Abstract
Purpose of review
Prior to 2010, docetaxel was the only treatment shown to prolong survival in metastatic castrate resistant prostate cancer (CRPC). In the past three years several therapeutic agents have demonstrated survival improvements for CRPC after receipt of prior docetaxel, leading to multiple approvals by the US Food and Drug Administration (FDA).
Recent findings
The development of these novel agents, each with a distinct mechanism of action, is the fruition of sedulous preclinical research and well-designed clinical trials. Cabazitaxel, a next generation taxane, was the first FDA-approved drug for the post-docetaxel setting. The recognition of sustained androgen-dependence of CRPC has led to identification of more potent and selective inhibitors of androgen synthesis and androgen-receptor signaling, such as abiraterone and enzalutamide, respectively. Radium-223, an α-emitting radionuclide still under regulatory review, recently showed a significant survival benefit for CRPC. Finally, sipuleucel-T, a form of immunotherapy, may benefit a subset of patients in the post-docetaxel setting.
Summary
Management of docetaxel failures has undergone a dramatic yet welcome paradigm change in the past three years. With multiple life-prolonging agents available, it now becomes imperative to coordinate how and when these new therapies should be used and sequenced to achieve optimal patient outcomes.
Keywords: castrate resistant prostate cancer, docetaxel, chemotherapy, hormonal therapy
INTRODUCTION
In 2004, the US Food and Drug Administration (FDA) approved docetaxel as a front line treatment for metastatic castrate-resistant prostate cancer (mCRPC), based on its survival benefit over mitoxantrone from two randomized phase III trials [1, 2]. Since then, a number of studies with docetaxel-based combinations have been undertaken but failed to show additional benefit in overall survival (OS) [3, 4]. Hence, docetaxel was the only treatment with survival benefit in mCRPC until 2010. Consequently, patient management after docetaxel has been primarily palliative. Since 2010, there has been emergence of multiple new therapies for mCRPC. Four agents, namely sipuleucel-T, cabazitaxel, abiraterone, and enzalutamide, gained FDA-approval on merits of overall survival benefit. In addition, radium-223 chloride (Alpharadin) has been granted Fast Track designation by the FDA for the same. Here, we will review results of recent trials of the new therapies, with particular emphasis of their role in the post-docetaxel setting.
CYTOTOXIC CHEMOTHERAPY
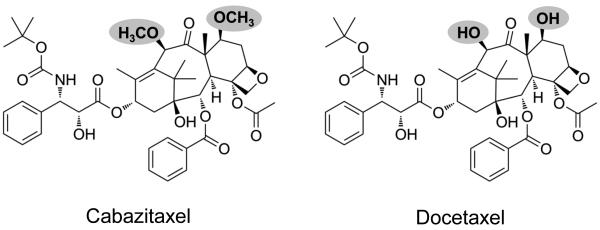
Cabazitaxel is a semisynthetic taxane with antitumor activity in preclinical models resistant to paclitaxel and docetaxel [5]. Unlike other taxanes, cabazitaxel has a low affinity to P-glycoprotein 1, an adenosine triphosphate-dependent drug efflux pump, which is believed to be responsible for resistance to docetaxel (Figure 1) [6, 7].
Figure 1. Structural basis for efficacy of cabazitaxel in docetaxel-resistant CRPC.
With methoxyl groups in place of hydroxyl groups as shown in the shaded areas, cabazitaxel is structurally unique from docetaxel. This accounts for a larger biochemical structure and cabazitaxel’s low affinity for membrane-associated P-glycoprotein drug efflux pump, which is believed to be a mechanism for docetaxel resistance.
Based on its activity in solid tumors, including docetaxel-pretreated mCRPC in a phase I study [8], the clinical efficacy and safety of cabazitaxel in post-docetaxel mCRPC were evaluated in a randomized open-label phase III trial (TROPIC)[9**]. Seven hundred fifty five patients were assigned to receive cabazitaxel plus prednisone or mitoxantrone plus prednisone. Patient eligibility was not restricted to 2nd line chemotherapy, and 22.9% of the patients were receiving 3rd line while 7.2% of the patients were receiving 4th line chemotherapy or beyond. Median OS was 15.1 months in the cabazitaxel group compared with 12.7 months in the mitoxantrone group [hazard ratio (HR) 0.70; 95% CI 0.59-0.83; P<0.0001]. Notably, subset analyses demonstrated survival benefit of cabazitaxel was more pronounced in patients who had received a longer course (≥ 12 vs. < 12 cycles) or had progressed within a shorter period after completion of docetaxel (< 3 vs. ≥ 3 months), underlining its efficacy in the patients likely truly refractory to docetaxel. Most secondary end points, including progression-free survival (PFS), time to progression (TTP), and response rate (RR) for patients with radiographic measurable disease, were in favor of cabazitaxel. PSA response rate was also significantly higher (39.2% vs. 17.8%, P= 0.0002). However, pain response rate and median time to pain were not significantly different between cabazitaxel and mitoxantrone. This may be due to the fact that mitoxantrone has active pain palliating properties [10, 11], yet the lack of improvement of this endpoint and the potential toxicities of cabazitaxel should be taken into account during the therapeutic agent selection process for each patient.
Compared with mitoxantrone, cabazitaxel was associated with increased incidences of grade 3 or 4 neutropenia, febrile neutropenia and diarrhea. Treatment-related mortality was considerably higher in the cabazitaxel arm (5% versus 2.5% in the mitoxantrone arm). Seven of 18 cabazitaxel-related deaths were due to neutropenia-associated sepsis, but it is important to note that primary prophylaxis with growth factors was not allowed during the first cycle of treatment, and all deaths on trial occurred during the first cycle. Hence, close surveillance is mandated and prophylactic use of granulocyte-colony-stimulating factors (G-CSF) is strongly recommended.
Cabazitaxel achieved regulatory approval by the FDA in 2010 for patients with mCRPC previously treated with docetaxel. Meanwhile, questions remain over its toxicities and potential role as a first-line agent. These will be addressed in two randomized phase III trials, PROSELICA (http://clinicaltrials.gov/ct2/show/NCT01308580) and FIRSTANA (http://clinicaltrials.gov/show/NCT01308567). The PROSELICA trial compares cabazitaxel at its currently approved dose (25 mg/m2) with a lower dose (20 mg/m2) to determine if efficacy is maintained along with better tolerability, whereas the FIRSTANA trial aims to determine if cabazitaxel is superior to docetaxel as a first-line drug in chemotherapy-naïve patients.
Mitoxantrone, one of the first cytotoxic drugs FDA-approved for mCRPC, improves quality of life and bone pain but not OS [10, 11]. As the new therapies with survival benefit become increasingly available, use of mitoxantrone will likely decrease. Nonetheless, when access to the new agents is limited or taxanes are poorly tolerated, mitoxantrone remains a viable option.
HORMONAL THERAPY
For many years, clinical observations have suggested an important role of androgen in CRPC. For instance, retrospective studies indicated that continuation of androgen deprivation therapy (ADT) was associated with a potential survival advantage in CRPC [12] and CRPC often responds to secondary hormonal manipulation [13]. Moreover, progression of CRPC is almost invariably accompanied with rising of PSA, an androgen-responsive gene, suggesting an active androgen-signaling pathway. In the past decade, this notion has been further validated by preclinical studies. A variety of mechanisms underlying castration-resistance have been delineated and exploited for drug development targeting androgen-signaling pathways [14, 15].
In a castrate state, androgen is primarily synthesized by adrenal glands and intratumorally by cancer cells [16-18]. Cytochrome P450-17 (CYP17) represents a class of enzymes crucial to de novo androgen synthesis. The efficacy of ketoconazole, a non-selective CYP17 inhibitor, was established in a randomized phase III trial that demonstrated objective response and PSA reduction in mCRPC by combining ketoconazole with antiandrogen withdrawal. However, no OS benefit was observed. Significant toxicities, related to its non-selective inhibition on both adrenal androgen and cortisol syntheses, were also of concern [13].
Abiraterone is an oral selective inhibitor of CYP17, 10 to 30 times more potent than ketoconazole [19]. The efficacy of abiraterone in docetaxel-pretreated CRPC was evaluated in a randomized double-blind placebo-controlled trial (COU-AA-301). A total of 1195 patients were assigned to receive abiraterone plus prednisone or placebo plus prednisone. After a median follow up of 12.8 months, an interim analysis demonstrated OS in the abiraterone group was significantly longer than in the control group (14.8 vs. 10.9 months; HR 0.65; 95% CI 0.54–0.77; P<0.0001). Secondary endpoints, including time to PSA progression (10.2 vs. 6.6 months; P<0.001), progression-free survival (5.6 vs. 3.6 months; P<0.001), and PSA reduction over 50% (29% vs. 6%, P<0.001), were all in favor of abiraterone. While mineralocorticoid excess-related adverse events, including fluid retention, hypertension, and hypokalemia, were seen more frequently with abiraterone, grade 3 or 4 events were rare [20**].
The potential use of abiraterone in chemotherapy-naïve patients has recently been assessed in a placebo-controlled phase III trial (COU-AA-302). Primary endpoints were OS and radiographic progression-free survival (rPFS). Results of an interim analysis after a median follow-up of 22.2 months were reported at ASCO 2012. Abiraterone with prednisone, compared to placebo with prednisone, led to significant improvement in rPFS (median not reached vs. 8.3 months, P<0.0001) and a strong trend for increased OS (median not reached vs. 27.2 months, P=0.0097). All secondary endpoints including time to chemotherapy initiation, time to PSA progression, and time to performance status deterioration, were also met. Again, typical mineralocorticoid side effects were well tolerated [21]. The FDA will now face the decision to consider expanding the label for abiraterone to include all patients with mCRPC, including those who are chemotherapy-naïve.
Enzalutamide, formally called MDV3100, is a second-generation oral androgen receptor (AR) antagonist. It has five- to eight-fold higher AR-binding affinity than bicalutamide, thereby more effectively blocking AR-binding by testosterone and preventing nuclear translocation, DNA binding of the ligand-AR complex and recruitment of coactivators. Unlike bicalutamide, enzalutamide is a pure antagonist without any agonist activity [22]. In a phase I/II trial involving 140 CRPC patients who progressed on ADT or docetaxel, enzalutamide showed promising antitumor effects [23], which led to two phase III trials in mCRPC evaluating efficacy in the post-docetaxel setting (AFFIRM) and the pre-docetaxel setting (PREVAIL).
In the AFFIRM trial, 1199 men with docetaxel-pretreated mCRPC were randomly assigned to receive enzalutamide or placebo. OS, as the primary end point, was significantly longer in the treatment arm than in the placebo arm (median 18.4 vs. 13.6 months, HR 0.63; 95% CI, 0.53 to 0.75; P<0.001). Enzalutamide was also superior in terms of secondary end points, including PSA decline by ≥50% (54% vs. 2%, P<0.001), soft-tissue response rate (29% vs. 4%, P<0.001), quality-of-life response rate (43% vs. 18%, P<0.001), time to PSA progression (8.3 vs. 3.0 months; HR 0.25; P<0.001), radiographic progression-free survival (8.3 vs. 2.9 months; HR 0.40; P<0.001), and time to the first skeletal-related event (16.7 vs. 13.3 months; HR 0.69; P<0.001). Side effects were mild, including fatigue, diarrhea, musculoskeletal pain and hot flashes, although seizure was rarely reported in the enzalutamide group (0.6%) but none in the placebo group. Notably, incidence of grade 3 or 4 events was lower in the enzalutamide group, suggesting most adverse events were disease-related [24**].
The role of enzalutamide in the chemotherapy-naïve patients will be addressed in the PREVAIL trial that has completed accrual of 1,680 patients worldwide. Primary endpoints include OS and rPFS (http://clinicaltrials.gov/show/NCT01212991).
Orteronel is an oral nonsteroidal CYP17 inhibitor. Because of its higher selectivity for 17,20-lyase over 17-hydroxylase, it is less likely to cause mineralocorticoid excess, hence requires no concomitant use of steroid when lower doses are used [25]. A phase I/II study of orteronel in chemotherapy-naïve CRPC patients showed encouraging results on PSA reduction and RECIST evaluation [26]. Of note, similar efficacy was seen in groups not receiving concomitant steroid and mineralocorticoid-related side effects were uncommon. The use of orteronel in chemotherapy-naïve and post-docetaxel CRPC is being evaluated in two randomized placebo-controlled phase III trials (http://clinicaltrials.gov/show/NCT01193244; http://clinicaltrials.gov/show/NCT01193257); however, both trials use higher doses of orteronel accompanied with steroids.
Resistance to abiraterone or enzalutamide has been observed in preclinical models and tumor-derived xenografts [27-29]. Unfortunately, no biomarker is available to predict response or resistance to either agent. Underlying biologic mechanisms of resistance in humans will need to be determined from tissue- and marker-based trials. Only then will optimal sequencing and combination of these agents be understood.
BONE-TARGETED THERAPY
Symptomatic bone metastases are a primary cause of morbidity and decreased quality of life in patients with mCRPC. Bone-targeted therapy, including bisphosphonates and denosumab, is now standard of care to reduce the risk of skeletal-related events (SREs) [30, 31]. Bone-seeking radiopharmaceuticals have been used primarily for refractory bone pain despite of systemic therapy and focally directed external beam radiation therapy. Samarium-153 and Strontium-89 are β-particle-emitting radionuclides approved by the FDA for pain palliation in mCRPC [32, 33]. However, their use has been limited by β-emission-induced myelosuppression. Moreover, none of β-emitters (e.g. strontium, samarium, rhenium) have been shown to prolong survival.
Radium-223 (Alpharadin) is a first-in-class α-particle-emitting radionuclide. Grouped in the same column as calcium on the periodic element table, radium shares similar chemical properties with calcium, thus serves as a calcium mimic by being incorporated in osteoblastic bone lesions, for instance metastatic sites from prostate cancer. Compared with β-emitters, it delivers higher energy with a shorter range (less than 100μm) thereby sparing surrounding bone marrow and reducing myelotoxicity. A randomized placebo-controlled phase III trial (ALSYMPCA) evaluated radium-223 in 921 patients with symptomatic bone metastatic CRPC either after docetaxel, or unfit for or refusing docetaxel. Results of an interim analysis showed radium-223 significantly improved OS compared with placebo (median 14.0 vs. 11.2 months; HR 0.695; 95% CI, 0.552-0.875; 2-sided P= 0.00185), and extended time to first SREs (median 13.6 vs. 8.4 months; HR 0.610; 95% CI, 0.461-0.807; P= 0.00046) [34*]. An updated analysis presented at ASCO 2012 further extended the median survival benefit to 3.6 months (14.9 vs. 11.3 months; HR 0.695; 95% CI, 0.581-0.832; P= 0.00007), as well as the median time to first SREs (15.6 vs. 9.8 months; HR 0.658; 95% CI, 0.522-0.830; P= 0.00037). Of note, significant OS benefit was demonstrated in both the chemotherapy naïve (median 16.1 vs. 11.5 months, HR 0.745, 95% CI 0.562-0.987; P=0.03932) and the post-docetaxel subgroup (median 14.4 vs. 11.3 months; HR 0.710; 95% CI, 0.565-0.891; P=0.00307). Safety profile of radium-223 remained favorable with a low risk of myelosuppression and diarrhea [35*]. The FDA will be evaluating this data in the near future.
IMMUNOTHERAPY
The role of immune-based therapy has been actively investigated in prostate cancer because of its long natural course and immunogenicity of prostate-specific antigens, e.g. PSA and prostatic acid phosphatase (PAP). Sipuleucel-T is an autologous dendritic cell product manufactured via activation in vitro with a recombinant fusion protein composed of granulocyte macrophage colony-stimulating factor (GM-CSF) and PAP [36].
The impact of sipuleucel-T on survival was confirmed in a double-blind placebo-controlled phase III trial (IMPACT), in which 512 patients with asymptomatic or minimally symptomatic mCRPC were randomized to receive either sipuleucel-T or placebo. The median OS was 25.8 months in the treatment group versus 21.7 months in the placebo group (HR 0.78; 95% CI 0.61-0.98; P= 0.03). Notably, in a subset analysis, benefit from sipuleucel-T treatment was seen in both those who did (only 14.4% of overall population) and those who did not receive prior docetaxel chemotherapy [37**]. The NCCN prostate cancer panel listed sipuleucel-T as a category 2A option “after failure of, or treatment with, chemotherapy in asymptomatic or minimally symptomatic patients with good performance status” [38].
Ipilimumab, a monoclonal antibody against a cytotoxic T-lymphocyte antigen, already FDA approved for metastatic melanoma, is being evaluated in a randomized double-blind placebo-controlled phase III trial in mCRPC patients pretreated with docetaxel (http://clinicaltrials.gov/show/NCT00861614).
NOVEL AGENTS IN PIPELINE
A number of unique novel agents are currently in phase III trials for post-docetaxel mCRPC. Among them are cabozantinib (XL-184) and custirsen (OGX-011).
Cabozantinib is a tyrosine kinase inhibitor of c-MET, VEGFR2 and multiple other kinases. Results from a randomized, discontinuation phase II trial presented at ASCO 2011 showed impressive response rates in bone scan resolution (86%), pain relief (64%) and overall disease control (71%), which led to discontinuation of randomization and expansion of the treatment cohort [39]. Data from the nonrandomized expansion cohort in 93 docetaxel-pretreated patients was reported at ASCO 2012 and showed again high rates of bone scan response (70%), durable pain reduction (49% ≥ 6 weeks), along with declines in bone turnover markers (74%, 67% and 47% in C-telopeptide, N-telopeptide, and bone alkaline phosphatase, respectively) and circulating tumor cells [40]. Treatment-related toxicity can be significant with fatigue, anorexia, weight loss and gastrointestinal disturbance, and discontinuation or dose reduction was required for many patients when 100 mg each day was used. Two randomized phase III trials, evaluating efficacy of cabozantinib 60 mg each day for OS (COMET-1, http://clinicaltrials.gov/ct2/show/NCT01605227) and pain response (COMET-2, http://clinicaltrials.gov/ct2/show/NCT01522443) after treatment with docetaxel, are ongoing.
Custirsen is an antisense inhibitor of clusterin, a chaperone protein whose increased expression promotes survival of cancer cells. It has been shown that custirsen, combined with docetaxel as a first line treatment, prolonged OS in mCRPC compared with docetaxel alone (median 23.8 vs. 16.9 months) [41]. In patients with progressive CRPC after first-line docetaxel, combination of docetaxel and custirsen showed an encouraging response rate in pain relief (77%), with interesting correlations between serum clusterin level and survival [42]. A randomized open-label phase III trial (SYNERGY) comparing docetaxel to docetaxel plus custirsen in chemotherapy-naïve patients is now fully accrued with results pending (http://clinicaltrials.gov/show/NCT01188187). A phase III trial (AFFINITY) has been recently initiated to compare cabazitaxel plus prednisone in combination with custirsen to cabazitaxel plus prednisone for patients who have receivd prior docetaxel (http://clinicaltrials.gov/ct2/show/NCT01578655).
RECOMMENDATIONS
It should be noted that prostate cancer, even at a castrate resistant stage, is a group of heterogeneous diseases. Thus, management of patients after docetaxel needs to be individualized. Nevertheless, due to lack of predictive biomarkers and comparative clinical trial data, therapeutic agent selection is generally based upon clinical parameters, e.g. age, performance status, symptoms, elapsed time from last treatment, pace of disease, location of metastases, and often, individual preferences.
We concur with NCCN recommendations that sipuleucel-T, while appropriate for asymptomatic or minimally symptomatic patients, should be avoided for those with rapidly progressive disease, visceral metastasis or life expectancy less than 6 months. Generally, it should be reserved for the chemotherapy-naïve patient; however, in unique situations where patients gain an impressive response to chemotherapy, deserving of a prolonged chemotherapy break, sipuleucel-T can be considered.
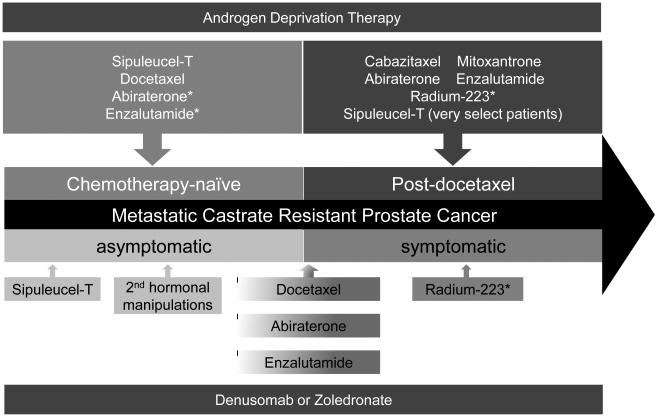
Cabazitaxel, abiraterone and enzalutamide are all reasonable options for patients with symptomatic progressive mCRPC in the post-docetaxel treatment paradigm. Abiraterone or enzalutamide may be reasonable agents immediately after docetaxel for the practical reason that the side-effect profiles are favorable, allowing patients to recover from chemotherapy toxicities. In addition, patients with limited performance status or intolerance to cabazitaxel will likely tolerate either abiraterone or enzalutamide. As mentioned above, both abiraterone and enzalutamide are being evaluated in chemotherapy-naïve patients. If trials are positive and indications are expanded, they may serve as a continuum of ADT in the pre-docetaxel setting, and consequently, chemotherapy such as docetaxel and cabazitaxel will be further deferred (Figure 2).
Figure 2. New treatment paradigm for metastatic castrate-resistant prostate cancer.
Treatment options have recently increased dramatically for patients with mCRPC post-docetaxel. This schematic separates treatment options based on important clinical parameters of whether the patient has received prior chemotherapy or whether the patient is symptomatic or not.
* The FDA has not provided a label for indication in this disease state
Radium-223, if approved by the FDA, will likely be an excellent selection for patients with symptomatic bone metastasis either prior to or after docetaxel. As a bone-seeking radionuclide, its survival benefit may be partly derived from targeting tumor microenvironment, which makes combinatorial use with other non-toxic agents an attractive option for future trials.
CONCLUSION
The emergence of new effective therapies has ushered in an exciting new era for prostate cancer therapy in the post-docetaxel setting. Patients have more options to help prolong survival. Yet clinicians are faced with an unprecedented challenge of maximizing benefit with the enriched armamentaria without any data to drive these decisions. Biomarker studies with tissue acquisition and molecular characterization of circulating tumor cells are logical research tools to aid in the understanding of drug sensitivity and resistance. Only with further such work can safe, intelligent sequencing and combinations be developed and introduced to earlier prostate cancer disease states with hope of even greater patient benefit. As a result, it cannot be overemphasized that participation in clinical trials is strongly encouraged to continue to facilitate more groundbreaking advances.
KEY POINTS.
Cabazitaxel prolongs overall survival in docetaxel-pretreated metastatic castrate resistant prostate cancer (mCRPC).
Abiraterone and enzalutamide, targeting androgen synthesis and androgen-receptor signaling respectively, represent a new generation of potent hormonal therapies that extend survival for docetaxel-pretreated mCRPC.
Radium-223, an α-particle-emitting radionuclide, is being evaluated by regulatory agencies for mCRPC with symptomatic bone metastasis, on the basis of its ability to prolong overall survival.
Sipuleucel-T, primarily indicated for asymptomatic or minimally symptomatic patients with mCRPC, should generally be reserved for the chemotherapy-naïve setting with the exception of a dramatic response to prior docetaxel treatment.
While emergence of novel agents has markedly changed landscape of mCRPC treatment, it has also created an urgent demand for generation of biologic and clinical data to help form guidelines for sequencing and combinations of established and new therapeutics.
Acknowledgements
None
Footnotes
Disclosures: None
Conflicts of interest
S.Z. has no conflict of interest to report.
E.Y. has received compensation for consultation with Amgen, Astellas, Dendreon, Janssen, and Medivation. E.Y. has received research funding from Dendreon, Janssen and OncoGeneX.
Contributor Information
Song Zhao, University of Washington/Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue North, D5-100, Seattle, WA 98109.
Evan Y. Yu, University of Washington School of Medicine, Seattle Cancer Care Alliance, 825 Eastlake Avenue East, G4-836, Box 358081, Seattle, WA 98109.
REFERENCES
- 1.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 2.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 3.Scher HI, Jia X, Chi K, et al. Randomized, open-label phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. J Clin Oncol. 2011;29:2191–2198. doi: 10.1200/JCO.2010.32.8815. [DOI] [PubMed] [Google Scholar]
- 4.Kelly WK, Halabi S, Carducci M, et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol. 2012;30:1534–1540. doi: 10.1200/JCO.2011.39.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attard G, Greystoke A, Kaye S, De Bono J. Update on tubulin-binding agents. Pathol Biol (Paris) 2006;54:72–84. doi: 10.1016/j.patbio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Morris PG, Fornier MN. Microtubule active agents: beyond the taxane frontier. Clin Cancer Res. 2008;14:7167–7172. doi: 10.1158/1078-0432.CCR-08-0169. [DOI] [PubMed] [Google Scholar]
- 7.Galsky MD, Dritselis A, Kirkpatrick P, Oh WK. Cabazitaxel. Nat Rev Drug Disc. 2010;9:677–678. doi: 10.1038/nrd3254. [DOI] [PubMed] [Google Scholar]
- 8.Mita AC, Denis LJ, Rowinsky EK, et al. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Clin Cancer Res. 2009;15:723–730. doi: 10.1158/1078-0432.CCR-08-0596. [DOI] [PubMed] [Google Scholar]
- 9**.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. The results of this clinical trial showed a survival benefit of cabazitaxel over mitoxantrone in the post-docetaxel setting, leading to the FDA approval of cabazitaxel in the USA. [DOI] [PubMed] [Google Scholar]
- 10.Kantoff PW, Halabi S, Conaway M, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: Results of the cancer and leukemia group b 9182 study. J Clin Oncol. 1999;17:2506–2513. doi: 10.1200/JCO.1999.17.8.2506. [DOI] [PubMed] [Google Scholar]
- 11.Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: A canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–1764. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 12.Taylor CD, Elson P, Trump DL. Importance of continued testicular suppression in hormone-refractory prostate cancer. J Clin Oncol. 1993;11:2167–2172. doi: 10.1200/JCO.1993.11.11.2167. [DOI] [PubMed] [Google Scholar]
- 13.Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583) J Clin Oncol. 2004;22:1025–1033. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 14.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 15.Eichholz A, Ferraldeschi R, Attard G, de Bono JS. Putting the brakes on continued androgen receptor signaling in castration-resistant prostate cancer. Mol Cell Endocrinol. 2012;360:68–75. doi: 10.1016/j.mce.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 16.Mohler JL, Gregory CW, Ford OH, 3rd, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 17.Mostaghel EA, Page ST, Lin DW, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potter GA, Barrie SE, Jarman M, Rowlands MG. Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J Med Chem. 1995;38:2463–2471. doi: 10.1021/jm00013a022. [DOI] [PubMed] [Google Scholar]
- 20**.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. The results of this clinical trial revealed abiraterone with prednisone to have a survival benefit over placebo and prednisone in a post-docetaxel metastatic CRPC patient population, leading to the FDA approval of abiraterone in the USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Ryan CJ, Smith MR, de Bono JS, et al. Interim analysis results of COU-AA-302, a randomized, phase III study of abiraterone acetate in chemotherapy-naive patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2012;30(suppl) LBA 4518. The results of this clinical trial will likely lead to FDA-approval of use of abiraterone in chemotherapy-naïve patients. [Google Scholar]
- 22.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24**.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. The results of this clinical trial revealed a survival benefit of enzalutamide over placebo in patients with mCRPC post-docetaxel, leading to the recent FDA approval of enzalutamide in the USA. [DOI] [PubMed] [Google Scholar]
- 25.Yamaoka M, Hara T, Kusaka M. Overcoming persistent dependency on androgen signaling after progression to castration-resistant prostate cancer. Clin Cancer Res. 2010;16:4319–4324. doi: 10.1158/1078-0432.CCR-10-0255. [DOI] [PubMed] [Google Scholar]
- 26.Agus DB, Stadler MW, Shevrin DH, et al. Safety, efficacy, and pharmacodynamics of the investigational agent orteronel (TAK-700) in metastatic castration-resistant prostate cancer: Updated data from a phase I/II study. J Clin Oncol. 2012;30(suppl 5) abstr 98. [Google Scholar]
- 27.Cai C, Chen S, Ng P, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71:6503–6513. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tzelepi V, Zhang J, Lu JF, et al. Modeling a lethal prostate cancer variant with small-cell carcinoma features. Clin Cancer Res. 2012;18:666–677. doi: 10.1158/1078-0432.CCR-11-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mostaghel EA, Marck BT, Plymate SR, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17:5913–5925. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 32.Serafini AN, Houston SJ, Resche I, et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double-blind placebo-controlled clinical trial. J Clin Oncol. 1998;16:1574–1581. doi: 10.1200/JCO.1998.16.4.1574. [DOI] [PubMed] [Google Scholar]
- 33.Lewington VJ, McEwan AJ, Ackery DM, et al. A prospective, randomised double-blind crossover study to examine the efficacy of strontium-89 in pain palliation in patients with advanced prostate cancer metastatic to bone. Eur J Cancer. 1991;27:954–958. doi: 10.1016/0277-5379(91)90257-e. [DOI] [PubMed] [Google Scholar]
- 34*.Parker C, Heinrich D, O'Sullivan JM, et al. Overall survival benefit and safety profile of radium-223 chloride, a first-in-class alpha-pharmaceutical: Results from a-phase-III randomized trial (ALSYMPCA) in patients with castration-resistant prostate cancer with bone metastases. J Clin Oncol. 2012;30(suppl 5) abstr 8. [Google Scholar]
- 35*.Parker C, Nilsson S, Heinrich D, et al. Updated analysis of the phase III, double-blind, randomized, multinational study of radium-223 chloride in castration-resistant prostate cancer patients with bone metastases (ALSYMPCA) J Clin Oncol. 2012;30(suppl) LBA4512. The results of this clinical trial (ref. 34 and 35) demonstrated significant survival benefit, at an interim analysis, along with pain palliation and excellent safety profile, based on which radium-223 chloride was granted Fast Track designation by the FDA. [Google Scholar]
- 36.Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 37**.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. The results of this randomized, phase 3 clinical trial and the trials from reference 36 revealed sipuleucel-T to offer a survival benefit in patients with relatively asymptomatic mCRPC, leading to the FDA approval of sipuleucel-T in the USA. [DOI] [PubMed] [Google Scholar]
- 38.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, Version 3.2012: featured updates to the NCCN guidelines. Journal of the National Comprehensive Cancer Network : JNCCN. 2012;10:1081–1087. doi: 10.6004/jnccn.2012.0114. [DOI] [PubMed] [Google Scholar]
- 39.Hussain M, Smith MR, Sweeney C, et al. Cabozantinib (XL184) in metastatic castration-resistant prostate cancer: Results from a phase II randomized discontinuation trial. J Clin Oncol. 29(suppl) 201. abstr 4516. [Google Scholar]
- 40.Smith MR, Sweeney C, Rathkopf DE, et al. Cabozantinib (XL184) in chemotherapy-pretreated metastatic castration resistant prostate cancer: Results from a phase II nonrandomized expansion cohort. J Clin Oncol. 2012;30(suppl) doi: 10.1200/JCO.2013.54.5954. abstr 4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chi KN, Hotte SJ, Yu EY, et al. Randomized phase II study of docetaxel and prednisone with or without OGX-011 in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:4247–4254. doi: 10.1200/JCO.2009.26.8771. [DOI] [PubMed] [Google Scholar]
- 42.Saad F, Hotte S, North S, et al. Randomized phase II trial of Custirsen (OGX-011) in combination with docetaxel or mitoxantrone as second-line therapy in patients with metastatic castrate-resistant prostate cancer progressing after first-line docetaxel: CUOG trial P-06c. Clin Cancer Res. 2011;17:5765–5773. doi: 10.1158/1078-0432.CCR-11-0859. [DOI] [PubMed] [Google Scholar]