Abstract
An implantable pediatric artificial lung (PAL) may serve as a bridge to lung transplantation for children with end-stage lung failure (ESLF); however, an animal model of pediatric lung failure is needed to evaluate a PAL’s efficacy before it can enter clinical trials. The objective of this study was to assess ligation of the right pulmonary artery (rPA) as a model for pediatric ESLF.
Seven 20-30kg lambs underwent rPA ligation and were recovered and monitored for up to 4 days. Intraoperatively, rPA ligation significantly increased physiologic deadspace fraction (Vd/Vt: baseline=48.6±5.7%, rPA ligation=60.1±5.2%, p=0.012), mean pulmonary arterial pressure (mPPA: baseline=17.4±2.2mmHg, rPA ligation=28.5±5.2mmHg, p<0.001), and arterial partial pressure of carbon dioxide (PaCO2: baseline=40.4±9.3mmHg, rPA ligation=57.3±12.7mmHg, p=0.026). Of the 7 lambs, 3 were unable to be weaned from mechanical ventilation post-operatively, 3 were successfully weaned but suffered cardiorespiratory failure within 4 days, and 1 survived all 4 days. All 4 animals that were successfully weaned from mechanical ventilation had persistent pulmonary hypertension (mPPA=28.6±2.2mmHg) and remained tachypneic (respiratory rate=63±21min−1). Three of the 4 recovered lambs required supplemental oxygen.
We conclude that rPA ligation creates the physiologic derangements commonly seen in pediatric end-stage lung failure and may be suitable for testing and implanting a PAL.
Keywords: Artificial lung, Pediatric, Pulmonary failure, Pulmonary artery ligation, Animal model, Sheep
Introduction
Pediatric end-stage lung failure (ESLF) presents a significant clinical challenge. Although lung transplantation remains the only definitive treatment for these patients, fewer than 100 pediatric lung transplantations are performed each year in the United States due to the limited availability of organs – a number that has plateaued even as adult lung transplant increases.(1) For every pediatric patient who receives a lung transplant, approximately one other patient dies while waiting for an organ, or is delisted due to illness.(2) As outcomes after lung transplant continue to improve, finding therapeutic interventions which allow patients to reach transplantation will become increasingly important.
Pediatric patients with ESLF have typically been supported up to transplantation using a combination of infusions, mechanical ventilation, and extracorporeal membrane oxygenation (ECMO).(3, 4) An implantable pediatric artificial lung (PAL) may serve as a bridge to transplantation for those patients. A PAL would permit ambulation, helping reverse the physical deconditioning caused by prolonged hospitalization and intubation, similar to the strategy currently in use for some adult patients.(5)
However, in order to translate an artificial lung’s theoretical advantages into practice, its efficacy in restoring normal physiology must first be studied in disease animal models. To date, these studies have been limited to healthy or acute respiratory failure models in adult-sized animals.(6-12). There has been no published long-term study of an artificial lung in a pediatric ESLF model.
Since pediatric lung disease is a group of diverse diseases, we attempted to model the most important common denominators. The diseases most frequently treated by lung transplant in children include cystic fibrosis (CF), idiopathic pulmonary arterial hypertension (IPAH), congenital heart disease (CHD), congenital diaphragmatic hernia (CDH) with associated pulmonary hypoplasia and pulmonary hypertension, idiopathic pulmonary fibrosis (IPF), and obliterative bronchiolitis.(1) Many of these patients also have some underlying bronchopulmonary dysplasia (BPD) resulting from premature birth and associated mechanical ventilation. Regardless of diagnosis, patients typically experience some or all of the following conditions: pulmonary arterial hypertension (PAH) with associated right ventricular (RV) failure, impaired oxygenation with refractory hypoxemia, increased dead space ventilation (Vd/Vt) with refractory hypercapnia, and shortness of breath (Table I).(13, 14) We hypothesized that rPA ligation would create a model with these characteristics, specifically PAH, hypoxemia, and hypercapnia.
Table I.
Disease Model Overview
| Disease Process | Unit Of Measurement | Potential Target Values For A Disease Model |
|---|---|---|
| Pulmonary Hypertension |
Mean PA Pressure (Mppa) | > 25 mmHg |
| Impaired Oxygenation |
Arteriolar Partial Pressure Of Oxygen (Po2) |
< 70 mmHg without supplemental O2 |
| Dead Space Ventilation |
Dead Space Fraction (Vd/Vt) |
> 50% |
| CO2 Retention (*Hypercapnia) |
Arterial Partial Pressure Of Carbon Dioxide (Pco2) |
> 45 mmHg |
| Dyspnea / Tachypnea |
Respiratory Rate (RR) | > 60 breaths per minute |
Materials and Methods
All animals received humane care in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Animal protocol was approved by the University of Michigan Committee on Use and Care of Animals.
Seven healthy lambs (20-30kg) were anesthetized with propofol (10mg/kg), intubated, placed in the right lateral decubitus position, and mechanically ventilated (MV) using a Narkomed 6000 ventilator (North American Dräger, Telford, PA) with: tidal volume 15mL/kg, frequency 12min−1, fraction of inspired oxygen (FiO2) 0.75. General anesthesia was maintained with inhaled isoflurane (1-3.5%). An arterial line was placed into the left carotid artery for continuous mean arterial pressure (mPART) monitoring and arterial blood gas (ABG) sampling. An 8Fr pulmonary artery catheter was placed for continuous monitoring of mean pulmonary artery pressure (mPPA), cardiac output (CO), and intravenous administration of medications and fluids. These lines were left for the duration of the study.
A left thoracotomy was performed and the fifth rib removed. The ligamentum arteriosum was ligated and divided. The rPA was ligated with a large Hem-o-lok (Teleflex Medical, Research Triangle Park, NC) polymer locking clip. Following rPA ligation, vasopressin (1-3u/hr) was used to maintain a mPART >50mmHg. Vasopressin was weaned and discontinued prior to attempted extubation in all animals.
Thoracostomy tubes were placed and attached to water-seal suction. A small “soaker catheter” with multiple side holes for distributing local anesthetic was placed within the chest wall for postoperative pain control. An additional 60mL 0.5% bupivacaine intercostal nerve block was performed over ribs 3-7 before closing. The chest was closed in three layers and stapled, lungs were recruited, and animals were weaned to extubation.
Following surgery, animals were monitored in our chronic care room for up to four days. Antibiotics (gentamicin 2.5mg/kg every 8 hours and nafcillin 500mg every 6 hours) were given intravenously. 0.5% Bupivacaine (0.5-1mg/kg every 6 hours) was injected through the chest wall catheter. Buprenorphine (0.3mg every 12 hours) and flunixin (1-2mg/kg every 12 hours) were given as needed, based on a formal pain assessment. Supplemental oxygen (Sup. O2) was provided via a nasal cannula to maintain PaO2>70mmHg.
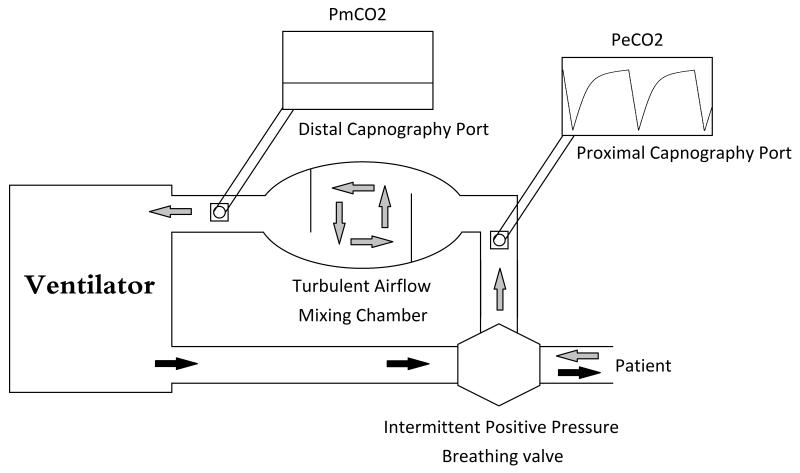
Intraoperatively, measurements of hemodynamics, ABGs, and dead space were collected at baseline (prior to rPA ligation and thoracotomy) and post-ligation (following chest closure and lung recruitment). Postoperatively, hemodynamics were recorded hourly and ABGs were drawn every 6 hours. A modified ventilator circuit was used to calculate dead space ventilation (Figure 1). During exhalation, an Intermittent Positive Pressure Breathing valve (CareFusion, Yorba Linda, CA) diverts exhaled gas through a proximal capnography port for end-tidal PCO2 (PETCO2) measurements, a 5L chamber, and a distal capnography port for mixed-expired PCO2 (PeCO2) measurements. The 5L chamber contains two baffles designed to obstruct laminar air flow and create turbulent, mixing air flow. Physiologic dead space (Vd/Vt) was calculated using the Bohr-Enghoff equation:
Figure 1. Modified Ventilation Circuit For Collection And Measurement Of Mixed Expired Gas.
Modified ventilation circuit for collection and measurement of mixed expired gas. The intermittent positive pressure breathing valve separates inspiratory and expiratory gases. The mixing chamber creates turbulent airflow. The distal capnograph measures partial pressure of mixed expired carbon dioxide (PmCO2).
Mean values for intraoperative baseline, intraoperative post-ligation, overall postoperative duration (beginning from time of chest closure), and final end-of-study measurements were calculated. The end-point of the study was defined as the time at which euthanization was performed. Animals were euthanized when they were either unable to be weaned from MV, when they exhibited signs of cardiorespiratory failure based on clinical assessment, and ABGs (MAP <50mmHg, pH <7.15, PaCO2 >60mmHg, lactate >10mmol/L), or when the goal study time of 4 days was reached. Data was expressed as mean ± standard deviation. Data analysis was performed using paired-sample t-tests in SPSS (IBM, Armonk, NY).
Results
Intraoperatively, rPA ligation significantly increased mPPA from 17.4±2.2mmHg to 28.5±5.2mmHg (p<0.001) (Table 2). Neither mPART (79.4±18.4mmHg vs 80.5±14.5mmHg, p=0.899) nor CO (4.12±1.41L/min vs 3.93±0.90L/min, p=0.727) were significantly changed by rPA ligation.
Table II.
Hemodynamics, Pulmonary Artery Hypertension, and Impaired Respiratory Function in a Lamb Model of Pediatric Lung Failure
| Intraoperative | Postoperative | P - values | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Ligation | Overall Duration |
Final End-Point |
Baseline vs. Ligation |
Baseline vs. Duration |
Baseline vs. End-Point |
|
| n = 7 | n = 7 | n = 7 | n = 7 | ||||
|
mPPA (mmHg) |
17.4 ± 2.20 | 28.5 ± 5.20 | 28.6 ± 2.20 | 29.4 ± 5.30 | < 0.001 | 0.001 | 0.001 |
|
mPART
(mmHg) |
79.4 ± 18.7 | 80.5 ± 14.5 | 76.6 ± 9.90 | 72.0 ± 11.0 | 0.899 | 0.772 | 0.485 |
|
CO
(L/min) |
4.12 ± 1.41 | 3.93 ± 0.90 | 3.79 ± 1.72 | 3.62 ± 1.43 | 0.727 | 0.885 | 1.000 |
| pH | 7.42 ± 0.11 | 7.30 ± 0.12 | 7.21 ± 0.17 | 7.20 ± 0.12 | 0.038 | 0.046 | 0.033 |
|
PaCO2
(mmHg) |
40.4 ± 9.30 | 57.3 ± 12.7 | 56.4 ± 16.5 | 61.1 ± 16.9 | 0.026 | 0.042 | 0.099 |
|
PaO2
(mmHg) |
378 ± 27.8 | 356 ± 49.6 | 111 ± 16.3 | 107 ± 14.9 | 0.327 | < 0.001 | < 0.001 |
|
Vd/Vt
(%) |
48.6 ± 5.70 | 60.1 ± 5.20 | Not Measured | 0.012 | -- | -- | |
|
RR
(min−1) |
15 ± 0 (MV support) | 63.3 ± 21.0 | 71.5 ± 34.9 | -- | -- | -- | |
|
Sup. O2
(L/min) |
1.1 ± 0.9 | 5.3 ± 4.1 | -- | -- | -- | ||
Values are expressed as means ± SD. mPART, mean arterial pressure; mPPA, mean pulmonary arterial pressure; CO, cardiac output; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; Vd/Vt, dead space fraction; RR, respiratory rate; Sup O2, supplemental oxygen; MV, mechanical ventilation.
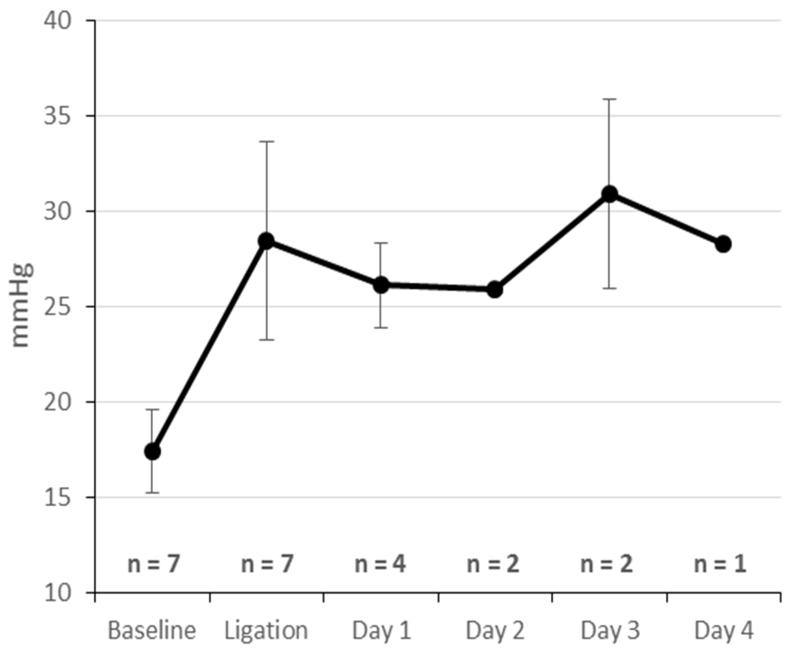
Postoperatively, mPPA remained consistently elevated at 28.6±4.0mmHg, while both mPPA and CO remained normal at 76.6±9.9mmHg and 3.79±1.72L/min respectively (Figure 2). During end-point measurements, hemodynamics remained stable in all animals. The mPPA had a mean of 29.4±5.2mmHg; average mPART was 72.0±11.0mmHg, and average CO was 3.62±1.43L/min.
Figure 2.
Intraoperative And Postoperative Mean Pulmonary Artery Pressure
In terms of gas exchange, the Vd/Vt increased from 48.6±5.7% to 60.1±5.2% (p=0.012) with rPA ligation. Additionally, PaCO2 increased from 40.4±9.3mmHg to 57.3±12.7mmHg (p=0.026) and pH decreased from 7.42±0.11 to 7.30±0.12 (p=0.038) at constant ventilator settings. Intraoperative PaO2 was not significantly altered; it was 356±49.6mmHg after ligation compared to 378±27.8mmHg before ligation (p=0.327).
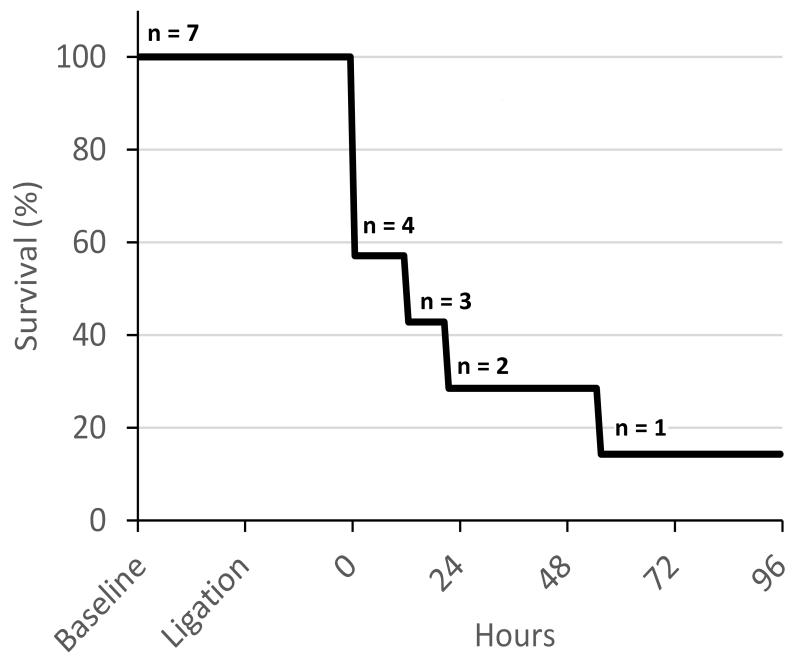
Following rPA ligation, 6 of 7 animals experienced respiratory failure within 4 days. Three lambs (43%) could not be weaned from MV (inadequate breathing after several hours of weaning attempts) and were euthanized (end-point pH range: 6.99-7.12, PaCO2 range: 62.2-84.4mmHg) (Figure 3). Three lambs (43%) were successfully extubated, but suffered cardiorespiratory failure and were euthanized at hours 12, 20, and 56 (end-point pH range: 7.14-7.38, PaCO2 range: 57.9-69.4mmHg). Each of these lambs appeared short of breath, were tachypneic, and had respiratory failure based on ABG’s. One lamb (14%) was successfully weaned and survived all 4 days (end-point pH 7.47, PaCO2 28.2mmHg). At end-point measurements, the PaCO2 averaged 61.1±16.9mmHg with a PaO2=107±14.9mmHg (on 5.3±4.1 L/min Sup. O2), and pH=7.20±0.12 (Table II). All 4 animals who were extubated were tachypneic throughout the experiment (average RR 63±21 min−1), and 3 of 4 required Sup. O2 to maintain a PaO2>70mmHg (average PaO2 before Sup. O2 was 56.1±8.8mmHg).
Figure 3. Kaplan-Meier Survival Curve.
All deaths were due to severe respiratory failure.
Discussion
The goal of this study was to develop an animal model of pediatric ESLF incorporating two of its key characteristics: impaired respiratory function and PAH. Animal models of acute and chronic pulmonary diseases that have been described previously are not suitable for testing an implantable PAL. Most of them were developed in adult-sized animals, and focus on modeling adult respiratory distress syndrome (ARDS), or hypoxia and acute lung injury. Models involving cotton-smoke inhalation,(7, 8) oleic acid instillation,(16) endotoxin injection (9, 17) and hydrochloric acid instillation (18, 19) induce lung injury and strongly activate the systemic inflammatory response, which is not characteristic of pediatric CLD. Saline lavage has been used in pediatric models of acute lung disease since it involves depletion of pulmonary surfactant (20, 21), but does not create PAH and is not generally performed in recovery experiments since severe lung injury is induced (9, 10, 21). Injecting substances into the pulmonary circulation, either beads (sephalex (11, 22) or ceramic (23)) or serial air embolisms (12), have been used to develop models of PAH. These methods often require weeks or months to develop, pushing the animal out of the appropriate size and age range. In addition, these models are expensive and effort intensive. Finally, the PAH induced by such models is often transient and does not change systemic blood gases and respiratory rate. After rPA ligation, we noted substantial and sustained PAH and respiratory failure without the inflammatory response typical of acute lung injury.
Our model caused a modest increase in Vd/Vt from 48.6±5.7% to 60.1±5.2% despite excluding the entire right lung from gas exchange. This increase in Vd/Vt may appear less than expected; this is a consequence of improved ventilation-perfusion (V/Q) matching in the left lung due to redistribution of pulmonary blood flow following rPA ligation (Figure 4). In our model, there was likely alveolar dead space in both lungs at baseline due to positive pressure ventilation, with a moderate fraction of tidal volume going to West Zone 1 regions of both lungs (drawn here in the human configuration for simplicity, although lungs in quadruped animals are anatomically rotated 90 degrees). After ligation of the rPA, redistribution of perfusion throughout the remaining left lung and associated PAH resulted in a significant reduction in West Zone 1 lung volume in the left lung. This improved V/Q matching in the left lung serves to offset the increased alveolar dead space in the right lung, resulting in less of an increase in Vd/Vt than expected.
Figure 4. Dead Space In An Animal Model Of Chronic Lung Disease.
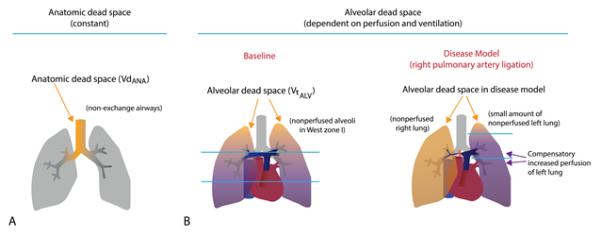
A: Anatomic dead space arising from non-exchange airways is constant. B: Alveolar dead space depends on perfusion of the lung. When the right pulmonary is ligated, alveolar dead space in that lung increases, but alveolar dead space in the left lung drops to compensate. (Note that this figure has been redrawn to correspond to human lung West zones; in quadruped animal models the lung zones are rotated 90°).
While dead space measurements are not routinely performed as part a pre-transplantation evaluation, ventilation-perfusion (V/Q) mismatching and impaired ventilation are important drivers of respiratory failure in pediatric ELSF. The respiratory impairment is created in our model through the large increase in alveolar dead space following ligation. When dead space rises acutely, as in pulmonary embolism, patients can initially compensate with a high respiratory rate, but eventually suffer respiratory fatigue. Our model is associated with an analogous respiratory failure: animals who could be extubated maintained a normal mean PaCO2 post-operatively by increasing respiratory rate, but subsequently developed acute respiratory failure when their compensatory mechanisms failed. While a right pnumonectomy in these lambs would improve the V/Q mismatch created by our model, it would not relieve the increased PAH; nor would it represent a viable treatment for children with ESLF, whose disease is diffuse and not limited to a single lung.
PAH is achieved in the model by abruptly decreasing the cross-sectional area of pulmonary blood flow by approximately one-half, which is sufficient to increase pulmonary vascular resistance in a lamb model. The increase in mPPA is sustained and reached a pressure consistent with clinically significant PAH in pediatric patients.(24) Although this increase in RV afterload causes right heart strain, this is not purely an RV-failure model because these animals did not have significant decreases in CO or mPART (systemic or pulmonary) at end-point measurement.
Several strategies are currently used to bridge patients with ESLF to transplantation, each with distinct limitations. Prolonged MV is not a suitable management strategy due to induction of lung trauma and is associated with increased mortality following lung transplantation in adults.(25) ECMO has been effective at treating acute lung disease, but is poorly suited as a long-term bridge for chronic disease and has a similar association with increased mortality.(26) Recent studies have demonstrated the feasibility of using artificial lungs as a bridge to transplantation in children.(27, 28) These artificial lungs are attached in a pumpless pulmonary-artery-to-left-atrium (PA to LA) configuration and are used in children with decompensating respiratory function and PAH. However, these artificial lungs are associated with high mortality and are too bulky to allow for ambulation. Studies from adult patients undergoing ECMO while awake have demonstrated the importance of physical reconditioning while awaiting lung transplantation.(5)
An implantable PAL would represent an advantageous bridge to transplantation. First, it would support respiratory function when the native lungs are insufficient. Second, the PAL would be attached in a PA to LA (parallel) configuration that would offload the RV by providing an alternative low-resistance flow pathway. Third, as a pumpless device, it would minimize blood trauma and allow for ambulation, physical reconditioning, and avoidance of debilitation. Fourth, it would allow for extubation of the patient, lowering the risk of complications that arise in the short term (pneumonia) and long term (subglottic stenosis) in patients who are intubated.(29, 30) All of these factors would prepare a patient for better post-transplant outcomes.
There were several limitations to our study. First, pediatric ESLF represents a diverse group of pathophysiologic derangements with common elements; while our model captures many of these elements, it does not accurately model any single disease process with high fidelity. For example, pediatric patients requiring lung transplantation from IPAH have primary RV failure, whereas a patient with obliterative bronchiolitis has primary respiratory failure. In order to test a PAL’s ability to treat either one of these, the disease model would need to incorporate the most clinically important aspects of these disorders. Relief of pulmonary hypertension is, therefore, as important to successful device testing as increased dead space and respiratory failure. A device capable of providing support for all of these pathophysiologic states would enjoy wide applicability.
Second, our model employs an acute, focal insult (rPA ligation), whereas ESLF may develops over months to years and is generally a diffuse process affecting the pulmonary and circulatory systems. In chronic lung diseases, this often leads to RV hypertrophy, which will tolerate PAL attachment differently than an unconditioned RV. Despite this limitation, we believe our model recreates the key physiologic derangements necessary to assess the value of a PAL. In fact, initial testing of a low resistance PAL with acute rPA ligation has shown significant decreases in pulmonary vascular resistance and impedance. Additionally, as discussed previously, there are no other models that recreate the pathophysiology observed in the setting of pediatric ESLF in the appropriate size animal.
Third, early mortality in our model. Our desire was to create an awake, spontaneously breathing model similar to what is seen in children with ESLF. However, about half of the animals were unable to independently support their own gas exchange, while the other half were able to do so temporarily. We hypothesize that this early mortality is caused by the combined insult of rPA ligation plus thoracotomy/anesthesia. We believe that by inducing the injury remote from anesthesia, we can achieve our goal. The point of this interim report is that, by an iterative process, we have identified the degree of vascular occlusion that results in an extubated model of hypercarbia and PAH. Our next step is to perform the procedure which allows induction of the rPA ligation injury remotely and asynchronous to the thoracotomy/anesthesia.
Finally, this model only focused on using 20-30 kg sheep, which was an appropriate size for model testing. In the clinical setting, pediatric patients could potentially be much smaller. While larger sheep were used in these experiments, this model should be applicable to smaller animals.
In conclusion, rPA ligation represents a promising model of pediatric ESLF. It incorporates multiple elements of the diverse pathophysiologic elements of end-stage lung failure including pulmonary artery hypertension, dead space ventilation, and refractory hypoxia.
Acknowledgment
Supported by National Institutes of Health Grant 2RO1 HD015434-29. University of Michigan Undergraduate Research Opportunity Program (UROP)
References
- 1.Benden C, Edwards LB, Kucheryavaya AY, Christie JD, Dipchand AI, Dobbels F, Kirk R, Lund LH, Rahmel AO, Yusen RD, Stehlik J, International Society for H. Lung T. The Registry of the International Society for Heart and Lung Transplantation: sixteenth official pediatric lung and heart-lung transplantation report--2013; focus theme: age. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32:989–997. doi: 10.1016/j.healun.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Valapour M, Paulson K, Smith JM, Hertz MI, Skeans MA, Heubner BM, Edwards LB, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2011 Annual Data Report: lung. Am J Transplant. 2013;13(Suppl 1):149–177. doi: 10.1111/ajt.12024. [DOI] [PubMed] [Google Scholar]
- 3.Casswell GK, Pilcher DV, Martin RS, Pellegrino VA, Marasco SF, Robertson C, Butt W, Buckland M, Gooi J, Snell GI, Westall GP. Buying time: The use of extracorporeal membrane oxygenation as a bridge to lung transplantation in pediatric patients. Pediatric transplantation. 2013;17:E182–188. doi: 10.1111/petr.12152. [DOI] [PubMed] [Google Scholar]
- 4.Strueber M. Bridges to lung transplantation. Current opinion in organ transplantation. 2011;16:458–461. doi: 10.1097/MOT.0b013e32834ac7ec. [DOI] [PubMed] [Google Scholar]
- 5.Turner DA, Cheifetz IM, Rehder KJ, Williford WL, Bonadonna D, Banuelos SJ, Peterson-Carmichael S, Lin SS, Davis RD, Zaas D. Active rehabilitation and physical therapy during extracorporeal membrane oxygenation while awaiting lung transplantation: a practical approach. Critical care medicine. 2011;39:2593–2598. doi: 10.1097/CCM.0b013e3182282bbe. [DOI] [PubMed] [Google Scholar]
- 6.Kirmse M, Fujino Y, Hess D, Kacmarek RM. Positive end-expiratory pressure improves gas exchange and pulmonary mechanics during partial liquid ventilation. American journal of respiratory and critical care medicine. 1998;158:1550–1556. doi: 10.1164/ajrccm.158.5.9708100. [DOI] [PubMed] [Google Scholar]
- 7.Kimura R, Traber LD, Herndon DN, Linares HA, Lubbesmeyer HJ, Traber DL. Increasing duration of smoke exposure induces more severe lung injury in sheep. J Appl Physiol (1985) 1988;64:1107–1113. doi: 10.1152/jappl.1988.64.3.1107. [DOI] [PubMed] [Google Scholar]
- 8.Alpard SK, Zwischenberger JB, Tao W, Deyo DJ, Traber DL, Bidani A. New clinically relevant sheep model of severe respiratory failure secondary to combined smoke inhalation/cutaneous flame burn injury. Critical care medicine. 2000;28:1469–1476. doi: 10.1097/00003246-200005000-00036. [DOI] [PubMed] [Google Scholar]
- 9.Odenstedt H, Aneman A, Karason S, Stenqvist O, Lundin S. Acute hemodynamic changes during lung recruitment in lavage and endotoxin-induced ALI. Intensive care medicine. 2005;31:112–120. doi: 10.1007/s00134-004-2496-x. [DOI] [PubMed] [Google Scholar]
- 10.Zick G, Frerichs I, Schadler D, Schmitz G, Pulletz S, Cavus E, Wachtler F, Scholz J, Weiler N. Oxygenation effect of interventional lung assist in a lavage model of acute lung injury: a prospective experimental study. Critical care. 2006;10:R56. doi: 10.1186/cc4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shelub I, van Grondelle A, McCullough R, Hofmeister S, Reeves JT. A model of embolic chronic pulmonary hypertension in the dog. Journal of applied physiology: respiratory, environmental and exercise physiology. 1984;56:810–815. doi: 10.1152/jappl.1984.56.3.810. [DOI] [PubMed] [Google Scholar]
- 12.Perkett EA, Brigham KL, Meyrick B. Continuous air embolization into sheep causes sustained pulmonary hypertension and increased pulmonary vasoreactivity. The American journal of pathology. 1988;132:444–454. [PMC free article] [PubMed] [Google Scholar]
- 13.Sweet SC. Pediatric lung transplantation. Proc Am Thorac Soc. 2009;6:122–127. doi: 10.1513/pats.200808-095GO. [DOI] [PubMed] [Google Scholar]
- 14.Dishop MK. Paediatric interstitial lung disease: classification and definitions. Paediatric respiratory reviews. 2011;12:230–237. doi: 10.1016/j.prrv.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Nunn JF, Holmdahl MH. Henrik Enghoff and the volumen inefficax. Ups J Med Sci. 1979;84:105–108. doi: 10.3109/03009737909179145. [DOI] [PubMed] [Google Scholar]
- 16.Overbeck MC, Pranikoff T, Yadao CM, Hirschl RB. Efficacy of perfluorocarbon partial liquid ventilation in a large animal model of acute respiratory failure. Critical care medicine. 1996;24:1208–1214. doi: 10.1097/00003246-199607000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Pacht ER, Kindt GC, Lykens MG. Increased antioxidant activity in bronchoalveolar lavage fluid after acute lung injury in anesthetized sheep. Critical care medicine. 1992;20:1441–1447. doi: 10.1097/00003246-199210000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. The Journal of clinical investigation. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabinovici R, Neville LF, Abdullah F, Phillip DR, Vernick J, Fong KL, Hillegas L, Feuerstein G. Aspiration-induced lung injury: role of complement. Critical care medicine. 1995;23:1405–1411. doi: 10.1097/00003246-199508000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs JR, Kaviani A, Watson K, Thompson J, Wilson JM, Fauza DO. Intratracheal pulmonary ventilation improves gas exchange during laparoscopy in a pediatric lung injury model. Journal of pediatric surgery. 2005;40:22–25. doi: 10.1016/j.jpedsurg.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Manaligod JM, Bendel-Stenzel EM, Meyers PA, Bing DR, Connett JE, Mammel MC. Variations in endexpiratory pressure during partial liquid ventilation: impact on gas exchange, lung compliance, and end-expiratory lung volume. Chest. 2000;117:184–190. doi: 10.1378/chest.117.1.184. [DOI] [PubMed] [Google Scholar]
- 22.Pohlmann JR, Akay B, Camboni D, Koch KL, Mervak BM, Cook KE. A low mortality model of chronic pulmonary hypertension in sheep. J Surg Res. 2012;175:44–48. doi: 10.1016/j.jss.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, Yung GL, Marsh JJ, Konopka RG, Pedersen CA, Chiles PG, Morris TA, Channick RN. Endothelin mediates pulmonary vascular remodelling in a canine model of chronic embolic pulmonary hypertension. The European respiratory journal. 2000;15:640–648. doi: 10.1034/j.1399-3003.2000.15d04.x. [DOI] [PubMed] [Google Scholar]
- 24.Rubin LJ, American College of Chest P Diagnosis and management of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:7S–10S. doi: 10.1378/chest.126.1_suppl.7S. [DOI] [PubMed] [Google Scholar]
- 25.Mason DP, Thuita L, Nowicki ER, Murthy SC, Pettersson GB, Blackstone EH. Should lung transplantation be performed for patients on mechanical respiratory support? The US experience. The Journal of thoracic and cardiovascular surgery. 2010;139:765–773. e761. doi: 10.1016/j.jtcvs.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Puri V, Epstein D, Raithel SC, Gandhi SK, Sweet SC, Faro A, Huddleston CB. Extracorporeal membrane oxygenation in pediatric lung transplantation. The Journal of thoracic and cardiovascular surgery. 2010;140:427–432. doi: 10.1016/j.jtcvs.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Hoganson DM, Gazit AZ, Boston US, Sweet SC, Grady RM, Huddleston CB, Eghtesady P. Paracorporeal lung assist devices as a bridge to recovery or lung transplantation in neonates and young children. The Journal of thoracic and cardiovascular surgery. 2014;147:420–426. doi: 10.1016/j.jtcvs.2013.08.078. [DOI] [PubMed] [Google Scholar]
- 28.Boston US, Fehr J, Gazit AZ, Eghtesady P. Paracorporeal lung assist device: an innovative surgical strategy for bridging to lung transplant in an infant with severe pulmonary hypertension caused by alveolar capillary dysplasia. The Journal of thoracic and cardiovascular surgery. 2013;146:e42–43. doi: 10.1016/j.jtcvs.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Elward AM, Warren DK, Fraser VJ. Ventilator-associated pneumonia in pediatric intensive care unit patients: risk factors and outcomes. Pediatrics. 2002;109:758–764. doi: 10.1542/peds.109.5.758. [DOI] [PubMed] [Google Scholar]
- 30.Schweiger C, Marostica PJ, Smith MM, Manica D, Carvalho PR, Kuhl G. Incidence of post-intubation subglottic stenosis in children: prospective study. The Journal of laryngology and otology. 2013;127:399–403. doi: 10.1017/S002221511300025X. [DOI] [PubMed] [Google Scholar]