJ. Regional differences in morphogenesis of the neuroepithelium suggest multiple mechanisms of spinal neurulation in the mouse. Anat. Embryol. 1996;194:65–73. doi: 10.1007/BF00196316. [DOI] [PubMed] [Google Scholar]
- Smith JL, Schoenwolf GC. Notochordal induction of cell wedging in the chick neural plate and its role in neural tube formation. J. Exp. Zool. 1989;250:49–62. doi: 10.1002/jez.1402500107. [DOI] [PubMed] [Google Scholar]
- Smithells RW, Sheppard S, Schorah CJ. Vitamin deficiencies and neural tube defects. Arch. Dis. Child. 1976;51:944–50. doi: 10.1136/adc.51.12.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithells RW, Sheppard S, Schorah CJ, Seller MJ, Nevin NC, et al. Apparent prevention of neural tube defects by periconceptional vitamin supplementation. Arch. Dis. Child. 1981;56:911–18. doi: 10.1136/adc.56.12.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Alvarez D, Siega-Riz AM, Herring AH, Carmichael SL, Feldkamp ML, et al. Maternal dietary patterns are associated with risk of neural tube and congenital heart defects. Am. J. Epidemiol. 2013;177(11):1279–88. doi: 10.1093/aje/kws349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover PJ. One-carbon metabolism-genome interactions in folate-associated pathologies. J. Nutr. 2009;139:2402–5. doi: 10.3945/jn.109.113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulik KK, Zuker RM, Dehart DB, Cessot F, Delezoide AL, et al. Normal patterns of neural tube closure differ in the human and the mouse. Proc. Greenwood Genet. Center. 1998;18:129–30. [Google Scholar]
- Takeuchi T, Kojima M, Nakajima K, Kondo S. jumonji gene is essential for the neurulation and cardiac development of mouse embryos with a C3H/He background. Mech. Dev. 1999;86(1-2):29–38. doi: 10.1016/s0925-4773(99)00100-8. [DOI] [PubMed] [Google Scholar]
- Tibbetts AS, Appling DR. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu. Rev. Nutr. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, et al. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119(4):555–66. doi: 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Vollset SE, Clarke R, Lewington S, Ebbing M, Halsey J, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50 000 individuals. Lancet. 2013;381:1029–36. doi: 10.1016/S0140-6736(12)62001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald N, Sneddon J, Densem J, Frost C, Stone R, MRC Vitamin Study Res Group Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–37. [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Xenopus Dishevelled signaling regulates both neural and mesodermal convergent extension: parallel forces elongating the body axis. Development. 2001;128(13):2581–92. doi: 10.1242/dev.128.13.2581. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129(24):5815–25. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang F, Guan J, Le J, Wu L, et al. Relation between hypomethylation of long interspersed nucleotide elements and risk of neural tube defects. Am. J. Clin. Nutr. 2010;91:1359–67. doi: 10.3945/ajcn.2009.28858. [DOI] [PubMed] [Google Scholar]
- Werth M, Walentin K, Aue A, Schönheit J, Wuebken A, et al. The transcription factor grainyhead-like 2 regulates the molecular composition of the epithelial apical junctional complex. Development. 2010;137(22):3835–45. doi: 10.1242/dev.055483. [DOI] [PubMed] [Google Scholar]
- Wlodarczyk BJ, Palacios AM, George TM, Finnell RH. Antiepileptic drugs and pregnancy outcomes. Am. J. Med. Genet. A. 2012;158A(8):2071–90. doi: 10.1002/ajmg.a.35438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LR, Smith MT. Generation of anencephaly: 1. Aberrant neurulation and 2. Conversion of exencephaly to anencephaly. J. Neuropath. Exp. Neurol. 1984;43:620–33. [PubMed] [Google Scholar]
- Xu WM, Baribault H, Adamson ED. Vinculin knockout results in heart and brain defects during embryonic development. Development. 1998;125:327–37. doi: 10.1242/dev.125.2.327. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Shinotsuka N, Nonomura K, Takemoto K, Kuida K, et al. Live imaging of apoptosis in a novel transgenic mouse highlights its role in neural tube closure. J. Cell Biol. 2011;195:1047–60. doi: 10.1083/jcb.201104057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Li X, Xu C, Eckert RL, Reece EA, et al. Maternal hyperglycemia activates an ASK1-FoxO3a-Caspase 8 pathway that leads to embryonic neural tube defects. Sci. Signal. 2013;6(290):ra74. doi: 10.1126/scisignal.2004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao TP, Oh SP, Fuchs M, Zhou ND, Ch’ng LE, et al. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell. 1998;93:361–72. doi: 10.1016/s0092-8674(00)81165-4. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Cogram P, Gerrelli D, Copp AJ. Sonic hedgehog and the molecular regulation of neural tube closure. Development. 2002;129:2507–17. doi: 10.1242/dev.129.10.2507. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Copp AJ. Bending of the neural plate during mouse spinal neurulation is independent of actin microfilaments. Dev. Dyn. 1999;215:273–83. doi: 10.1002/(SICI)1097-0177(199907)215:3<273::AID-AJA9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Gaston-Massuet C, Girdler G, Klingensmith J, Arkell R, et al. Neural plate morphogenesis during mouse neurulation is regulated by antagonism of Bmp signalling. Development. 2007a;134:3203–11. doi: 10.1242/dev.008177. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Savery D, Gerrelli D, Signore M, Mitchell CE, et al. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development. 2007b;134:789–99. doi: 10.1242/dev.000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Smallwood PM, Wang Y, Vidaltamayo R, Reed R, Nathans J. Frizzled 1 and frizzled 2 genes function in palate, ventricular septum and neural tube closure: general implications for tissue fusion processes. Development. 2010;137:3707–17. doi: 10.1242/dev.052001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Hoover AN, Liu A. PCP effector gene Inturned is an important regulator of cilia formation and embryonic development in mammals. Dev. Biol. 2010;339:418–28. doi: 10.1016/j.ydbio.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Zohn IE, Sarkar AA. Does the cranial mesenchyme contribute to neural fold elevation during neurulation? Birth Defects Res. A Clin. Mol. Teratol. 2012;94(10):841–48. doi: 10.1002/bdra.23073. [DOI] [PMC free article] [PubMed] [Google Scholar]