Abstract
The current investigation presents an exploration in phase behaviour of carboxymethyl cellulose (CMC) produced from pomegranate seed pips compared to low and high viscosity CMCs (LMCMC and HMCMC) primarily at low solid concentrations. Cellulose was extracted with 10 % NaOH at 35 °C for 22 h from pomegranate seed pips and converted to CMC by etherification process. Thermomechanical analysis and micro-imaging were carried out using small deformation dynamic oscillation in shear, modulated differential scanning calorimetry (MDSC) and scanning electron microscopy (SEM). The results emphasize the importance of molecular interaction and the degree of substitution in produced CMC. Thermal gravimetric analysis (TGA) thermograms showed an initial weight loss in pomegranate seed pips CMC (PSCMC) sample, which we attribute to presence of amount of moisture in sample powder. MDSC analysis of PSCMC showed five different peaks at 84, 104, 173, 307 and 361 °C. Moreover, G’ and G” changes were found to be dependent on both concentration and frequency. The results of frequency sweep and tan δ indicate that PSCMC solutions can be classified as weak gels.
Keywords: Pomegranate seed pips, Carboxymethyl cellulose, Thermomechanical analysis, Tan δ, Intrinsic viscosity
Introduction
The hydrocolloids are widely used in various food industries to improve the sensory, mechanical and technological characteristics of products (Gomez-Díaz and Navaza 2003; Hegedusic et al. 2000). Depending on the kind of substances and reactions, polysaccharides such as cellulose, starch, chitin, gellan and pullulan can be alternated to new hydrocolloids with higher functional properties (Silva et al. 2004). Structure-functionality of these modified hydrocolloids has delivered a promising entry to many products in the food industry.
Carboxymethyl cellulose (CMC) is synthesized commercially in the so-called slurry process by the reaction of alkali cellulose with sodium chloroacetate. CMC is a copolymer of β-D-glucose and 2-O-(carboxymethyl) monosodium salts, allocating randomly along the macromolecule. Commercial CMC samples show DS in the range of 0.4–1.4 and the degree of polymerization (DP) ranging from 20 to 100 (Kotz et al. 2001; Toğrul and Arslan 2003). High water solubility permits vast application of CMC in many industries such as food industry, cosmetics, pharmaceuticals, detergents, textiles, paper and drugs. Although CMC is produced historically from cotton linters and wood recourses, new environmental regulations, growing environmental awareness and societal concerns have raised many concerns on industries, resulting in the search for new products and processes compatible with the environment. Moreover, availability, cost and easy of delignification of conventional sources of cellulose from cotton linters and wood pulp are discouraged now-a-days. Plant wastes contain more than 90 % (w/w) of polysaccharides which can be used in chemical and biochemical modifications. In this respect, several studies have been conducted with non-wood/cotton resources such as sago waste, cavendish banana pseudo stem, milox pulp, orange peel, cashew tree and beet pulp as alternative chemical feedstock (Adinugraha et al. 2005; Dapia et al. 2003; Pushpamalar et al. 2006; Silva et al. 2004; Toğrul and Arslan 2003, 2004; Yasar et al. 2007).
Pomegranate (Punica granatum) fruit is one of the most important commercial fruits in the Middle East and USA with total production of more than 1,000,000 tons per year (Fadavi et al. 2005). Pomegranates are a major source of phenolic compounds, vitamin B, pectin, tannin, cellulose. The fruit contents are 45–61 % juice, 12–26 % outer rind, 0.05 % central lamella and 4–9.5 % seeds. Based on the type of pomegranate cultivars, pips (woody part) contain 27.2 % lipid, 13.2 % crude protein, 4.7 % total sugar, 25.52 % hemicellulose, 39.67 % lignin and 26.98 % cellulose (dry wt. basis) (El-Nemr et al. 1990; Ucar and Karagoz 2009). In previous studies, evaluation of antioxidant properties of pomegranate pulp and peel and total lipid content and fatty acid composition of pomegranate oilseed have been investigated (Li et al. 2006; Melgarejo and Artes 2000). Therefore, isolation and characterization of the functional properties of these fractions could increase the value to pomegranate seeds over its value as an animal feed.
Some studies have been conducted with the rheological behaviour of wood and non-wood CMC in aqueous solutions (Silva et al. 2004; Toğrul and Arslan 2003; Tsujiyama and Miyamori 2000; Yasar et al. 2007). In spite of the great interest in this group of substances, there are no reports in the literature on the thermal and viscoelastic properties of CMC from pomegranate seed pips. Therefore, the objectives of this study were: (a) to extract the cellulose from pomegranate seed pips and produce CMC, (b) to investigate the thermal behaviour of produced CMC, (c) to determine the average molecular weight and intrinsic viscosity of produced CMC, and (d) to evaluate the oscillatory small deformation properties of pomegranate seed pips CMC solutions at different concentrations. Using a systematic study on the structural and thermal properties of the gel systems, this study demonstrates how the pomegranate seed pips CMC gel is affected by temperature variations and different range of frequencies. In addition, modulated differential scanning calorimetry (MDSC), thermal Gravimetric Analysis (TGA) and U-tube viscometry methodologies were used to compare the obtained results.
Materials and methods
Materials
Ripened pomegranates were obtained from Tehran (Saveh) province of Iran. The edible parts containing juice and seeds were separated manually using a hand press. The seeds were washed and dried overnight in a cabinet drier (Dongguan Jianqiao Testing Equipment Co., Ltd., China, Model JQ-9023A) at 60 °C. Dried seeds were milled using a laboratory mill (A 11 basic Analytical mill, IKA-Werke Staufen, Germany) and were sieved through a no 60 mesh (251 μm) to obtain fine particles. Preparation of CMC from pomegranate seed pips was performed according to the method previously described by Savadkoohi et al. (2013a) and Toğrul and Arslan (2003) with minor modification. Seven grams of powder were defatted with ethanol and chloroform (1:3) and dried in room temperature for 3 hr. Defatted powder was mixed with 350 ml of phosphate buffer 0.1 M Na3PO4 (pH, 7.5) with 35 mg trypsin (2,000 E/g) and stirred overnight at 37 °C to remove proteinous compounds. To separate the hemicellulose compounds, 500 ml NaOH (10 % w/w) was added to deproteinated powder and stirred in a bench top shaking incubator (SI4, SL Sheldon manufacture Ltd.) at 35 °C for 22 h followed by washing with distilled water to remove the remained base. To remove the lignin compounds, 100 ml distilled water, 5 ml acetic acid (10 % w/w) and 2 g NaCl was added to the paste and stirred for 60 min at 75 °C. Then, the dispersion was filtered and washed using ethanol and distilled water. The extracted cellulose was dried in a cabinet drier at 55 °C for 16 h.
To produce CMC from the extracted cellulose, 500 ml isobutyl alcohol and 100 ml NaOH (30 % w/w) were added to 10 g of dried cellulose powder. This suspension was stirred mechanically at 25 °C for 90 min and filtered. Then, 15 g monosodium chloroacetate was added to the slurry and stirred in a reaction vessel (stainless steel) at 75 °C for 360 min. Processed slurry was washed with acetic acid (90 %, w/w) and filtered. Filtered paste was washed with ethanol (70 %, w/w) and dried in an oven at 55 °C for 16 h. The produced powder was kept in propylene containers until further experiments. All samples were produced in triplicate.
Low viscosity (product no. 217277) and high viscosity (product no. 217274) lyophilized powders of sodium carboxymethyl cellulose were purchased from Merck KGaA, Darmstadt, Germany. According to manufacturer specifications, these types of CMC were prepared from cotton linters with the purity of more than 90 % in the form of white and free flowing solid. The viscosities of aqueous solutions (2 %, w/w) of low viscosity CMC and high viscosity CMC at 25 °C were 25–75 and 800–3,100 mPa.s, respectively. Three replicates of dried materials, including pomegranate seed pips (PSP), pomegranate seed pips CMC (PSCMC), commercial low viscosity CMC (LMCMC) and commercial high viscosity CMC (HMCMC) were analyzed to determine moisture, protein and fat contents using AOAC methods (AOAC, 1995). The degree of substitution (DS) and purity of CMC samples were determined according to ASTM standard method (ASTM, 1973).
Previous studies indicated that the preparation of CMC solutions requires a minimum time to fully dissolve the CMC powder, depending on the polymer concentration and the temperature (Edali et al. 2001; Savadkoohi et al. 2013a). Therefore, PSCMC, LMCMC and HMCMC dispersions (0.5, 1, 3, 5 and 7 % w/w) were prepared by dissolving the powder in deionized water at neutral pH and ambient temperature. Exceptionally, the high CMC concentration solutions (5 and 7 %, w/w) were prepared by using water heated at 50 °C as described by Benchabane and Bekkour (2008). In addition, adequate gentle stirring period (approximately 48 h) was allowed to achieve complete hydration and avoiding air bubbles formation.
Modulated differential scanning calorimetry (MDSC) and thermal gravimetric analysis (TGA)
MDSC and TGA thermograms were obtained using a Perkin-Elmer Pyris Diamond DSC calibrated for temperature and enthalpy with indium and zinc over a range 25–500 °C. Powders of produced cellulose, PSCMC, LMCMC and HMCMC were used for analysis. Samples of 1–8 mg weighted to an accuracy of 0.01 mg on DSC pan. The scanning run was carried out at a heating rate of 10 °C/min from 0 to 500 °C. The reference empty pan was used to approximate the heat capacity of samples. The process of each sample was measured in triplicate.
Scanning electron microscopy (SEM)
To study the microscopic structure and of the CMC samples network, a CAM SCAN SERIES-2 (Cambridge Scanning Company, UK) was used. Frozen powders (produced cellulose, PSCMC, LMCMC and HMCMC) were attached on sample holder followed by gold plating for the analysis. Samples were attached by double sided electrically conductive carbon tape then coated with a thin (~200 Å) layer of gold. Images of several gold plated samples under conditions of high vacuum and an accelerating voltage of 30 KV was recorded.
Intrinsic viscosity
Molecular structure in terms of shape, size and molecular weight of polymers is defined by intrinsic viscosity in fully solubilised dilute solutions (Harding 1997). Produced cellulose, PSCMC and commercial CMC dispersions of 0.001, 0.005, 0.01, 0.03, 0.05 and 0.07 % (w/v) solids were prepared by mixing the powder in 1 M NaNO3. Solutions were stirred for 1 hr using a magnetic stirrer to ensure proper dissolution (Nayak and Singh 2001). To evaluate the solubility of each sample in NaNO3, the light transmittance of each solution was considered at 640 nm against the blank (1 M NaNO3) and the percentage of transmittance was determined (Harding 1997). The intrinsic viscosity of the sample solutions were carried out with a U-tube viscometer with the inside diameter of 0.5 mm at 22 ± 0.1 °C. The concepts of relative, reduced, and intrinsic viscosities were defined using the Eqs. 1–4, respectively (Harding 1997).
| 1 |
where ηrel is relative viscosity, t and t0 are the times required for the sample and solvent (1 M NaNO3) to pass through the U-tube, respectively. Values of ρ and ρ0 are the densities of sample solution and solvent, respectively.
Also reduced viscosity, ηred was determined according to the Eq. (2):
| 2 |
where C is the concentration of the sample. Then intrinsic viscosity, [η] was determined using the Eq. (3):
| 3 |
The intrinsic viscosity as a function of average molecular weight was measured by Mark-Houwink-Sakurada empirical Eq. (4): (Toğrul and Arslan, 2003)
| 5 |
where K and α were constant and suitable for such systems (2.313 × 10−6 m3/kg and 0.7665, respectively). All measurements were performed in triplicate.
Rheological measurements
A controlled-stress rheometer (Model SR-5000, 176 Rheometrics Scientific, Piscataway, NJ) with a 25 mm standard steel parallel plate geometry was employed to obtain viscoelastic properties (storage modulus G’, loss modulus G”, tan δ) of CMC samples. The gap between the two plates was set to 1 mm for all samples. The static plate was equipped with a Pletier heating and cooling system with a temperature variation of ± 0.2 °C. The sample solutions were degassed under vacuum in a rotary vacuum evaporator (INGOS, RVO 400, INGOS Ltd.) until no visible air bubbles were observed. Adequate amount of sample was loaded onto the Peltier plate of the instrument and allowed to equilibrate for 10 min at 25 °C. A thin layer of silicone oil (Sigma-Aldrich Co., Ltd., Gillingham, UK) was placed on the exposed edges of the samples to minimize variation in the water content.
Dynamic measurements for all samples were carried out at the predetermined linear viscoelasticity region (LVR). The LVR was determined using a strain sweep at a frequency of 1 rad.s−1 where the sample was sinusoidally deformed with increasing amplitude/strain (0.001–10 % strain, 1–50 mm amplitude) (Sun et al. 2007). By analyzing the results of oscillatory stress-sweep tests for all systems, the strain amplitude of 3 % (within the LVR) was chosen to apply in all experiments. At this strain, the gel network was not impaired by the strain imposed during the measurements.
Temperature dependence of storage (G’) and loss (G”) moduli as well as tan delta (tan δ) were measured by using a temperature ramp (heating) from 25 to 95 °C at a constant rate of 3 °C/min with a strain of 1 % (within the LVR) and a frequency of 1 rad.s−1. The loss modulus (G”) was also monitored during all the measurements (data not shown). To examine the rheological behaviour of the PSCMC, the storage modulus, loss modulus and tan δ variations were determined as a function of dynamic frequency (0.1–100 rad.s−1) at 20 °C with a constant strain of 1 %. All measurements were performed in triplicate.
Statistical analysis
Analysis of variance was performed on all variables measured using the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL. version 19). The chemical composition and rheological measurements were analyzed using one-way ANOVA procedure. In addition, the statistical significance of differences between mean values was determined by using the Duncan’s multiple range test (p < 0.05).
Results and discussion
Chemical composition of CMC
Table 1 shows chemical composition of pomegranate seed pips and CMC samples. The moisture, protein and fat levels of pomegranate seed pips were 5.34, 6.22, and 26.14 % (wet wt. basis), respectively. In addition, the yield value of cellulose production was found to be 24.13 % as compared with total amount of cellulose (26.98 %) in pips. The results of seed compositions were similar to the values reported by other researchers (El-Nemr et al. 1990; Li et al. 2006; Melgarejo and Artes 2000). In the case of DS and purity, although PSCMC showed significantly (p < 0.05) lower value of DS compared to HMCMC, the produced CMC had high purity of 85.94 %. In general, CMCs with DS ranges of 0.4 to 1.5 are used in food and pharmaceutics industry; therefore, PSCMC with DS of 0.59 will be in the required range. As discussed in the literature, at low DS values (less than 0.3), majority of etherification occurs in the amorphous and the crystalline surface regions of the cellulose, resulting in a product with a high residual degree of chain-chain association and poor water solubility. In contrast, at higher DS (more than 0.4), the inter-chain associations are more fractured with partially soluble material (Stephen et al. 2006).
Table 1.
Chemical composition of pomegranate seed pips and CMC samples
| Characteristic | |||||
|---|---|---|---|---|---|
| Hydrocolloids | Moisture (%) | Protein (%) | Fat (%) | DS | Purity (%) |
| PSP | 5.34 ± 0.5b | 6.22 ± 0.2a | 26.14 ± 0.12a | ---- | ---- |
| PSCMC | 6.01 ± 0.3b | 0.73 ± 0.1b | 0.26 ± 0.11b | 0.59 ± 0.04a | 85.94 ± 2.51b |
| LMCMC | 0.32 ± 0.1a | 0.14 ± 0.2c | 0.12 ± 0.09b | 0.66 ± 0.03a | 91.23 ± 2.84a |
| HMCMC | 0.25 ± 0.2a | 0.15 ± 0.1c | 0.14 ± 0.08b | 0.91 ± 0.02b | 90.96 ± 3.48a |
Composition of the dried CMC powder after an average of triplicate trial ± standard deviation at p < 0.05; PSP, pomegranate seed pips, PSCMC, pomegranate seed CMC, LMCMC, low viscosity CMC, HMCMC, high viscosity CMC; different letters mean significant differences in each column
Thermal behaviour of the CMC system
The amount of changes in weight thermobalance related to the disturbance of conformational arrangements can be readily captured in heating runs of the material. Figure 1a depicts the TGA thermograms of CMC samples during heating from 25 to 450 °C at a scan rate of 10 °C/min.
Fig. 1.
TGA thermograms of PSCMC (Ο), LMCMC (▲) and HMCMC (■) (a) and MDSC analysis of pomegranate seed pips cellulose (i), PSCMC (ii), LMCMC (iii) and HMCMC (iv) (b) obtained under N2 atmosphere during heating from 25 to 500 °C at a heating rate of 10 °C/min
Heating run for PSCMC showed non-monotonic trace characteristic of weight changes as a function of temperature compared to commercial samples which can be possibly related to involving amounts of water (approximately 6 % w/w) in the dried PSCMC. As described by Li et al. (2009), water molecules formed strong hydrogen bonds with hydrophilic OH− and COO−Na+ group of CMC within polymer chains. Thus, the initial observed weight loss in CMC samples can be due to presence of moisture in the produced CMC sample. The second detected weight changes are probably related to the loss of CO2 from decarboxylation of COO− group of the polysaccharide (Biswal and Singh 2004). Previous studies showed that thermal study stability of hydrocolloids increases after acetylation (Li et al. 2009; Xu et al. 2010). Therefore, the slight decrease of stability of PSCMC with lower DS value (0.59) might be due to removal of carboxyl groups from the CMC molecule.
In order to examine the thermal properties and structural and conformational changes of PSCMC during the heating, modulated differential scanning calorimetry (MDSC) was used. Figure 1b shows the MDSC curves of the CMC samples with demonstrating of two endothermic and two exothermic peaks at 104, 173, 307 and 361 °C, respectively. In the case of PSCMC, there is another peak at 84 °C, which may represent the denaturation of the protein fractions of pomegranate seed. Noticeably, the first peak at 104 °C is corresponding to the complete loss of water molecules as TGA curves shown in Fig. 1a, whereas the second peak is related to the structural transitions of the polymer chains (Li et al. 2009). During the heating process the non-substituted O6H6 groups in the CMC chains are oxidized, resulting in a peak around 173 °C (Fig. 1b, ii-iii). As discussed earlier, the number of carboxyl groups increases with the increase in DS value. Therefore, disappearance of the second peak in the HMCMC sample is due to high DS value (0.91) of this CMC.
Two main combustion peaks were observed at CMC samples (Fig. 1b, i-iv) approximately 307 and 361 °C. In addition, cellulose revealed one endothermic at 102 °C and two exothermic peaks at 334, 475 °C and all CMC samples had an exothermic peak at around 361 °C. Tsujiyama and Miyamori (2000) reported that wood cellulose has two exotherms at 336 and 488 °C. The difference between peaks of wood and pomegranate seed pips cellulose is probably due to differences in preparation methods and cellular structure of each sample. As seen in Fig. 1b (i-iii), the main transition of CMC for pomegranate seed pips and commercial CMC samples were approximately at 307 and 361 °C. These two peaks are caused by two different regions present crystalline and amorphous forms of the cellulose (Tsujiyama and Miyamori 2000). However, the last peak (at 361 °C) in PSCMC sample (Fig. 1b, ii) showed higher change of enthalpy compared to commercial samples (LMCMC and HMCMC), possibly due to contamination with hemicelluloses. As discussed in the literature, hemicellulose typically reveals transition at 300–350 and 450–500 °C in other systems (Reh et al. 1987).
Scanning electron micrographs
Figure 2 shows the scanning electron micrographs of produced cellulose, produced CMC and commercial CMCs. Surface morphology of cellulose before carboxy methylation (Fig. 2a) shows a fibrillar form, which has been changed to granular structure after methylation. As shown in Fig. 2b, the PSCMC particles are of a uniform rod, compact surface and granular-like structure with a diameter of 20 μm. Thus, comparison of these figures reveals that methylation has taken place.
Fig. 2.
Scanning electro-micrograms of pomegranate seed pips cellulose (a), PSCMC (b), LMCMC (c) and HMCMC (d)
Determination of intrinsic viscosity and average molecular weight
The molecular weight of the CMC polymer, average number of carboxyl content per anhydroglucose unit and the distribution of carboxyl substituent along the polymer chains are the main factors to confine the various properties of CMC (Biswal and Singh 2004). Intrinsic viscosity is an indication of molecular structure of CMC in terms of molecular weight, size and shape. Determination of solubility shows that all samples had the solubility of greater than 95 % under the conditions used for CMC samples.
In a research, fifty different sources of cellulose were used such as textile ryon, tire cord, wood pulp, hydrolyzed cotton linter. The molecular weight ranges of these sources are form 27,000 to 900,000 kDa (Immergut et al. 1953). Table 2 illustrates the intrinsic viscosity and molecular weight of the pomegranate seed pips and commercial CMCs samples. As seen, PSCMC displays the lower values of intrinsic viscosity and molecular weight compared to HMCMC solutions. The decrease in intrinsic viscosity means a decrease of the hydrodynamic volume of the macromolecular chain (Toğrul and Arslan 2003). Intrinsic viscosity is a characteristic of macromolecules, describing the ability of molecules to disturb flow and explaining the size and shape of them. In the case of PSCMC, lower intrinsic viscosity is due to lower DS of the produced CMC, leading to the reduction in the hydrodynamic volume the CMC molecules. However, intrinsic viscosity of PSCMC was not significantly different from LMCMC, while its molecular weight showed a significant lower value than the other CMC samples. Previous studies showed that softwood, hardwood, beet pulp, rayon fiber and cotton linter have a molecular weight of 645745, 640995, 894500, 219128 and 360535, respectively (Silva and Laver 1997; Toğrul and Arslan 2003). Therefore, pomegranate seed pips cellulose with molecular weight of 728439 is approximately similar to wood cellulose.
Table 2.
Intrinsic viscosity and average molecular weight of pomegranate seed pipis and CMC solutions
| Hydrocolloids | Intrinsic viscosity (mL/g) | Molecular weight (kg/kg mol) |
|---|---|---|
| PSP | 72.06 ± 1.12a | 728439 ± 134.31a |
| PSCMC | 82.50 ± 2.01b | 869145 ± 152.89b |
| LMCMC | 86.99 ± 3.30b | 931347 ± 101.03c |
| HMCMC | 100.27 ± 2.24c | 1120973 ± 112.17d |
Values are reported as means (average of triplicate trial) ± standard deviation at p < 0.05; different letters mean significant differences in each column
Oscillatory small deformation of CMC samples
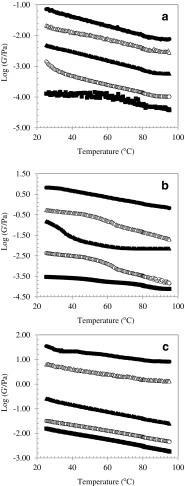
Figure 3 illustrates the mechanical properties of CMC solutions as a function of temperature. The results obtained for the heat treatment from 25 to 95 °C showed that both the storage modulus and loss modulus (data not shown) decreased significantly with an increase in the temperature. Likewise, G’ and G” increased with the increase in concentration for all samples. Moreover, stronger temperature dependency was obtained for the 7 % CMC solutions for both G’ and G”. In the whole temperature ramp, G’ remained greater than G” (data not shown), indicating a viscoelastic system dominated by the elastic component. The thermal treatments lead to losses in the network aggregates and alterations in structural conformation of the sequence segments in the bonding zones (Angioloni and Collar 2009). In the case of PSCMC (Fig. 3a), storage modulus was almost constant in low concentrations (0.5 %), probably relating to less DS of the produced CMC. The increase in the viscoelasticity of CMC gels with increasing DS is due to a transition from largely insoluble, compact, cellulose-like structures with microcrystalline portions to more readily soluble particles with microgel character. These results are in line with the observable transition from turbid to clear solution in the wood CMC with DS less than 1 (Kulicke et al. 1996).
Fig. 3.
Variation of storage modulus (G’) of 0.5 (■), 1 (Ο), 3 (▲), 5 (∆) and 7 % (●) (w/w) CMC solutions during heating ramp as a function of temperature at ramp rate 3 °C/min, frequency of 1 rad.s−1 and strain 1 %; PSCMC (a), LMCMC CMC (b) and HMCMC (c)
The reverse relationship between temperature and storage modulus is related to the incidence of a freer molecule to molecule interaction at raised temperatures. This could be related to the interactions of hydrogen bonds with hydroxyl groups and the alteration in the velocity pattern of the liquid by hydrated molecules of solute. At higher concentration, the polyanion undertakes a less extended conformation, leading to decrease the amount of interactions with the solvent. Moreover, intermolecular associations between solute molecules may occur at higher concentrations, thus the number of available hydrophilic groups decrease for solute-solvent interactions (Kumshah et al. 1976).
Figure 4 shows the relationship between tan δ and temperature in 7 % solution of CMC. As can be seen from 25 to 60 °C, tan δ (G”/G’) is close to 1 for PSCMC and LMCMC, which indicates fluid-like behaviour. Additionally, an increase in temperature from 60 to 95 °C led to a decrease in tan δ of PSCMC for the temperature region considered. The results obtained for commercial CMC were in line with the results reported for wood and non-wood CMC (Angioloni and Collar 2009; Barba et al. 2002). The profile observed in PSCMC sample indicates that the elastic character dominates to the viscous part. As mentioned earlier (Table 1), PSCMC had a purity of 85.94 % compared to 90 % purity of commercial CMCs. Non-CMC components can be protein, cellulose or even lignin. Therefore, the difference between tan δ in PSCMC and commercial CMCs at high temperature may be due to denaturation of remained proteins. Generally, heat treatment induces the denaturation and aggregation of protein molecules. The denaturation of proteins leads to changes in the native conformation of the molecule and the eventual unfolding of the protein. Hence, the decrease in tan δ can be likely caused by the formation of more cross-links between the protein particles.
Fig. 4.
Tan δ variations of 7 % (w/w) PSCMC (■), LMCMC (Ο) and HMCMC (▲) as a function of temperature at a ramp rate 3 °C/min, frequency 1 rad.s−1 and strain 1 %
The frequency measurements explain the viscous and elastic behavioural changes of material at the rate of application of strain while amplitude of the signal is constant. The analysis of the frequency is a precise technique to determine gelling properties. The dynamic storage and loss moduli reveal a dependency on the frequency. G’ and G” were determined at strain of 1 % as a function of the oscillation frequency at 25 °C (Fig. 5a-c). The logarithmic angular frequency varied from −1 to 2 rad.s−1. In accordance to the theory of gel systems described by Clark and Ross-Murphy (1987) and Ross-Murphy (1995), the mechanical spectra of the gels at different concentrations can indicate true gel strength. The samples with values of G’ lower than G” within all tested frequencies are classified as dilute solutions. In the case of semi-dilute or entanglement solution, G’ is below G” at low frequencies and both G’ and G” moduli increase with oscillation frequency increasing and cross over at a certain frequency. This type of spectrum is closer to a gel and could be described as “highly elastic solutions” (Zhang et al. 2007). Gel-like or strong materials disclose a dominant, plateau and frequency independent storage modulus over the complete range of frequencies, describing characteristic of gels.
Fig. 5.
Variation of G’ (closed symbol) and G” (open symbol) of PSCMC (a), LMCMC (b) and HMCMC (c) as a function of frequency of oscillation at 25 °C and strain 1 %; the bottom curve was taken at 0.5 (■,□), 1 (♦,♢), 3 (▲,∆), 5 ( ,✚) and 7 % (●,Ο) w/w of CMC
,✚) and 7 % (●,Ο) w/w of CMC
Figure 5a shows the variation of both storage and loss moduli as a function of frequency in PSCMC solutions. The dispersion of 5 and 7 % (w/w) of PSCMC behaved as gels where G” was below G’ within all frequencies. The samples with lower concentrations (1 and 3 %) behaved as semi-diluted or entangled solutions where G’ and G” crossed over at frequency 1.5 rad.s−1. The sample with 0.5 % solid content behaved as dilute solution where G’ was below G” within all frequencies. The slopes of the storage moduli of PSCMC gels were relatively independent of frequency (0.11 < slope < 0.22) while the loss moduli were slightly dependent on frequency (0.07 < slope < 0.10). Therefore, CMC gel behaviour against various frequencies demonstrates that PSCMC creates a weak gel at high solids content (greater than 1 %).
To compare the obtained results from PSCMC with other systems, frequency sweep test was also carried out for LMCMC and HMCMC solutions. The dispersion of 3, 5 and 7 % (w/w) of LMCMC behaved as gels, while the concentration 1 and 0.5 % behave as entangled and diluted solutions, respectively. The slopes of the G’ for LMCMC gels were between 0.14 and 0.3 while the slopes of loss moduli were between 0.06 and 0.18. As seen in Fig. 5c, HMCMC behave as a semi-diluted solution at 0.5 % solid content and the other concentrations show gel-like behaviour. The slopes of the G’ for HMCMC gels were between 0.1 and 0.21 while the loss moduli slopes were between 0.01 and 0.07. These results are in line with the Maxwellian explanation of the viscoelastic materials response to oscillatory stress. As the oscillatory frequency is decreased, the samples disclose properties characteristic of both solids and liquids, corresponding to greater time to dashpot extension. At high frequencies, the gels behave as elastic solids, due to elongation with sufficient time to enable spring elongation and contraction under the imposed oscillatory shear. However, there is insufficient time to allow for dashpot movement in high frequencies (Jones et al. 1997).
To examine the gel strength of PSCMC, LMCMC and HMCMC, the changes in tan δ versus the angular frequency was plotted in Fig. 6 for different concentrations at 25 °C and strain of 1 %. According to tan δ, polymer systems are classified into dilute solutions (very high tan δ), amorphous polymers (0.2–0.3) and glassy crystalline polymers and gels (near 0.01) (Savadkoohi et al. 2013b). As seen in Fig. 6a, the tan δ values of PSCMC gels were greater than 1 for concentrations of 0.5, 1 and 3 % (w/w), while the tan δ of 5 and 7 % solutions were less than 1. The tan δ of 5 and 7 % gels decreased from 0.95 at low frequency to a value of 0.52–0.55. The high concentration PSCMC solutions showed a frequency dependence of tan δ commensurate with a weak gel. As the frequency increased, an initial decrease in tan δ was detected, whereas the values of tan δ of low concentration solutions were approximately constant over the examined frequency range. At higher frequencies, a plateau region was observed for high concentrations followed by a slight increase in tan δ, suggesting damage to the gel network. This behaviour is typical for a weak gel and a cross-linked polymer in other systems reported by Mleko and Foegeding (2000) and Savadkoohi and Farahnaky (2012). The same profile was observed for concentrations of 3, 5 and 7 % of LMCMC (Fig. 6b) as well as 1, 3, 5 and 7 % of HMCMC (Fig. 6c). The weak gel structure of TSP is similar to the weak gels of other systems such as gellan gum, κ-carrageenan, κ-carrageenan/LBG, psyllium and abaca CMC gels, each of which has a tan δ greater than 0.1 (Andrade et al. 2000; Barba et al., 2002; Farahnaky et al. 2010; Fernandes et al. 1991; Miyoshi et al. 1994).
Fig. 6.
Effect of various concentrations of PSCMC (a), LMCMC (b) and HMCMC (c) on tan δ as a function of frequency of oscillation at 25 °C and strain 1 %; the top curve was taken at 0.5 (▲), 1 (□), 3 (■), 5 (Ο) and 7 % (●) w/w of CMC
Conclusion
The findings of the present study indicated that the produced pomegranate seed pips CMC solutions revealed three endothermic and two exothermic MDSC peaks. Thermomechanical spectra along with micrographs demonstrated that PSCMC sample exhibits more weight loss and less degree of substitution as compared to commercial CMCs. PSCMC displays values of intrinsic viscosity and molecular weight of 72 mL/g and 728439, respectively. Moreover, according to the rheological experiments, PSCMC showed lower elasticity and intrinsic viscosity. The dynamic rheological results demonstrated that PSCMC can create a gel at critical concentration of 3 % w/w and an increase in concentration led to increase in storage modulus. For all critical concentrations in CMC samples, G’ was always superior to G”, i.e. 7 % PSCMC solutions showed mainly elastic properties. The maximum functional properties were seen for 7 % HMCMC. In addition, the gels formed by PSCMC were classified as weak gels based on rheological features such as frequency sweep and tan δ. Further investigations into the applications of pomegranate seed pips cellulose and CMC should be undertaken with a view to extending the knowledge gained presently to specific product concepts with improved nutritional profile and mouth feel. These characteristics exhibited by PSCMC make this functional dietary hydrocolloid as a suitable ingredient for some food applications. In addition, knowledge on the effect of concentration and temperature on functional properties of this study can be useful for the evaluation and design of CMC processing equipment.
References
- Adinugraha MP, Marseno DW, Haryadi M. Synthesis and characterization of sodium carboxymethyl cellulose from Cavendish banana pseudo stem (Musa cavendishii lambert) Carbohyd Polym. 2005;62(2):164–169. doi: 10.1016/j.carbpol.2005.07.019. [DOI] [Google Scholar]
- Andrade CT, Azero EG, Luciano L, Goncalves MP. Rheological properties of mixtures of κ-carrageenan from Hypnea musciformis and galactomannan from Cassia javanica. Int J Biol Macromol. 2000;27:349–353. doi: 10.1016/S0141-8130(00)00139-2. [DOI] [PubMed] [Google Scholar]
- Angioloni A, Collar C. Small and large deformation viscoelastic behavior of selected fibre blends with gelling properties. Food Hydrocolloid. 2009;23:742–748. doi: 10.1016/j.foodhyd.2008.04.005. [DOI] [Google Scholar]
- AOAC (1995) Official Methods of Analysis, AOAC International Arlington, TX. Methods: 920.39; 925.09 and 967.06
- ASTM (1973) Standard methods of testing sodium carboxymethyl cellulose. D1439-72, 104–113
- Barba C, Montane D, Fariol X, Desbrieres J, Rinaudo M. Systhesis and characterization of carboxymethyl cellulose from non-wood pulps II. Rheological behaviour of CMC in aqueous solution. Cellulose. 2002;9:327–335. doi: 10.1023/A:1021136626028. [DOI] [Google Scholar]
- Benchabane A, Bekkour K. Rheological properties of carboxymethyl cellulose (CMC) solutions. Colloid Polym Sci. 2008;286(10):1173–1180. doi: 10.1007/s00396-008-1882-2. [DOI] [Google Scholar]
- Biswal DR, Singh RP. Characterization of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbohyd Polym. 2004;57:379–387. doi: 10.1016/j.carbpol.2004.04.020. [DOI] [Google Scholar]
- Clark AH, Ross-Murphy SB. Structural and mechanical properties of biopolymer gels. Adv Polym Sci. 1987;83:57–192. doi: 10.1007/BFb0023332. [DOI] [Google Scholar]
- Dapia S, Santos V, Parajo JC. Carboxymethylcellulose from totally chlorine free bleached milox pulp. Bioresource Technol. 2003;89:289–296. doi: 10.1016/S0960-8524(03)00066-X. [DOI] [PubMed] [Google Scholar]
- Edali M, Esmail MN, Vatistas GH. Rheological properties of high concentrations of carboxymethyl cellulose solutions. J Appl Polym Sci. 2001;79:1787–1801. doi: 10.1002/1097-4628(20010307)79:10<1787::AID-APP70>3.0.CO;2-2. [DOI] [Google Scholar]
- El-Nemr SE, Ismail IA, Ragab M. Chemical composition of juice and seeds of pomegranate fruit. Die Nahrung. 1990;34(7):601–606. doi: 10.1002/food.19900340706. [DOI] [Google Scholar]
- Fadavi A, Barzegar M, Azizi H, Bayat M. Note. Physicochemical composition of Ten pomegranate cultivars (Punica granatum L.) grown in Iran. Food Sci Technol Int. 2005;11:113–119. doi: 10.1177/1082013205052765. [DOI] [Google Scholar]
- Farahnaky A, Askari H, Majzoobi M, Mesbahi G. The impact of concentration, temperature and pH on dynamic rheology of psyllium gels. J Food Eng. 2010;100:294–301. doi: 10.1016/j.jfoodeng.2010.04.012. [DOI] [Google Scholar]
- Fernandes PB, Goncalves MP, Doublier JL. A rheological characterization of kappa-carrageenan/galactomannan mixed gels: a comparison of locust bean gum samples. Carbohydr Polym. 1991;16:253–274. doi: 10.1016/0144-8617(91)90112-P. [DOI] [Google Scholar]
- Gomez-Díaz D, Navaza JM. Rheology of aqueous solutions of food additives: effect of the concentration, temperature and blending. J Food Eng. 2003;56:387–392. doi: 10.1016/S0260-8774(02)00211-X. [DOI] [Google Scholar]
- Harding SE. The intrinsic viscosity of biological macromolecules; progress in measurement, interpretation and application to structure in dilute solution. Prog Biophys Mol Biol. 1997;68:207–262. doi: 10.1016/S0079-6107(97)00027-8. [DOI] [PubMed] [Google Scholar]
- Hegedusic V, Herceg Z, Rimac S. Rheological properties of carboxymethyl cellulose and whey model solutions before and after freezing. Food Technol Biotech. 2000;38(1):19–26. [Google Scholar]
- Immergut EH, Ranby BG, Mark HF. Recent work on molecular weight of cellulose. J Ind Eng Chem. 1953;45(11):2483–2490. doi: 10.1021/ie50527a036. [DOI] [Google Scholar]
- Jones DS, Woolfson AD, Brown AF. Textural, viscoelastic and mucoadhesive properties of pharmaceutical gels composed of cellulose polymers. Int J Pharm. 1997;151:223–233. doi: 10.1016/S0378-5173(97)04904-1. [DOI] [Google Scholar]
- Kotz J, Bogen TH, Heinze U, Heinze WM. Peculiarities in the physico-chemical behaviour of non-statistically substituted carboxymethylcelluloses. Colloid Surface. 2001;184:621–633. doi: 10.1016/S0927-7757(01)00518-0. [DOI] [Google Scholar]
- Kulicke WM, Kull AH, Kull W, Thielking H, Engelhardt J, Pannek JB. Characterization of aqueous carboxymethyl cellulose solutions in terms of their molecular structure and its influence on rheological behavior. Polym. 1996;37(13):2723–2731. doi: 10.1016/0032-3861(96)87634-8. [DOI] [Google Scholar]
- Kumshah CA, Pass G, Philips GO. The interaction between sodium carboxymethyl cellulose and water. J Solution Chem. 1976;5(11):799–806. doi: 10.1007/BF00651490. [DOI] [Google Scholar]
- Li Y, Guo C, Yang J, Wei J, Xu J, Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006;96:254–260. doi: 10.1016/j.foodchem.2005.02.033. [DOI] [Google Scholar]
- Li W, Sun B, Wu P. Study on hydrogen bond of carboxymethyl cellulose sodium film with two-dimensional correlation infrared spectroscopy. Carbohyd Polym. 2009;78:454–461. doi: 10.1016/j.carbpol.2009.05.002. [DOI] [Google Scholar]
- Melgarejo P, Artes F. Total lipid content and fatty acid composition of oilseed from lesser known sweet pomegranate clones. J Sci Food Agr. 2000;80:1452–1454. doi: 10.1002/1097-0010(200008)80:10<1452::AID-JSFA665>3.0.CO;2-L. [DOI] [Google Scholar]
- Miyoshi E, Takaya T, Nishinari K. Gel–sol transition in gellan gum solutions. II. DSC studies on the effects of salts. Food Hydrocolloid. 1994;8:529–542. doi: 10.1016/S0268-005X(09)80063-5. [DOI] [Google Scholar]
- Mleko S, Foegeding EA. pH induced aggregation and weak gel formation of whey protein polymers. J Food Sci. 2000;65(1):139–143. doi: 10.1111/j.1365-2621.2000.tb15969.x. [DOI] [Google Scholar]
- Nayak BR, Singh RP. Development of graft copolymer flocculating agents based on hydroxypropyl guar gum and acrylamide. J Appl Polym Sci. 2001;81:1776–1785. doi: 10.1002/app.1610. [DOI] [Google Scholar]
- Pushpamalar V, Langford M, Ahmad M, Lim YY. Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohyd Polym. 2006;64:312–318. doi: 10.1016/j.carbpol.2005.12.003. [DOI] [Google Scholar]
- Reh U, Kraepelin G, Lamprecht I. Differential scanning calorimetry as a complementary tool in wood biodegradation studies. Thermochim Acta. 1987;119(1):143–150. doi: 10.1016/0040-6031(87)88015-2. [DOI] [Google Scholar]
- Ross-Murphy SB. Rheological characterisation of gels. J Texture Stud. 1995;26(4):391–400. doi: 10.1111/j.1745-4603.1995.tb00979.x. [DOI] [Google Scholar]
- Savadkoohi S, Farahnaky A. Dynamic rheological and thermal study of the heat-induced gelation of tomato-seed proteins. J Food Eng. 2012;113(3):479–485. doi: 10.1016/j.jfoodeng.2012.06.010. [DOI] [Google Scholar]
- Savadkoohi S, Mesbahi G, Niakousari M, Farahnaky A (2013a) A new study on the steady shear flow, thermal and functional properties of beet pulp carboxymethyl cellulose. J Food Process Pres doi: 10.1111/jfpp.12192
- Savadkoohi S, Shamsi K, Hoogenkamp H, Javadi A, Farahnaky A. Mechanical and gelling properties of comminuted sausages containing chicken MDM. J Food Eng. 2013;117:255–263. doi: 10.1016/j.jfoodeng.2013.03.004. [DOI] [Google Scholar]
- Silva AA, Laver M. Molecular weight characterization of wood pulp cellulose: dissolution and size exclusion chromatographic analysis. Tappi J. 1997;80(6):173–180. [Google Scholar]
- Silva DA, Paula RCM, Feitosa JPA, Brito ACF, Maciel JS, Paula HCB. Carboxymethylation of cashew tree exudate polysaccharide. Carbohyd Polym. 2004;58:163–171. doi: 10.1016/j.carbpol.2004.06.034. [DOI] [Google Scholar]
- Stephen AM, Phillips GO, Williams PA. Food polysaccharides and their applications. (2nd ed.) London: Taylor and Francis Group LLC; 2006. pp. 147–179. [Google Scholar]
- Sun N, Das S, Frazier E. Dynamic mechanical analysis of dry wood: linear viscoelastic response region and effects of minor moisture changes. Holzforschung. 2007;61(1):28–33. doi: 10.1515/HF.2007.006. [DOI] [Google Scholar]
- Toğrul H, Arslan N. Production of carboxymethyl cellulose from sugar beet pulp cellulose and rheological behavior of carboxymethyl cellulose. Carbohyd Polym. 2003;54:73–82. doi: 10.1016/S0144-8617(03)00147-4. [DOI] [Google Scholar]
- Toğrul H, Arslan N. Carboxymethyl cellulose from sugar beet pulp cellulose as a hydrophilic polymer in coating of mandarin. J Food Eng. 2004;62:271–279. doi: 10.1016/S0260-8774(03)00240-1. [DOI] [Google Scholar]
- Tsujiyama S, Miyamori A. Assignment of DSC thermograms of wood and its components. Thermochim Acta. 2000;351:177–181. doi: 10.1016/S0040-6031(00)00429-9. [DOI] [Google Scholar]
- Ucar S, Karagoz S. The slow pyrolysis of pomegranate seeds: the effect of temperature on the product yields and bio-oil properties. J Anal Appl Pyrol. 2009;84:151–156. doi: 10.1016/j.jaap.2009.01.005. [DOI] [Google Scholar]
- Xu C, Leppänen AS, Eklund P, Holmlund P, Sjöholm R, Sundberg K, Willför S. Acetylation and characterization of spruce (Picea abies) galactoglucomannans. Carbohyd Res. 2010;345(6):810–816. doi: 10.1016/j.carres.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Yasar F, Toğrul H, Arslan N. Flow properties of cellulose and carboxymethyl cellulose from orange peel. J Food Eng. 2007;81:187–199. doi: 10.1016/j.jfoodeng.2006.10.022. [DOI] [Google Scholar]
- Zhang T, Jiang B, Wang Z. Gelation properties of chickpea protein isolates. Food Hydrocolloid. 2007;21(2):280–286. doi: 10.1016/j.foodhyd.2006.04.005. [DOI] [Google Scholar]