Abstract
The effect of three different coatings; resin wax (Britex Ti), carnauba wax (Xedasol M14), and chitosan (1 and 2 % w/v) on postharvest quality of pomegranate fruits were investigated. Fruits quality characteristics and bioactive compounds were evaluated during 40, 80 and 120 days storage at 4.5 °C and 3 additional days at 20 °C. The results showed that uncoated fruits showed higher respiration rate, weight loss, L* and b* values of arils, total soluble solids (TSS)/titratable acidity (TA), and pH than coated fruits during storage. Coating treatments could delay declining TSS and TA percent, a* value of arils, as well as bioactive compounds such as total phenolics, flavonoids and anthocyanins content and antioxidant activity. The coated fruits with commercial resin and carnauba waxes showed significantly lower respiration rate and weight loss than other treatments, however carnauba wax could maintain considerably higher fruits quality and bioactive compounds than other coating treatments. The results suggested that postharvest application of carnauba wax have a potential to extend storage life of pomegranate fruits by reducing respiration rate, water loss and maintaining fruit quality.
Keywords: Pomegranate, Coating, Shelf life, Antioxidant compounds
Introduction
Pomegranate (Punica granatum L.) is one of the most important fruit crops which grown on commercial scale in Iran (Fadavi et al. 2005). Although total pomegranate production in Iran changes from 1 year to another, but the recent production has reached to 900,000 tones (Anonymous 2011). The edible parts of pomegranates (called arils) make up approximately 50 % of the fruit weight and are made up of 76–85 % juice and 15–24 % seeds (Varasteh et al. 2012). Pomegranate fruits are mainly used fresh and mostly for making juice, jelly and grenadine (Elyatem and Kader 1984). It is a good source of natural antioxidants (Gil et al. 2000), such as anthocyanins, flavonoids, and phenolic acids (Zaouay et al. 2012). In addition, it is rich in the usual nutrients such as vitamins and minerals (Fawole and Opara 2013).
In spite of the low respiration rate in pomegranate fruits, it is a highly perishable commodity (Barman et al. 2011), because of fruit peel has numerous minute openings that permit free movement of water vapor, and make fruit highly susceptible to water loss (Fawole and Opara 2013). Furthermore, it is sensitive to low temperatures and cold storage at 5 °C or lower resulted in fruits chilling injury (Elyatem and Kader 1984). However, storage at higher temperature leads to reduction of shelf life by acceleration of physiological and pathological activities (Barman et al. 2011).
A potential postharvest treatment that preserves fruit quality is the use of surface coatings. They are usually used for fresh fruits to provide alternative modified atmosphere storage by reducing quality changes and quantity losses through modification and control of the internal atmosphere of the individual fruits (Park 1999). The performance of different types of coatings is dependent on their composition. Chitosan (a high molecular weight cationic polysaccharide) is soluble in dilute organic acids and could theoretically be used as a preservative coating material for fruits (Jiang and Li 2001). Previous studies showed that chitosan coating delayed the decline of anthocyanin content and chroma value in pomegranate fruit (Varasteh et al. 2012), exhibited antifungal activity in citrus fruits (Chien et al. 2007), retarded weight loss and the decline in sensory quality of litchi fruits (Dong et al. 2004) and reduced respiration rate and delayed the increase in polyphenol oxidase (PPO) activity and the changes in colour in longan fruit (Jiang and Li 2001). Carnauba wax is another natural edible coating material, which is recovered from the underside of the leaves of a Brazilian palm tree (Copernica cerifera). It reduced chilling injury, weight and firmness loss in pomegranate fruit (Barman et al. 2011) and incidence of fungal disease in nectarines and plums during storage (Goncalves et al. 2010). Formerly, the effect of carnauba wax (Barman et al. 2011) and chitosan coating (Varasteh et al. 2012) have been studied on cold storage of pomegranate fruit, but they haven’t been compared with another and other coatings in one study. Therefore, the main purpose of this study is to compare the effects of three different coatings on maintaining some quality parameters and bioactive compounds of pomegranate fruit cv. Malase Torshe Saveh stored at 4.5 °C followed by 3 days at 20 °C under marketing condition.
Materials and methods
Plant material
Pomegranate (Punica granatum L. cv. Malase Torshe Saveh) fruits were harvested at the commercial ripening stage (180 days after fruit set) from a commercial orchard located at Saveh, Markazi province, Iran. Immediately, the same day fruits were transported by a ventilated car to the laboratory in University of Guilan, Iran. Pomegranate fruits were selected for uniformity in size (300–350 g), shape and colour. Diseased, sunburn, bruised and injured fruits were discarded. The remaining fruits were randomized and divided into five lots of 45 fruits for the following treatments in three replicates (each replicate contained 15 individual fruits).
Treatments and storage conditions
Fruits were coated with three different coatings, chitosan (medium molecular weight, Fluka, Buchs, Switzerland) at 1 and 2 % (w/w), carnauba wax (Shellac solution in ammonia + carnauba emulsion 27 % + food additives; commercial name: Xedasol M14; Xeda International Co. France) and resin wax (Wood resin wax 18 % + Imazalil 0.2 % + Tiabendazol 5 %; commercial name: Britex Ti; Brodex Co. Spain). Dipping fruits in distilled water was used as a control. The coating treatment of resin and carnauba waxes were manually applied by brush at ambient temperature. For chitosan treatment, fruits were dipped for 2 min in solution of 1 & 2 % (w/v) chitosan with 1 % acetic acid (v/v), which was prepared according to the method described by Jiang et al. (2005). Coated fruits allow drying at ambient temperature. Thereafter, fruits were stored at 4.5 ± 0.5 °C with 90 ± 5 % relative humidity.
Fruits sampling and evaluation
Five fruits from each replicate were randomly sampled every 40 days interval at cold storage and 3 additional days at 20 °C. Respiration rate and weight loss were assayed in intact fruits. Thereafter, fruits husk was carefully cut at the equatorial zone with sharpened knives, peels and arils were manually separated. Aril colour values from sampled fruits were measured according to Fawole and Opara (2013). The aril juice was extracted using a garlic press to evaluate quality parameters [total soluble solid (TSS), titratable acidity (TA), TSS/TA and pH], antioxidant activity and total phenol, flavonoid and anthocyanin content. The same parameters were also measured at harvest time and considered as a day 0. For quality parameters data are shown only at day 0 and last sampling date.
Fruits weight loss and respiration rate
Weights of individual replicate were recorded at harvest time (day 0) and every 40 day intervals at cold storage and 3 additional days at 20 °C over 120 days storage. Cumulative weight losses were expressed as a percentage loss of original weight (Fawole and Opara 2013).
Respiration rate was measured by placing one fruit in 1 L flask and capped with a rubber stopper for 3 h. Then, 1 mL gas samples were withdrawn from the headspace by syringe to determine carbon dioxide (CO2) using a gas chromatograph (Model: Agilent 7890A) equipped with a Poropak column and thermal conductivity detector. The column, injector and detector temperatures were 90, 120 and 100 °C, respectively. Helium was used as the carrier gas at a flow rate 60 mL min−1. Respiration rate was expressed as mg CO2 kg−1 h−1 on three replicates.
Aril colour values
Pomegranate aril colour was measured using a colourimeter (Chroma Meter, CR 400-Minolta, Japan). Aril colour was assessed according to the Commission International del’Eclairage (CIE) and expressed as L*, a* and b* values. Aril colour from sampled fruit was measured on the 20 g arils placed in a colourless glass petri dish (Fawole and Opara 2013).
TSS, TA, TSS/TA and pH
Total soluble solids (TSS) was determined with a digital refractometer (Euromex RD 635, Holland) at 20 °C, and expressed as % (°Brix). Titratable acidity (TA) was determined by titrating with 0.1 N NaOH up to pH 8.2, using 5 mL of diluted juice in 35 mL distilled water, and results expressed as % citric acid. TSS to TA ratio was calculated by dividing TSS to TA percent. The pH was measured at room temperature using a Metron model pH meter (WTW 526, Germany).
Juice total phenolics and flavonoids content
Total phenolic content (TPC) in pomegranate juice were determined by using Folin–Ciocalteu method as described by Ghasemnezhad et al. (2013) with a slight modification. Briefly, 300 μL of diluted pomegranate juice in the ratio of 1:100 with methanol was mixed with 1.5 mL diluted Folin–Ciocalteu reagent with water (1:10 v/v) and 1.2 mL of 7.5 % of sodium carbonate. The absorbance was measured by a UV-visible spectrophotometer (T80+, PG Instruments) at 760 nm. The results were expressed as mg gallic acid equivalent in 100 mL of juice (mg GAE/100 mL of juice).
Total flavonoids content (TFC) in juice was determined by a colourimeteric method described by Park et al. (2008). In a 10 mL tube, 0.3 mL pomegranate juice, 3.4 mL 30 % ethanol, 0.15 mL of 0.5 mol L−1 sodium nitrite (NaNO2) and 0.15 mL of 0.3 mol L−1 aluminium chloride (AlCl3. 6H2O) were added and mixed. After 5 min, 1 mL of 1 mol L−1 Sodium hydroxide (NaOH) was added and the mixture was measured at 506 nm. The results were expressed as mg catechin equivalents (RE) per 100 mL of juice.
Juice total anthocyanins content and antioxidant activity
Total anthocyanins content (TAC) was determined spectrophotometrically by the pH differential method as described by Ghasemnezhad et al. (2013). Absorbance was measured at 510 and 700 nm in buffers at pH 1.0 and 4.5 using a UV–visible spectrophotometer, and then calculated according to following equation: A = [(A510 – A700)pH1.0 − (A510 – A700)pH4.5]. Results were expressed as mg of cyanidin-3-glucoside per 100 mL of juice, using a molar absorptive coefficient (ε) of 26,900 and a molecular weight of 449.2.
The antioxidant activity (AA) of pomegranate juices was measured according to the DPPH method reported by Brand-Williams et al. (1995) with modifications. Briefly, 100 μL of juice diluted with methanol in the ratio of 1:10 was mixed with 1.9 mL of 0.1 mM DPPH in methanol. The absorbance was measured at 515 nm using a UV-visible spectrophotometer. For each sample, three separate determinations were recorded. Antioxidant activity was expressed as the percentage decline in absorbance relative to the control, corresponding to the percentage of DPPH scavenged (%DPPHsc), which was calculated as: %DPPHsc = (1 − Asample/Acontrol) × 100.
Statistical analysis
The experimental design was two-factorial, sources of variation were time storage (40, 80 and 120 days) and coating treatments. Data were analyzed using ANOVA procedure SAS software Version 9.1. Mean comparisons were performed using least significant difference (LSD) tests at p < 0.05 level. The results were presented as mean values ± SE. All the treatments were replicated three times.
Results and discussion
Fruit weight loss
As the results showed (Fig. 1), the weight loss of coated and uncoated pomegranate fruits significantly increased over cold storage and 3 additional days in shelf life condition. However, weight loss percent was significantly affected by coating treatments and the higher value was found in control fruits (Fig. 1). At the end of storage time, the highest weight loss (≈18 %) was recorded in control fruits, while this one was (≈10 %) for resin wax, which was the most effective treatment on reducing weight loss. Furthermore, there was a significant difference among resin wax and other coated fruits except for carnauba wax at the end of storage (Fig. 1). No significant difference was found between 1 and 2 % chitosan concentrations.
Fig. 1.
Effect of different coatings on the weight loss of pomegranate fruits during 120 days storage at 4.5 °C and 3 additional days at 20 °C. The value indicates the mean ± standard error
Fawole and Opara (2013), found that water loss in pomegranate fruits during storage is mainly related to the peel, up to the arils. This is due to high porosity of peel that permits free movement of water vapor (Elyatem and Kader 1984). Consequently, coating treatments will serves as a gas barrier to oxygen, carbon dioxide and water vapor, which can reduce water loss in fruits (Park 1999). The reduction of weight loss by coating treatment was reported in pomegranate (Barman et al. 2011), and other fruits such as mango (Dang et al. 2008), citrus (Chien et al. 2007) and longan (Jiang and Li 2001) fruits. Resin based waxes, such as Britex Ti formulated for shine surface fruits, have relatively low permeability that restricted gas exchange (Petracek et al. 1998). Hence, the lower weight loss in coated fruits during storage could be related to formation of a barrier to gas diffusion between pomegranate fruits and atmosphere.
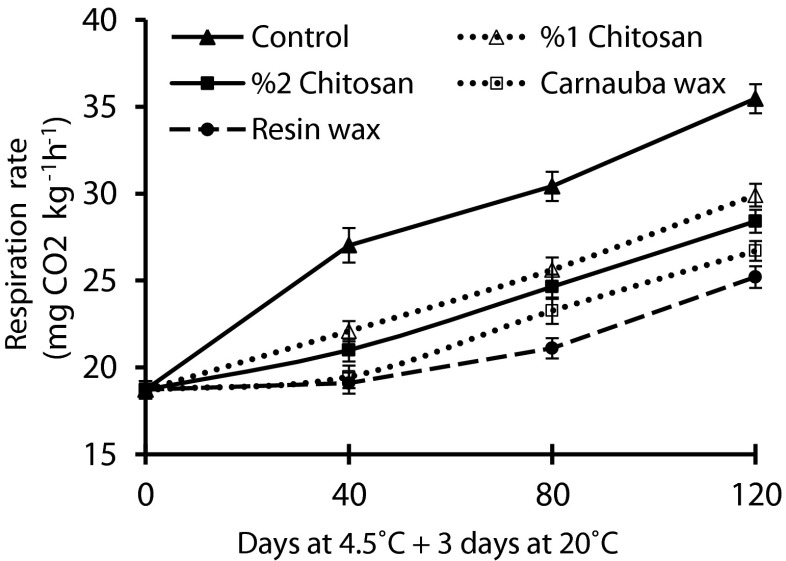
Fruit respiration rate
Respiration rate of coated and control fruits continuously increased during storage time and shelf life condition; however fruits respiration rate was significantly affected by coating treatments (Fig. 2). It was significantly higher over storage time in control fruits (Fig. 2). At the end of 120 days cold storage and 3 additional days in shelf life, the highest (35.5 ± 0.8 mg CO2 kg−1 h−1) and lowest (25.2 ± 0.9 mg CO2 kg−1 h−1) respiration rate was recorded in control and resin wax coated fruits, respectively (Fig. 2). The tendency of higher water loss of uncoated pomegranate was accompanied with the increase of respiration rate over storage (Figs. 1 and 2). After 120 days cold storage with 3 additional days in shelf life, resin wax had significantly lower respiration rate than 1 and 2 % chitosan, but no significant difference was showed with carnauba wax (Fig. 2).
Fig. 2.
Effect of different coatings on the respiration rate of pomegranate fruits during 120 days at 4.5 °C and 3 additional days at 20 °C. The value indicates the mean ± standard error
We found that respiration rate of coated fruits and control increased during storage time and shelf life. This was in contrast with finding of Elyatem and Kader (1984), who reported that respiration rate in pomegranate fruits declined over cold storage period. According to previous studies, the increment of respiration rate and weight loss under chilling inducing storage condition, could be due to chilling injury (Mirdehghan et al. 2007b) or disease development (Jiang and Li 2001). But in this study, we didn’t find any chilling damages and disease incidence in fruits. Therefore, it could be due to high porosity of pomegranate peel that naturally permits free movement of water vapor and other gases. Therefore, the increment of fruits respiration rate and weight loss could be related to increasing peel porosity with extending storage time. Coating treatments could serve as a gas barrier to oxygen, carbon dioxide and water vapor, which can reduce both water loss and respiration rate. This result is in agreement with previous studies on pomegranate (Barman et al. 2011) and longan fruit (Jiang and Li 2001), that showed respiration rate of coated fruits was significantly lower than control under cold storage. The lower respiration rate in coated fruits attributed due to the less gas interchange and consequently the lower oxygen availability to the fruit tissues for respiration (Barman et al. 2011). Surface coatings block pores of the peel and lessen permeability to water vapor and gas exchanges (Varasteh et al. 2012). Jiang and Li (2001) also indicated that chitosan coating decelerated respiration rate of longan fruits and its effect increased with higher chitosan concentration.
Aril colour values
The changes of aril color values in coated and uncoated pomegranate fruits are presented in Table 1. The results showed that arils L* and b* values significantly decreased the first 40 days cold storage and 3 additional days in shelf life, however no significant differences was found between coated and control fruits. Meanwhile, the L* and b* values significantly increased to the end of storage. Both L* and b* values was significantly affected by coating treatments. Coated fruits showed the lower arils L* and b* values compare to control, though no significant differences was observed among coating treatments (Table 1). In contrast, a* increased during the first 40 days storage. Thereafter, a* values decreased gradually to the end of the experiment (Table 1). At the end of storage, the highest and lowest a* were recorded with carnauba wax and control fruits with mean 20.77 ± 0.6 and 17.37 ± 0.5, respectively. Additionally, there was no significant differences among fruits coated with carnauba wax and 1 and 2 % chitosan concentration for a* at the late sampling date (Table 1).
Table 1.
Changes in arils colour parameters (L*, a* and b*) in coated and uncoated pomegranate fruits over 120 days storage at 4.5 °C and 3 additional days at 20 °C
| Parameter | Treatment | Day storage | |||
|---|---|---|---|---|---|
| 0 | 40 | 80 | 120 | ||
| L* | Control | 23.1 ± 0.71 gh | 19.0 ± 0.87 j | 25.4 ± 0.85 cde | 30.7 ± 1.13 a |
| Chitosan 1 % | 23.1 ± 0.71 gh | 20.5 ± 0.85 ij | 25.1 ± 0.73 def | 27.2 ± 0.68 bc | |
| Chitosan 2 % | 23.1 ± 0.71 gh | 20.0 ± 0.82 ij | 22.1 ± 0.79 hi | 26.7 ± 0.76 bcd | |
| Carnauba wax | 23.1 ± 0.71 gh | 19.5 ± 1.05 j | 23.5 ± 0.84 fgh | 25.8 ± 0.71 bcde | |
| Resin wax | 23.1 ± 0.71 gh | 20.3 ± 0.62 ij | 24.5 ± 0.78 efg | 27.5 ± 1.16 b | |
| a* | Control | 22.6 ± 0.56 e | 25.4 ± 0.30 a | 20.9 ± 0.27 fg | 17.4 ± 0.46 j |
| Chitosan 1 % | 22.6 ± 0.56 e | 23.7 ± 0.31 cd | 22.0 ± 0.38 e | 19.8 ± 0.55 hi | |
| Chitosan 2 % | 22.6 ± 0.56 e | 24.1 ± 0.45 bc | 22.7 ± 0.24 de | 20.2 ± 0.35 gh | |
| Carnauba wax | 22.6 ± 0.56 e | 24.9 ± 0.24 ab | 22.0 ± 0.37 e | 20.8 ± 0.59 fgh | |
| Resin wax | 22.6 ± 0.56 e | 24.3 ± 0.37 bc | 21.7 ± 0.18 ef | 19.0 ± 0.48 i | |
| b* | Control | 8.1 ± 0.25 de | 6.8 ± 0.37 g | 8.8 ± 0.37 bcd | 10.0 ± 0.27 a |
| Chitosan 1 % | 8.1 ± 0.25 de | 7.3 ± 0.43 fg | 8.1 ± 0.43 cde | 8.8 ± 0.29 bc | |
| Chitosan 2 % | 8.1 ± 0.25 de | 7.1 ± 0.30 g | 8.2 ± 0.30 cde | 9.0 ± 0.35 b | |
| Carnauba wax | 8.1 ± 0.25 de | 6.9 ± 0.38 g | 8.0 ± 0.38 e | 8.6 ± 0.29 bcde | |
| Resin wax | 8.1 ± 0.25 de | 7.1 ± 0.29 g | 8.0 ± 0.29 ef | 9.2 ± 0.30 b | |
Values with different letters across coating treatment and storage time for each parameter are significantly different at p < 0.05. Data are the mean ± SE (n = 3)
These results are in agreement with previous reports, in which coating treatment delayed the changes in color values of fruit (Jiang et al. 2005; Varasteh et al. 2012). Although, no significant changes were observed for a* in the arils of ‘Ruby’ pomegranate cultivar stored under 2 and 5 °C for 16 weeks (Fawole and Opara 2013). In general, the changes of aril color values during cold storage could be related to synthesis or discoloration of anthocyanin pigments, because the correlation between color parameters and anthocyanin levels has been previously reported in pomegranate fruit (Zaouay et al. 2012). Therefore, it seems that initial increasing in a* and reduction in the lightness (L*) observed for the arils of coated and uncoated fruits, could be due to anthocyanin synthesis in the arils. Jiang et al. (2005) reported that the maintenance of skin color of the litchi fruit by chitsoan coating may be related to the higher level of anthocyanin content in the skin. The increase in L*and b* and decrease in a* during 40 to 120 days storage is probably related to discoloration or degradation anthocyanin pigment by enzyme activity (Jiang and Li 2001). Our results showed lower variation for arils color values in coated fruits than control, probably due to the less activity of PPO enzyme that correlated with anthocyanin discoloration (Jiang et al. 2005). Furthermore, the discrepancies between this study and others previous could be due to cultivar and experimental conditions.
TSS, TA, TSS/TA and pH
Quality parameters of pomegranate fruits at harvest time and after 120 days cold storage with 3 additional days at 20 °C are summarized in the Table 2. There was a decrease in TSS and TA percent at the end of storage in coated and control fruits. After 120 days storage, the highest reduction of TSS (5.8 %) and TA (25.1 %) were recorded in control fruits (Table 2). A significant difference was found for TA in coating and control fruits. The changes in TA content were more slowly in coated than in control fruits. The coating treatments tend to maintain significantly (P < 0.05) higher levels of TA than compared with the control, while there were no significant differences for TSS between control and coating treatments (P < 0.05) at the end of the storage period. The highest level of TA was recorded in fruits coated with resin wax after 120 days storage (Table 2). The TSS/TA ratio of pomegranate fruits is shown in Table 2. A significant increase of TSS/TA was found in both coated and control fruits at the end of the storage as compared to harvest time. The highest increase was observed in control fruits (25.8 %).
Table 2.
Changes in quality parameters (TSS, TA, TSS/TA, pH) in coated and uncoated pomegranate fruits immediately after harvest and after 120 days storage at 4.5 °C and 3 additional days at 20 °C
| Treatment | TSS (°Brix) | TA (%) | TSS/TA | pH |
|---|---|---|---|---|
| At harvest day 0 | 17.7 ± 0.3 | 1.34 ± 0.02 | 13.2 ± 0.30 | 3.34 ± 0.04 |
| 120 + 3 days | ||||
| Control | 16.7 ± 0.2 a | 1.01 ± 0.04 c | 16.6 ± 0.6 a | 3.59 ± 0.04 a |
| Chitosan 1 % | 17.0 ± 0.3 a | 1.08 ± 0.04 bc | 14.4 ± 0.7 c | 3.51 ± 0.02 a |
| Chitosan 2 % | 17.1 ± 0.4 a | 1.11 ± 0.03 ab | 15.0 ± 0.5 bc | 3.49 ± 0.08 a |
| Carnauba wax | 17.3 ± 0.3 a | 1.14 ± 0.03 ab | 15.7 ± 0.6 abc | 3.44 ± 0.06 a |
| Resin wax | 17.3 ± 0.3 a | 1.19 ± 0.02 a | 15.9 ± 0.3 ab | 3.46 ± 0.09 a |
Values within the same column with different superscripts are significantly different at p < 0.05. Data are the mean ± S.E. (n = 3)
The changes of TSS and TA during storage in pomegranate fruits could be the result from ripening process, as previously reported (Mirdehghan et al. 2007b; Sayyari et al. 2011). Furthermore organic acids are the main respiratory substrates during pomegranate postharvest storage (Sayyari et al. 2011). These results are also in agreement with previous reports in coated pomegranate (Nanda et al. 2001), longan (Jiang and Li 2001) and litchi fruits (Jiang et al. 2005), in which TSS and TA reduced during cold storage and the coating treatment could significantly reduce it. In contrast, Ghasemnezhad et al. (2013), found that TSS and TA significantly increased in chitosan coated pomegranate arils during storage for 12 days at 4 °C. The increase in TSS/TA during storage was attributed to higher decrease in TA in comparison with the TSS.
The juice pH at harvest time was 3.34 ± 0.04 and increased at the end of experiment in the control and coated fruits, reaching maximum values at the end of storage with mean 3.59 ± 0.04 in control fruits but without significant differences with coating treatments (Table 2). In accordance, Elyatem and Kader (1984) also reported an increase in juice pH of pomegranate stored at 0–10 °C for 16 weeks. The change in pH is associated with number of reasons; it might be due to the effect of treatment on the biochemical condition of the fruit and slower rate of respiration and metabolic activity (Jitareerat et al. 2007).
Total phenolic content
TPC of coated and control pomegranate fruits were summarized in the Table 3. Non-significant enhancement was observed in TPC up to 40 days storage, but thereafter its content declined to the end of storage (Table 3). The lowest TPC was found in control (84.8 ± 3.5 mg GAE 100 mL−1) as compared to coating treatments. Coating treatments, significantly suppressed declining in TPC during storage. Fruits coated with chitosan and carnauba wax, significantly maintained the higher TPC as compared to resin wax and control (Table 3).
Table 3.
Changes in total phenolics (TPC), flavonoids (TFC) and anthocyanins content (TAC) and antioxidant activity (AA) in coated and uncoated pomegranate fruits over 120 days storage at 4.5 °C and 3 additional days at 20 °C
| Parameters | Treatment | Day storage | |||
|---|---|---|---|---|---|
| 0 | 40 | 80 | 120 | ||
| TPC | Control | 135.6 ± 4.9 abc | 135.7 ± 5.1 ab | 112.7 ± 2.5 h | 84.8 ± 3.5 i |
| Chitosan 1 % | 135.6 ± 4.9 abc | 135.8 ± 4.4 abc | 127.8 ± 3.0 bcde | 113.5 ± 4.0 fg | |
| Chitosan 2 % | 135.6 ± 4.9 abc | 137.4 ± 4.3 ab | 125.4 ± 5.3 cde | 118.8 ± 4.9 ef | |
| Carnauba wax | 135.6 ± 4.9 abc | 138.0 ± 5.8 ab | 129.9 ± 6.1 abcd | 123.5 ± 4.5 def | |
| Resin wax | 135.6 ± 4.9 abc | 139.4 ± 5.2 a | 121.9 ± 3.9 def | 106.6 ± 4.2 gh | |
| TFC | Control | 83.5 ± 2.6 cde | 89.8 ± 2.1 ab | 73.3 ± 1.9 gh | 62.6 ± 1.6 i |
| Chitosan 1 % | 83.5 ± 2.6 cde | 87.4 ± 2.3 abc | 83.6 ± 1.9 cde | 71.9 ± 1.8 gh | |
| Chitosan 2 % | 83.5 ± 2.6 cde | 88.1 ± 3.0 abc | 81.2 ± 2.4 def | 73.6 ± 1.3 g | |
| Carnauba wax | 83.5 ± 2.6 cde | 91.4 ± 1.5 a | 83.5 ± 2.1 cde | 76.8 ± 1.9 fg | |
| Resin wax | 83.5 ± 2.6 cde | 85.1 ± 3.2 bcd | 79.2 ± 2.5 ef | 68.2 ± 1.9 h | |
| TAC | Control | 147.6 ± .04de | 166.3 ± 0.3 a | 142.3 ± 0.7 ef | 106.7 ± 0.5 i |
| Chitosan 1 % | 147.6 ± 0.04 de | 159.1 ± 0.4 abc | 152.7 ± 0.7 cd | 131.5 ± 0.5 g | |
| Chitosan 2 % | 147.6 ± 0.04 de | 163.4 ± 0.3 ab | 159.8 ± 0.5 abc | 134.5 ± 0.3 fg | |
| Carnauba wax | 147.6 ± 0.04 de | 165.2 ± 0.3 ab | 156.9 ± 0.5 abcd | 141.7 ± 0.5 efg | |
| Resin wax | 147.6 ± 0.04 de | 162.4 ± 0.4 abc | 155.6 ± 0.3 bcd | 120.4 ± 0.4 h | |
| AA | Control | 65.7 ± 1.5 def | 68.6 ± 1.4 bcd | 56.8 ± 1.8 h | 48.7 ± 1.6 i |
| Chitosan 1 % | 65.7 ± 1.5 def | 70.7 ± 2.4 bcd | 67.2 ± 2.4 cde | 58.4 ± 1.7 gh | |
| Chitosan 2 % | 65.7 ± 1.5 def | 73.3 ± 2.0 ab | 65.5 ± 1.4 cde | 60.5 ± 1.6 gh | |
| Carnauba wax Carnauba wax | 65.7 ± 1.5 def | 76.4 ± 1.9 a | 67.5 ± 1.5 bcd | 62.6 ± 2.1 ef | |
| Resin wax | 65.7 ± 1.5 def | 72.0 ± 2.2 abc | 61.1 ± 2.7 fgh | 58.6 ± 1.7 h | |
Values with different letters across coating treatment and storage time for each parameter are significantly different at p < 0.05. Data are the mean ± S.E. (n = 3). TPC, (mg GAE 100 mL−1 juice); TFC, (mg RE 100 mL−1 juice); TAC, (mg L−1); AA, (% DPPHSC)
Our findings are in agreement with those reported by Fawole and Opara (2013), who reported a decline in TPC in pomegranate fruit (‘Ruby’ and ‘Bhagwa’) stored under 5–7 °C for 16 weeks and Ghasemnezhad et al. (2013), in pomegranate arils (‘Tarom’) coated with chitosan stored at 4 °C for 12 days. In contrast, Sayyari et al. (2011), found a significant increase of TPC in control and treated pomegranate fruits over 84 days storage. The changes of TPC during cold storage may be related to fluctuations of phenylalanine ammonia-lyase (PAL) enzyme activity, the key enzyme in the first step of the phenylpropanoid pathway directly involved in the biosynthesis of phenolic compounds (Sayyari et al. 2011). In addition, the latter reduction in TPC could be related to enzymatic degradation (Jiang et al. 2005). The inhibition of PPO and peroxidase (POD) activity by coating treatment has also been observed with longan and litchi fruits stored at low temperature (Dong et al. 2004; Jiang et al. 2005).
Total flavonoids content
The change of TFC during storage was similar to the phenolic content. TFC increased significantly during the first 40 days, but thereafter its content declined both coated and control fruits (Table 3). The level of TFC at harvest time, recorded 83.5 ± 3.7 mg RE 100 mL−1 that after 40 days storage reached to the highest level in coated fruits with carnauba wax (91.4 ± 2.1 mg RE 100 mL−1 juice). The lowest TFC was recorded in control fruits after 120 days storage. However, no significant differences observed among coating treatments except for resin wax which had significantly less value.
This result is in contrast with Ghasemnezhad et al. (2013), which observed a significantly increase in TFC in the arils pomegranate stored at 4 °C for 12 days. The amount of flavonoids depends on many factors such as genotype, environmental conditions, production methods, transportation, handling system and storage conditions postharvest of fruit (Ghasemnezhad et al. 2013). The initial increases were probably related to synthesis of flavonoids compounds in pomegranate fruit during postharvest storage (Varasteh et al. 2012), and the latter decline could be correlated to flavonoids degradation as previously found in phenolic content (Table 3). However, Wang and Gao (2013), found a declining of flavonoids content in chitosan coated apple during cold storage and the rate of reduction decelerated with higher chitosan concentration.
Total anthocyanins content
Changes of TAC was recorded in coated and control fruits over cold storage time and 3 additional days in shelf life (Table 3). The results showed that the TAC significantly increased in coated and control fruits during the first 40 days, but thereafter decreased up to the end of storage. Fruit coating treatments could significantly maintain higher TAC than control during postharvest periods. However, fruits coated with commercial carnauba wax showed the highest TAC with 141.7 ± 4.8 mg L−1 after 120 days storage at 4.5 °C plus 3 days at 20 °C (Table 3).
A similar result has been previously reported by Barman et al. (2011), and Fawole and Opara (2013), on pomegranate fruits, while Mirdehghan et al. (2007a), observed significant increases in anthocyanins in putrescine-treated pomegranate fruits over 60 days storage period at 2 °C plus 3 days at 20 °C. With comparing these results and a* values, it could be seen that a decrease in pigments coincides with reduction in TAC for all treatments (Tables 2 and 3). Several studies have shown that the anthocyanin content of pomegranate fruit could increase or decrease depending on storage conditions and postharvest treatments (Fawole and Opara 2013; Mirdehghan et al. 2007a; Sayyari et al. 2011; Varasteh et al. 2012). Furthermore, the effect of different treatments could be related to changes in the fruit internal atmosphere (Miguel et al. 2004). The increase in TAC during the first sampling date of storage may be pertained to the anthocyanin synthesis in pomegranate fruits during storage (Miguel et al. 2004; Varasteh et al. 2012). The increase of anthocyanin concentration after harvest was previously reported in pomegranates (Varasteh et al. 2012; Fawole and Opara 2013), cherries (Goncalves et al. 2007) and strawberry (El Ghaouth et al. 1991). The increasing of TAC during postharvest might result from activation of its related enzymes as anthocyanins are the major phenolic compound being synthesize in mature pomegranate fruit during storage time (Miguel et al. 2004). Additionally, our results showed that coating treatment reduced degradation of anthocyanins over storage period. It was most probably due to that coating treatment reduces the activity of PPO and POD enzymes in response to changes in the internal atmosphere of coated fruit (Varasteh et al. 2012). Dong et al. (2004), also reported that degradation of anthocyanin in litchi fruit caused by PPO and POD, but application chitosan coating treatment decreased enzyme activity over storage time.
Antioxidant activity
The AA of both coated and control pomegranate fruits, increased during the first 40 days storage and declined gradually up to the end of storage (Table 3). Higher antioxidant activity was found in coated fruits than control fruits. The highest AA (76.4 ± 2.2 DPPHsc), was recorded in carnauba wax after 40 days storage, but the lowest value (48.7 % DPPHsc) was found in control fruits after 120 days cold storage with 3 additional days in shelf life condition (Table 3). The results also showed that AA changes are parallel to phenolics, flavonoids and anthocyanins during postharvest storage (Table 3).
Antioxidant capacity of plant products is mainly because of the presence of pigments, vitamins and phenolic compounds (Barman et al. 2011). In addition, a positive correlation between antioxidant activity and phenolic compounds in pomegranate fruit has previously reported (Mirdehghan et al. 2007a). Therefore, total anthocyanin and other phenolic compounds have effective role in antioxidant activity. In agreement with our results, Ghasemnezhad et al. (2013) also found a significant decrease in antioxidant activity of coated and uncoated pomegranate arils over cold storage at 4 °C; however this decrease was lower in coated treatment. The reason for higher antioxidant activity in coated fruits may be explained with the reduced losses of anthocyanins, total phenols and flavonoids content (Table 3). In contrast, Mirdehghan et al. (2007a) found an increase in antioxidant activity in polyamine-treated pomegranate fruits during 60 days storage at 2 °C with 3 additional days at 20 °C.
Conclusions
In conclusion, the data presented here unequivocally suggest that postharvest physiological responses, quality parameters and bioactive compounds of pomegranate fruits during cold storage and shelf life, are affected by coating treatments. It also suggested that total antioxidant activity of pomegranate juice is related to variations of total phenol, flavonoid and anthocyanin content over storage time. Coating treatment significantly maintained these functional compounds during storage in pomegranate fruits. The result also showed that a* (redness) and other color parameters changed with fluctuations of total anthocyanin content during storage period. Commercial resin and carnauba waxes were more effective than chitosan in reducing fruits water loss and respiration rate. Indeed, carnauba wax was the most effective treatment in maintaining quality parameters, total phenolics, flavonoids and anthocyanins content and color parameters of pomegranate fruit over storage period.
Acknowledgments
The authors would like to thank the University of Guilan, Rasht, Iran for funding this research.
References
- Anonymous . Statistical book of agricultural of Iran. Tehran: Iranian Statistical Centre; 2011. [Google Scholar]
- Barman K, Asrey R, Pal RK. Putrescine and carnauba wax pretreatments alleviate chilling injury, enhance shelf life and preserve pomegranate fruit quality during cold storage. Sci Hortic. 2011;130:795–800. doi: 10.1016/j.scienta.2011.09.005. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Chien PJ, Sheu F, Lin HR. Coating citrus (Murcott tangor) fruit with low molecular weight chitosan increases postharvest quality and shelf life. Food Chem. 2007;100:1160–1164. doi: 10.1016/j.foodchem.2005.10.068. [DOI] [Google Scholar]
- Dang KTH, Singh Z, Winny AES. Edible coatings influence fruit ripening, quality, and aroma biosynthesis in mango fruit. J Agric Food Chem. 2008;56:1361–1370. doi: 10.1021/jf072208a. [DOI] [PubMed] [Google Scholar]
- Dong H, Cheng L, Tan J, Zheng K, Jiang Y. Effects of chitosan coating on quality and shelf life of peeled litchi fruit. J Food Eng. 2004;64:355–358. doi: 10.1016/j.jfoodeng.2003.11.003. [DOI] [Google Scholar]
- El Ghaouth A, Ponnampalam R, Boulet M. Chitosan coating effect on storability and quality of fresh strawberries. J Food Sci. 1991;56:1618–1621. doi: 10.1111/j.1365-2621.1991.tb08655.x. [DOI] [Google Scholar]
- Elyatem SM, Kader AA. Postharvest physiology and storage behavior of pomegranate fruits. Sci Hortic. 1984;24:287–298. doi: 10.1016/0304-4238(84)90113-4. [DOI] [Google Scholar]
- Fadavi A, Barzegar M, Azizi MH, Bayat M. Physicochemical composition of ten pomegranate cultivars (Punica granatum L.) grown in Iran. Food Sci Technol Int. 2005;11:113–119. doi: 10.1177/1082013205052765. [DOI] [Google Scholar]
- Fawole OA, Opara UL. Effects of storage temperature and duration on physiological responses of pomegranate fruit. Ind Crop Prod. 2013;47:300–309. doi: 10.1016/j.indcrop.2013.03.028. [DOI] [Google Scholar]
- Ghasemnezhad M, Zareh S, Rassa M, Hassan-Sajedi R. Effect of chitosan coating on maintenance of aril quality, microbial population and PPO activity of pomegranate (Punica granatum L. cv. Tarom) at cold storage temperature. J Sci Food Agric. 2013;93:368–374. doi: 10.1002/jsfa.5770. [DOI] [PubMed] [Google Scholar]
- Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- Goncalves B, Silva AP, Moutinho-Pereira J, Bacelar E, Rosa E, Meyer AS (2007). Effect of ripeness and postharvest storage on the evolution of colour and anthocyanins in cherries (Prunus avium L.). Food Chem 103: 976-984 [DOI] [PubMed]
- Goncalves FP, Martins MC, Silva-Junior GJ, Lourenco SA, Amorim L. Postharvest control of brown rot and Rhizopus rot in plums and nectarines using carnauba wax. Postharvest Biol Technol. 2010;58:211–217. doi: 10.1016/j.postharvbio.2010.08.004. [DOI] [Google Scholar]
- Jiang Y, Li Y. Effects of chitosan coating on postharvest life and quality of longan fruit. Food Chem. 2001;73:139–143. doi: 10.1016/S0308-8146(00)00246-6. [DOI] [Google Scholar]
- Jiang Y, Li J, Jiang W. Effects of chitosan coating on shelf life of cold-stored litchi fruit at ambient temperature. LWT Food Sci Technol. 2005;38:757–761. doi: 10.1016/j.lwt.2004.09.004. [DOI] [Google Scholar]
- Jitareerat P, Paumchai S, Kanlayanarat S. Effect of chitosan on ripening enzymatic activity, and disease development in mango (Mangifera indica L.) fruit. N Z J Crop Hortic Sci. 2007;35:211–218. doi: 10.1080/01140670709510187. [DOI] [Google Scholar]
- Miguel G, Fontes C, Antunes D, Neves A, Martins D. Anthocyanin concentration of ‘Assaria’ pomegranate fruits during different cold storage conditions. J Biomed Biotechnol. 2004;5:338–342. doi: 10.1155/S1110724304403076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirdehghan SH, Rahemi M, Serrano M, Guillén F, Martínez-Romero D, Valero D. The application of polyamines by pressure or immersion as a tool to maintain functional properties in stored pomegranate arils. J Agric Food Chem. 2007;55:755–760. doi: 10.1021/jf062985v. [DOI] [PubMed] [Google Scholar]
- Mirdehghan SH, Rahemi M, Castillo S, Martínez-Romero D, Serrano M, Valero D. Pre-storage application of polyamines by pressure or immersion improves shelf-life of pomegranate stored at chilling temperature by increasing endogenous polyamine levels. Postharvest Biol Technol. 2007;44:26–33. doi: 10.1016/j.postharvbio.2006.11.010. [DOI] [Google Scholar]
- Nanda S, Rao DVS, Krishnamurthy S. Effects of shrink film wrapping and storage temperature on the shelf life and quality of pomegranate fruits cv. Ganesh. Postharvest Biol Technol. 2001;22:61–69. doi: 10.1016/S0925-5214(00)00181-2. [DOI] [Google Scholar]
- Park HJ. Development of advanced edible coatings for fruits. Trends Food Sci Technol. 1999;10:254–260. doi: 10.1016/S0924-2244(00)00003-0. [DOI] [Google Scholar]
- Park YS, Jung ST, Kang SG, Heo BG, Arancibia-Avila P, Toledo F, Drzewiecki J, Namiesnik J, Gorinstein S. Antioxidants and proteins in ethylene-treated kiwifruits. Food Chem. 2008;107:640–648. doi: 10.1016/j.foodchem.2007.08.070. [DOI] [Google Scholar]
- Petracek PD, Dou H, Pao S. The influence of applied waxes on postharvest physiological behavior and pitting of grapefruit. Postharvest Biol Technol. 1998;14:99–106. doi: 10.1016/S0925-5214(98)00018-0. [DOI] [Google Scholar]
- Sayyari M, Babalar M, Kalantari S, Martínez-Romero D, Guillén F, Serrano M, Valero D. Vapour treatments with methyl salicylate or methyl jasmonate alleviated chilling injury and enhanced antioxidant potential during postharvest storage of pomegranates. Food Chem. 2011;124:964–970. doi: 10.1016/j.foodchem.2010.07.036. [DOI] [Google Scholar]
- Varasteh F, Arzani K, Barzegar B, Zamani Z. Changes in anthocyanins in arils of chitosan-coated pomegranate (Punica granatum L. cv. Rabbab-e-Neyriz) fruit during cold storage. Food Chem. 2012;130:267–272. doi: 10.1016/j.foodchem.2011.07.031. [DOI] [Google Scholar]
- Wang SY, Gao H. Effect of chitosan -based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria x aranassa Duch.) LWT Food Sci Technol. 2013;52:71–79. doi: 10.1016/j.lwt.2012.05.003. [DOI] [Google Scholar]
- Zaouay F, Mena P, Garcia-Viguera C, Mars M. Antioxidant activity and physico-chemical properties of Tunisian grown pomegranate (Punica granatum L.) cultivars. Ind Crops Prod. 2012;40:81–89. doi: 10.1016/j.indcrop.2012.02.045. [DOI] [Google Scholar]