Abstract
The presented paper shows the influence of temperature increase in the range typically used during antioxidant measurements (15–35 °C) on the estimation of antioxidant properties of phenolic compounds (caffeic acid, ferulic acid, gallic acid, trolox, butylhydroxyanisole, butylhydroxytoluene and 2,6-diisopropylphenol) in associating and non-associating solvents. A significant influence of temperature on the DPPH•/antioxidant reaction kinetic is observed for strongly associating solvents (e.g. methanol). This trend is less marked for non-associating solvents (e.g. dioxane, ethyl acetate). The performed experiments prove that the change of solvent structure, caused by temperature increase, influences the estimation of antioxidant properties of phenolic compounds much more than the increase of kinetic energy of reacting molecules and/or the increase of the dissociation degree of hydroxyl groups occurring in antioxidants.
Keywords: Antioxidant properties, DPPH method, Temperature impact, Phenolic compounds, Solvent clusters
Introduction
In the last two decades there has been an explosive interest in the role of oxygen-free radicals, more generally known as “reactive oxygen species” (ROS). ROS are produced in the body as the by-products of normal cellular aerobic respiration and as a consequence of exposure to environmental factors such as ultraviolet sunlight, X-rays and gamma rays radiation, smoking, pollution, ozone, and certain drugs, chemicals or pesticides (Yanai et al. 2008; Sen et al. 2010). Although ROS are known to have a beneficial role in biological systems (Andrè et al. 2010), their harmful activity in living systems rivets the attention of researchers due to their capability of damaging crucial biomolecules such as nucleic acids, lipids, proteins, polyunsaturated fatty acids, and carbohydrates, and—consequently—evoking many human ailments: atherosclerosis, cancer, diabetes, inflammation, cardiovascular diseases, and neurological disorders (Halliwell et al. 1995; Noguchi and Niki 2000; Brannan et al. 2001; Grune et al. 2001). Oxidation of unsaturated fatty acids caused by ROS is also considered as one of the most important processes of food deterioration (Moure et al. 2001; Cosio et al. 2006). Hence, the investigation of the already known compounds exhibiting antioxidant properties and searching for new ones is still an important scientific challenge.
A number of methods have been developed and applied for the measurement of antioxidant capacity (Schlesier et al. 2002; Prior et al. 2005; Karadag et al. 2009). Among them, the methods applying chromogen compounds are commonly used due to their ease, speed and sensitivity (Arnao 2000; Villaňo et al. 2004). The method employing stable 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) is one of the most popular (Molyneux 2004; Huang et al. 2005). DPPH• assay is routinely practiced for the assessment of the antioxidant activity of pure compounds and complex mixtures, e.g. plant extracts. Moreover, it is not specific to any particular antioxidant component, but applies to the overall antioxidant capacity of the sample.
The antioxidant properties in the DPPH method are determined by monitoring the reaction kinetics between the examined antioxidant and the DPPH radical (Brand-Williams et al. 1995; Bondet et al. 1997). As it was shown earlier (Dawidowicz and Olszowy 2010, 2011; Dawidowicz et al. 2012), the reaction kinetics between butylated hydroxytoluene (BHT) and DPPH radical in DPPH method or between BHT and 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt radical cation (ABTS•+) in ABTS method or between BHT and peroxyl radicals (LOO•) in β-carotene bleaching assay depends on the metal and hydrogen ion concentration and solvent type. Hence, the mentioned factors account for the estimation of antioxidant properties of BHT using DPPH, ABTS and β-carotene bleaching assays. It should be stressed, however, that temperature is one of the crucial factors influencing the reaction kinetic, including the reaction between antioxidant and scavenged radicals. The main reason of the reaction kinetics increase with temperature growth is that more of the reacting particles will have the necessary energy resulting in more successful reaction. The influence of temperature is described by the Arrhenius equation (Laidler 1987). The kinetic of the reaction depends also on the properties of its environment (e.g. association degree of the solvent in which reaction proceeds). When the properties of the reaction medium strongly depend on the temperature, the observed temperature kinetic changes results both from the energy changes of reacting molecules and from the changes of medium properties. Methanol is one of the most popular solvents applied for the estimation of antioxidant properties of phenolic compounds by DPPH• method. As it is known the literature (Ludwig 1999; Borowski et al. 2003), this solvent is composed of hydrogen bonded clusters that remain in thermodynamic equilibrium. According to Borowski et al. (Borowski et al. 2003), bulk methanol is mostly composed of hepta-, hexa-, penta-, tetra- and trimeric cluster structures and monomeric methanol molecules constitute only its minor fraction. It is also well known that the increase of temperature shifts the equilibrium towards small methanol clusters—large associates disintegrate into smaller associates and single molecules.
The presented paper shows that the temperature changes of methanol structure (in the temperature range typically used during measurements, 15–35 °C) can have stronger influence on the estimation of antioxidant properties of phenolic compounds than the energy changes of reagents resulting from temperature variation. The experiments were performed using a few chosen compounds differing in antioxidant activity: caffeic acid, ferulic acid, gallic acid, trolox, butylhydroxyanisole (BHA), butylhydroxytoluene (BHT), and 2,6-diisopropylphenol (Propofol). As results from the literature (Dawidowicz et al. 2012), the DPPH assay is frequently applied in comparing the antioxidant activities of the plant extracts, for example of the same plant material, differing not only in the qualitative and quantitative composition but also in the type of extracting solvent, which is the main extract component. Hence, beside methanol (ψ = 1.90, δ = 14.50, β = 0.41) four additional solvents exhibiting different self-association ability and different hydrogen bond acceptor ability were used—n-propanol (ψ = 1.50, δ = 12.18), dioxane (ψ = 1.07, δ = 9.7, β = 0.47), ethyl acetate (ψ = 1.00, δ = 8.91, β = 0.48), carbon tetrachloride (ψ = 1.00, δ = 8.55, β = 0.05), where: ψ—dimensionless association coefficient of solvent; δ—solubility parameter in cal1/2 × cm−3/2 (Miyabe and Isogai 2011); β—hydrogen bond acceptor ability in Abraham scale (Kamlet et al. 1983).
Experimental
Reagents
n-propanol, carbon tetrachloride, ethyl acetate and 1,4-dioxane were supplied by the Polish Chemical Plant—POCh (Gliwice, Poland). 2,2′-diphenylpicrylhydrazyl (DPPH), butylhydroxtoluene (BHT), butylhydroxyanisole (BHA), 2,6-diisopropylphenol (Propofol), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), ferulic acid, caffeic acid, gallic acid and methanol were purchased from Sigma Aldrich (Poznań, Poland). To remove peroxides, dioxane was distillated before using.
Methods
The estimation of the unreacted DPPH• concentration in DPPH•/antioxidant systems was performed by the slightly modified Brand-Williams method (Brand-Williams et al. 1995). BHT, BHA Propofol, Trolox, ferullic acid, caffeic acid, gallic acid were used as antioxidants. DPPH• solutions in appropriate solvent (without antioxidant) were applied as controls. Methanol, n-propanol, carbon tetrachloride, 1,4-dioxane and ethyl acetate were used as DPPH• and antioxidant solvents. The DPPH• concentrations in control and in measuring system with examined antioxidant were the same. To zero the spectrophotometer, the appropriate solvent without DPPH• and the antioxidant was used. The assays were carried out in triplicate and the data points are expressed as average values. The measurements were performed in the temperature range from 15 to 35 °C (most frequently with changes every 5 °C).
The aliquot (2.94 ml) of DPPH• solution was mixed in a 4 ml test tube with 60 μl of the antioxidant dissolved in the same solvent as DPPH•. The concentration of the used antioxidants were different, their values are given in captions to the figures. The mixture was vigorously shaken during 30 s and then poured into an optical glass cuvette (1 cm × 1 cm × 3.5 cm) and immediately placed in a spectrophotometer. The decrease in absorbance at 516 nm was registered in a continuous manner during 30 or 180 min, employing a UV Probe –1800 Spectrophotometer equipped with thermostatic cell (Shimadzu, Kyoto, Japan). Subsequent readings were taken at regular intervals (60 s).
The percent of the remaining DPPH• (% DPPH•rem) was calculated from the following equation:
where: A t = 0 and At are the values of absorbance at 0 min and at time equal to (t) min, respectively.
The antioxidant activity expressed as inhibition percent [I (%)] was established using the following equation:
Statistical analysis
Results are presented as mean values ± SD. In order to determine the measurements reproducibility, each antioxidant activity assay was done three times. RSD of all measurements were lower than 9 %. p < 0.05 was assumed as statistical differences between experimental points. The kinetic curves were drown using all experimental data; however, only a few experimental points (mean values ± SD) were put in the figures for their clarity.
Results and discussion
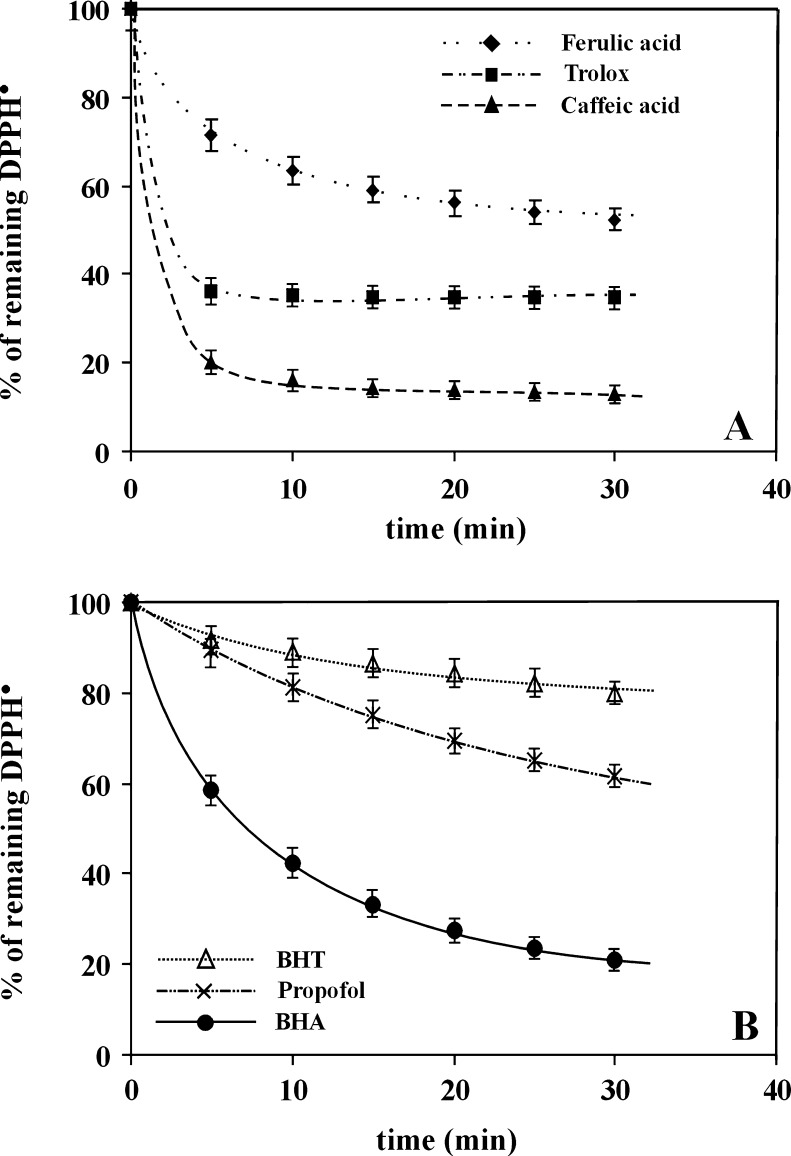
As appears from the literature, the reaction mechanism between the antioxidant and DPPH radical depends on the antioxidant structure (Brand-Williams et al. 1995). Figure 1 shows changes of the remaining DPPH• in measuring systems during 30 min in the presence of the applied antioxidants differing in chemical structure—see Fig. 2. In these experiments methanol played the role of solvent. To avoid too quick or too slow kinetics of the scavenging process of DPPH•, the used antioxidants were divided into two concentration groups due to significant differences in their antioxidant activity. More active ones—caffeic acid, ferulic acid, and Trolox—were applied at the concentration equaling 0.25 mg/ml (see Fig. 1a), whereas less active ones—BHA, BHT and Propofol—at the concentration of 0.50 mg/ml (see Fig. 1b). The presented dependences allow for an easy determination of the relative antioxidant properties of the used antioxidants. As results from the figure, the highest antioxidant properties are exhibited by caffeic acid, and the lowest ones—by BHT. Following the literature (Janeiro and Brett 2004; Ndhlala et al. 2010), the multiple hydroxyl groups in the chemical structure of phenolic compounds make them ideal for the free radical scavenging process. The arrangement of hydroxyl groups around phenolic molecules is also important for antioxidant reactions. The antioxidant activity of phenolic acids improves with the increase of number of hydroxyl and methoxyl groups. Hence, the highest antioxidant activity of caffeic acid is obvious. o-dihydroxylation enhances antioxidant properties of phenolic compounds (Namicki 1990). The lowest antioxidant activity of BHT results not only from the presence of only one OH group but also from its hindrance by the neighbouring tertbuthyl groups. Better antioxidant properties of Propofol is connected with lower hindrance of hydroxyl groups in this molecule. Availability of OH group in BHA is greater and BHA exhibits still higher antioxidant properties. Moreover, better antioxidant properties of BHA results from para position of OH groups in relation to methoxy groups. Such position stabilizes phenoxy anions taking part in electron transfer to DPPH radical. The lower antioxidant activity of ferulic acid in relation to caffeic acid and Trolox can be explained by the lower activity of hydroxyl group in ferulic acid due to its engagement in the formation of intra-molecular hydrogen bond with oxygen of the neighbouring methoxyl group. Moreover, it was shown that methoxylation of hydroxyl group drastically reduces antioxidant activity of phenolic compounds (Eskin and Robinson 2001).
Fig. 1.
The changes of remaining DPPH• in measuring systems during 30 min of the reaction between DPPH• and: a Caffeic acid or Ferulic acid or Trolox (for each antioxidant the concentration equals 0.25 mg/ml), b BHA or BHT or Propofol (for each antioxidant the concentration equals 0.50 mg/ml). The measurements were performed in methanol at 25 °C
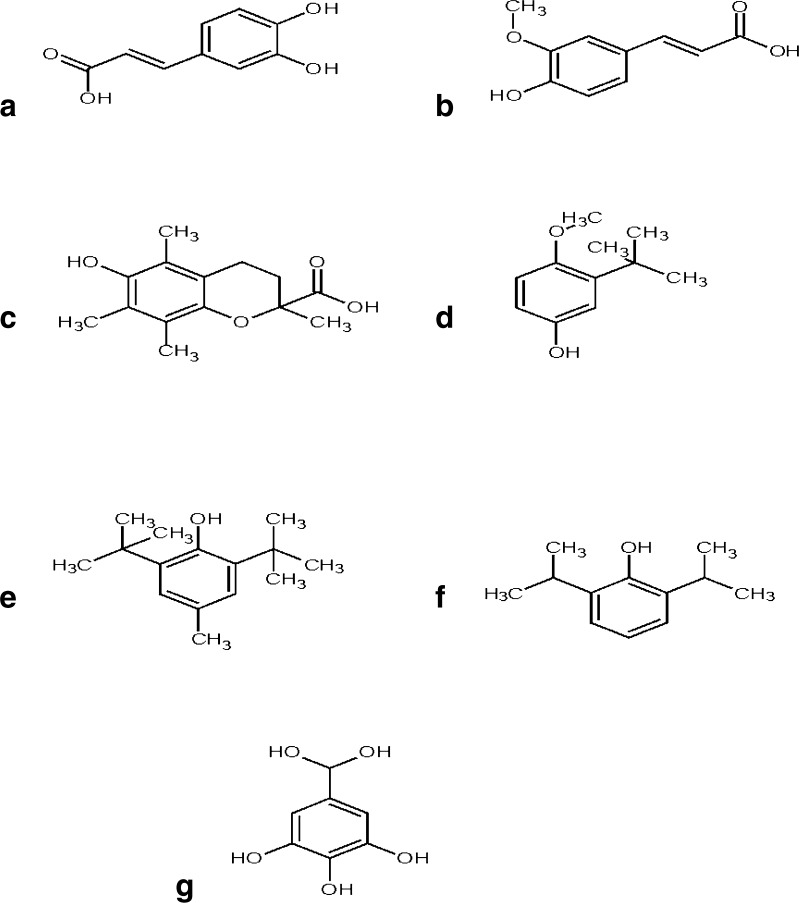
Fig. 2.
Molecular structures of the applied antioxidants: a Caffeic acid; b Ferulic acid; c Trolox; d BHA; e BHT; f Propofol; g Gallic acid
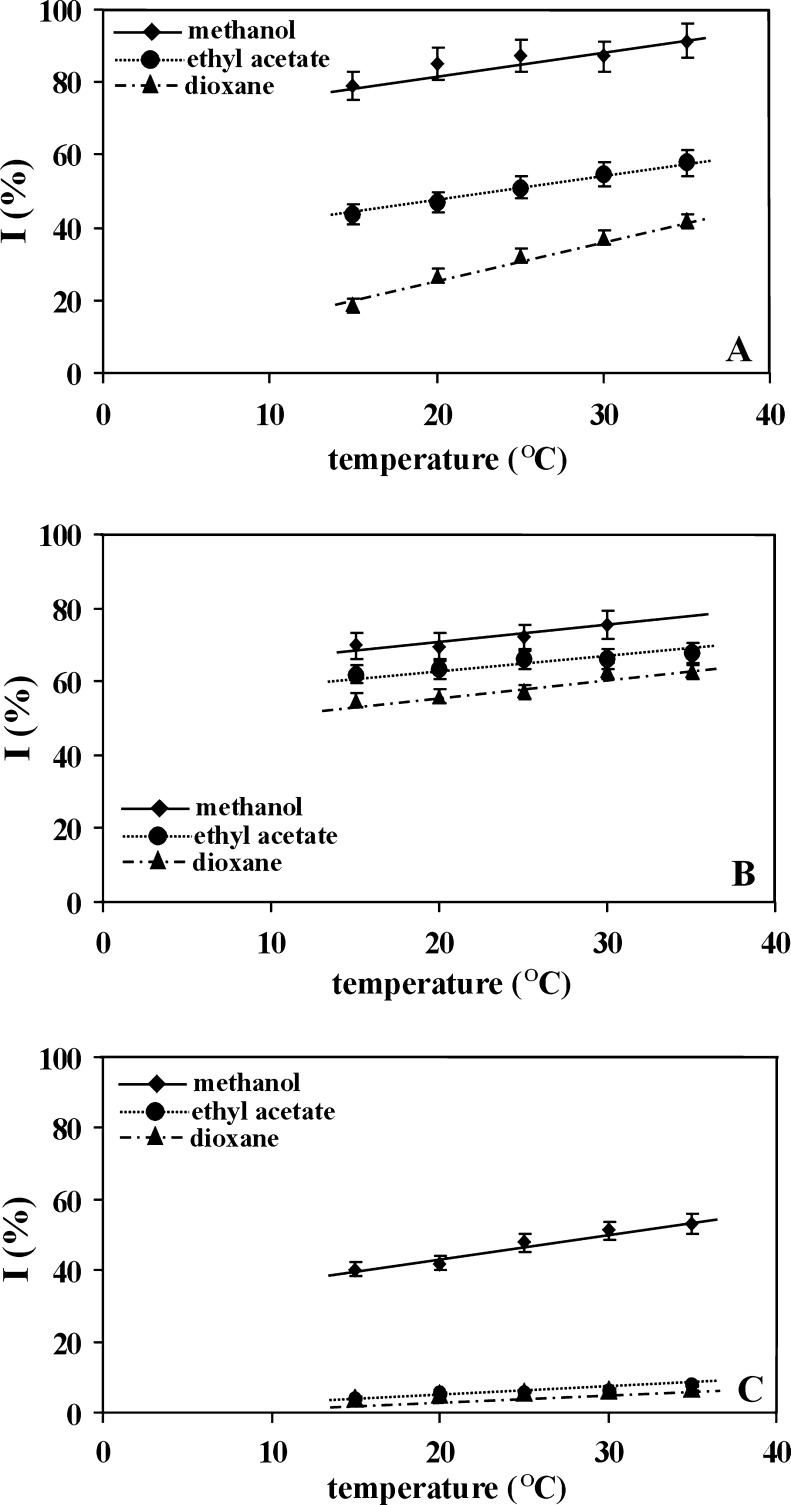
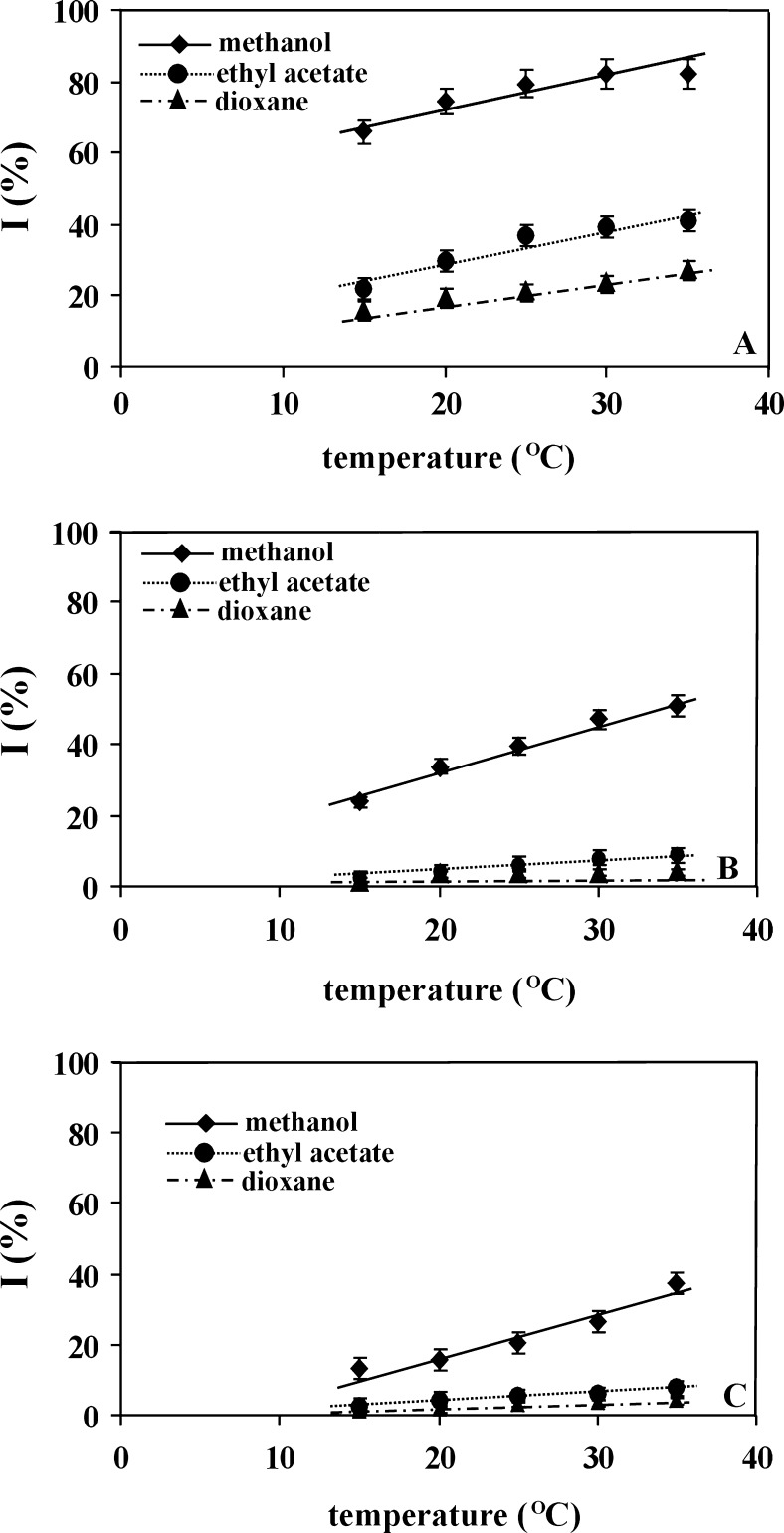
Figures 3 and 4 present the influence of temperature on the antioxidant properties [expressed as inhibition percent (I)] of the used antioxidants, estimated in systems differing in solvent type. According to Litwinienko and Ingold (2007) in solvents of high dielectric constant and of high basicity, the reaction between phenolic antioxidants and DPPH radical occurs according to the Sequential Proton Loss Electron Transfer (SPLET) mechanism. Due to strong hydrogen acceptor properties, dioxane (β = 0.47) and ethyl acetate (β = 0.48) are classified as aprotic solvents which disturb the SPLET mechanism. This explains the observed lower inhibition percent of DPPH• in these two solvents.
Fig. 3.
The influence of temperature on the inhibition percent of DPPH• estimated in methanol or ethyl acetate or dioxane for: a Caffeic acid, b Trolox, c Ferulic acid. All antioxidants were used in concentration equal to 0.25 mg/ml
Fig. 4.
The influence of temperature on the inhibition percent of DPPH• estimated in methanol or ethyl acetate or dioxane for: a BHA, b Propofol, c BHT. All antioxidants were used in concentration equal to 0.50 mg/ml
As appears from Figs. 3 and 4, the increase of measurement temperature causes the increase of inhibition percent for each applied antioxidant: the higher the temperature, the higher the antioxidant properties exhibited by the examined antioxidant. This general rule is more visible in weaker antioxidants (see Fig. 4) and less evident for antioxidants characterizing by higher antioxidant properties (see Fig. 3). Moreover, it is observed for each solvent used, but, the intensity of the changes clearly depends on the solvent type. In the case of weaker antioxidants, the greatest temperature influence on the estimation of antioxidant properties (the greatest slope of I vs. T dependence) is observed when methanol is used as solvent, while smaller influence is seen when dioxane and ethyl acetate are applied—see Fig. 4. For stronger antioxidants, this rule is less evident, possibly because their concentration in the measuring system was two times lower than of the weaker ones.
Considering the influence of temperature on the reaction between DPPH• and antioxidant, it should be mentioned that the temperature increase leads to the increase of kinetic energy of the reacting molecules and to the increase of dissociation degree of hydroxyl groups occurring in the used antioxidants. Hence, the observed increase of antioxidant properties of the examined compounds with temperature is logical. On the other hand, the temperature increase leads to the change of the solvent structure. Among the solvents used, methanol structure seems to be most temperature—sensitive. As it was stated in Introduction this solvent is known as solvent composed of hydrogen bonded clusters that remain in thermodynamic equilibrium. Its association coefficient (ψ) and solubility parameter (δ) are the greatest among the used solvents and equal 1.90 and 14.50, respectively. The increase of temperature shifts the equilibrium towards small methanol clusters—large associates disintegrate into smaller associates and single molecules (Borowski et al. 2003). It is possible that single methanol molecules exhibit higher ability of proton and electron transfer and, in consequence, are responsible for the estimation of higher antioxidant properties of the examined substances in relation to those observed for two other solvents or for strongly clustered methanol existing in low temperature.
In the case of dioxane and ethyl acetate, the increase of inhibition percent with the increase of measurement temperature is clearly weaker and similar. It is worth noticing that the both solvents do not exhibit association ability. The association coefficient (ψ) and solubility parameter (δ) for dioxane equal 1.07 and 9.7, respectively and for ethyl acetate 1.00 and 8.91, respectively. It can be supposed that the visible but small increase of inhibition percent with the increase of measurement temperature results from the increase of kinetic energy of the reacting molecules and from the increase of dissociation degree of hydroxyl groups occurring in the used antioxidants.
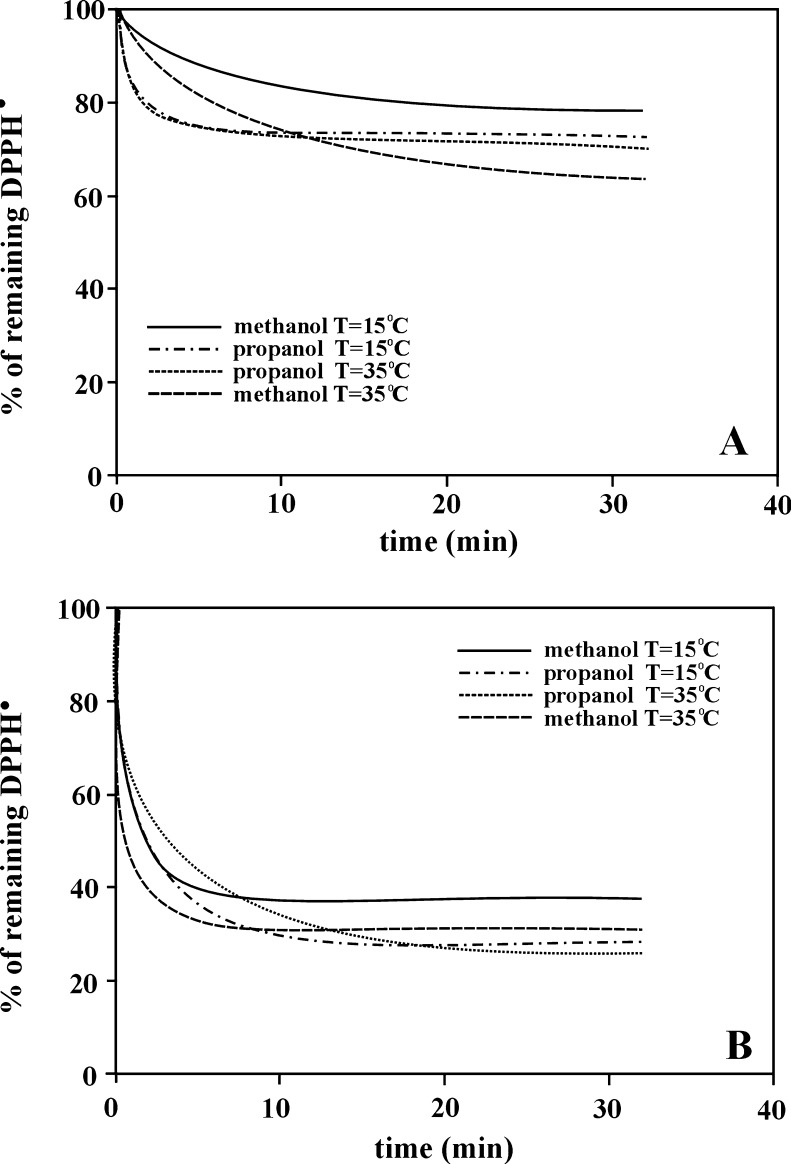
The results presented so far were obtained measuring DPPH• concentration after 30 min incubation time,—i. e. the so-called simplified way of the estimation of antioxidant properties was used. According to Brand-Williams et al. (1995), the DPPH•/antioxidant reactions should be monitored till the value of DPPH• concentration reaches a steady state. Although DPPH radical is recognized as stable, experimental results report its slow disappearance in time; therefore the incubation time should not be too long. Moreover, in the case of volatile solvents, concentration changes in the measuring system are observed at long incubation times. To reach the steady state in a reasonable time and to avoid the mentioned problems, gallic acid, a very strong and quickly reacting antioxidant, was used in subsequent experiments. Figure 5a shows kinetic curves for the DPPH•/gallic acid reaction obtained at two different temperatures (15 and 35 °C) and using two different alcohols, strongly associating methanol (ψ = 1.90, δ = 14.50) or less associating n-propanol (ψ = 1.50, δ = 12.18), as the reaction environment. As results from the figure, the steady state of the DPPH•/gallic acid reaction was reached in all the examined systems. It should be noticed, however, that the concentration of the remaining DPPH• in the measuring systems at two different temperatures is almost the same in the case of n-propanol and different in the case of methanol. In other words, the antioxidant properties of gallic acid depend more on the temperature when estimated in methanol than in n-propanol. This fact additionally proves the influence of the cluster structure of bulk alcohols. The increase of temperature in the measuring system shifts the equilibrium towards single methanol molecules. Of course, bulk n-propanol is also composed of clusters, however, they are smaller than in methanol. Moreover, more single alcohol molecules exist in propanol than in methanol. If monomeric and smaller alcohol structures are better carriers of electrons and protons from antioxidant to DPPH•, it is not surprising that the antioxidant properties of gallic acid are stronger in n-propanol than in methanol.
Fig. 5.
Kinetic curves for the reaction: a DPPH•/gallic acid and b DPPH•/Trolox, obtained at two different temperatures (15 and 35 °C) and using two different alcohols, methanol or n-propanol, as the reaction environment. The concentration of gallic acid and Trolox were 0.025 and 0.25 mg/ml, respectively. For clarity of the figure, the error bars were omitted. RSD of experimental data was lower than 9 %—see Experimental
Figure 5b presents analogous results obtained for the reaction between DPPH radicals and Trolox. Although in these cases the steady states were not reached, the results can also be explained by various thermal changes of cluster structures of bulk alcohols, methanol and n-propanol.
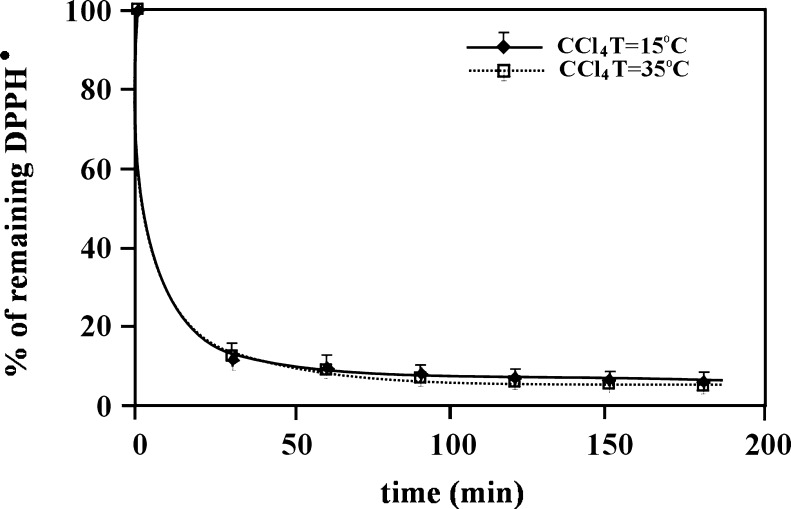
If the observed temperature differences in reaction kinetics are connected with the changes in solvent structure, they should be absent or negligible in aprotic solvents. Figure 6 shows the kinetic curves for DPPH•/antioxidant reaction performed at two different temperatures (15 and 35 °C) with carbon tetrachloride as the reaction environment—an aprotic solvent (β = 0.05) which does not form clusters (ψ = 1.00, δ = 8.55). BHA was used in these experiments as, of all the applied antioxidants, it is the only one soluble in CCl4. The temperature increase should accelerate the DPPH•/BHA reaction kinetic as the result of the kinetic energy increase of the reacting molecules and of the dissociation degree increase of hydroxyl groups of the phenolic compound. The convergence (the coverage) of the curves obtained in two different temperatures indicates that the influence of temperature increase on the reaction kinetic was very small in the used temperature range. Relating these results to the previously discussed ones, the importance of the solvent structure on the estimation of antioxidant properties is evident.
Fig. 6.
Kinetic curves for the DPPH•/BHA reaction obtained at two different temperatures (15 and 35 °C) and using carbon tetrachloride as the reaction environment. The concentration of BHA was 0.5 mg/ml
Conclusions
The presented results show that the temperature increase influences the antioxidant properties of phenolic compounds: its increase leads to the increase of antioxidant properties. A significant influence of temperature on the DPPH•/antioxidant reaction kinetic is observed for strongly associating solvents (e.g. methanol). This trend is less marked for non-associating solvents (e.g. dioxane, ethyl acetate). The performed experiments prove that the change of solvent structure, caused by temperature increase, influences the estimation of antioxidant properties of phenolic compounds much more than the increase of kinetic energy of reacting molecules and/or the increase of the dissociation degree of hydroxyl groups occurring in antioxidants.
References
- Andrè CM, Larondelle Y, Evers D. Dietary antioxidants and oxidative stress from human and plant perspective: a review. Curr Nutr Food Sci. 2010;6:2–12. doi: 10.2174/157340110790909563. [DOI] [Google Scholar]
- Arnao MB. Some methological problems in the determination of antioxidant using chromogen radicals: a practical case. Trends Food Sci Technol. 2000;11:419–421. doi: 10.1016/S0924-2244(01)00027-9. [DOI] [Google Scholar]
- Bondet V, Brand-Williams W, Berset C. Kinetics and mechanisms of antioxidant activity using the DPPH• free radical method. LWT Food Sci Technol. 1997;30:609–615. doi: 10.1006/fstl.1997.0240. [DOI] [Google Scholar]
- Borowski P, Jaroniec J, Janowski T, Woliński K. Quantum cluster equilibrium theory of hydrogen – bonded liquids: water, methanol and ethanol. Mol Phys. 2003;101(10):1413–1421. doi: 10.1080/0026897031000085083. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Brannan RG, Connolly BI, Decker EA. Peroxynitrite: a potential initiator of lipid oxidation in food. Trends Food Sci Technol. 2001;12:164–173. doi: 10.1016/S0924-2244(01)00073-5. [DOI] [Google Scholar]
- Cosio MS, Buratti S, Mannino S, Benedetti S. Use of an electrochemical method to evaluate the antioxidant activity of herb extracts from the Labiatae family. Food Chem. 2006;97:725–731. doi: 10.1016/j.foodchem.2005.05.043. [DOI] [Google Scholar]
- Dawidowicz AL, Olszowy M. Influence of some experimental variables and matrix components in the determination of antioxidant properties by β-carotene bleaching assay: experiments with BHT used as standard antioxidant. Eur Food Res Technol. 2010;231:835–840. doi: 10.1007/s00217-010-1333-4. [DOI] [Google Scholar]
- Dawidowicz AL, Olszowy M. Antioxidant properties of BHT estimated by ABTS assay in systems differing in pH or metal ion or water concentration. Eur Food Res Technol. 2011;232:837–840. doi: 10.1007/s00217-011-1451-7. [DOI] [Google Scholar]
- Dawidowicz AL, Wianowska D, Olszowy M. On practical problems in estimation of antioxidant activity of compounds by DPPH* method. Food Chem. 2012;131:1037–1104. doi: 10.1016/j.foodchem.2011.09.067. [DOI] [Google Scholar]
- Eskin NAM, Robinson DS. Food shelf life stability; chemical, biochemical and microbiological changes. Washington: CRC Press; 2001. [Google Scholar]
- Grune T, Schringarpure R, Sitte N, Davies K. Age-related changes in protein oxidation and proteolysis in mammalian cells. J Gerontol. 2001;56A:B459–B467. doi: 10.1093/gerona/56.11.B459. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Aeschbach R, Lőliger I, Aruoma OI. The characterization of antioxidants. Food Chem Toxicol. 1995;33:601–617. doi: 10.1016/0278-6915(95)00024-V. [DOI] [PubMed] [Google Scholar]
- Huang DJ, Ou BX, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- Janeiro P, Brett AMO. Catechin electrochemical oxidation mechanisms. Anal Chim Acta. 2004;518:109–115. doi: 10.1016/j.aca.2004.05.038. [DOI] [Google Scholar]
- Kamlet MJ, Abboud JLM, Abraham MH, Taft RW. Linear salvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters, pi∗, alpha, and beta, and some methods for simplifying the generalized solvatochromic equation. J Org Chem. 1983;48:2877–2887. doi: 10.1021/jo00165a018. [DOI] [Google Scholar]
- Karadag A, Ozcelik B, Saner S. Review of methods to determine antioxidant capacities. Food Anal Methods. 2009;2:41–60. doi: 10.1007/s12161-008-9067-7. [DOI] [Google Scholar]
- Laidler KJ. Chemical kinetics. 3. New York: Harper & How; 1987. [Google Scholar]
- Ludwig R. Quantum clusters equilibrium theory of liquids: molecular clusters and thermodynamics of liquid ethanol. Mol Phys. 1999;97:465–477. doi: 10.1080/00268979909482847. [DOI] [Google Scholar]
- Litwinienko G, Ingold KU. Solvent effects on the rates and mechanisms of the reaction of phenols with free radicals. Account Chem Res. 2007;40:222–230. doi: 10.1021/ar0682029. [DOI] [PubMed] [Google Scholar]
- Miyabe K, Isogai R. Estimation of molecular diffusivity in liquid phase systems by the Wilke-Chang equation. J Chromatogr A. 2011;1218:6636–6645. doi: 10.1016/j.chroma.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Molyneux P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol. 2004;26:211–219. [Google Scholar]
- Moure A, Cruz JM, Franco D, Dominguez JM, Sineiro J, Dominguez H. Natural antioxidant from residual sources. Food Chem. 2001;72:145–171. doi: 10.1016/S0308-8146(00)00223-5. [DOI] [Google Scholar]
- Namicki M. Antioxidants/antimutagens in food. Food Sci Nutr. 1990;29:273–300. [Google Scholar]
- Ndhlala AR, Moyo M, Staden JV. Natural antioxidants: fascinating or mythical biomolecules? Molecules. 2010;15:6905–6930. doi: 10.3390/molecules15106905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi N, Niki E. Phenolic antioxidants: a rationale for design and evolution of novel antioxidant drug for atherosclerosis. Free Radic Biol Med. 2000;28:1538–1546. doi: 10.1016/S0891-5849(00)00256-2. [DOI] [PubMed] [Google Scholar]
- Prior RL, Wu X, Schaich K. Standardized methods for determination of antioxidant capacity and phenolics in food and dietary supplements. J Agric Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Schlesier K, Harwat M, Bohm V, Bitsch R. Assessment of antioxidant activity by using different in vitro methods. Free Radic Res. 2002;36:237–242. doi: 10.1080/10715760290006411. [DOI] [PubMed] [Google Scholar]
- Sen S, Chakraborty R, Sridhar C, Reddy YSR, De B. Free radicals, antioxidants, diseases and phytomedicines: current status and future prospect. Int J Pharm Sci Rev Res. 2010;3:91–100. [Google Scholar]
- Villaňo D, Fernández- Pachoń MS, Troncoso AM, Garcia-Parrilla MC. The antioxidant activity of wines determined by the ABTS •+ method: influence of sample dilution and time. Talanta. 2004;64:501–509. doi: 10.1016/j.talanta.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Yanai N, Shiotani S, Hagiwara S, Nabetani H, Nakijma M. Antioxidant combination inhibits reactive oxygen species mediated damage. Biosci Biotechnol Biochem. 2008;72:3100–3106. doi: 10.1271/bbb.80159. [DOI] [PubMed] [Google Scholar]