Abstract
The aim of this study was to evaluate the influence of additives of Jerusalem artichoke (JA), fermented with P. acidilactici KTU05-7, P. pentosaceus KTU05-9, L. sakei KTU05-6, on the quality and safety parameters of ready – to cook – minced pork (RCMP). Fermented JA additives reduced pH of the meat products and decreased water holding capacity (WHC) from 2.01 till 2.93 %. Concentrations of biogenic amines in RCMP with additives of the lactic acid bacteria (LAB) - fermented JA were significantly lower comparing with control sample. The number of pathogenic bacteria in artificially contaminated meat samples was significantly reduced in case of LAB-fermented JA additives. The highest antimicrobial activity was obtained using P. acidilactici fermented JA additives. The amounts of microbial pathogens E. coli and Ent. faecalis, S. aureus and Streptococcus spp. were determined 3.41, 3.38, 3,96 and 4.74 log CFU/g correspondingly, whereas without LAB-fermented JA additives were 8.94, 7.75, 8.82 and 8.58 log CFU/g, correspondingly. A possibility to improve sensory properties (flavor) of RCMP using LAB fermented JA additives was investigated. The composition of volatile compounds of RCMP without additive and with LAB-fermented JA additives was analyzed using gas chromatography–mass spectrometry (GC-MS). The results of sensory evaluation of meat products supplemented with fermented JA additives revealed specific odor, which is pleasant and acceptable for consumers might be explainable that LAB-fermented JA additives have shown considerable differences mainly due to the accumulation of volatiles such as toluene, ethylbenzene, decane, undecane, 2 methyl undecane. N-morpholinomethyl-isopropyl-sulfide, 6-undecilamine and N,N-dimethyl-1-pentadecanamine were not determined in RCMP with LAB-fermented JA additives. The results obtained show, that P. acidilactici fermented JA 5 % additive is most suitable for the RCMP processing in order to prevent microbiological spoilage, increase volatile compounds and acceptability of the products.
Keywords: Ready-to-cook minced pork meat, Jerusalem artichoke, Lactic acid bacteria fermentation, Biogenic amines, Bacteriocin like inhibitory substances
Introduction
Meat and meat products are important sources for proteins, fats, essential amino acids, minerals, vitamins and other nutrients (Biesalski 2005). In recent years, much attention has been paid to develop meat and meat products with physiological functions, promote health and to reduce the risk of diseases. The consumers demand healthier meat and meat products with improved functional value. Health enhancing ingredients such as vegetable proteins, dietary fibers, herbs and spices, probiotics are rapidly increasing worldwide (Wangang et al. 2010) similar to natural bio-preservatives.
Lactic acid bacteria (LAB) as bio-preservation organisms play a key role in food fermentations where they not only contribute to the development of desired sensory properties in the final product but also to their microbiological safety (Smaoui et al. 2010; Cizeikiene et al. 2013). The antimicrobial effect of LAB is related to the production of organic acids, short chain fatty acids, hydrogen peroxide and proteinaceous compounds – bacteriocins and bacteriocin-like inhibitory substances (BLIS) (Dalié et al. 2010). During recent years an interest in BLIS producing LAB increased considerably (Narbutaite et al. 2008; Digaitiene et al. 2012) because they produce natural antimicrobial agents those enhance safety of the food products. In previous studies, we described the antimicrobial activity of Pediococcus acidilactici KTU05-7, Pediococcus pentosaceus KTU05-9, Lactobacillus sakei KTU05-6 produced BLIS against ropes producing B. subtilis. L. sakei KTU05-6 produced sakacin demonstrated high antibacterial activity and thermal stability. It remains active after the heat treatment at 100 °C for 60 min (Digaitiene et al. 2012). It was determined, that organic acids and BLIS of those LAB show fungicidal and fungistatic activities against yeast and fungi such as Fusarium culmorum, Penicillium chrysogenum, Aspergillus fumigatus, A. versicolor, P. expansum, A. niger, Debaryomyces hansenii and Candida parapsilosis (Cizeikiene et al. 2013).
Fresh meat is a highly perishable product due to its biological composition, whereas bacteriocins or BLIS producing LAB presence in meat products is significant from food safety point. Helianthus tuberosus L (Jerusalem artichoke (JA)) gains increasing interest in food applications. JA is usually used as a root vegetable and has been promoted as a healthy choice for diabetics. The tubers of JA have high content (up to 70 % from the total carbohydrates) of β-(2.1)-linked fructans of varied (3–30) degree of polymerization (Saengthongpinit and Sajjaanantakul 2005). The nutritional value of JA is contributed by proteins, plant lipids, macro- and microelements as minor constituents of potent physiological activity (Bekers et al. 2005; Nemeth and Izsaki 2006; Paseephol et al. 2007). Several researchers reported that JA has effects of reducing glucose and improving insulin secretion (Park 2011; Yang et al. 2012) and revealed excellent antioxidant activity (Kim et al. 2010). Moreover, the JA stimulates the growth of beneficial endogenous LAB and bifidobacteria (Gibson and Wang 1994; Roberfroid et al. 1998), therefore fermented JA products using BLIS producing LAB is a good source for meat functional value improvement.
However, the production of fermented foods involves the activity of a variety of microorganisms, not only associated with the desired technological fermentative properties but also undesired contaminants that can produce biogenic amines (BAs) and thus might have strong affect on food safety and human health. In this respect, the control of BAs in fermented foods is needed and it is one of the present challenges of the food industry (Vidal-Carou et al. 2007; Latorre-Moratalla et al. 2012).
The aim of this study was to evaluate an influence of the additives of JA tubers, fermented with BLIS producing LAB strains (Pediococcus acidilactici KTU05-7, Pediococcus pentosaceus KTU05-9, Lactobacillus sakei KTU05-6), on RCMP quality and safety parameters.
Methods
Materials
The Jerusalem artichoke (JA) tubers were obtained from the Lithuanian Institute of Horticulture (Babtai, Lithuania) in 2012. The lactic acid bacteria (LAB) Pediococcus acidilactici KTU05-7, P. pentosaceus KTU05-9, Lactobacillus sakei KTU05-6, previously isolated from spontaneous rye sourdoughs (Digaitiene et al. 2005) showing antimicrobial activity against undesirable microorganisms in the food industry by producing organic acids and BLIS (Narbutaite et al. 2008; Digaitiene et al. 2012; Cizeikiene et al. 2013) were used for JA tuber mash fermentation. The LAB were stored at −70 °C and cultured at temperatures 35 °C (KTU05-9 and KTU05-7) or 30 °C (KTU05-6) for 48 h in MRS broth (CM0359, Oxoid Ltd, Hampshire, UK) supplemented with 40 mmol × L−1 fructose and 20 mmol × L−1 maltose.
Solid state fermentation (SSF) of JA tubers
JA tuber mash (300 g) and LAB cell suspension (10 g), containing ca. 10.2 log10 of colony-forming units (cfu) per g of the above individual LAB strains, were used to prepare fermented JA products following the fermentation at appropriate temperatures for 24 h. Final LAB count in the fermented JA was ca. 7.28 log10 cfu/g.
Preparation of ready- to-cook minced meat products (RCMP)
Fresh pork loin was obtained from the local market and used for preparation of RCMP. Meat was minced with a hole diameter of 4.0 mm, and mixed with 5 % of JA product, fermented with different LAB. Control samples were prepared using minced meat without an additive of fermented JA. RCMP with and without fermented JA additives were covered with plastic film and were stored until analyses in a refrigerator at +4 °C for 24 h.
Analysis of fermented JA and ready- to-cook minced meat products (RCMP)
The pH value of fermented JA and RCMP was measured and recorded by a pH electrode (PP – 15, Sartorius, Goettingen, Germany). The moisture content was determined according to the AOAC 950.46 method (AOAC 2006). Each sample (5 g) was oven-dried (UNB400, Memmert, Germany) at 103–104 °C temperature to constant weight. The moisture content was calculated dividing the mass change of the sample by the initial mass and expressed in percents. Water holding capacity (WHC) was determined using compression method, described by Grau and Hamm (1956).
Sensory evaluation
The panelist evaluation was carried out in area separated from the food preparation which was free of extraneous cooking odors. Sensory evaluations were conducted using a 7-point rating scale for the cooked minced pork meat products attributes (odor, spice odor, flavor, spice flavor, firmness) by fifteen judges. Number 1 corresponded to the lowest intensity and number 7 to the highest intensity of the attribute. Coded samples were served and water was provided for rinsing between the sensory procedures of the samples.
Acceptability of RCMP was carried out according to the ISO 8586-1 method (ISO 1993) by fifteen judges for preliminary sensory acceptability using a 7 scores hedonic line scale ranging from 7 (extremely like) to 1 (extremely dislike). Coded samples were served and water was provided for rinsing between the sensory procedures of the samples.
Determination of biogenic amines (BAs) in ready-to-cook meat products (RCMP)
Extraction of samples and determination of BAs were carried out according to the procedures developed by Ben-Gigirey et al. (1999).
Microbiological analysis of ready-to-cook minced meat products (RCMP)
To evaluate the antimicrobial LAB effect in RCMP, the 5 % of fermented JA with BLIS producing LAB was added as additive to minced meat and separate samples were artificially contaminated with ∼1 × 104 CFU per gram Escherichia coli, Enterococcus faecalis, Staphylococcus aureus and group D Streptococcus spp. previously cultivated in appropriate media (Tryptone bile X-glucuronide medium (TBX, UK), Macconkey agar (Oxoid, UK), Baird parker agar (Oxoid, UK), Bile Aesculin Azide agar (Gifco, Paisley, UK), respectively) and carefully mixed. Escherichia coli, Enterococcus faecalis, Staphylococcus aureus and group D Streptococcus spp. were previously isolated from spoiled meat samples. As a control RCMP without fermented JA was carried out.
Artificially contaminated RCMP samples were kept at room temperature (18 °C) for 12 h. Serial dilutions, plating and counting of live bacteria were used to determine the total number of bacteria in the RCMP. The diluted samples were placed on the nutrition agar and incubated at 30 °C for 72 h. Under these conditions, all bacteria rapidly grow in the presence of adequate food to form visible colonies. For the detection of total count of enterobacteria the diluted samples were placed on Macconkey agar. The samples were placed and on selective and differential diagnostic media. For determination of Staphylococcus aureus number the Baird parker agar, for group D Streptococcus the Bile Aesculin Azide agar (Gifco, Paisley, UK) and for Escherichia coli the Tryptone bile X-glucuronide medium were used. The plates were incubated at 37 °C under aerobic conditions for 2 days. A final number of bacteria was expressed as the cell forming units per gram (cfu/g) and recalculated as log 10 CFU/g.
Analysis of volatile compounds using GC-MS
Samples for gas chromatographic analysis were prepared using solid-phase microextraction (SPME) device equipped with Supelco 57750-U Stableflex™ fiber, coated with 65-μm PDMS-DVB layer (Sigma-Aldrich, St. Louis, MO, USA). For extraction, 0.01 g of sample was placed in a 10-mL glass vial with the PTFE-lined silicone septa. The fiber was placed in the headspace of the sample at 25 °C for 1 h. The injection was performed by thermal desorption of the volatiles in the injection port of the gas chromatograph at 230 °C. For GC-MS measurements a model GCMS-QP2010 gas chromatograph with a mass spectrometric detector (Shimadzu, Tokyo, Japan) was used. The ionization of the analytes was performed using an electron ionization mode at 70 eV. For the separation of volatiles a low polarity Rtx®-5MS column (Restek Corporation, Bellefonte, PA, USA) (length 30 m, coating thickness 0.25 μm, 0.25 mm i.d.) was used. Ion source temperature was set at 220 °C and interface temperature was 260 °C. Sample injection was carried out for 1 min in order to ensure full desorption of volatiles from the SPME fibre. Split mode injection (1:10) was used. Temperature gradient program was set as follows: from 30 to 200 °C at 5 °C/min and up to 280 °C at 20 °C/min, then maintained for 2 min. The carrier gas was 99.999 % helium (AGA, Vilnius, Lithuania) with a pressure of 90 kPa at the column head, and the column flow of 1.61 mL/min was used. The compounds were identified according to the mass spectral library NIST 8.0 (The National Institute of Standards and Technology, Gaithersburg, MD, USA). Five identical samples were repeatedly measured for solid-phase microextraction coupled with GC-MC. The relative standard deviation (RSD) for peak area did not exceed 5.15 %.
RCMP shelf life evaluation
The thiobarbituric acid (TBA) assay was performed following the method proposed by Raharjo et al. (1993). The color characteristics of RCMP were measured using a chromameter (MiniScan XE Plus, Reston, USA). Values for L* (brightness or whiteness), a* (greenness to redness), and b* (yellowness to blueness) were determined.
Statistical analysis
All analytical determinations were performed at least in triplicate. Data obtained were analyzed using statistical package SPSS for Windows XP V15.0 (SPSS Inc., Chicago, IL, USA, 2007). Significance of differences between treated samples was evaluated using Duncan’s multiple range tests at a 5 % confidence level.
Results and discussion
Influence of the fermented JA additives on quality of ready-to- cook minced meat products (RCMP)
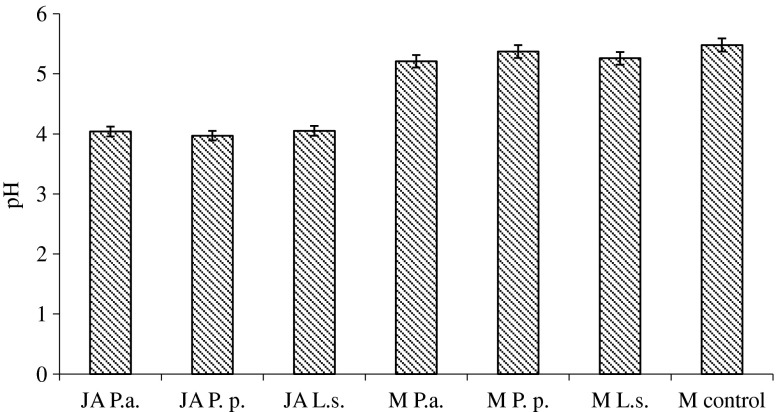
The pH of JA tuber mash before fermentation with LAB was 6.21, whereas after 24 h JA fermentation using BLIS producing LAB, fermented JA pH values varied from 3.97 ± 0.02 to 4.05 ± 0.01 (Fig. 1). There was not statistically significant pH differences observed between different LAB strains used for JA fermentation. Previously for meat product fermentation the selection of starter cultures was based on limited number of properties regarding rapid acidification, fast growth and phage resistance (Leroy and De Vuyst 2004). Decreasing of pH in meat occurs when meat glycogen turns into lactic acid, which increases a sourness. In fermented meat products the decrease of pH is also due to the action of LAB, making the meat proteins coagulate and generating texture (Ravyts et al. 2012). Other studies (Huff-Lonergan et al. 2002; Bryhni et al. 2003; Bidner et al. 2004) have shown, that higher ultimate pH tended to be associated with juicier meat and the lowest juiciness was observed at approximately 5.4 (Bidner et al. 2004). Since 1940 lactobaccilli and pediococci are used as acidifiers, usually in combination with Gram-positive, catalase positive cocci (Ravyts et al. 2012).
Fig. 1.
pH values of fermented JA and ready-to-cook minced meat products. Samples: JA P.a. – fermented with P. acidilactici JA; JA P.p. – fermented with P. pentosaceus JA; JA L.s. – fermented with L. sakei JA; ready-to-cook minced meat products: M P.a. – with fermented with P. acidilactici JA;: M P.p. – with fermented with P. pentosaceus JA; M L.s. – with fermented with L. sakei JA; M control – without fermented JA
In this study a decrease in pH was probably due to an increased population of LAB, which are the major producers of lactic acid and are responsible for the decrease of pH during meat products manufacturing (Aro Aro et al. 2010). There are the significant differences in pH values between the different starter culture treatments (Aro Aro et al. 2010). This is in agreement with our results. We found, that P. acidilactici fermented JA significantly reduced meat pH, whereas L. sakei and P. pentosaceus fermented JA additives did not significantly decrease pH of the RCMP.
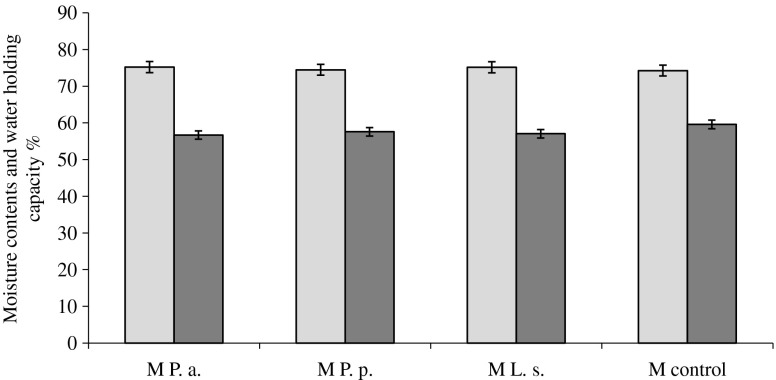
The percentage of naturally occurring water in meat varies with the type of muscle, the kind of meat, the season of the year, and the pH of the meat. In fact some of water in muscle cells is very closely bound to proteins (Huff-Lonergan and Lonergan 2005). Our study showed that the moisture content of RCMP with fermented JA additives was by 0.2–0.93 points higher than in the control samples (Fig. 2).
Fig. 2.
Moisture contents ( ) and water holding capacity (WHC) (
) and water holding capacity (WHC) ( ) of ready-to-cook minced meat products (M P.a. – with fermented with P. acidilactici JA; M P.p. – with fermented with P. pentosaceus JA; M L.s. – with fermented with L. sakei JA; M control – without fermented JA)
) of ready-to-cook minced meat products (M P.a. – with fermented with P. acidilactici JA; M P.p. – with fermented with P. pentosaceus JA; M L.s. – with fermented with L. sakei JA; M control – without fermented JA)
Small differences of moisture content were found between RCMP with JA additives and the control sample. This can be due to the ability of LAB strains to decrease meat pH during the fermentation. The correlation between pH and moisture content was statistically insignificant (r = 0.53; p = 0.28) and there was no statistically significant differences found between all groups (p > 0.05). The highest moisture content was of RCMP with P. acidilactici fermented JA additive (0.73 points higher compared to RCMP sample with P. pentosaceus fermented JA additive (p > 0.05)).
Water holding capacity (WHC) is one of the most important quality characteristics of meat products. Early postmortem events, including rate and extent of pH decrease, ionic stench, proteolysis and protein oxidation can affect meat ability to retain moisture (Huff-Lonergan and Lonergan 2005). Low WHC and pH resulted in high cooking loss of pork while no difference in cooking loss was observed between meat having medium or high WHC and pH (Aaslyng et al. 2003). Other researches (Puolanne and Peltonen 2013) reported that WHC depends on the pH and salt content in the fermented meat products.
In the present study WHC decreased from 2.01 to 2.93 % compared with the control meat product (Fig. 2). WHC was dependent on the LAB strain. The highest WHC occurred in RCMP with P. pentosaceus fermented JA additive and the lowest – in RCMP with P. acidilactici fermented JA. The differences between these groups were statistically significant (p < 0.02). The results showed that only P. acidilactici fermented JA significantly decreased (p < 0.05) WHC of RCMP compared with the control group.
Usually during the conversion of muscle to meat lactic acid is formed in the tissue leading to a reduction pH of the meat (Huff-Lonergan and Lonergan 2005). During this process myosine was found partially denaturated. The end of this process reduced the space within myofibrills, and WHC decreased. In our study, the lacto fermentation treated JA additives to RCMP significantly decreased the pH of meat. This study confirms that WHC correlates with pH values of RCMP (r = 0.72; p = 0.044).
The influence of fermented JA additive on biogenic amines (BAs) formation in ready-to-cook minced meat products (RCMP)
The formation and accumulation of BAs in foods is the result of the enzymatic amino acids decarboxylation due to microbial enzymes and tissue activity. Determination of these compounds is of a great interest, not only for their potential risk to human health, but also because they can be considered indicators of food quality and freshness. The content and composition of BAs is associated with the degree of food fermentation or degradation (Favaro et al. 2007). The principal BAs found in fresh and processed meat are putrescine (PUT), cadaverine (CAD), histamine (HIS) and tyramine (TYR), while natural polyamines levels, such as spermidine (SPD) and spermine (SPM) slightly change during storage or processing (Favaro et al. 2007).
The results of BAs analysis in RCMP samples are presented in Table 1. The BAs found in RCMP were phenylethylamine, histamine, tyramine and tryptamine. The highest total BAs content was found in RCMP with JA additives treated with P. pentosaceus (6.25 mg/kg). The total BAs contents in RCMP with JA additives fermented with L. sakei and P. acidilactici were lower by 20.6 % and 71.8 %, respectively. The highest histamine and tyramine contents of 5.01 and 0.30 mg/kg, respectively were determined in RCMP with P. pentosaceus fermented JA additives, and the highest tryptamine concentration of 4.82 mg/kg was determined in RCMP with L. sakei fermented JA additives (Table 1).
Table 1.
BAs contents (mg kg−1 d. m.) in ready-to-cook minced meat products
| BAs | M L.s. | M P.a. | M P.p. | M control |
|---|---|---|---|---|
| Phenylethylamine | – | 0.92 ± 0.14a | – | 2.1 ± 0.15b |
| Histamine | 0.15 ± 0.04a | – | 5.01 ± 0.17b | 5.07 ± 0.10b |
| Tyramine | – | – | 0.30 ± 0.05a | 0.4 ± 0.9b |
| Tryptamine | 4.82 ± 0.11d | 0.84 ± 0.09a | 0.94 ± 0.03b | 1.3 ± 0.18c |
| Total | 4.96 | 1.76 | 6.25 | 8.87 |
Data are represented as mean values ± SD. a–d Mean values listed in rows with different letters are significantly different (p ≤ 0.05). Ready-to-cook minced meat products: M control – without fermented JA; M P.a. – with fermented with P. acidilactici JA; M P.p. – with fermented with P. pentosaceus JA; M L.s. – with fermented with L. sakei JA
Different concentrations of these compounds in fresh and processed meat can be explained by different properties of meat substrates from various sources and by microbial floras with different biochemical potentials regarding metabolism of amino acids. A big part of Enterobacteriaceae, Pseudomonas spp. and certain Lactobacilli, Enterococci and Staphylococci are particularly involved in BAs formation. These amine-positive microorganisms can be naturally present in meat products or can be introduced by contamination before, during or after processing (Rokka et al. 2004). We found that the BAs concentrations in all analyzed RCMP were far below those levels associated with a health risk. According to Bentling (1996), slight responds are possible at concentrations in food of 10–40 mg histamine, 5–10 mg tyramine, 25 mg tryptamine, or 5 mg phenylathylamine, while for a toxic response 80–100 mg histamine or 25–250 mg tyramine are Inavitable.
Results of microbiological analysis of ready-to-cook minced meat products (RCMP)
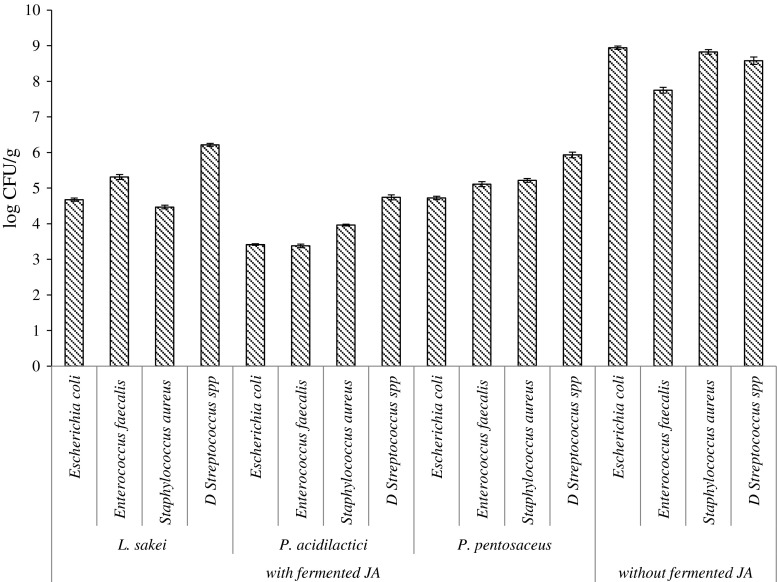
Different strains of used LAB showed different antimicrobial activity in RCMP with fermented JA (Fig. 3.). It was found that fermented JA additives inhibited the growth of Escherichia coli, Enterococcus faecalis, Staphylococcus aureus, D Streptococcus spp bacteria, in RCMP at the levels depending on the used LAB. The highest antimicrobial activity was demonstrated by P. acidilactici fermented JA additives. The numbers of E. coli in meat samples with P. acidilactici, L. sakei and P. pentosaceus fermented products were 3,41, 4.68 and 4.72 log CFU/g correspondingly, while the number of E. coli in RCMP without fermented JA was 8.94 log cfu/g. Moreover Staphylococcus aureus growth was reduced using BLIS producing LAB. Using P. acidilactici, L. sakei and P. pentosaceus fermented products values 3,96, 4.47 and 5.22 log CFU/g were determined, while the number of S. aureus in RCMP without fermented JA was 8.82 log cfu/g. The lowest LAB affect was observed against D Streptococcus spp. Using P. acidilactici, L. sakei and P. pentosaceus fermented products the values 4.79, 6.21 and 5.93 log CFU/g were determined, while the number of D Streptococcus spp in RCMP without fermented JA was 8.58 log cfu/g.
Fig. 3.
The influence of LAB-fermented JA additives on the microbiological safety of ready-to-cook minced meat products
LAB affect the growth of pathogenic microorganisms in 2 ways. They produce lactic acid and reduce pH (Daeschel 1989). In addition, they produce various antimicrobial compounds and bacteriocins (Ammor et al. 2008). Due to these properties, bacteriocins-producing strains or purified bacteriocins have a great potential of application in biologically based food preservation systems (De Martinis et al. 2002).
In the literature the LAB inhibition process on bacteria growth in meat is presented contradictory. According to Bredholt et al. (2001), all tested 5 LAB strains inhibited the growth of Listeria monocytogenes and E. coli O157:H7 in cooked sliced, vacuum or gas packed meat. According Tantillo et al. (2002) and Ammor et al. (2008) LAB inhibit Listeria inocua while strains of E. coli, Pseudomonas aeroginosa, Serratia marcescens are resistant.
Other research studies confirm, that LAB strains can inhibit the growth of Staphylococcus spp. (carnosus and aureus) (Amor and Mayo 2007). Among 141 of LAB isolates, 27 showed antimicrobial activity against Listeria innocua, while strains of E. coli, Pseudomonas aeroginosa, Serratia marcescens were resistant.
RCMP shelf life evaluation
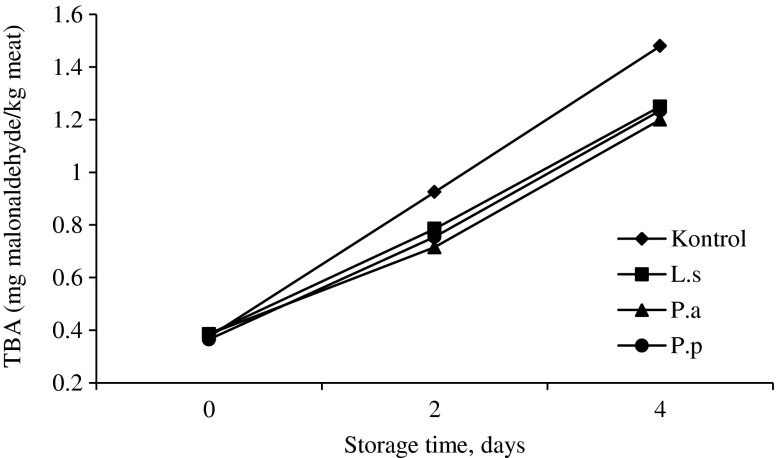
Presence of TBA is considered as lipid oxidation marker in meat and meat products (Shahidi et al. 1987). It was found, that the LAB-fermented JA additives in RCMP significantly increase the shelf life – TBA content after 2 and 4 days storage at 4 °C was 15.5–18.9 and 15.1–22.7 % lower, respectively, in comparison with RCMP without LAB-fermented JA (Fig. 4). Additionally, fermented products have influence on the color coordinates of the RCMP during the storage (Table 2). All samples with fermented JA additives show lower values of brightness (L*), yellowness (b*) and redness (a*).
Fig. 4.
Content of TBA (expressed as malonaldehyde) in RCMP stored at 4 °C temperatures (used LAB: L.s. – L. sakei; P.a. – P. acidilactici; P.p. – P. pentosaceus)
Table 2.
RCMP colour coordinates
| Storage time (days) | RCMP | Colour coordinates | ||
|---|---|---|---|---|
| L* | a* | b* | ||
| 0 | Control | 65.74 ± 1.78b | 3.93 ± 0.21a | 15.19 ± 0.71d |
| L.s. | 61.01 ± 1.02a | 3.25 ± 0.54b | 13.62 ± 0.54b | |
| P.a. | 59.51 ± 1.89c | 3.41 ± 0.38c | 13.59 ± 0.27b | |
| P.p. | 59.36 ± 0.97c | 3.21 ± 0.42b | 14.57 ± 0.16c | |
| 2 | Control | 63.14 ± 1.24b | 3.05 ± 0.31a | 14.06 ± 0.63d |
| L.s. | 59.62 ± 1.14a | 2.94 ± 0.51a | 12.52 ± 0.44b | |
| P.a. | 59.51 ± 1.89a | 2.81 ± 0.48b | 12.59 ± 0.37b | |
| P.p. | 57.16 ± 1.37c | 2.82 ± 0.32b | 13.53 ± 0.26c | |
| 4 | Control | 60.05 ± 1.12b | 2.73 ± 0.21a | 12.29 ± 0.51d |
| L.s. | 54.32 ± 1.14a | 2.65 ± 0.53b | 11.02 ± 0.64b | |
| P.a. | 57.62 ± 1.74c | 2.61 ± 0.64b | 11.09 ± 0.27b | |
| P.p. | 56.02 ± 1.33c | 2.53 ± 0.42b | 12.17 ± 0.36d | |
Control – RCMP without fermented JA additives. LAB used for JA fermentation: L.s. – L. sakei; P.a. – P. acidilactici; P.p. – P. pentosaceus; Data are the mean ± SD of three analyses. a–d Means within a column with different superscript letters are significantly different (p < 0.05)
Conversion of volatile compounds in ready-to-cook meat products (RCMP)
Flavor is a very important component of the acceptability of meat products. The desirable characteristics of flavor have also been sought in the production if simulated meat flavorings, which are of considerable importance in convenience of processed savory foods (Mottram 2012). LAB affect a flavor of fermented foods (Feeteers 2004) through the release and degradation of free amino acids and the oxidation of unsaturated free fatty acids isprevented (Fadda et al. 2010). The higher product acceptability relates with aroma compounds changes during the fermentation.
Addition of fermented JA to RCMP increased concentrations of toluene, undecane and 2-methyl undecane by 59.8, 44.6 and 44.1 %, respectively depending on LAB used for JA fermentation (Table 3). The concentrations of these compounds did not change in the RCMP samples with L. sakei and P. acidilactici fermented JA additives. Hexanal and 2,4-dimethyl-pentane were found in small quantities in all tested RCMP samples. Additionally the contents of pentylbenzene, p-xylene and o-xylene higher by 45.5, 51 and 62 %, respectively and significantly lower sulfide (isopropyl-morpholinomethyl sulfide) and ammines (6-undekilamine, N, N-dimethyl-1-pentadekanamine) contents were determined compared with the control sample (Table 3). These changes can be associated with volatile compounds formation in a minced pork meats (Martin et al. 2009). The results of sensory evaluation of meat products supplemented with fermented JA showed, that fermented JA provides the specific odor, which is pleasing and acceptable for consumers. This can be explained that LAB-fermented JA additives, which considerable differences in flavor mainly due to the accumulation of volatiles such as toluene, ethylbenzene, decane, undecane, ethylbenzene, 2 methyl undecane and suppression of N-morpholinomethyl-isopropyl-sulfide, 6-undecilamine and N,N-dimethyl-1-pentadecanamine formation in the RCMP with LAB-fermented JA additives.
Table 3.
The composition of volatiles formed in ready-to-cook meat products
| Compounds | Time, min. | w(volatile)/% | |||
|---|---|---|---|---|---|
| M P. a. | M P. p. | M L. s. | M control | ||
| toluene | 3.27 | 3.53 | 3.62 | 4.36 | 1.57 |
| hexanal | 3.80 | 0.00 | 4.85 | 3.40 | 0.00 |
| ethylbenzene | 5.04 | 6.43 | 5.85 | 6.58 | 2.43 |
| o-xylene | 5.22 | 37.70 | 36.30 | 45.99 | 15.44 |
| p-xylene | 5.78 | 11.18 | 9.68 | 10.04 | 5.08 |
| 2,4-dimethyl-pentane | 5.90 | 0.00 | 1.03 | 1.07 | 0.71 |
| 2-methyl-2-propenoic acid, butyl ester | 8.13 | 2.47 | 1.86 | 2.37 | 2.30 |
| 2,2-dimethyl-heptane | 8.35 | 0.00 | 1.08 | 1.48 | 1.21 |
| decane | 8.63 | 3.98 | 3.70 | 2.83 | 2.57 |
| undecane | 11.63 | 6.55 | 9.34 | 5.06 | 5.17 |
| pentyl benzene | 13.41 | 0.00 | 2.02 | 0.00 | 1.10 |
| 2-methyl-undecane | 14.63 | 7.82 | 11.56 | 6.88 | 6.46 |
| 2,2-dimethyl-butane | 17.53 | 4.30 | 6.09 | 3.09 | 3.39 |
| N-morpholinomethyl-isopropyl-sulfide | 22.13 | 0.00 | 0.00 | 0.00 | 3.77 |
| 6-undecilamine | 31.80 | 0.00 | 0.00 | 0.00 | 4.41 |
| N,N-dimethyl-1-pentadecanamine | 32.20 | 2.01 | 0.00 | 0.00 | 31.00 |
Ready-to-cook minced meat products: M control – without fermented JA; M P.a. – with fermented with P. acidilactici JA; M P.p. – with fermented with P. pentosaceus JA; M L.s. – with fermented with L. sakei JA
Sensory attributes and acceptability of meat products supplemented with fermented JA
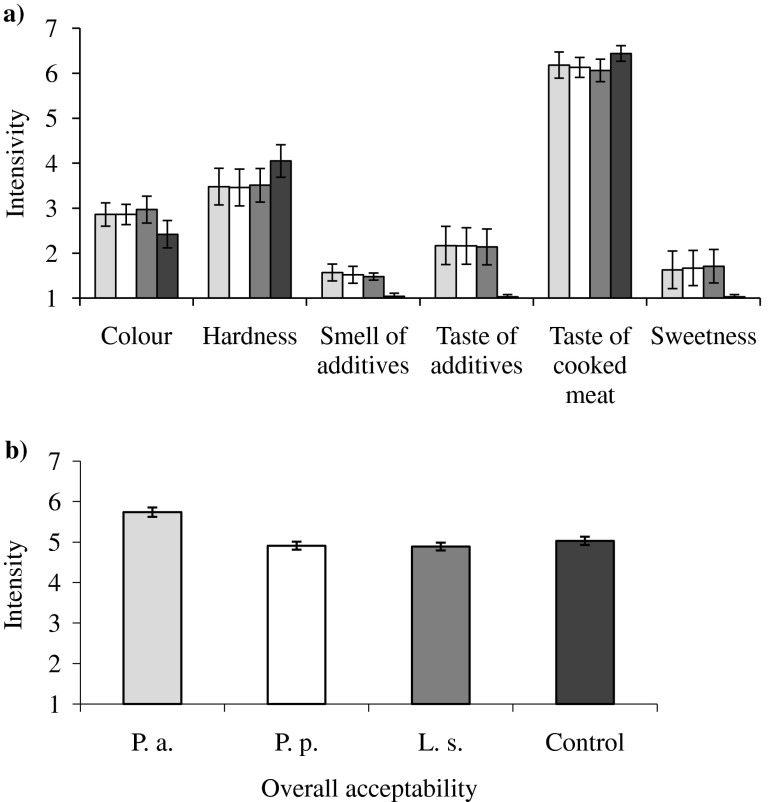
In this study the sensory properties (flavor) of meat products supplemented with fermented JA using BLIS producing LAB have been studied (Fig. 5a). The results of sensory evaluation of fermented products showed that fermented JA provides the specific odor, which is pleasing and acceptable for consumers. The sweet and delicate odour has been achieved using fermented JA, however the intensity of the odor was low. This study revealed that JA fermented with LAB could be a suitable additive for RCMP.
Fig. 5.
The influence of JA on cooked meat sensory attributes (a) and overall acceptability (b) of JA fermented with P.a – P. acidilactici ( ), P.p – P. pentosaceus (
), P.p – P. pentosaceus ( ) and L.s – L. sakei (
) and L.s – L. sakei ( ) and control meat products without JA additives (
) and control meat products without JA additives ( )
)
The significant differences in the acceptability of the RCMP with different LAB fermented JA and control samples were found (Fig. 5b). The highest acceptability showed the RCMP with P. acidilactici fermented JA (an average 5.74 scores). Control samples were found to have lower acceptability (on average 5.03 scores). RCMP with L. sakei and P. pentosaceus fermented JA were found less acceptable than control sample and sampleswith P. acidilactici treated JA (4.89 and 4.91 scores, respectively).
Conclusions
LAB used for fermentation of JA reduce the pH of the substrate from 3.97 to 4.05, and decrease WHC ca. by 2.5 %. Results showed strong relation (r = 0.72; p = 0.044) between WHC and pH values. The BAs concentrations in RCMP were measured far below those levels associated with a health risk (1.76–6.25 mg/kg). The use of LAB fermented JA additives allowed to inhibit the growth of Ent. faecalis, S. aureus, E. coli and Staphylococcus spp. family bacteria thus ensuring the microbiological safety of processed meat products. The addition of fermented JA to RCMP increased the formation of volatile compounds and somewhat improved the acceptability of RCMP.
P. acidilactici fermented JA tuber additives at a level of 5 % are recommended for meat processing in order to prevent microbiological spoilage, increase the acceptable odor (volatile compounds concentration), acceptability and shelf-life of meat products.
Acknowledgments
The authors gratefully acknowledge the Research Council of Lithuania for the financial support (Project BIOFITAS No. SVE-09/2011).
References
- Aaslyng MD, Bejerholm C, Ertbjerg P, Bertram HC. Cooking loss and juiciness of pork in relation to raw meat quality and cooking procedure. Food Qual Prefer. 2003;14(4):277–288. doi: 10.1016/S0950-3293(02)00086-1. [DOI] [Google Scholar]
- Ammor MS, Michaelidis C, Nychas GJE. Insights into the role of quorum sensing in food spoilage. J Food Prot. 2008;71(7):1510–1525. doi: 10.4315/0362-028x-71.7.1510. [DOI] [PubMed] [Google Scholar]
- Amor MS, Mayo B. Selection criteria for lactic acid bacteria to be used as functional starter cultures in dry sausage production. Meat Sci. 2007;76(1):138–146. doi: 10.1016/j.meatsci.2006.10.022. [DOI] [PubMed] [Google Scholar]
- AOAC. Official method 950.46 (Ch. 39.1.02), moisture in meat, B. Air drying (2006). In: Horwitz W (ed) Official methods of analysis of AOAC International, 18th edn., AOAC International, Maryland
- Aro Aro JM, Nyam OP, Tsuji K, Shimada K, Fukushima M, Sekihawa M. The effect of starter cultures on proteolytic chantes and amino acid content in fermented sausages. Food Chem. 2010;119:279–285. doi: 10.1016/j.foodchem.2009.06.025. [DOI] [Google Scholar]
- Bekers M, Grube M, Upite D, Kaminska E, Linde R, Danilevich A (2005). The effect of exo-inulinase and dehydration temperature on carbohydrates of Jerusalem artichoke. Intrafood 876–870
- Ben-Gigirey B, De Sousa JMVB, Villa TG, Barros-Velazquez J. Histamine and cadaverine production by bacteria isolated from fresh and frozen albacore (Thunnus alalunga) J Food Prot. 1999;68(8):933–939. doi: 10.4315/0362-028x-62.8.933. [DOI] [PubMed] [Google Scholar]
- Bidner BS, Ellis M, Brewer MS, Campion D, Wilson ER, McKeith FK. Effect of ultimate pH on the quality characteristics of pork. J Muscle Foods. 2004;15(2):139–154. doi: 10.1111/j.1745-4573.2004.tb00717.x. [DOI] [Google Scholar]
- Biesalski HK. Meat as a component of a healthy diet - Are there any risks or benefits if meat is avoided in the diet? Meat Sci. 2005;70(3):509–524. doi: 10.1016/j.meatsci.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Bredholt S, Nesbakken T, Holch A. Protective cultures inhibit growth of Listeria monocytogenes and Escherichia coli O157:H7 in cooked, sliced, vacuum- and gas-packaged meat. Int J Food Microbiol. 2001;53(1):43–52. doi: 10.1016/S0168-1605(99)00147-6. [DOI] [PubMed] [Google Scholar]
- Bryhni EA, Byrne DV, Rødbotten M, Møller S, Claudi-Magnussen C, Karlsson A, Agerhem H, Johansson M, Martens M. Consumer and sensory investigations in relation to physical/chemical aspects of cooked pork in Scandinavia. Meat Sci. 2003;65(2):737–748. doi: 10.1016/S0309-1740(02)00276-0. [DOI] [PubMed] [Google Scholar]
- Cizeikiene D, Juodeikiene G, Paskevicius A, Bartkiene E. Antimicrobial activity of lactic acid bacteria against pathogenic and spoilage microorganism isolated from food and their control in wheat bread. Food Control. 2013;31(2):539–545. doi: 10.1016/j.foodcont.2012.12.004. [DOI] [Google Scholar]
- Daeschel MA. Antimicrobial substances from lactic acid bacteria for use as food preservatives. Food Technol. 1989;38:426–430. [Google Scholar]
- Dalié DKD, Deschamps AM, Richard-Forget F. Lactic acid bacteria – potential for control of mould growth and mycotoxins: a review. Food Control. 2010;21(4):370–380. doi: 10.1016/j.foodcont.2009.07.011. [DOI] [Google Scholar]
- De Martinis ECP, Alves VF, Franco BDGM. Fundamentals and perspectives for the use of bacteriocins produced by lactic acid bacteria in meat products. Food Rev Int. 2002;18(2):191–208. doi: 10.1081/FRI-120014688. [DOI] [Google Scholar]
- Digaitiene A, Hansen A, Juodeikiene G, Josepsen J. Microbial population in Lithuanian spontaneus rye sourdougs. Ekologia i Technika. 2005;13(5):193–198. [Google Scholar]
- Digaitiene A, Hansen A, Juodeikiene G, Eidukonyte D, Josephsen J. Lactic acid bacteria isolated from rye sourdoughs produce bacteriocin-like inhibitory substances active against Bacillus subtilis and fungi. J Appl Microbiol. 2012;112(4):732–742. doi: 10.1111/j.1365-2672.2012.05249.x. [DOI] [PubMed] [Google Scholar]
- Fadda S, Anglade P, Baraige F, Zagorec M, Talonc R, Vignolo G, Champomier-Verges MC. Adaptive response of Lactobacillus sakei 23K during growth in the presence of meat extracts: A proteomic approach. Int J Food Microbiol. 2010;142(1–2):36–43. doi: 10.1016/j.ijfoodmicro.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Favaro G, Pastore P, Saccani G, Cavalli S. Determination of biogenic amines in fresh and processed meat by ion chromatography and integrated pulsed amperometric detection on Au electrode. Food Chem. 2007;105(4):1652–1658. doi: 10.1016/j.foodchem.2007.04.071. [DOI] [Google Scholar]
- Feeteers MC. Fermentation Microorganisms and Flavor changes in fermented foods. J Food Sci. 2004;69(1):35–38. [Google Scholar]
- Gibson GR, Wang X. Bifidogenic properties of different types of fructo-olygosacharides. Food Microbiol. 1994;11(6):491–492. doi: 10.1006/fmic.1994.1055. [DOI] [Google Scholar]
- Grau R, Hamm R. Die Bestimmung des Wasserbindung des Fleisches mittels der Pressmethode. Fleischwirtschaft. 1956;36:733–736. [Google Scholar]
- Huff-Lonergan E, Lonergan SM. Mechanisms of water holding capacity of meat: the role of postmortem biochemicaland structural changes. Meat Sci. 2005;71(1):194–204. doi: 10.1016/j.meatsci.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Huff-Lonergan E, Baas TJ, Malek M, Dekkers JCM, Prusa K, Rothschild MF. Correlations among selected pork quality traits. J Anim Sci. 2002;80(3):617–627. doi: 10.2527/2002.803617x. [DOI] [PubMed] [Google Scholar]
- ISO (1993) Sensory analysis; general guidance for selection, training and monitoring of assessors. Part I: Selected assessors. ISO 8586-1:1993, ISO, Geneva, Switzerland, p. 26
- Kim D, Fan JP, Chung HC, Han GD. Changes in extractability and antioxidant activity of Jerusalem artichoke (Helianthus tuberosus L.) tubers by various high hydrostatic pressure treatments. Food Sci Biotechnol. 2010;19(5):1365–1371. doi: 10.1007/s10068-010-0194-8. [DOI] [Google Scholar]
- Latorre-Moratalla ML, Bover-Cid S, Veciana-Nogués MT, Vidal-Carou MC. Control of biogenic amines in fermented sausages: role of starter cultures. Front Microbiol. 2012;7(3):169. doi: 10.3389/fmicb.2012.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy F, De Vuyst L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci Tech. 2004;12(2):67–78. doi: 10.1016/j.tifs.2003.09.004. [DOI] [Google Scholar]
- Martin D, Antequera T, Muriel E. Cured loin as affected by dietary conjugate linoleic acid and monosaturated fatty acids. Meat Sci. 2009;81:549–556. doi: 10.1016/j.meatsci.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Mottram D. Flavour formation in meat and meat products. Food Chem. 2012;62(4):415–424. doi: 10.1016/S0308-8146(98)00076-4. [DOI] [Google Scholar]
- Narbutaite V, Fernandez A, Horn N, Juodeikiene G, Narbad A. Influence of baking enzymes on antimicrobial activity of five bacteriocin-like inhibitory substances produced by lactic acid bacteria isolated from Lithuanian sourdoughs. Lett Appl Microbiol. 2008;47(6):555–60. doi: 10.1111/j.1472-765X.2008.02466.x. [DOI] [PubMed] [Google Scholar]
- Nemeth G, Izsaki Z. Macro- and micro-element content and uptake of Jerusalem artichoke (Helianthus tuberosus L.) Cereal Res Commun. 2006;34(1):597–600. doi: 10.1556/CRC.34.2006.1.149. [DOI] [Google Scholar]
- Park BS. Effect of oral administration of Jerusalem artichoke inulinon reducing blood lipid and glucose in STZ-induced diabetic rats. J Anim Vet Adv. 2011;10(19):2501–2507. doi: 10.3923/javaa.2011.2501.2507. [DOI] [Google Scholar]
- Paseephol T, Small D, Sherkat F. Process optimization for fractionating Jerusalem artichoke fructans with ethanol using response surface methodology. Food Chem. 2007;104(1):73–80. doi: 10.1016/j.foodchem.2006.10.078. [DOI] [Google Scholar]
- Puolanne E, Peltonen J. The effects of high salt and low pH on the water-holding of meat. Meat Sci. 2013;93(2):67–70. doi: 10.1016/j.meatsci.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Raharjo S, Sofos JN, Schmidt GR. Solid-phase acid extraction improves thiobarbituric acid method to determine lipid oxidation. J Food Sci. 1993;4:921–924. doi: 10.1111/j.1365-2621.1993.tb09391.x. [DOI] [Google Scholar]
- Ravyts F, de Vuyst L, Leroy F. Bacterial diversity and functionalities in food fermentation. Eng Life Sci. 2012;12(4):356–367. doi: 10.1002/elsc.201100119. [DOI] [Google Scholar]
- Roberfroid MB, Van Loo JAE, Gibson GR. The bifidogenic nature of chicory inulin and its hydrolysis products. J Nutr. 1998;128(1):11–19. doi: 10.1093/jn/128.1.11. [DOI] [PubMed] [Google Scholar]
- Rokka M, Eerola S, Smolander M, Alakomi HL, Ahvenainen R. Monitoring of the quality of modified atmosphere packaged broiler chicken cuts stored in different temperature conditions B. Biogenic amines as quality-indicating metabolites. Food Control. 2004;15(8):601–607. doi: 10.1016/j.foodcont.2003.10.002. [DOI] [Google Scholar]
- Saengthongpinit W, Sajjaanantakul T. Influence of harvest time and storage temperature on characteristics of inulin from Jerusalem artichoke (Helianthus tuberosus L.) tubers. Postharvest Biol Technol. 2005;37(1):93–100. doi: 10.1016/j.postharvbio.2005.03.004. [DOI] [Google Scholar]
- Shahidi F, Yun J, Rubin LJ, Wood DF. The hexanal content as an indicator of oxidative stability and flavor acceptability in cooked ground pork. CIFST. 1987;20:104–106. [Google Scholar]
- Smaoui S, Elleuch L, Bejar W, Karray-Rebai I, Ayadi I, Jaouadi B, et al. Inhibition of fungi and gram-negative bacteria by bacteriocin BacTN635 produced by Lactobacillus plantarum sp. TN635. Appl Biochem Biotechnol. 2010;162(4):1132–1146. doi: 10.1007/s12010-009-8821-7. [DOI] [PubMed] [Google Scholar]
- Tantillo M, Di GPA, Novello L. Bacteriocin – producing Lactobacillus sakei as starter culture in dray sausages. New Microbiol. 2002;25(1):45–49. [PubMed] [Google Scholar]
- Vidal-Carou MC, Latorre-Moratalla ML, Veciana-Nogués MT, Bover-Cid S. Biogenic amines: risks and control. In: Todrà F, Hui YH, Astiasarán I, Nip Wai-Kit, Sebranek JG, Silveira ETF, Stahnke LH, Talon R, editors. Handbook of fermented meat and poultry. Oxford: Blackwell; 2007. pp. 455–468. [Google Scholar]
- Wangang Z, Shan X, Himali S, Eun JL, Dong UA. Improving functional value of meat products. Meat Sci. 2010;86(1):15–31. doi: 10.1016/j.meatsci.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Yang HJ, Kwon DY, Kim MJ, Kang S, Kim DS, Park S. Jerusalem artichoke and cheonggukjang additively improve insulin secretion and sensitivity in diabetic rats. Nutr Metab. 2012;9:112–124. doi: 10.1186/1743-7075-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]