Abstract
Delirium commonly occurs during myeloablative hematopoietic stem cell transplantation (HCT). Little is known about how delirium during the acute phase of HCT affects long-term distress, health related quality of life (HRQOL), and neurocognitive functioning. This prospective, cohort study examines these outcomes at 6 months and 1 year in 90 patients undergoing HCT. Patients completed a battery assessing distress, HRQOL, and subjective neuropsychological functioning before receiving their first HCT as well as at 6 months and 1 year. Patients with a delirium episode within the 4 weeks after HCT had significantly more distress and fatigue at 6 months (P<.004) and at 1 year (P<.03), compared with patients without delirium. At one year, patients with delirium also had worse symptoms of depression and post traumatic stress (P<.03). Patients with delirium had worse physical health on the SF-12 at 6 months (P<.03) and worse mental health on the SF-12 at 1 year (P<.03). At both 6 months and 1 year, patients with delirium after HCT reported worse memory (P<.009) and executive functioning (P<.006). Delirium during the acute phase of HCT is significantly associated with persistent distress, decreased HRQOL and subjective neurocognitive dysfunction at both 6 months and 1 year.
Introduction
Delirium is a common neuropsychiatric complication of hematopoietic stem-cell transplantation (HCT), reported in up to 50% of patients in the first four weeks post-transplantation.1,2 The high rates of delirium in patients undergoing HCT appear linked to having a severe acute systemic disease and being exposed to treatment interventions with deliriogenic or cognitive effects. Potential treatments that may precipitate delirium include: psychoactive medications such as opioid analgesics, sedatives, corticosteroids, and anticholinergics3 as well as total body irradiation 4 and resultant infections5.
By definition, delirium is characterized by a reversible disturbance of consciousness and change in cognition or perception.6 It is independently associated with significant morbidity, mortality, and functional decline across multiple populations.7 Deleterious effects on mortality8,9, length of hospital stay10, and performance status8 have been noted in hospitalized cancer patients who develop delirium. To our knowledge, no studies have examined the effects of delirium on longer-term outcomes of cognition, distress, and health-related quality of life (HRQOL) in patients with either cancer or those who received HCT.
A prospective, longitudinal study of patients undergoing HCT indicates that physical, psychological, and vocational recovery begins during the first year post-transplantation and continues over at least the next three to five years.11 The same research group also found that patients in the first post-HCT year had persistent declines in grip strength and motor dexterity but otherwise returned to pretransplantation levels of cognition.12 In contrast, a prospective study of neurocognitive changes in the first 20 months in patients with hematological malignancies being treated with HCT compared to a reference group of patients receiving nonmyeloablative therapies found persistent declines in attention and executive function in addition to psychomotor function in both groups.13 It remains unclear whether delirium contributes to the risk of persistent functional and cognitive decrements in HCT patients.
The goal of this study was to investigate the association of delirium during acute phase treatment with distress, health-related quality of life (HRQOL) and cognition 6 months and 1 year after HCT. Fifty percent of this previously described cohort had a delirium episode during the first four weeks after HCT.2 Previous outcome results from this cohort have demonstrated delirium's adverse impact on depression, anxiety, and fatigue at 30 days as well as worse anxiety, fatigue, distress, HRQOL, executive functioning, attention, and processing speed at 80 days.2 In the current analysis, we hypothesized that patients who had experienced a delirium episode in the acute phase of HCT would have worse cognitive functioning, distress, and HRQOL at the extended follow-up points of 6 months and 1 year compared to patients who had not experienced delirium.
Patients and Methods
Patients
Ninety patients between the ages of 22 to 62 were recruited before their first myeloablative allogenic or autologous bone marrow or peripheral blood HCT at the Fred Hutchinson Cancer Research Center (FHCRC).
Procedures
Study procedures for this cohort have been previously reported.2 The FHCRC institutional review board approved the protocol and all study procedures. All study patients received and signed written informed consent. Patients completed a comprehensive battery before transplantation measuring distress, HRQOL, and neuropsychological functioning. The results of the readministration of a subset of this battery at 30 days and the full battery again at 80 has been previously reported.14 At 6 months and 1 year, patients again completed a subset of this battery. From 7 days pretransplantation until 30 days post-transplantation, study investigators or trained research nurses screened patients 3 times per week with a brief delirium assessment battery, targeted to the same time each day (Monday, Wednesday, and Friday).
Independent Variables
We measured delirium using the Delirium Rating Scale (DRS), a 10-item, clinician-rated scale (score range, 0 to 32) that rates delirium severity over 24 hours using sources including patient interview, mental status examination, medical history and tests, and collateral observation from nursing staff and family.15 We defined a delirium episode as a DRS score >12 on at least two of three consecutive assessments.
Dependent Variables
Distress
The Symptom Checklist-90-R (SCL-90) is a standardized self-report inventory of psychological symptoms (score range, 0 to 4; higher scores = greater distress) that has been used with cancer patients.16,17 It has been shown to have high reliability and validity in medical patients. We report on the depression and anxiety subscales.
The Profile of Mood States (POMS) Fatigue subscale is the seven item subset of a valid and reliable scale of mood disturbance (score range, 0 to 4; higher scores = greater fatigue) widely used in cancer patients.18
The Cancer and Treatment Distress Scale is the mean of a 29-item self-report questionnaire (score range, 0 to3; higher scores = greater distress), developed in the HCT population, that measures cancer specific distress distinct from general anxiety or depression.11
The Post Traumatic Stress scale was developed by the fourth author (KLS) for use in HCT patients. It is similar to the Patient Health Questionnaire (PHQ)19 as a self-report of symptoms for diagnosing PTSD corresponding to Diagnostic and Statistical Manual IV psychiatric criteria6. The measure includes 11 symptom items scored for frequency from 0 = “not at all” to 6 = “several times a day,” and one item on overall impact rated from 0 = “these things haven't happened to me” to 5 = “severely disturbing, they really impact how I feel and what I do.”
Health Related Quality of Life (HRQOL)
The 12 item version of the Short-Form Health Survey (SF-12) measures physical and mental HRQOL using two standardized summary measures of physical and mental health (higher scores = better functioning).20
Neuropsychological Functioning
The Neurobehavioral Rating Scale (NBRS) is a 27-item structured self-report questionnaire of executive functioning measuring behavioral disturbance including insight, disinhibition, and impaired attention (score range, 0 to 10; higher scores = more severe behavioral disturbance).21
The Modified Memory Questionnaire (MMQ) is a 35-item measure of subjective memory function (score range, 0 to 4; higher scores = greater memory dysfunction).22
Potential Confounders
Potential confounders (See Table 1) obtained from the centralized medical database were categorized into the following four blocks of variables:
-
1)
Demographics included age and gender.
-
2)
Baseline medical and transplantation variables included severity of disease (cancer diagnosis and stage), donor cell type, and graft-versus-host disease (GVHD) prophylaxis.
-
3)
Systemic chemotherapy before transplantation and radiation included type of prior chemotherapy and dose of conditioning with total body irradiation..
-
4)
Long-term Post-transplantation Complications included extent of chronic graft vs. host disease.
Table 1.
Characteristics of Patients Available at 1 year (n=52)
| No Delirium Episode (n=29) n (%) | Delirium Episode1 (n=23) n (%) | P Value | |
|---|---|---|---|
| Age at study entry | 0.28 | ||
| 22-35 | 12 (41.4) | 5 (21.7) | |
| 36-45 | 9 (31.0) | 7 (30.4) | |
| 46-55 | 6 (20.7) | 6 (26.1) | |
| 56-62 | 2 (6.9) | 5 (21.7) | |
| Gender | 0.11 | ||
| Male | 19 (65.5) | 10 (43.5) | |
| Female | 10 (34.5) | 13 (56.5) | |
| Race | 0.30 | ||
| White | 27 (93.1) | 23 (100%) | |
| Hispanic | 1 (3.4) | 0 (0) | |
| Other | 1 (3.4) | 0 (0) | |
| Diagnosis | 0.01 | ||
| CML | 17 (58.6) | 10 (43.5) | |
| ALL or AML | 5 (17.2) | 3 (13.0) | |
| BR or OV | (3.4) | 7 (30.4) | |
| MDS or MM | 4 (13.8) | 0 (0) | |
| NHL | 2 (6.9) | 3 (13.0) | |
| Disease stage2 | 0.47 | ||
| Less advanced | 15 (51.7) | 10 (43.5) | |
| More advanced | 13 (44.8) | 13 (56.5) | |
| Source of Stem Cells | 0.06 | ||
| Bone Marrow | 24 (82.8) | 13 (56.5) | |
| Stem Cell | 5 (17.2) | 10 (43.5) | |
| Donor Type | 0.02 | ||
| Allogeneic | 27 (93.1) | 15 (65.2) | |
| Autologous | 2 (6.9) | 8 (34.8) | |
| GVHD Prophylaxis | 0.03 | ||
| CSP or Tacrolimus and Methotrexate | 22 (75.9) | 12 (52.2) | |
| Methotrexate alone | 2 (6.9) | 8 (43.8) | |
| RFT5.dgA | 3 (10.3) | 3 (13.0) | |
| BC-3 | 2 (6.9) | 0 (0) | |
| Systemic Chemotherapy before HCT | 0.34 | ||
| Hydroxyurea only or none | 13 (44.8) | 7 (30.4) | |
| Interferon with or without hydroxyurea | 6 (20.7) | 4 (17.4) | |
| Chemo other than interferon or hydroxyurea | 9 (31.0) | 12 (52.2) | |
| Cranial Irradiation or Intrathecal Chemotherapy before HCT | 0.80 | ||
| Yes | 3 (10.3) | 3 (13.0) | |
| None | 25 (86.2) | 20 (87.0) | |
| Conditioning with Total Body Irradiation | 0.62 | ||
| ≤ 1200 cGy | 7 (24.2) | 7 (30.4) | |
| > 1200 cGy | 9 (31.0) | 6 (26.1) | |
| None | 13 (44.8) | 10 (43.5) | |
| Chronic GVHD Extent | 0.22 | ||
| Extensive | 23 (79.3) | 14 (60.9) | |
| Limited or None | 6 (20.7) | 9 (39.1) | |
| GVHD Treatment Medications | 0.48 | ||
| Glucocorticoids | 22 (75.9) | 20 (87.0) | |
| Other or none | 7 (24.1) | 3 (13.0) | |
| Relapse at 6 months | 0.69 | ||
| Relapse since baseline | 2 (6.9) | 1 (4.3) | |
| No relapse | 27 (93.1) | 22 (95.7) | |
| Relapse at 1 year | 0.62 | ||
| Relapse since 6 months | 3 (10.3) | 1 (4.3) | |
| No relapse | 26 (89.7) | 22 (95.7) | |
| Mean (SD) | Mean (SD) | ||
| Pretransplantation Charlson Comorbidity Score | 2.3 (1.1) | 2.4 (1.3) | 0.72 |
CML = chronic myeloid leukemia, ALL = acute lymphoblastic leukemia, AML = acute myeloid leukemia, BR = breast carcinoma, OV = ovarian carcinoma, MDS = myelodysplasia, MM = multiple myeloma, NHL = non-Hodgkin's lymphoma, GVHD = Graft-versus-host disease, CSP = cyclosporine, RFT5.dgA = CD25-specific immunotoxin, BC-3 = CD3-specific antibody
Delirium episode group includes patients with 2 out of 3 consecutive assessments with DRS>12.
Less advanced disease was defined as acute lymphoblastic leukemia or acute myeloid leukemia in first remission; chronic myeloid leukemia in a chronic phase; non-Hodgkin's lymphoma in first remission, untreated first recurrence, or second remission. More advanced disease included all other stages of these diseases, all other types of hematologic malignancies, breast carcinoma ≥ stage II, and ovarian carcinoma
Statistical Analyses
To assess whether differential attrition biased the study results, t-tests or chi square tests evaluated whether there were demographic or clinical differences, including delirium episode status, between patients who were present in the current analyses and those who had dropped out of the study.
Next, we used t tests to compare unadjusted means on outcomes for the delirium episode and no delirium episode groups. Finally, we used linear regression modeling to calculate betas (B), standard errors (SEs), and 95% confidence intervals (CI) for the relationships between delirium and 6 month and 1 year outcomes, adjusting for potential confounding factors. Confounding was evaluated separately for each model by sequentially entering the four blocks of potential confounders (demographics, baseline medical and transplantation variables, pretransplantation chemotherapy and radiation, and long-term post-transplantation complications) and then iteratively eliminating non-confounding variables from each block by monitoring the change in the beta coefficient for delirium for ≥ 10%.23,24 Models did not adjust for the pretransplantation level of the outcome variable for two reasons; (1) potential effects of delirium on outcomes would have been obscured by shared variation between delirium and the pretransplantation level of each outcome and by the high correlations (e.g., 0.7) between the 6 month or 1 year outcomes and the pretransplantation level of each outcome; and (2) recent work has suggested that adjustment for baseline levels of outcome variables can induce bias in observational studies.25
Results
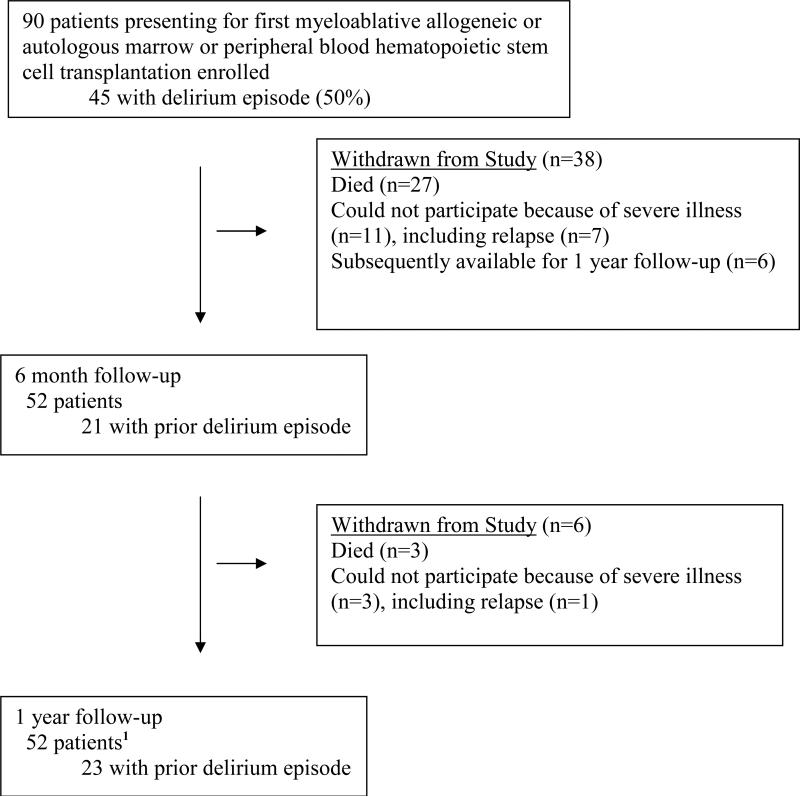
Of the 90 original patients enrolled, 30 patients had died and 8 patients were unavailable because of relapse by one year (Figure 1). Six of the patients unavailable for follow-up at 6 months were subsequently tested at one year. Few significant differences were found between patients present in the current analyses compared to those who had dropped out. There was a non-significant trend for a lower percent of patients who had experienced a delirium episode to be available for testing at one year compared to those who had not experienced a delirium episode (64.4% vs. 51.6%; p=.20). The two groups differed significantly in diagnosis (p=.009): a higher percent of survivors with chronic myeloid leukemia, breast carcinoma, ovarian carcinoma, or non-Hodgkin's Lymphoma were available at one year (71.1%, 72.7%, 83.3%) while a lower percent of those surviving after acute lymphoblastic or myeloid leukemia, and myelodysplasia or multiple myeloma were available (32.0%, 40.0%). Patients tested at one year were also significantly more likely to have extensive graft versus host disease (GVHD) than those unavailable (75.5% vs 24.5%, p=.03).
Figure 1.
Patient Participation in Study
1 Six of the patients unavailable for follow-up at 6 months because of severe illness were subsequently available at one year.
The baseline demographic and medical characteristics of the 52 patients who were available for follow-up at 6 months and 1 year are listed in Table 1. The sample was predominantly white, younger than 55, and included more men than women. The most common diagnosis was CML (51.9%). Most patients received allogeneic bone marrow transplants. More than half of the sample had received pre-transplant conditioning with total body irradiation and over 70% of patients had extensive chronic GVHD. Diagnosis, donor type, and GVHD prophylaxis were significantly different between patients with a delirium episode and patients without a delirium episode.
The unadjusted means on 6 month and 1 year outcomes by delirium episode status are presented in Table 2. Without adjustment for confounding, the delirium group had more fatigue and cancer and treatment distress, and worse neuropsychological functioning at 6 months compared to patients who had not experienced a delirium episode. The delirium group also showed similar differences in unadjusted scores at one year.
Table 2.
Unadjusted 6 Month and 1 Year Outcome Means by Delirium Episode Status during Acute Treatment
| 6 Month Outcomes | 1 Year Outcomes | |||||
|---|---|---|---|---|---|---|
| No Delirium Episode (n=31) Mean (SD) | Delirium Episode (n=21) Mean (SD) | P value1 | No Delirium Episode (N=29) Mean (SD) | Delirium Episode (N=23) Mean (SD) | P value1 | |
| Distress | ||||||
| Symptoms Checklist Depression subscale | .51 (.53) | .29 (.45) | .09 | .23 (.32) | .35 (.51) | .31 |
| Symptoms Checklist Anxiety subscale | .29 (.45) | .41 (.62) | .29 | .35 (.37) | .58 (.60) | .10 |
| Profile of Mood States Fatigue subscale | .85 (.70) | 1.50 (1.00) | .009 | .68 (.62) | 1.08 (.82) | .05 |
| Cancer & Treatment Distress | .57 (.41) | .90 (.54) | .02 | .45 (.38) | .67 (.48) | .08 |
| Post-Traumatic Stress Disorder Scale | --- | --- | --- | .41 (.49) | .94 (1.18) | .05 |
| Health-related Quality of Life | ||||||
| SF-12 Physical Component Summary | 43.72 (11.94) | 37.55 (9.78) | .06 | 45.79 (12.44) | 43.60 (10.56) | .50 |
| SF-12 Mental Component Summary | 52.29 (8.48) | 50.45 (8.01) | .44 | 55.06 (5.80) | 51.06 (9.48) | .50 |
| Neuropsychological Functioning | ||||||
| Neurobehavioral Rating Scale | 1.03 (.99) | 1.88 (1.47) | .02 | .82 (.79) | 1.65 (1.38) | .01 |
| Modified Memory Questionnaire | .45 (.43) | .81 (.52) | .009 | .42 (.36) | .77 (.49) | .005 |
P values are for unadjusted t-test models
The results of linear regression models predicting outcomes at both time points, adjusted for potential confounders are listed in Table 4. At 6 months post-transplantation, after adjusting for confounding factors, patients who had experienced a previous delirium episode reported significantly more fatigue (p =.004) and cancer and treatment distress (p = .002), and worse physical HRQOL scores (p = .03) compared to those who had not experienced a delirium episode. Patients who had experienced a delirium episode reported significantly worse neurocognitive functioning as measured by both the NBRS (p = .006) and the MMQ (p = .009).
At 1 year following transplantation, after adjusting for confounding factors, patients who had experienced a previous delirium episode reported greater mental health distress including depression (p = .03), and post traumatic stress disorder (PTSD) symptoms ( p = 0.03), and lower scores on the mental component of the SF-12 ( p = .04) as well as greater fatigue (p = .02) and cancer and treatment distress (p = .03). Patients who had experienced a previous delirium episode also reported significantly worse neurocognitive functioning on the NBRS (p = .004) and the MMQ (p = .005).
Discussion
The present study examines the association of delirium in the acute phase of myeloablative HCT with later outcomes of distress, HRQOL, and neurocognitive functioning, in this case at 6 months and 1 year after transplantation. Small but statistically significant differences in specific domains of all three categories of outcomes emerged at both 6 months and 1 year for patients experiencing delirium, thus supporting our initial hypotheses.
Patients in the current study with a delirium episode reported significantly decreased memory and neurobehavioral functioning at both 6 months and, to a lesser magnitude, 1 year compared to those without delirium. The clinical significance of the NBRS and MMQ scores seen in this study is not well-established. However, comparison of the 6-month and 1-year scores to 80-day scores seen in our earlier work14, (delirium and non delirium groups at 80 days with respective NBRS scores of 1.8 and 1.2 and MMQ scores of 0.7 and 0.4), indicates that the magnitude of all the scores between delirium and no delirium groups remains consistent or increases across time.
Previous research of long-term delirium outcomes on cognition has largely focused on older populations and has shown a robust association between delirium and subsequent cognitive deterioration including dementia in patients hospitalized for general medical conditions over 1 year,26 2 years27 and 3 years,28 as well as elderly patients hospitalized for hip fracture over the two subsequent years 29. Interestingly, analyses30,31 of two of the aforementioned studies26,29 found that patients with recovery of delirium symptoms at 2 months had no increased risk of cognitive decline
Though patients undergoing HCT appear to report baseline mild to moderate worsening of distress, HRQOL, and/or cognition,32 their younger age is associated with a lower prevalence of dementia then the older study populations. The continuing aggressiveness of the cancer and intensity of treatment and supportive care following resolution of a delirium episode may be contributory factors for the persistent cognitive declines in patients undergoing HCT following resolution of their acute delirium episode.
Similar to the pathophysiology of delirium itself, the etiology of its association with persistent cognitive decline remains unclear but is likely multifactorial. Possible mechanisms with both acute and chronic impacts include oxidative stress,33 exacerbation in inflammatory cascades,34 and decreased brain perfusion35. Ongoing use of medications including opioid analgesics, sedatives, corticosteroids, calcineurin inhibitors, and anticholinergics could both cause or worsen delirium as well as possibly have long term toxic effects on the brain, thereby affecting cognition.36 The diffuse range of differences in distress, HRQOL, and cognition reported by patients following delirium in HCT does not definitively localize the pathophysiological mechanisms to specific brain regions, circuits or neurotransmitter systems. Our results are consistent with a growing body of evidence associating depressive and cognitive symptoms, potentially through the link between executive dysfunction37 and decreased prefrontal lobe perfusion38.
We found delirium to be associated with cancer and treatment distress as well as fatigue at both time points, though of decreased magnitude at one year. We similarly found delirium status to be associated with reports of significantly more severe depression and PTSD related symptoms at 1 year. These results correspond to a recent review paper reporting a relationship between inhospital delirium and subsequent depression and/or anxiety in 5 of 8 studies of multiple patient populations39
Though the underlying etiology for the emergence of greater depression symptoms in our study at one year is unclear, it is possibly related to worse mental rather than physical functioning. Patients experiencing delirium during acute phase treatment reported worse physical HRQOL at 6 months compared to patients in the no-delirium group, but by 1 year there was no significant difference between the groups. Conversely, patients with a delirium episode during acute phase treatment reported worse mental functioning at 1 year but not at 6 months.
Past studies examining the impact of delirium on HRQOL have largely focused on functional outcomes in older populations hospitalized with a medical condition or hip fracture, with outcomes including ambulation, activities of living, and independent activities of daily living. Results have indicated a largely negative impact of delirium on one or more of these outcomes at time lengths varying from 6 months to 2 years26,29,31 though one study showed no relationship40.
Our study had several limitations. Though follow-up at both 6 months and 1 year was high for patients still healthy enough to participate, the study population had severe illness related to hematological malignancies. By 6 months, almost half of the original 90 patients were unavailable for follow-up because of unavoidable reasons including death, severe illness, or relapse. Patients available at 1 year in both the delirium and no delirium groups were significantly more likely to have chronic GVHD, and there may have been other unmeasured factors leading to information bias. All outcome measures are standardized, valid, and reliable, but also subjective. We lack objective or observational measures for neurocognitive outcomes. Except for PTSD, which was only measured at 12 months, we measured all outcomes at 6 months and 12 months. Additional unmeasured variables affecting vulnerability for delirium, e.g. personality traits, physical/emotional reserve, cerebral atherosclerosis, as well as delirium precipitating factors, such as long-term effects of anticholinergic medications, could be associated with residual confounding at both outcome points.
Our findings suggest that the occurrence of delirium during the acute phase of HCT may have adverse long-term effects on distress, HRQOL, and cognition. These findings further highlight the importance of recognizing and treating delirium as a complication of HCT with potential long-term consequences, rather than as a strictly acute and transient side effect of HCT. Future research should aim to replicate these findings in larger myeloablative and non-myeloablative HCT populations and examine in more detail a broader range of conditioning regimens. The mechanisms by which delirium may affect long-term outcomes needs further study. Our results add to the literature suggesting the importance of more attention to detection and treatment of acute delirium as a focus of sound clinical care for survivors of HCT.
Table 3.
Adjusted Linear Regression Models Predicting 6 month and 1 year Outcomes from Delirium Episode Status During Acute Treatment
| 6 Month Outcomes | 1 Year Outcomes | |||||
|---|---|---|---|---|---|---|
| Delirium Episode vs. No Delirium Episode B (SE)1 | 95 % Confidence Interval | P value | Delirium Episode vs. No Delirium Episode B (SE) 1 | 95 % Confidence Interval | P value | |
| Distress | ||||||
| Symptoms Checklist Depression subscale | 0.26 (0.15) | −0.05, 0.57 | 0.09 | 0.31 (0.14) a,h | 0.04, 0.59 | 0.03 |
| Symptoms Checklist Anxiety subscale | 0.20 (0.15) a | −0.11, 0.51 | 0.20 | 0.17 (0.12) a,h | −0.07, 0.41 | 0.17 |
| Profile of Mood States Fatigue subscale | 0.72 (0.24) a | 0.24, 1.20 | 0.004 | 0.49 (0.21) d | 0.08, 0.91 | 0.02 |
| Cancer & Treatment Distress | 0.42 (0.13) a | 0.17, 0.68 | 0.002 | 0.28 (0.12) a | 0.03, 0.52 | 0.03 |
| Post-Traumatic Stress Disorder Scale | --- | --- | --- | 0.66 (0.33) g | 0.13, 1.20 | 0.02 |
| Health-related Quality of Life | ||||||
| SF-12 Physical Component Summary | − 7.26 (3.31) d | −13.92, −0.60 | 0.03 | −2.40 (3.01) b,d,f,g | −8.47, 3.66 | .43 |
| SF-12 Mental Component Summary | −1.47 (2.5) a,f,h | −6.59, 3.66 | 0.57 | − 4.75 (2.21) a | −9.19, −0.32 | 0.04 |
| Neuropsychological Functioning | ||||||
| Neurobehavioral Rating Scale | 1.00 (0.35) a | 0.30, 1.70 | 0.006 | 0.60 (0.31) a | 0.33, 1.59 | 0.004 |
| Modified Memory Questionnaire | 0.36 (0.13) | 0.09, 0.63 | 0.009 | 0.31 (0.12) | 0.11, 0.59 | 0.005 |
B=beta coefficient, SE=standard error for the beta coefficient; Results in boldface type are statistically significant at p < 0.05.
a-hPotential confounders were evaluated in blocks. The superscript letters a-h denotes which variables were found to be significant confounders and were adjusted for in the indicated model:
Demographics:
age
gender
Baseline medical & transplantation variables:
severity of disease
cell type
GVHD prophylaxis
Total body irradiation
or systemic chemotherapy before transplantation
Long-term Posttransplantation Complications:
Extent of chronic graft versus host disease
Acknowledgements
Funded by grants from the American Cancer Society (RPG-97-035-01-PBR) and the University of Washington Royalty Research Fund. Part of Dr. Syrjala's effort was funded by grants from the National Cancer Institute (CA63030, CA78990, CA112631).
The authors wish to thank Bart Burington, MS for statistical assistance, and Sari Roth-Roemer, PhD, Kathy Beach, RN and Wendy Brown, RN for their invaluable assistance in carrying out the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contribution: WJK, KLS, and JRF designed the research; JRF collected and assembled data; JRB, CMA, KLS, and JRF analyzed and interpreted data; CMA and JRF performed statistical analysis; JRB, CMA, and JRF wrote the manuscript; WJK and KLS edited manuscript
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Beglinger LJ, Duff K, Van Der Heiden S, Parrott K, Langbehn D, Gingrich R. Incidence of delirium and associated mortality in hematopoietic stem cell transplantation patients. Biol Blood Marrow Transplant. 2006;12:928–935. doi: 10.1016/j.bbmt.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Fann JR, Roth-Roemer S, Burington BE, Katon WJ, Syrjala KL. Delirium in patients undergoing hematopoietic stem cell transplantation. Cancer. 2002;95:1971–1981. doi: 10.1002/cncr.10889. [DOI] [PubMed] [Google Scholar]
- 3.Han L, McCusker J, Cole M, Abrahamowicz M, Primeau F, Elie M. Use of medications with anticholinergic effect predicts clinical severity of delirium symptoms in older medical inpatients. Arch Intern Med. 2001;161:1099–1105. doi: 10.1001/archinte.161.8.1099. [DOI] [PubMed] [Google Scholar]
- 4.Andrykowski MA, Altmaier EM, Barnett RL, Burish TG, Gingrich R, Henslee-Downey PJ. Cognitive dysfunction in adult survivors of allogeneic marrow transplantation: relationship to dose of total body irradiation. Bone Marrow Transplant. 1990;6:269–276. [PubMed] [Google Scholar]
- 5.Julin JE, van Burik JH, Krivit W, et al. Ganciclovir-resistant cytomegalovirus encephalitis in a bone marrow transplant recipient. Transpl Infect Dis. 2002;4:201–206. doi: 10.1034/j.1399-3062.2002.02005.x. [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association . American Psychiatric Association. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. ed 4th American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 7.Fann JR. The epidemiology of delirium: a review of studies and methodological issues. Semin Clin Neuropsychiatry. 2000;5:64–74. doi: 10.153/SCNP00500064. [DOI] [PubMed] [Google Scholar]
- 8.Caraceni A, Nanni O, Maltoni M, et al. Impact of delirium on the short term prognosis of advanced cancer patients. Italian Multicenter Study Group on Palliative Care. Cancer. 2000;89:1145–1149. doi: 10.1002/1097-0142(20000901)89:5<1145::aid-cncr24>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 9.Lawlor PG, Gagnon B, Mancini IL, et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer: a prospective study. Arch Intern Med. 2000;160:786–794. doi: 10.1001/archinte.160.6.786. [DOI] [PubMed] [Google Scholar]
- 10.Ljubisavljevic V, Kelly B. Risk factors for development of delirium among oncology patients. Gen Hosp Psychiatry. 2003;25:345–352. doi: 10.1016/s0163-8343(03)00070-7. [DOI] [PubMed] [Google Scholar]
- 11.Syrjala KL, Langer SL, Abrams JR, et al. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA. 2004;291:2335–2343. doi: 10.1001/jama.291.19.2335. [DOI] [PubMed] [Google Scholar]
- 12.Syrjala KL, Dikmen S, Langer SL, Roth-Roemer S, Abrams JR. Neuropsychologic changes from before transplantation to 1 year in patients receiving myeloablative allogeneic hematopoietic cell transplant. Blood. 2004;104:3386–3392. doi: 10.1182/blood-2004-03-1155. [DOI] [PubMed] [Google Scholar]
- 13.Harder H, Van Gool AR, Duivenvoorden HJ, et al. Case-referent comparison of cognitive functions in patients receiving haematopoietic stem-cell transplantation for haematological malignancies: two-year follow-up results. Eur J Cancer. 2007;43:2052–2059. doi: 10.1016/j.ejca.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Fann JR, Alfano CM, Roth-Roemer S, Katon WJ, Syrjala KL. Impact of delirium on cognition, distress, and health-related quality of life after hematopoietic stem-cell transplantation. J Clin Oncol. 2007;25:1223–1231. doi: 10.1200/JCO.2006.07.9079. [DOI] [PubMed] [Google Scholar]
- 15.Trzepacz PT, Baker RW, Greenhouse J. A symptom rating scale for delirium. Psychiatry Res. 1988;23:89–97. doi: 10.1016/0165-1781(88)90037-6. [DOI] [PubMed] [Google Scholar]
- 16.Derogatis LR. Clinical Psychometrics Research. Baltimore: 1975. The SCL-90. [Google Scholar]
- 17.Derogatis LR, Morrow GR, Fetting J, et al. The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249:751–757. doi: 10.1001/jama.249.6.751. [DOI] [PubMed] [Google Scholar]
- 18.Cella DF, Jacobsen PB, Orav EJ, Holland JC, Silberfarb PM, Rafla S. A brief POMS measure of distress for cancer patients. J Chronic Dis. 1987;40:939–942. doi: 10.1016/0021-9681(87)90143-3. [DOI] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Levin HS, High WM, Goethe KE, et al. The neurobehavioural rating scale: assessment of the behavioural sequelae of head injury by the clinician. J Neurol Neurosurg Psychiatry. 1987;50:183–193. doi: 10.1136/jnnp.50.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sunderland A HJ, Baddely A. Do laboratory tests predict everyday memory? A neuropsychological study. J Verb Learn Verb Behav. 1983;22:341–357. [Google Scholar]
- 23.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 24.Thompson WD. Statistical analysis of case-control studies. Epidemiol Rev. 1994;16:33–50. doi: 10.1093/oxfordjournals.epirev.a036143. [DOI] [PubMed] [Google Scholar]
- 25.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162:267–278. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
- 26.McCusker J, Cole M, Dendukuri N, Belzile E, Primeau F. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ. 2001;165:575–583. [PMC free article] [PubMed] [Google Scholar]
- 27.Francis J, Kapoor WN. Prognosis after hospital discharge of older medical patients with delirium. J Am Geriatr Soc. 1992;40:601–606. doi: 10.1111/j.1532-5415.1992.tb02111.x. [DOI] [PubMed] [Google Scholar]
- 28.Rockwood K, Cosway S, Carver D, Jarrett P, Stadnyk K, Fisk J. The risk of dementia and death after delirium. Age Ageing. 1999;28:551–556. doi: 10.1093/ageing/28.6.551. [DOI] [PubMed] [Google Scholar]
- 29.Dolan MM, Hawkes WG, Zimmerman SI, et al. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci. 2000;55:M527–534. doi: 10.1093/gerona/55.9.m527. [DOI] [PubMed] [Google Scholar]
- 30.Cole MG, You Y, McCusker J, Ciampi A, Belzile E. The 6 and 12 month outcomes of older medical inpatients who recover from delirium. Int J Geriatr Psychiatry. 2008;23:301–307. doi: 10.1002/gps.1878. [DOI] [PubMed] [Google Scholar]
- 31.Gruber-Baldini AL, Zimmerman S, Morrison RS, et al. Cognitive impairment in hip fracture patients: timing of detection and longitudinal follow-up. J Am Geriatr Soc. 2003;51:1227–1236. doi: 10.1046/j.1532-5415.2003.51406.x. [DOI] [PubMed] [Google Scholar]
- 32.Andrykowski MA, Schmitt FA, Gregg ME, Brady MJ, Lamb DG, Henslee-Downey PJ. Neuropsychologic impairment in adult bone marrow transplant candidates. Cancer. 1992;70:2288–2297. doi: 10.1002/1097-0142(19921101)70:9<2288::aid-cncr2820700913>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 33.Seaman JS, Schillerstrom J, Carroll D, Brown TM. Impaired oxidative metabolism precipitates delirium: a study of 101 ICU patients. Psychosomatics. 2006;47:56–61. doi: 10.1176/appi.psy.47.1.56. [DOI] [PubMed] [Google Scholar]
- 34.Fricchione GL, Nejad SH, Esses JA, et al. Postoperative delirium. Am J Psychiatry. 2008;165:803–812. doi: 10.1176/appi.ajp.2008.08020181. [DOI] [PubMed] [Google Scholar]
- 35.Alsop DC, Fearing MA, Johnson K, Sperling R, Fong TG, Inouye SK. The role of neuroimaging in elucidating delirium pathophysiology. J Gerontol A Biol Sci Med Sci. 2006;61:1287–1293. doi: 10.1093/gerona/61.12.1287. [DOI] [PubMed] [Google Scholar]
- 36.Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14:87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 37.Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58:204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 38.Levy-Cooperman N, Burhan AM, Rafi-Tari S, et al. Frontal lobe hypoperfusion and depressive symptoms in Alzheimer disease. J Psychiatry Neurosci. 2008;33:218–226. [PMC free article] [PubMed] [Google Scholar]
- 39.Davydow DS. Symptoms of depression and anxiety after delirium. Psychosomatics. 2009;50:309–316. doi: 10.1176/appi.psy.50.4.309. [DOI] [PubMed] [Google Scholar]
- 40.Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618–624. doi: 10.1111/j.1532-5415.2000.tb04718.x. [DOI] [PubMed] [Google Scholar]