Abstract
Noodles are staple cereal food in many countries; however addition of encapsulated probiotics into noodle formulation, its effect on noodle quality and cell viability has not yet been reported. The aim of this study was to prepare microencapsulated Lactobacillus plantarum (MTCC 5422) by freeze drying with wall material combinations such as fructooligosaccharide (FOS), FOS + whey protein isolate (WPI), and FOS + denatured whey protein isolate (DWPI) to evaluate best wall system. Results showed that FOS + DWPI wall system provided better protection to cells after drying, during storage (60 days, 4 °C) and in simulated acidic and bile conditions. Further, FOS + DWPI encapsulates were incorporated into noodle formulation and evaluated the noodle quality and probiotic cell viability of cooked noodle obtained from two different production methods: (i) fresh and (ii) dried (room temperature dried - RTD, 28 °C and high temperature dried - HTD, 55 °C). The quality characteristics (cooking time, solid loss, texture, colour and sensory profiles) of FOS + DWPI encapsulates incorporated cooked noodles (both fresh and dried) were found to be acceptable. On evaluation of encapsulated probiotic bacteriaL. plantarum cell viability, 93.63 % and 62.42 % cell survival was obtained in fresh noodles before and after cooking respectively. However, 80.29 % (RTD) and 64.74 % (HTD) of encapsulated cells were viable in dried noodles, after cooking there was complete survival loss. This study suggested that fresh noodle was found to be a suitable carrier system to deliver viable cells. This is first report on influence of probiotic microcapsules in noodle processing.
Keywords: Probiotic, Prebiotic, Synbiotic, Whey protein, Freeze-drying, Noodle
Introduction
Worldwide market of functional food is dominated by gut health products particularly foods incorporated with probiotics (Cruz et al. 2010). Probiotics are live microbial food supplements, several studies have shown that certain strains of bacteria belongs to genera Lactobacillus and Bifidobacterium confer health benefits such as control of diarrhoea, lactose intolerance, intestinal infections, immune system modulation and risk associated with carcinogenicity (Shah 2007). In addition, the probiotic approach of directly introducing live cells as dietary supplements another possible way to promote the growth of probiotic bacteria is through supplementation with prebiotics (Cruz et al. 2010). Prebiotics are non-digestible short chain carbohydrates (e.g. fructooligosaccharide) that support the selective growth and/or activity of one or limited number of probiotics in the colon thus improving the intestinal health. Due to the potential synergy between probiotics and prebiotics, a combination of these two ingredients is stated as synbiotics (Gibson and Roberfroid 1995).
Freeze-drying is a commonly used method for preservation of probiotics into dried powder on a large-scale, which are easy to handle, storage and transportation. Sugars are usually incorporated as cryoprotectants to minimise the damage to the cell wall and cell membrane during freeze drying. Ability of sugars to form glassy matrices enhances the cell survival during dehydration (Santivarangkna et al. 2011). However these matrices permeate water vapour, gases and small organic molecules during storage that affects the cell viability. The probiotic bacteria in the food product should remain metabolically stable, active and the viability should be minimum of 106 cfu/g of a product for its optimum functionality (Shah 2007). To improve the stability and functionality of the probiotics in the food products as well as targeted release of cells in specific parts of the gut, microencapsulation has been extensively studied for the past two decades (Champagne et al. 2005; Chávez and Ledeboer 2007).
Microencapsulation is a technology that applies physical barrier to protect the microbial cells against adverse environmental conditions such as temperature and gastric solutions (Champagne et al. 2005; Bajaj et al. 2010). Wall materials like maltodextrin, starch, carbohydrates, and proteins were found to be suitable for microencapsulation by freeze drying (Ezhilarasi et al. 2013). The prebiotic, fructooligosaccharide (FOS) is the most studied low molecular weight short-chain carbohydrate. Only few researchers attempted the production of synbiotic microcapsules using microencapsulation technique. Stickiness behaviour limits the application of FOS for microencapsulation using drying methods, mainly due to its low glass transition temperature. To reduce the stickiness and caking of particles during drying, appropriate blends of protein and carbohydrate based wall system has been used for microencapsulation (Chávez and Ledeboer 2007). Whey proteins are globular proteins having excellent functional properties such as water binding, foaming, emulsification, gelation and film forming has found wide applications in the food industry (Perez‐gago and Krochta 2001; Dissanayake and Vasiljevic 2009). The major component of whey protein includes β-lactoglobulin, α-lactalbumin, bovine serum albumin and immunoglobulin. The tertiary structure of whey protein is stabilised by non-covalent interaction forces (hydrogen, ionic and hydrophobic bonding) and covalent cross-linking. Heat denaturation is a significant change that occurs in the structure of compactly folded protein molecules into an unfolded state that allows the hydrophobic interactions, disulphide cross-linking and hydrogen-bonding thus resulting in aggregation, coagulation and precipitation (Pérez‐Gago et al. 1999; Anandharamakrishnan et al. 2007). These structural changes results in superior film strength, elasticity and less degree of solubility offers options for controlled release applications (Nicolai et al. 2011; Parthasarathi et al. 2013).
Recently, increasing consumer demand for new food products has resulted incorporation of microencapsulated probiotics into various food formulations including baby foods, cereal products, chocolates, meat products, soy milk, fruit drinks, and vegetable juices such as tomato, cabbage, beet and carrot juices (Champagne et al. 2005; Rivera-Espinoza and Gallardo-Navarro 2010). Noodles are staple cereal food that contributes the 50 % of wheat flour consumption in many countries. Noodle processing includes various stages such as mixing, sheeting, and cutting into thin strands. Processed noodles are marketed by fresh or dried forms. Fresh noodles are of raw wet noodles; to increase the shelf life, fresh noodles are dried by either natural or controlled temperature drying (Fu 2008). Various ingredients such as oat flour, banana flour, soy flour, fish protein concentrates, legumes, Amaranthus betacyanin pigments, broccoli has been incorporated in the noodle formulations in order to provide different flavours, colours, additional nutrients and health benefits; however, the addition of microencapsulated probiotic bacteria in noodle formulation and its effect on noodle quality characteristics and probiotic cell viability has not yet been reported. The application of temperature can influence the viability of encapsulated probiotic cells in the fresh or dried noodles during cooking. Factors such as simultaneous starch gelatinization and protein coagulation during cooking, causes major structural changes that affect the cooking quality of fresh or dried noodles (Torres et al. 2007; Cubadda et al. 2007; Choy et al. 2013; Silva et al. 2013). Hence, this work is focused on the effect of noodle processing conditions on noodle quality and encapsulated cell viability.
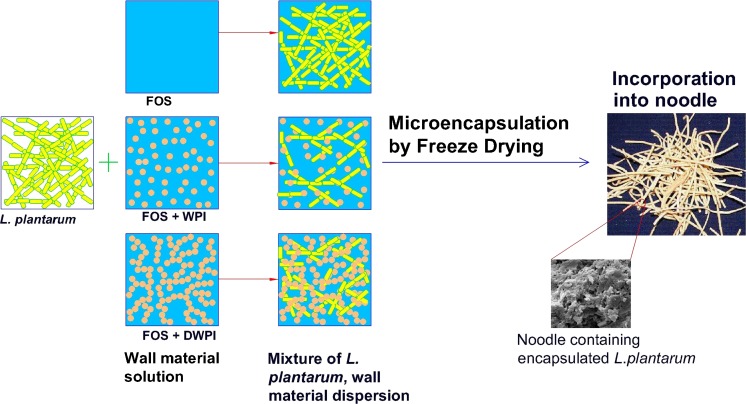
The objective of the study was to investigate the effectiveness of encapsulating materials such as fructooligosaccharide (FOS), partial replacement (50 %) of FOS with whey protein isolate or denatured whey protein isolate on survival of Lactobacillus plantarum (MTCC 5422), after freeze drying, storage and in simulated acidic and bile conditions. This has been graphically explained in Fig. 1. Further the best wall system for microencapsulation of probiotic has been identified and incorporated in noodle formulation to examine the impact of noodle processing conditions on qualitative characteristics, and encapsulated probiotic cell viability.
Fig. 1.
Schematic illustration of microencapsulation of L.plantarum and incorporation into noodles
Materials and methods
Materials
The probiotic bacterial strain Lactobacillus plantarum (MTCC 5422), isolated from fermented batter of cereal/legume (Roopashri and Varadaraj 2009) was used in this study. MRS broth, MRS agar, extra pure sodium chloride, methanol and bile salt were procured from HiMedia Laboratories, Mumbai, India. Whey protein isolate was obtained from Ultimate Nutrition (Farmington, CT, USA). Fructooligosaccharide (purity of FOS 96.2 %, Glucose + Fructose + Sucrose 3.8 %) was obtained from Meiji Food Materia Co., Ltd, (Japan). Pepsin (1:3000 μ/g, extra pure) was procured from SD fine chemicals, Boisar, India. Refined wheat flour (Triticum durum) was procured from the local market. All the chemicals used in this study were of analytical grade.
Microencapsulation of L. plantarum by freeze drying
All the glass wares used in this experiment were sterilized at 121 °C for 15 min. For the preparation of microcapsules, probiotic bacteria Lactobacillus plantarum (MTCC 5422) encapsulated by three different combinations of wall materials such as (i) Fructooligosaccharide (FOS), (ii) FOS + whey protein isolate (WPI) and (iii) FOS + denatured whey protein isolate (DWPI). The probiotic cell concentrate was collected according to the procedure described in our previous study (Rajam et al. 2012) and the cell pellets were used on the same day for microencapsulation. FOS powder (25 g) was dissolved in 200 g sterile milli-Q water using a magnetic stirrer and the freshly harvested cell concentrate of L. Plantarum (25 g) was mixed thoroughly with FOS solution to obtain 1:1 core - to - wall ratio (20 % w/w). WPI powder (12.5 g) was mixed with sterile milli-Q water (112.5 g), and the solution (10 % w/w) was stirred gently using a magnetic stirrer to dissolve the WPI. To denature the whey protein, the whey protein isolate solution (10 % w/w) was kept at 90 °C for 30 min in a water bath (Dissanayake and Vasiljevic 2009) then cooled to room temperature. Denatured whey protein isolate (DWPI) solution was homogenised using high speed homogenizer (IKA-Ultra-Turrax T18 basic, Germany) for 5 min at 7,000 rpm and water loss during denaturation was taken into account. FOS powder (12.5 g) dissolved in 87.5 g sterile milli-Q water was blended with WPI or DWPI solution and homogenized at the same rpm for further 5 min. The freshly harvested cell concentrate of L. Plantarum (25 g) was mixed with WPI + FOS or DWPI + FOS solution in order to obtain a desired core - to - wall ratio of 1:1 (20 % w/w). All the sample solution were homogenised for 60 s at 7,000 rpm before freeze drying. The samples were freeze dried in a pilot scale freeze drier (Lyophilization Systems, Inc, USA) at - 40 to 30 °C and the entire freeze drying process was carried out in 20 h. The freeze dried microcapsules were collected, packed in polythene bags, sealed in aluminium foil and stored at 4 °C.
Moisture content analysis
The moisture content (% wet basis) of the encapsulated powder was measured gravimetrically according to International Dairy Federation. A known mass of sample (0.5 g) was placed in aluminium pan and dried in a hot air oven (Industrial and laboratory tools corporation, Chennai, India) at 102 ± 2 °C until it reaches constant weight. The initial and final weights were used to calculate the wet basis moisture contents. The experiments were carried out in triplicates and average values were taken.
Particle size distribution and volume-average particle size (D4,3)
Particle analyser (S3500, Microtrac Inc., USA) was used to determine the particle size and size distribution of the freeze dried microcapsules by laser diffraction method using methanol as dispersing medium. Particle size distributions based on volume fraction were calculated with a refractive index of 1.33 for methanol and reported by listing D10, D50, D90, which correspond to the diameters at which the cumulative sample volume was under 10 %, 50 % and 90 % respectively. Particle size measurement tests were replicated three times.
Morphology of microcapsules
Scanning electron microscope (SEM) (Leo 435 VP, Leo Electronic Systems, Cambridge, UK) was used to study the morphology of microcapsules. The encapsulated samples were mounted on the specimen holder by using double-sided adhesive tape. The mounted samples were then coated with gold in a sputter-coater under high vacuum (2 min, 2 mbar). The coated samples were transferred to the SEM where its images were observed at 15 kV and a vacuum of 9.75 × 10−5 Torr.
Enumeration of encapsulated cells and determination of survival percentage
To determine the viable counts of encapsulated bacteria, 0.5 g of microcapsules were dispersed in 2 g of 0.85 % (w/v) saline (NaCl, pH 7.0) in order to make the initial solid content of 20 % w/w. The rehydrated samples were kept in a shaker (100 rpm) at 37 °C for 30 min to release the cells from the dried microcapsules. Cell viability was determined by using the standard plate count method. The rehydrated samples (1 mL) were pour plated on MRS agar plates after appropriate 10-fold serial dilutions in 0.85 % sterile saline solution. Colony forming units (CFU) were enumerated manually after incubation at 37 °C for 24 h. Plates containing 20–350 colonies were counted and recorded as CFU per mL of solution.
The percentage of survival with respect to initial counts before drying was calculated as follows:
| 1 |
Where, N is log cell number of viable bacteria per mL after drying and N0 is log cell number of bacteria per mL prior to freeze drying.
The survival percentage of encapsulated probiotic cells at different storage times were calculated by Eq.2
| 2 |
Where Ni and Nt is the log cell number of viable bacteria per g at the time of storage and at various storage times (t).
Survival of encapsulated bacteria in simulated acidic and bile condition
Simulated gastric juice (SGJ) was prepared according to the method of Dolly et al. (2011) to determine the survival of encapsulated probiotics in simulated acidic condition. MRS broth was adjusted to pH 2.0 with 1 M HCl and sterilized by autoclaving at 121 °C for 15 min. Pepsin (3,000 μ/g) was filtered through a 0.22 μm sterile membrane filter then added to sterile MRS broth for a final concentration of 0.3 % (v/v). Simulated intestinal juice (SIJ) was prepared by dissolving bile salt in MRS broth to a final concentration of 2 % (w/w) and sterilized by autoclaving at 121 °C for 15 min. Microcapsules (0.5 g) were added to 9.5 g of sterile SGJ or SIJ and incubated at 37 °C for 4 h at constant agitation (100 rpm). The samples were taken at 0, 1, 2, 3 and 4 h, and the relative cell viability (a ratio of viable cells at time ‘t’ to that at time zero h) was evaluated in simulated acidic or bile conditions.
Incorporation of probiotic microcapsules in noodle
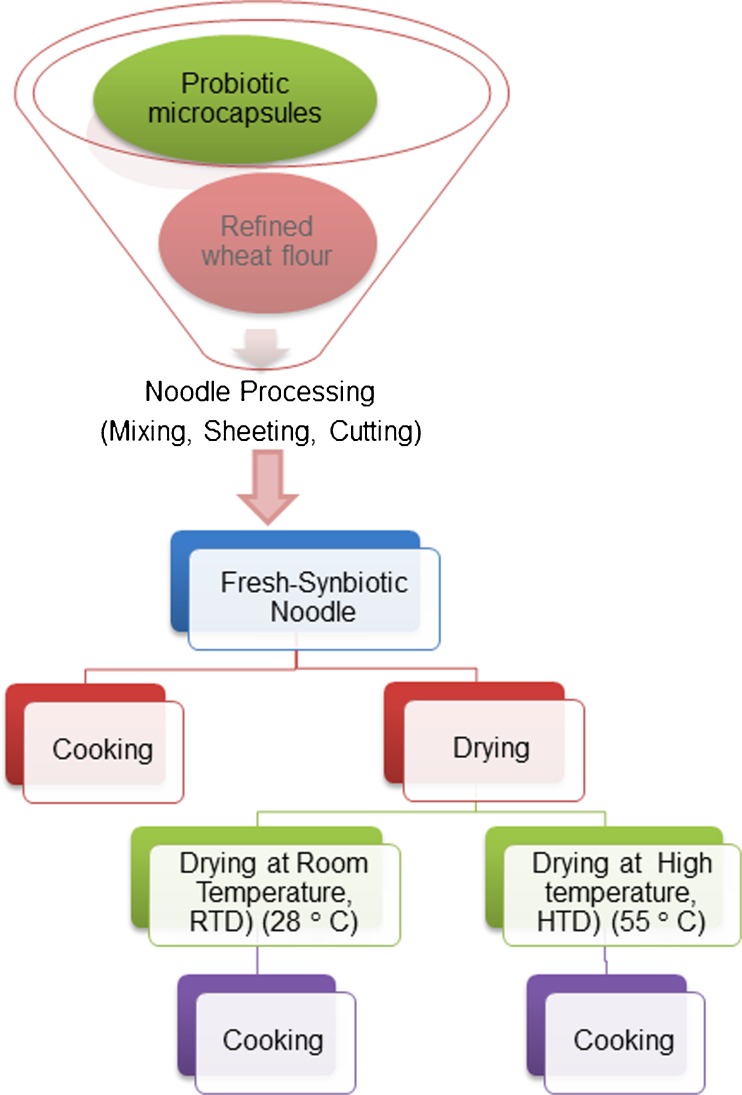
Probiotic microcapsule (50 g) was incorporated with refined wheat flour (450 g) for the preparation of synbiotic noodle (SN) and only 100 % refined wheat flour (500 g) was used for the preparation of control noodle (CN). Water at a temperature of 40 ° C was mixed with flour (350–400 gkg−1 of flour) to form firm dough in a noodle mixer. The dough was allowed to rest for 15–20 min then sheeted to 5 mm thickness in dough sheeter. Sheeting involved 5–6 steps to reduce the desired thickness. The sheeted dough was cut into appropriate length and then cut into noodles using noodle cutter, and it was referred as fresh noodles (FN) in this study. Fresh noodles are raw, wet noodles. Dried noodles are produced from drying of uncooked raw, wet noodle strands. Samples of wet noodle strands were dried by two methods: room temperature (RTD) drying (28 °C) and high temperature (HTD) drying (cabinet tray drier, 55 °C) for 4 h and 2 h respectively. Schematic diagram for the preparation of fresh and dried noodles is given in Fig. 2. Dried noodles were packed in polythene covers, sealed in aluminium foil, and stored at 4 °C for further analysis.
Fig. 2.
Schematic diagram of noodle process flow chart
Analysis of noodle quality characteristics
The cooking quality of SN (fresh and dried) was evaluated by optimal cooking time, solid loss, and texture of cooked noodles. Noodle samples (25 g) were cut into approximately 5 cm length, and immersed in 250 mL of boiling water. The optimal cooking time was determined by the time at which the white core in the central portion of noodle strand disappeared when squeezed between a pair of glass plates (AACC 2000). Weight of total solids leached into the cooking water is expressed as a percentage of solid loss. The TA-XDi texture analyser (Stable Micro Systems, Guildford, UK) equipped with Warner Bratzler Blade was used to evaluate the texture profile analysis of cooked noodles. Five noodle strands were arranged adjacent to each other on the base plate. Test was performed with 25 kg load cell and the crosshead speed of 10 mm/min (five replicates per sample). From the force time curves of texture profile, the shear strength (height of the peak) and adhesiveness (negative area between the first and second peak) were determined. The colour of noodles in terms of L*, a* and b* values were measured by Hunter lab colorimeter (colour-flex, USA) colour measuring system equipped with D65 illuminant. Three measurements were taken for each sample, and the average value was reported. Colour index of noodles was calculated using the equation adopted Cavazza et al. (2012) for pasta colour index determination. The moisture content of noodle samples (2–3 g) was determined according to AACC (2000) method and performed in triplicates. Quantitative Descriptive Analysis (QDA) method was used to evaluate the colour, distinct strands, firmness, chewiness, aroma, sweetness and overall quality of cooked noodles (Stone et al. 1974). The trained and semi-trained panellists (15–20) marked the perceived intensity (0 for very low intensity and 15 for very high intensity) of each attribute listed on the scorecard by drawing a vertical line on the line scale (15 cm). Finally, mean value was taken for each attribute of a sample graphically represented as ‘Sensory Profile’.
Survival percentage of encapsulated probiotic cells in noodle
To assess the viability of microencapsulated probiotic bacteria in noodles, 3 g of the sample was weighed and rehydrated in 97 g sterile saline (0.85 % w/v), then homogenized aseptically using high speed homogenizer for one minute. Further, samples were kept in a shaker (100 rpm) at 37 °C for 30 min to release the cells from the microcapsules and cell viability was determined by using the standard plate count method as described above.
The survival percentage of microencapsulated probiotic cells in noodles were obtained by Eq. 3
| 3 |
Where, Np is the log cell number of viable probiotic bacteria per g of microcapsule incorporated in the product (dough, fresh, cooked and dried noodles) and Ns is log cell number of viable bacteria per g of microcapsule.
Statistical analysis
Results were statistically analysed by t test (test of significance for the difference between two means) using Microsoft excel software and the differences at P < 0.05 were considered as significant.
Results and discussion
The effect of wall materials on cell viability, influence of probiotic microcapsules on noodle quality are discussed in the following sub-sections.
Moisture content
The residual moisture content highly determines the powder quality, cell survival rate and storage stability of encapsulated probiotics (Chávez and Ledeboer 2007; Fu and Chen 2011). The results of the moisture content values are given in Table 1. The residual moisture content of freeze dried microcapsules produced with three different wall materials varied from 4.34 to 6.67 %. Microcapsules produced with FOS alone as wall material resulted in higher (6.67 %) moisture content (P < 0.05) than the microcapsules produced with FOS + WPI (4.34 %) and FOS + DWPI (5.07 %). This can be due to structural collapse of sugars that leads to sealing of capillaries which in turn reduces the dehydration rate (Levi and Karel 1995) and results in higher residual moisture content during freeze drying. de Valdéz et al. (1983) stated that, residual moisture content varies with the composition of wall material in which the microorganisms were dried. Zayed and Roos (2004) reported that microcapsules with 5.6 % moisture content improved the survival rate for encapsulated L. salivarius than its lower residual moisture content. Ananta et al. (2005) also reported that final moisture content of less than 7 % after drying minimised the risk of storage related defects. Hence the residual moisture content of the freeze dried microcapsules (4.34 % to 6.67 %) obtained in our study is in agreement with the moisture content usually recommended for good storage stability of dried encapsulated powders.
Table 1.
Effect of encapsulation material on final moisture content, particle size distribution and cell viability
| Wall material | Final moisture contenta, % | Volume diameters, μm ± S.D. | Cell viabilitya (Log10 CFU/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| D[4,3] | D10 | D50 | D90 | Span | Before drying | After drying | ||
| FOS | 6.67 ± 0.71 | 63.72 | 10.58 | 38.07 | 154.3 | 3.77 | 10.68 ± 0.03 | 10.54 ± 0.03 |
| FOS + WPI | 4.34 ± 0.53 | 72.23 | 21.20 | 55.27 | 140.5 | 2.15 | 10.68 ± 0.02 | 10.57 ± 0.08 |
| FOS + DWPI | 5.07 ± 0.46 | 89.71 | 25.72 | 68.82 | 174.6 | 2.16 | 10.68 ± 0.04 | 10.52 ± 0.05 |
FOS, fructooligosaccharide; WPI, whey protein isolate; DWPI, denatured whey protein isolate; a Results are expressed as mean ± standard deviation (n = 3); D[4,3] equivalent mean volume diameter (De Brouckere Mean Diameter) n = 3
D10, D50, D90 = 10 %, 50 % and 90 % of particles have diameter less that stated respectively. Span = (D90 – D10)/D50
Particle size and morphology of microcapsules
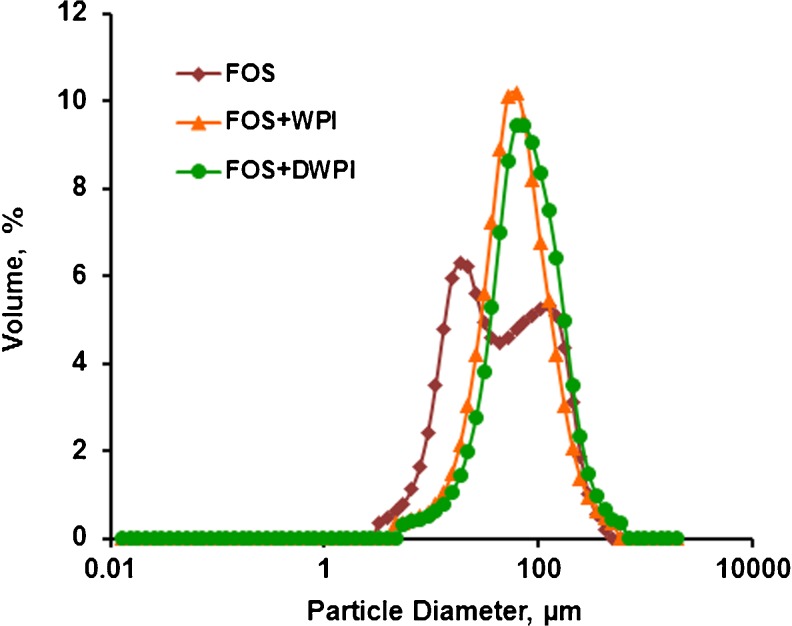
Particle size is an important parameter that determines the encapsulated powder quality and potential application of the micro particles to various foods. The use of different combinations of wall material had significant influence on particle size of encapsulated powder. The volume mean diameter D[4,3] and the size distributions (D10, D50, D90) of freeze dried microcapsules were presented in Table 1. The highest value of D[4,3] was observed for microcapsules obtained with FOS + DWPI (89.71 μm). The width of particle size distribution was determined by span and a smaller span value indicated narrow particle size distribution and uniform size. FOS + WPI and FOS + DWPI encapsulate exhibited similar range of span values (Table 1). Volume based particle size distribution curves for the microcapsules produced with three different wall materials are shown in Fig. 3. Probiotic Cells encapsulated with FOS + WPI and FOS + DWPI wall material exhibited monomodal particle size distribution whereas, FOS encapsulated powder showed bimodal distribution, thus indicating the presence of aggregated particles.
Fig. 3.
Particle size distribution of encapsulated powders. FOS, Fructooligosaccharide; WPI, Whey protein isolate; DWPI, denatured whey protein isolate
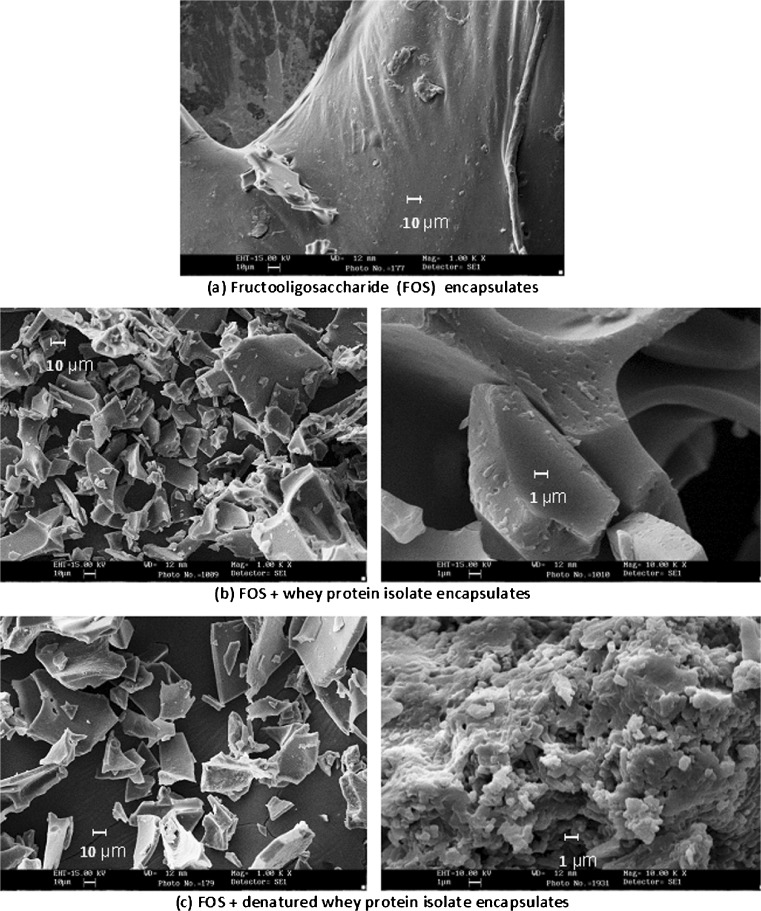
The scanning electron microscopy images (Fig. 4) showed the morphological characteristics of the freeze dried microcapsules. FOS encapsulated samples resembled gummy, cake like mass, with structural collapse as shown in Fig. 4a. This can be due to the hygroscopic nature of FOS, its tendency to aggregate and fuse together while loading sample in the adhesive tape for SEM study. This may be attributed to the decrease of glass transition temperature of FOS alone encapsulated sample at higher moisture content and water activity during freeze drying. Moreover, structural collapse occurred as a result of water plasticization effect of FOS at higher moisture content and water activity. This hygroscopicity of FOS encapsulated micro particles can be reduced by the addition of high molecular weight materials (Tsourouflis et al. 1976). In this study, an attempt has been made with the partial (50 %) replacement of FOS with WPI or DWPI in the wall system. Cells encapsulated with WPI and DWPI exhibited variations in microstructure, due to the differences in viscoelastic and film forming properties of each wall material. FOS + WPI encapsulates exhibited flake like structure with smooth surface whereas FOS + DWPI had a porous, spongy flakes. The Fig. 4b shows that probiotic cells were randomly distributed throughout the undenatured WPI wall matrix whereas DWPI film partially wrapped the bacterial cells (Fig. 4c) within the gel network due to its increased film strength and stretchability (Pérez‐Gago et al. 1999).
Fig. 4.
SEM images of microcapsules prepared by freeze drying method
Survival of probiotic cells after encapsulation and during storage
Survival of probiotic bacteria after encapsulation and their viability during storage is the foremost concern in the development of microencapsulation technique. Factors that affects the stability of dried probiotic cells are type of probiotic strain, method of drying, particle size, composition of wall material used for microencapsulation, residual moisture content and storage temperature (Chávez and Ledeboer 2007; Ananta et al. 2005). Table 1 shows the viable cell counts of L. plantarum in the feed formulations before and after freeze-drying. During freeze-drying intracellular ice formation reduces the cell viability due to membrane injury. In this study, there was no significant reduction in the cell viability for all the encapsulated samples (p < 0.05). Interaction between the cell membrane and fructons of the FOS maintains the membrane fluidity (Schwab et al. 2007), thus prevents membrane damage during dehydration and results in higher cell viability. All the three encapsulates yielded almost higher cell survival rate (above 98 %). This can be due to low temperature application (−40 to 30 °C) in all the three stages of freeze-drying and encapsulation with protective wall material.
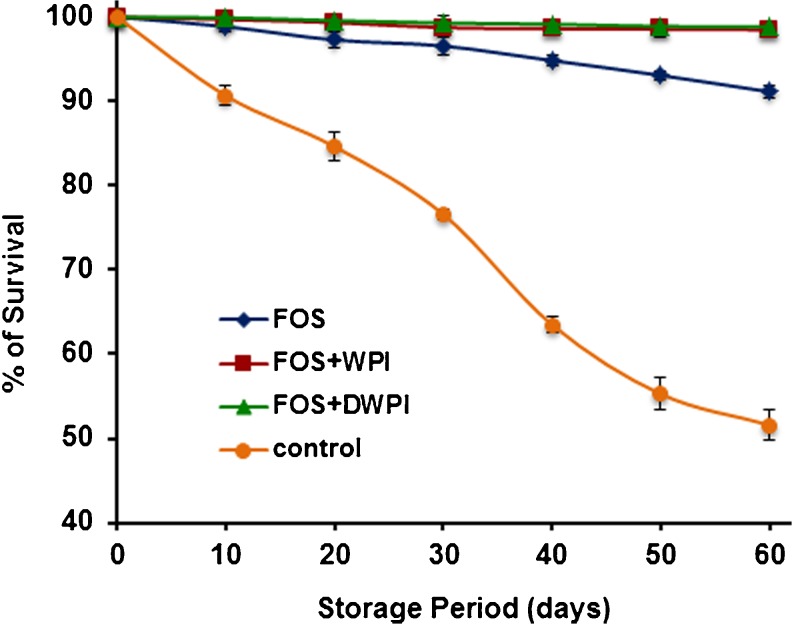
Survival of encapsulated probiotic bacteria during storage is one of the most important aspects. The viability of encapsulated cells during the storage period is shown in Fig. 5. Results indicated that L. plantarum encapsulated in FOS with either WPI or DWPI had an excellent viability at the end of storage period. Comparatively, cells encapsulated with FOS alone showed 9 % survival loss after 60 days of storage indicating the poor protection of FOS. The similar findings were reported by Capela et al. (2006) where the addition of FOS to fresh yoghurt retained the probiotic cells viability during freeze drying, but had a negative effect on freeze dried yoghurt after 4 weeks of storage at 4 °C. Loss of viability during storage of encapsulated probiotic cells in the dried state was also reported (Capela et al. 2006; Santivarangkna et al. 2011). Dehydration suppress the metabolic activity of cells by removal of moisture, but insufficient removal of moisture may allow the resumption of metabolism at low rate, thus leads to cell death (Fu and Chen 2011). However, addition of WPI or DWPI along with FOS to the wall system showed good stability and exhibited slight loss (2 %) of viability of L. plantarum during the storage period. Low water vapour and oxygen permeability of whey protein films (Perez‐gago and Krochta 2001) can limit the diffusion of substances through the whey protein wall could have improved the stability of encapsulated probiotic cells during storage.
Fig. 5.
Effect of wall materials on viability of L. plantarum during storage of freeze dried microcapsules at 4 °C. Significant difference in the viability was not obtained between FOS + WPI and FOS + DWPI wall systems (p < 0.05). Error bars represent standard deviation of means (n = 3)
Survival of encapsulated probiotic cells in simulated acidic and bile condition
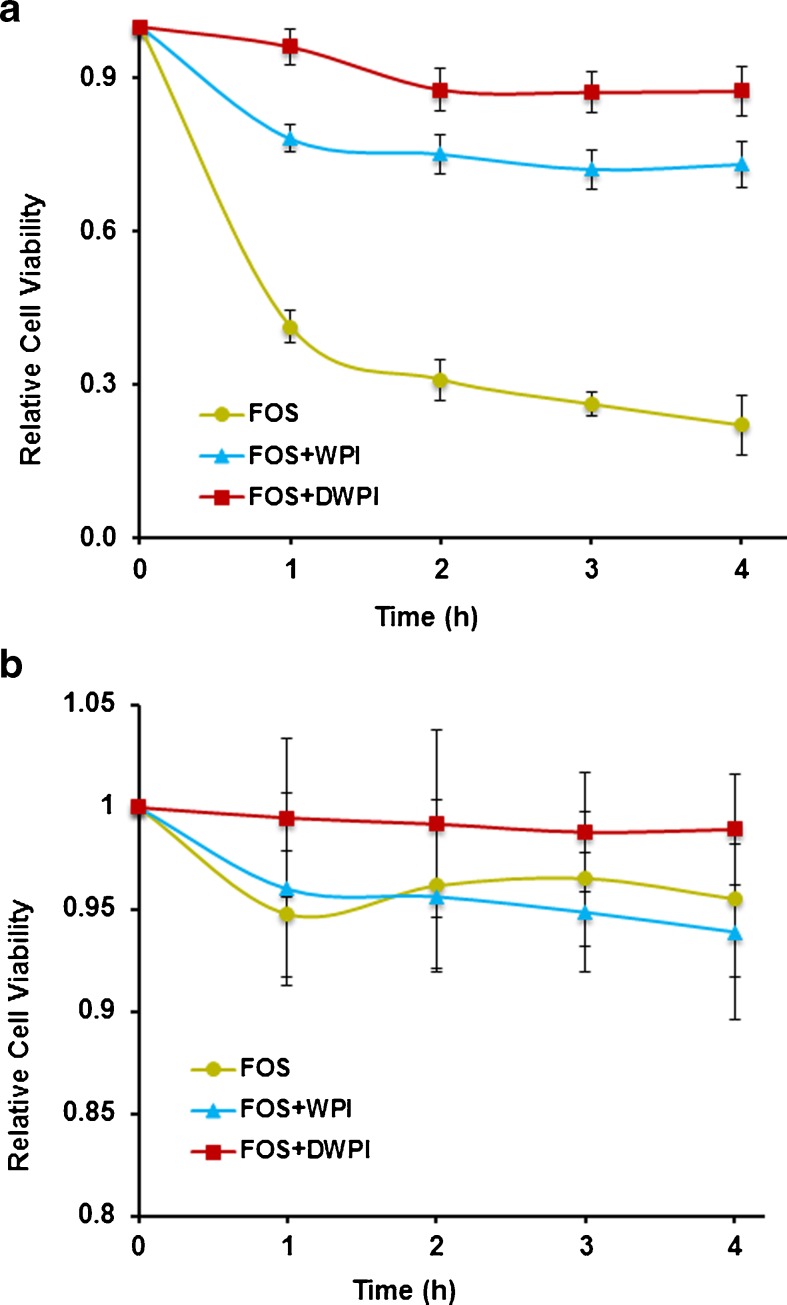
The relative viability of microencapsulated L. plantarum was evaluated in MRS broth containing low pH (2.0) with 0.3 % pepsin (Fig. 6a). Statistical analysis revealed significant difference (p < 0.05) in the relative viability for the cells encapsulated with all the combination of wall materials. Higher reduction in the relative viability was observed for cells encapsulated with FOS alone. Microcapsules produced with FOS alone completely released the cells due to its solubility when exposed to gastric medium thus lead to viability loss. On contradict to FOS, combination of FOS with WPI or DWPI showed less reduction in the relative cell viability during the 4 h exposure to gastric condition. This can be attributed to the partial resistance offered by wall matrix for the diffusion of gastric medium. Moreover, FOS + DWPI wall matrix showed minimal reduction in the relative cell viability, due to the denaturation of proteins that resulted in more exposure of hydrophobic sites on its surface (Pérez‐Gago et al. 1999) which in turn exhibited poor diffusion of gastric medium. This study clearly indicated that the FOS + DWPI is an effective wall material to protect the sensitive bacterial cells from the acidic environment.
Fig. 6.
(a) Relative cell viability of L. plantarum after exposure to simulated gastric juice at pH 2.0 and pepsin (0.3 %) for 4 h at 37 °C. Significant difference in the relative cell viability was obtained between wall systems (p < 0.05). (b) Relative cell viability of L. plantarum after exposure to simulated bile juice (2 %) for 4 h at 37 °C. Significant difference in the relative cell viability was not obtained between wall systems (p < 0.05). Error bars represent standard deviation of means (n = 3)
Effect of bile salt (2 %) on relative viability of microencapsulated L. plantarum is shown in Fig. 6b. FOS alone and FOS + WPI encapsulated cells showed reduction in the relative viability during the first hour and then remained stable over 4 h. However relative viability of FOS + DWPI encapsulated cells remained stable during the 4 h exposure to bile juice. This higher stability of wall material can be due to the incorporation of DWPI with FOS. Denatured whey protein prevented the permeation of bile fluid through the wall matrix and reduced the release of cells during 4 h incubation due to its reduced solubility. Further, statistical analysis revealed no significant difference (p < 0.05) for the relative viability of cells in all the three encapsulates. The results are consistent with our previous research work (Rajam et al. 2012), where DWPI wall matrix exhibited better stability and protected the cells in both simulated acidic and bile conditions. This study shows that water insolubility, higher tensile properties and lower oxygen permeability of DWPI (Perez‐gago and Krochta 2001) wall matrix effectively protected the probiotic cells from adverse acidic and bile conditions. From the results of the simulated acidic and bile conditions it can be concluded that FOS + DWPI wall system can be effective to deliver viable cells in the intestine for further colonisation.
Effect of incorporation of microcapsules on noodle quality
Microcapsules prepared with FOS + DWPI wall material exhibited better protection after drying, during storage and in simulated acidic and bile conditions. Hence, FOS + DWPI encapsulates has been incorporated in noodles and evaluated the impact of noodle processing conditions on qualitative characteristics of noodle and encapsulated cell viability.
Moisture content
The moisture content of the (i) fresh and (ii) dried noodles (SN - synbiotic noodle; CN - control noodle) before and after cooking are given in Table 2. The average moisture content of fresh SN was 2.15 % higher than CN, may be due to higher water absorption of microcapsules during noodle preparation. Similarly, in the case of fresh SN noodle after cooking had 11.39 % higher moisture uptake than fresh CN due to the higher water holding capacity of the DWPI (Perez‐gago and Krochta 2001) present in the noodle matrix. Among the dried noodles, HTD (High temperature dried) noodles retained lower moisture than RTD (room temperature dried) noodles, is obviously due to the higher dehydration rate of noodles dried at HTD (55 °C) than RTD (28 °C). Although dried SN retained significantly higher moisture content compared to CN, there was no significant difference in the moisture uptake of dried SN and CN after cooking.
Table 2.
Cooking quality and cell viability analysis of noodles with probiotic microcapsule incorporation
| Noodle type | Sample name | Moisture content | Optimum Cooking timea (min) | Solid lossa (%) | Texturea (N) | Coloura | Noodle colour index | Cell viability (log10 CFU/g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before cookinga | After cookinga | SS | ADH | L* | a* | b* | Before cookinga | After cookinga | |||||
| Fresh noodle | SN | 36.28 ± 0.02 | 71.34 ± 0.88 | 3.45 ± 0.11 | 5.8 ± 0.1 | 0.9 ± 0.1 | −0.5 ± 0.1 | 41.2 ± 0.8 | 3.9 ± 0.3 | 17.2 ± 0.7 | 44.7 | 9.85 ± 0.02 | 6.57 ± 0.32 |
| CN | 34.13 ± 0.50 | 59.95 ± 1.74 | 5.15 ± 0.09 | 3.3 ± 0.2 | 0.8 ± 0.2 | −0.4 ± 0.1 | 68.7 ± 0.2 | 0.3 ± 0.1 | 18.0 ± 0.4 | 71.1 | - | - | |
| Dried noodle | SN-RTD | 14.30 ± 0.80 | 69.07 ± 0.14 | 5.12 ± 0.09 | 7.0 ± 0.4 | 1.1 ± 0.1 | −0.6 ± 0.2 | 48.2 ± 2.9 | 4.1 ± 0.3 | 13.2 ± 0.8 | 50.0 | 8.45 ± 0.20 | NIL |
| SN-HTD | 9.82 ± 0.39 | 69.62 ± 3.17 | 4.37 ± 0.14 | 6.5 ± 0.5 | 1.2 ± 0.1 | −0.8 ± 0.4 | 50.9 ± 1.4 | 4.3 ± 0.2 | 14.2 ± 0.6 | 52.8 | 6.81 ± 0.40 | NIL | |
| CN-RTD | 13.26 ± 0.20 | 70.63 ± 0.44 | 5.43 ± 0.02 | 4.7 ± 0.2 | 0.9 ± 0.1 | −0.5 ± 0.1 | 62.7 ± 2.5 | −0.4 ± 0.1 | 13.0 ± 0.6 | 64.0 | - | - | |
| CN-HTD | 7.55 ± 0.24 | 66.50 ± 1.49 | 4.30 ± 0.15 | 4.4 ± 0.0 | 1.4 ± 0.1 | −0.5 ± 0.1 | 63.5 ± 2.6 | −0.7 ± 0.2 | 12.7 ± 1.1 | 64.8 | - | - | |
SS - Shear strength; ADH – Adhesiveness; SN, synbiotic noodle; CN, control noodle; RTD, room temperature dried (28 ° C); HTD, high temperature dried (55 ° C); a Results are presented as mean ± standard deviation (n = 3); For texture analysis: n = 5
Optimum cooking time and solid loss
Cooking time and solid loss are the two important factors determining the noodles cooking quality (Fu 2008). Mean values of optimal cooking time (min) and solid loss (%) of cooked noodles are summarized in Table 2. Shorter cooking time was required for fresh SN (3.45 min) compared to CN (5.15 min). Investigations have shown that gluten content governs the optimum cooking time (Cubadda et al. 2007; Dipin et al. 2012). Incorporation of probiotic microcapsules decrease the gluten level that allows the rapid gelatinization, and reduces the cooking time (Cubadda et al. 2007). Choy et al. (2013) reported that significant reduction in the cooking time for noodles containing buckwheat flour due to the physical disruption of the gluten matrix which in turn provided a path for rapid water absorption into noodle. Moreover, RTD noodle (both SN and CN) exhibited considerably increased cooking time than the HTD noodle.
On the evaluation of solid loss, higher solid loss was obtained for both fresh and dried SN compared to their respective control samples (p < 0.05). However, fresh synbiotic noodle had lowest solid loss (5.8 %) than the RTD (7 %) and HTD (6.5 %) synbiotic noodle. Addition of probiotic microcapsules could have disturbed the gluten network thus resulted in discontinuous protein matrix that allows more amounts of solids leached into cooking water. Our results are in agreements with various reports. Choy et al. (2013) also reported that instant noodle made from wheat flour enriched with buck-wheat flour exhibited significant decrease in optimal cooking time and increased cooking loss. These results are comparable with the reports of Tudorica et al. (2002), where the higher solid loss of pasta enriched with inulin was observed during cooking due to the disruption of the protein network. Torres et al. (2007) also confirmed that addition of legume flour diluted the gluten and resulted in higher solids leaches into cooking water. Although incorporation of probiotic microcapsules had resulted significant solid loss (5.8–7 %) than control sample, Oh et al. (1985) stated that solid loss values of wheat flour noodles in the range of 6.5–7.6 % were acceptable.
Texture and colour analysis of noodle
Texture of cooked noodle is the essential quality characteristic that determines the consumer acceptability of the product (Bharath Kumar and Prabhasankar 2013). Texture profile parameters such as shear strength and adhesiveness of cooked noodle made from fresh and dried noodles were measured and summarised in Table 2. Statistical analysis indicated that incorporation of probiotic microcapsules caused no significant effect on the shear strength and adhesiveness of cooked noodles compared with control. Similar findings were reported by Silva et al. (2013) for the addition of broccoli particles to noodle. The colour of the noodle is an important quality parameter that strongly influences the consumer preference (Bharath Kumar and Prabhasankar 2013). The results (Table 2) showed that the addition of probiotic microcapsules significantly (P < 0.05) affected the L* and a* values of noodles after cooking. However, no significant difference in the yellowness (b*) value compared with control. The L* value of fresh and dried SN noodle were less than their respective control. Comparatively, a* value of fresh and dried SN noodle were higher than their control. These observed changes in the colour can be attributed to the influence of coloured components present in the commercial WPI (visually brown in colour). Overall colour of the noodle was expressed by the term colour index, and it was found to be decreased with the addition of probiotic microcapsules. Overall colour index value of fresh and dried SN ranged from 44 to 52, whereas CN exhibited from 64 to 71.
Sensory analysis of noodle
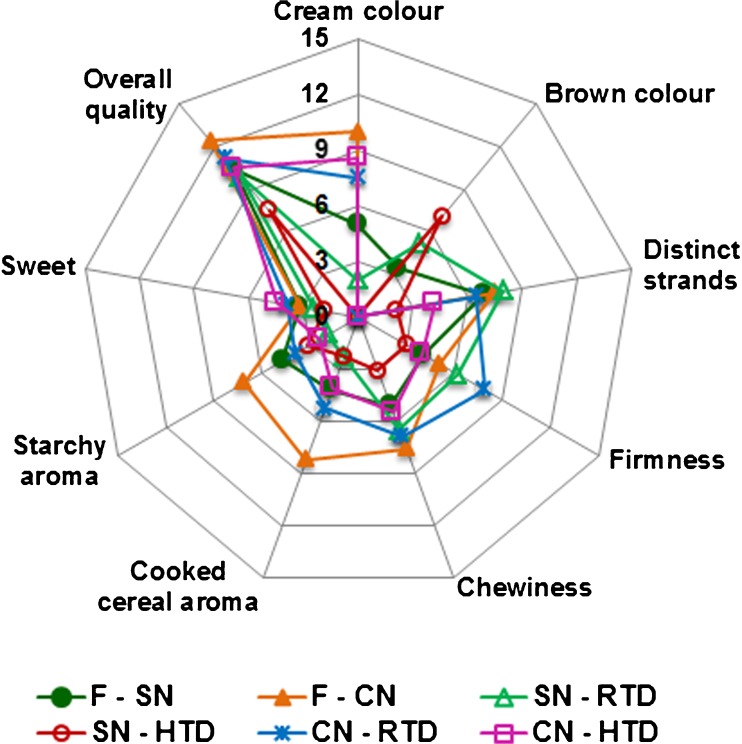
The sensory analysis gives idea about the acceptance of product by consumers. The sensory profile of fresh and dried noodles after cooking is given in Fig. 7. Quantitative Descriptive Analysis revealed that, there was no significant difference in the quality attributes (distinct strands, firmness, chewiness, and sweet) of fresh noodle containing probiotic microcapsules in comparison to control. Overall quality score of probiotic incorporated fresh noodles was above 10, indicating its good acceptability (Fig. 7). The appealing light brown colour of the noodles was due to the incorporation of probiotic microcapsules which was visually brown in colour. Moreover overall quality score of all the fresh and dried SN noodles were more than 7.5 on a 15 cm QDA scale, which indicates the product was acceptable with the incorporation of probiotic microcapsules.
Fig. 7.
Sensory profile of fresh and dried noodles after cooking. F, fresh; SN, synbiotic noodle; CN, control noodle; RTD, room temperature dried; HTD, high temperature dried
Effect of cooking on viability of L. plantarum in fresh and dried noodles
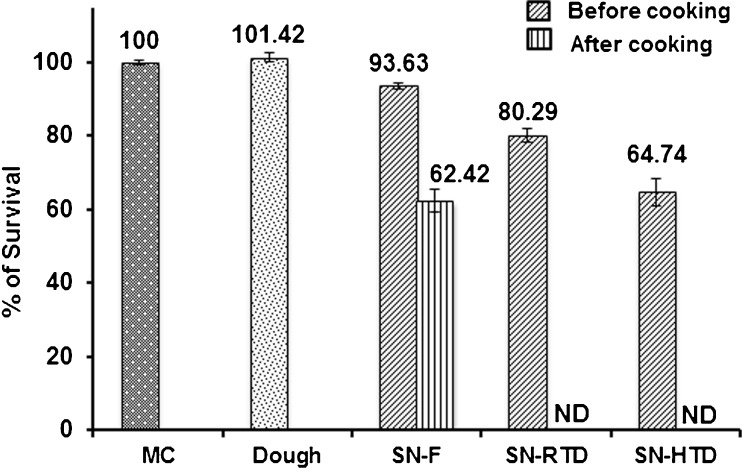
Viability of encapsulated L. plantarum cells per gram of microcapsules incorporated in the noodle matrix is presented in Table 2. Effect of noodle processing conditions on survival percentage of FOS + DWPI encapsulated probiotic cells are given in Fig. 8. Higher moisture content of dough and readily available substrate (FOS) present in the wall matrix resulted around 1.5 % increased percentage of survival in dough compared to FOS + DWPI microcapsules. In the fresh noodles 93.63 % of encapsulated cells remained viable, which may be due to microencapsulation that protected the cells during noodle processing. In the case of dried noodles, RTD retained up to 80.29 % cell viability, whereas HTD retained only 64.74 % viability. This reduction in the viability may be attributed to higher temperature application during drying at 55 °C. Heat stress during dehydration leads to viability loss due to the cell wall and cell membrane damage (Santivarangkna et al. 2008). On comparison encapsulated probiotic cell viability in fresh noodles had relatively high than dried noodles. After cooking, complete viability loss was observed in the dried noodles. This can be attributed to thermal inactivation occurred during rehydration of dried cells due to leakage of essential cellular components (Santivarangkna et al. 2008). However, fresh noodles retained 62.42 % encapsulated cell viability after cooking due to the presence of initial moisture (36 %) in the fresh noodles that protected the cell membrane from osmotic shock and thermal injury during rehydration. Besides this, microencapsulation had offered partial protection to the cells and helped to retain the cell viability in cooked fresh noodles. This result indicated that moisture content of noodles, rehydration temperature and rate of rehydration could have influenced the viability of probiotic cells in dried noodles during cooking. On a comparison, it was found that after cooking only fresh noodles retained the cell viability. This is the first report on the incorporation of probiotic microcapsules to noodles and further studies are needed to find the factors influencing loss of cell viability in dried noodles during cooking.
Fig. 8.
Effect of noodle processing conditions on survival of microencapsulated L. plantarum. Significant difference in the encapsulated L. plantarum cell viability was obtained before and after cooking of fresh and dried noodles (p < 0.05). Error bars represent standard deviation of means (n = 3). MC, FOS + DWPI microcapsule; SN, synbiotic noodle; F, fresh; RTD, room temperature dried; HTD, high temperature dried; ND, cell viability not detected after cooking
Conclusions
In the present study probiotic bacteria L. plantarum was microencapsulated with three different wall materials. All the three encapsulates yielded almost higher rate of cell survival (above 98 %). However FOS + DWPI wall matrix provided adequate protection to probiotic cells in simulated acidic and bile environment. Thus, FOS + DWPI found to be suitable to incorporate in to noodle formulation. Incorporation of probiotic microcapsules reduced the cooking time with slight increase in solid loss (5.8–7 %). However, shear strength, adhesiveness and colour of cooked noodles was not much affected due to incorporation of probiotic microcapsules. The sensory characteristics of the cooked noodles were also found to be acceptable. On the evaluation of encapsulated cell viability, fresh noodles retained 93.63 % of viability, whereas RTD retained up to 80.29 % and HTD retained only 64.74 % viability. However, after cooking fresh noodles alone retained cell viability (62.42 %). This research showed a novel way to incorporate microencapsulated probiotics in noodle formulation, and the fresh type of noodle can meet the consumers demand as a healthy functional food.
Acknowledgments
The authors wish to thank Prof. Ram Rajasekharan, Director, CSIR-CFTRI for the support and encouragement. We gratefully acknowledge the Department of Science and Technology (DST), Government of India for the financial support and Women Scientist Fellowship (WOS-A) to Rajam, which enabled this work to be carried out. Authors wish to thank Dr. M. C. Varadaraj, Chief Scientist, FM, CFTRI, and Mr.K.Anbalagan, CIFS, CFTRI for their help.
References
- AACC (2000) Approved Methods of the American Association of Cereal Chemists, Ed. AACC.
- Anandharamakrishnan C, Rielly C, Stapley A. Effects of process variables on the denaturation of whey proteins during spray drying. Dry Technol. 2007;25:799–807. doi: 10.1080/07373930701370175. [DOI] [Google Scholar]
- Ananta E, Volkert M, Knorr D. Cellular injuries and storage stability of spray-dried Lactobacillus rhamnosus GG. Int Dairy J. 2005;15:399–409. doi: 10.1016/j.idairyj.2004.08.004. [DOI] [Google Scholar]
- Bajaj PR, Survase SA, Bule MV, Singhal RS. Studies on viability of Lactobacillus fermentum by microencapsulation using extrusion spheronization. Food Biotechnol. 2010;24:150–164. doi: 10.1080/08905436.2010.482010. [DOI] [Google Scholar]
- Bharath Kumar S, Prabhasankar P. A study on noodle dough rheology and product quality characteristics of fresh and dried noodles as influenced by low glycemic index ingredient. J Foos Sci Technol. 2013 doi: 10.1007/s13197-013-1126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capela P, Hay TKC, Shah NP. Effect of cryoprotectants, prebiotics and microencapsulation on survival of probiotic organisms in yoghurt and freeze-dried yoghurt. Food Res Int. 2006;39:203–211. doi: 10.1016/j.foodres.2005.07.007. [DOI] [Google Scholar]
- Cavazza A, Corradini C, Rinaldi M, Salvadeo P, Borromei C, Massini R. Evaluation of Pasta Thermal Treatment By Determination of Carbohydrates, Furosine, and Color Indices. Food Bioprocess Technol. 2012;6:2721–2731. doi: 10.1007/s11947-012-0906-6. [DOI] [Google Scholar]
- Champagne CP, Gardner NJ, Roy D. Challenges in the addition of probiotic cultures to foods. Crit Rev Food Sci Nutr. 2005;45:61–84. doi: 10.1080/10408690590900144. [DOI] [PubMed] [Google Scholar]
- Chávez BE, Ledeboer AM. Drying of Probiotics: Optimization of Formulation and Process to Enhance Storage Survival. Dry Technol. 2007;25:1193–1201. doi: 10.1080/07373930701438576. [DOI] [Google Scholar]
- Choy A-L, Morrison PD, Hughes JG, Marriott PJ, Small DM. Quality and antioxidant properties of instant noodles enhanced with common buckwheat flour. J Cereal Sci. 2013;57:281–287. doi: 10.1016/j.jcs.2012.11.007. [DOI] [Google Scholar]
- Cruz AG, Cadena RS, Walter EHM, Mortazavian AM, Granato D, Faria JAF, Bolini HMA. Sensory Analysis: relevance for Prebiotic, Probiotic, and Synbiotic Product Development. Comp Rev Food Sci Food Saf. 2010;9:358–373. doi: 10.1111/j.1541-4337.2010.00115.x. [DOI] [PubMed] [Google Scholar]
- Cubadda RE, Carcea M, Marconi E, Trivisonno MC. Influence of Gluten Proteins and Drying Temperature on the Cooking Quality of Durum Wheat Pasta. Cereal Chem. 2007;84:48–55. doi: 10.1094/CCHEM-84-1-0048. [DOI] [Google Scholar]
- de Valdéz GF, de Giori GS, de Ruiz Holgado AA, Oliver G. Protective effect of adonitol on lactic acid bacteria subjected to freeze-drying. App Env Microbiol. 1983;45:302–304. doi: 10.1128/aem.45.1.302-304.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipin S, Pillai DS, Prabhasankar P, Jena BS, Anandharamakrishnan C. Microencapsulation of Garcinia cowa fruit extract and effect of its use on pasta process and quality. Int J Food Prop. 2012;15:590–604. doi: 10.1080/10942912.2010.494756. [DOI] [Google Scholar]
- Dissanayake M, Vasiljevic T. Functional properties of whey proteins affected by heat treatment and hydrodynamic high-pressure shearing. J Dairy Sci. 2009;92:1387–1397. doi: 10.3168/jds.2008-1791. [DOI] [PubMed] [Google Scholar]
- Dolly P, Anishaparvin A, Joseph G, Anandharamakrishnan C. Microencapsulation of Lactobacillus plantarum (mtcc 5422) by spray-freeze-drying method and evaluation of survival in simulated gastrointestinal conditions. J microencapsul. 2011;28:568–574. doi: 10.3109/02652048.2011.599435. [DOI] [PubMed] [Google Scholar]
- Ezhilarasi PN, Indrani D, Jena BS, Anandharamakrishnan C. Freeze drying technique for microencapsulation of Garcinia fruit extract and its effect on bread quality. J Food Eng. 2013;117:513–520. doi: 10.1016/j.jfoodeng.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Fu BX. Asian noodles: History, classification, raw materials, and processing. Food Res Int. 2008;41:888–902. doi: 10.1016/j.foodres.2007.11.007. [DOI] [Google Scholar]
- Fu N, Chen XD. Towards a maximal cell survival in convective thermal drying processes. Food Res Int. 2011;44:1127–1149. doi: 10.1016/j.foodres.2011.03.053. [DOI] [Google Scholar]
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Levi G, Karel M. Volumetric shrinkage (collapse) in freeze-dried carbohydrates above their glass transition temperature. Food Res Int. 1995;28:145–151. doi: 10.1016/0963-9969(95)90798-F. [DOI] [Google Scholar]
- Nicolai T, Britten M, Schmitt C. β-Lactoglobulin and WPI aggregates: formation, structure and applications. Food Hydrocoll. 2011;25:1945–1962. doi: 10.1016/j.foodhyd.2011.02.006. [DOI] [Google Scholar]
- Oh N, Seib P, Deyoe C, Ward A. Noodles II. The surface firmness of cooked noodles from soft and hard wheat flours. Cereal Chem. 1985;62:431–436. [Google Scholar]
- Parthasarathi S, Ezhilarasi PN, Jena BS, Anandharamakrishnan C. A comparative study on conventional and microwave-assisted extraction for microencapsulation of Garcinia fruit extract. Food Bioprod Process. 2013;91:103–110. doi: 10.1016/j.fbp.2012.10.004. [DOI] [Google Scholar]
- Perez‐gago M, Krochta J. Denaturation time and temperature effects on solubility, tensile properties, and oxygen permeability of whey protein edible films. J Food Sci. 2001;66:705–710. doi: 10.1111/j.1365-2621.2001.tb04625.x. [DOI] [Google Scholar]
- Pérez‐Gago M, Nadaud P, Krochta J. Water Vapor Permeability, Solubility, and Tensile Properties of Heat‐denatured versus Native Whey Protein Films. J Food Sci. 1999;64:1034–1037. doi: 10.1111/j.1365-2621.1999.tb12276.x. [DOI] [Google Scholar]
- Rajam R, Karthik P, Parthasarathi S, Joseph G, Anandharamakrishnan C (2012) Effect of whey protein–alginate wall systems on survival of microencapsulated Lactobacillus plantarum in simulated gastrointestinal conditions. J Func Foods 4:891–898
- Rivera-Espinoza Y, Gallardo-Navarro Y. Non-dairy probiotic products. Food Microbiol. 2010;27:1–11. doi: 10.1016/j.fm.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Roopashri AN, Varadaraj MC. Molecular characterization of native isolates of lactic acid bacteria, bifidobacteria and yeasts for beneficial attributes. Appl Microbiol Biotechnol. 2009;83:1115–1126. doi: 10.1007/s00253-009-1991-y. [DOI] [PubMed] [Google Scholar]
- Santivarangkna C, Kulozik U, Foerst P. Inactivation mechanisms of lactic acid starter cultures preserved by drying processes. J Appl Microbiol. 2008;105:1–13. doi: 10.1111/j.1365-2672.2008.03744.x. [DOI] [PubMed] [Google Scholar]
- Santivarangkna C, Aschenbrenner M, Kulozik U, Foerst P. Role of glassy state on stabilities of freeze-dried probiotics. J Food Sci. 2011;76:R152–156. doi: 10.1111/j.1750-3841.2011.02347.x. [DOI] [PubMed] [Google Scholar]
- Schwab C, Vogel R, Ganzle MG. Influence of oligosaccharides on the viability and membrane properties of Lactobacillus reuteri TMW1.106 during freeze-drying. Cryobiol. 2007;55:108–114. doi: 10.1016/j.cryobiol.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Shah NP. Functional cultures and health benefits. Int Dairy J. 2007;17:1262–1277. doi: 10.1016/j.idairyj.2007.01.014. [DOI] [Google Scholar]
- Silva E, Sagis LMC, van der Linden E, Scholten E. Effect of matrix and particle type on rheological, textural and structural properties of broccoli pasta and noodles. J Food Eng. 2013;119:94–103. doi: 10.1016/j.jfoodeng.2013.05.003. [DOI] [Google Scholar]
- Stone H, Sidel J, Singleton RC. Sensory evaluation by QDA. J Food Technol. 1974;28:24–34. [Google Scholar]
- Torres A, Frias J, Granito M, Guerra M, Vidal-Valverde C. Chemical, biological and sensory evaluation of pasta products supplemented with α-galactoside-free lupin flours. J Sci Food Agric. 2007;87:74–81. doi: 10.1002/jsfa.2673. [DOI] [Google Scholar]
- Tsourouflis S, Flink JM, Karel M. Loss of structure in freeze‐dried carbohydrates solutions: effect of temperature, moisture content and composition. J Sci Food Agric. 1976;27:509–519. doi: 10.1002/jsfa.2740270604. [DOI] [Google Scholar]
- Tudorica C, Kuri V, Brennan C. Nutritional and physicochemical characteristics of dietary fiber enriched pasta. J Agric Food Chem. 2002;50:347–356. doi: 10.1021/jf0106953. [DOI] [PubMed] [Google Scholar]
- Zayed G, Roos YH. Influence of trehalose and moisture content on survival of Lactobacillus salivarius subjected to freeze-drying and storage. Process Biochem. 2004;39:1081–1086. doi: 10.1016/S0032-9592(03)00222-X. [DOI] [Google Scholar]