Abstract
In this study, the effects on antioxidant activity and structure change of corn peptides (CPS) with 10 to 30 kDa molecular weight (MW) treated by pulsed electric field (PEF) technology were investigated. 2, 2–diphenyl–1–picrylhydrazyl (DPPH) inhibition was used to evaluate the antioxidant activity of CPS. Response surface methodology (RSM) was used to investigate the effects of PEF treatment parameters on antioxidant activity of CPS. The optimal conditions were as follows: concentration of CPS 10 mg mL−1, electric field intensity 15 kV cm−1, and pulse frequency 2,000 Hz. Under the optimized conditions, the DPPH inhibition of CPS increased 32.1 %, compared to the sample untreated. And mid–infrared spectroscopy (MIR) was used for analyzing the structure change of CPS. The results showed that PEF technology could obviously increase the DPPH inhibition of CPS under the optimized conditions (P < 0.05).
Keywords: Corn peptides (CPS), Pulsed electric field (PEF), 2, 2–diphenyl–1–picrylhydrazyl (DPPH) inhibition, Response surface methodology (RSM), Mid–infrared spectroscopy (MIR)
Introduction
Corn peptides (CPS) are obtained by enzyme hydrolysis from corn gluten meal, which is a by–product of the starch industry containing approximately 60–65 % protein (Li et al. 2007). Corn gluten meal is poor in nutritional quality and usually used as animal feed due to its deficiency of essential amino acids (such as lysine and tryptophan), insolubility in water, and peculiar smell (Dai et al. 2008). However, in recent years, it was reported that CPS have some high bioactivities (Miao et al. 2010), such as accelerating ethanol metabolism and reducing its concentration in blood plasma (Yamaguchi et al. 1996; Yamaguchi et al. 1997a), affecting mammary tumor progression in female rats and displaying anti–breast cancer activity (Yamaguchi et al. 1997b), and demonstrating resistance lipid peroxidation (Xu et al. 2002). The current studies have focused on the various protein sources of peptides. Yet a thorough study on the antioxidant capacity of CPS is relatively less reasearched. It was reported that, CPS had higher antioxidative property and solubility than corn gluten, and the sequence of the purified peptide was found (Zheng et al. 2006), Li et al. (2009) investigated that, CPS showed the same notable antioxidant activities, free radical scavenging and reducing activities in vitro as those of zein peptides (ZPS). And the effect of CPS on removing superoxide anion in vitro was also reported (Li et al. 2007).
In view of food deterioration associated with free radical generation, our study attempted to measure the antioxidant activities of CPS by the 2,2–diphenyl–1–picrylhydrazyl (DPPH) inhibition assay (Miliauskas et al. 2004). In previous investigations, the DPPH inhibition assay had high reproducibility, standard equipment, and correlation for determining antioxidant activities in food ingredients (Prior et al. 2003; Thaipong et al. 2006; Wong et al. 2006). Pulsed electric field (PEF) processing is an environmentally friendly and newly developing technique in food industry. This growing trend is due to its advantages of non–thermal processing, low energy consumption and short processing time. It has been shown to accelerate the extraction process, extend product quality, and provide flavor (Balasa et al. 2008). In our study, the improvement of DPPH inhibition of CPS treated by PEF was researched. Optimal conditions for PEF using response surface methodology (RSM) have been published previously (Wang et al. 2012a). RSM is an effective statistical technique for optimizing complex processes. The main advantage of RSM is the reduced number of experimental trials needed to evaluate multiple parameters and their interactions (Lin et al. 2012a). Therefore, it is less laborious and time consuming than other approaches to optimize a process. Therefore, the aim of this research was to investigate the effects of CPS of molecular weight (MW) 10 to 30 kDa treated by PEF technology on antioxidant activity.
Materials and methods
Materials and reagents
Corn gluten meal (protein content of 66.40 %) was purchased from Dacheng Co. (Changchun, China). Alcalase (enzymic activity 20,000 U g−1) was purchased from Novozymes Co. (Denmark). DPPH was obtained from Sigma Aldrich Co. (St Louis, MO, USA). Potassium bromide powder was obtained from PIKE Technologies (Madison, WI, USA). Methanol, ethanol, sodium hydroxide and all other chemical reagents were purchased from Peking Chemical Plant (Beijing, China) and were all of analytical grade purity.
Instruments
The PEF system was self–designed by professor Yongguang Yin, and was described in previous studies (Lin et al. 2011; Lin et al. 2012b). μQuant microplate reader (Bio–Tek Instrument, USA.) was used to measure the absorbance, ultra filtration (UF) equipment (Millipore, Bedford, MA) was used to separated CPS, IRPrestige–21 Fourier transform infrared spectroscopy (Shimadzu, Japan) were used to measure Mid–infrared spectroscopy (MIR).
PEF treatment
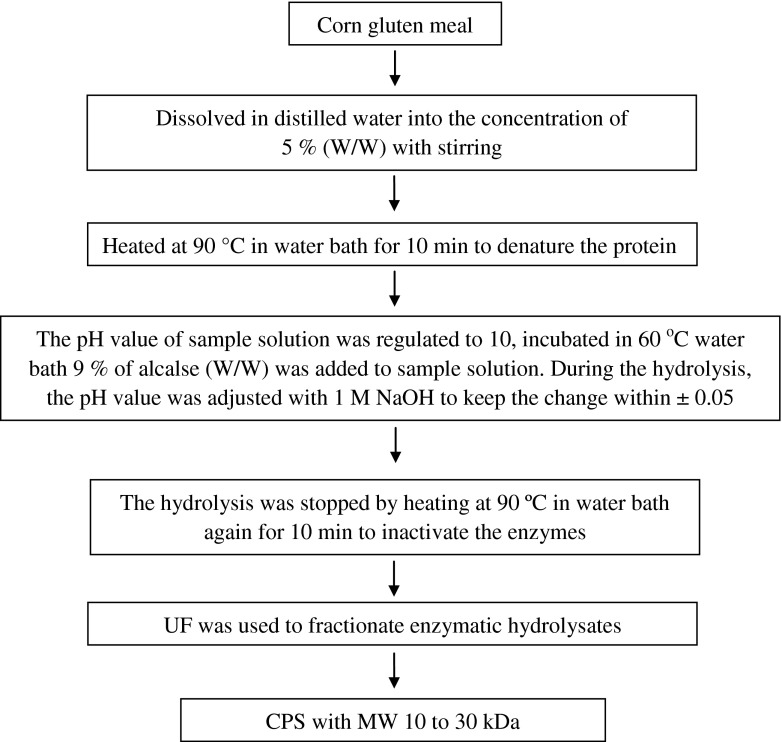
The preparation process for CPS was shown in Fig. 1. The pump and circuit of the PEF system were washed with deionized water and ethanol 2 or 3 times. The CPS powder was mixed with deionized water to result in different concentrations of peptide solution for experimental optimization. Afterwards, the peptide solutions were pumped into the PEF system at a flow velocity of 1.60 mL min−1. And then, the charge voltage and pulse frequency were adjusted to the desired experimental levels. The CPS sample circulated in the process chamber for the time required. Then the peptide solutions were incubated in the refrigerator of 5 °C for 30 min and saved standby.
Fig. 1.
Preparation process of CPS
Determination of antioxidant activity
The DPPH inhibition of each peptide solution was determined according to the method modified from Brand-Williams et al. (1995) and Katalinic et al. (2006) The DPPH solution in methanol (6 × 10−5 M) was prepared daily. An aliquot of 100 μL of the solution was mixed with 100 μL of peptide solution at different concentrations (6, 8 and 10 mg mL−1) and 100 μL of methanol in 96–well microplate. The samples were incubated for 1.50 h at 37 °C in a water bath. The DPPH radical has an absorption maximum at 520 nm, and the absorbance of DPPH was decreased by reduction of the antioxidant compound. The absorbance was measured by a μQuant Bio–Tek microplate reader at 520 nm at 20 °C. There were four cells made up for each treatment concentration, and each cell was measured once. Data were expressed as means ± standard deviation (SD). All measurements were performed under reduced light. The decrease in absorbance at 520 nm was measured (AE). A blank sample containing 100 mL of methanol in the DPPH solution was prepared daily, and its absorbance was measured (AB). The DPPH experiment was performed in triplicate. DPPH inhibition was calculated using the following formula:
Where AB is the absorbance of the blank sample at 520 nm, and AE is the absorbance of the sample at 520 nm.
One–factor–at–a–time tests (OFAT) of PEF technology
First, the effect of concentration on antioxidant activity was studied. The PEF–treated peptide solution was prepared in a series of concentrations of 6, 8, and 10 mg mL−1 respectively, were tested under the electric field intensity of 10 kV cm−1, frequency of 2,000 Hz. Hereafter, the effect of electric field intensity on improving polypeptide antioxidant activity was studied. Three levels, 5, 10, and 15 kV cm−1 electric field intensity were used, while concentration and pulse frequency were fixed at 8 mg mL−1 and 2,000 Hz. Lastly, the effect of pulse frequency on antioxidant activity was studied. The sample solutions were prepared at a concentration of 8 mg mL−1, and then treated by PEF under the electric field intensity of 10 kV cm−1 with the frequency of 2,000, 2,350, and 2,700 Hz, respectively.
Experimental design of RSM
In this study, RSM was used to evaluate the effect of the variables that might have impacts on the DPPH inhibition of CPS, according to the results of the independent variable experiments. Calculations were performed using the Design Expert Software (Trial Version 8.0.0, Stat–Ease Inc., USA). The variables and their ranges were chosen on the basis of the preliminary experiment results. A total of 15 experimental runs were performed for optimizing the three independent variables in the Box–Behnken design (BBD). These three independent variables were: peptide solution concentration (A), electric field intensity (B), and frequency (C). These three variables, labeled as X1, X2 and X3 respectively, were used to express the DPPH inhibition as shown in Table 1.
Table 1.
Independent variables and their levels used for Box–Behnken rotatable design
| Independent variable | Code | Variable level | ||
|---|---|---|---|---|
| −1 | 00 | 1 | ||
| Concentration (mg mL−1) | X1 | 6 | 88 | 10 |
| Electric field intensity (kV cm−1) | X2 | 5 | 110 | 15 |
| Electric field frequency (Hz) | X3 | 2,000 | 22350 | 2,700 |
MIR spectroscopy of functional groups
MIR was performed with a resolution of 4 cm−1, over the range (4,000–400 cm−1, using a ultraviolet spectrometer). The KBr was dried at 130 ºC and its spectrum was taken as the background. The CPS was treated by PEF under the optimized condition, freeze–dried, placed in sealed bags, and stored in desiccators. The KBr sample pellets were prepared by mixing 1 mg of sample with 200 mg of KBr. Treated sample and control were placed sample compartment with automatic accessory recognition at the scanning speed of 2.80 mm s−1. After background correction, all spectra were baseline corrected, and then samples spectra were obtained.
Statistical analysis
Analysis of variance (ANOVA) was performed using the SPSS 13.0 software package (SPSS Inc., Chicago, Illinois, USA). Results were expressed as means ± SD of three replicated measurements. The coefficients of the second polynomial model and the responses obtained from each set of experimental design (Table 2) were subject to multiple nonlinear regressions using Design–Expert 8.0 software (Static Made Easy, Minneapolis, MN, USA.). The significance of the regression coefficients were also tested using an F–test. The quality of the fit of the polynomial model equation was expressed by the coefficient of correlation (R2). The regression coefficients were used for statistical calculation to generate dimensional and contour maps from the regression models. Significant differences were determined with 95 % confidence intervals (P < 0.05).
Table 2.
Experimental data for DPPH inhibition (%) from the Box–Behnken design
| Experimental number | X1 | X2 | X3 | DPPH inhibition (%) | |
|---|---|---|---|---|---|
| Actual value | Predicted value | ||||
| 1 | 1 | −1 | 0 | 69.93 ± 3.38 | 66.79 |
| 2 | 0 | 0 | 0 | 70.07 ± 3.23 | 69.39 |
| 3 | 1 | 0 | −1 | 61.12 ± 3.36 | 62.62 |
| 4 | 0 | 0 | 0 | 68.03 ± 2.53 | 69.39 |
| 5 | −1 | 1 | 0 | 59.37 ± 3.90 | 62.51 |
| 6 | 0 | −1 | 1 | 57.47 ± 2.75 | 59.27 |
| 7 | 1 | 0 | 1 | 60.09 ± 9.89 | 61.43 |
| 8 | 0 | 1 | 1 | 68.29 ± 2.93 | 66.65 |
| 9 | −1 | 0 | 1 | 51.47 ± 5.06 | 49.97 |
| 10 | 0 | 0 | 0 | 70.07 ± 3.58 | 69.39 |
| 11 | 0 | −1 | −1 | 57.51 ± 2.51 | 59.15 |
| 12 | 0 | 1 | −1 | 69.45 ± 3.33 | 67.65 |
| 13 | 1 | 1 | 0 | 70.70 ± 4.34 | 71.00 |
| 14 | −1 | 0 | −1 | 50.99 ± 3.87 | 49.65 |
| 15 | −1 | −1 | 0 | 51.14 ± 9.38 | 50.84 |
Results and discussion
Effects of concentration on antioxidant activity
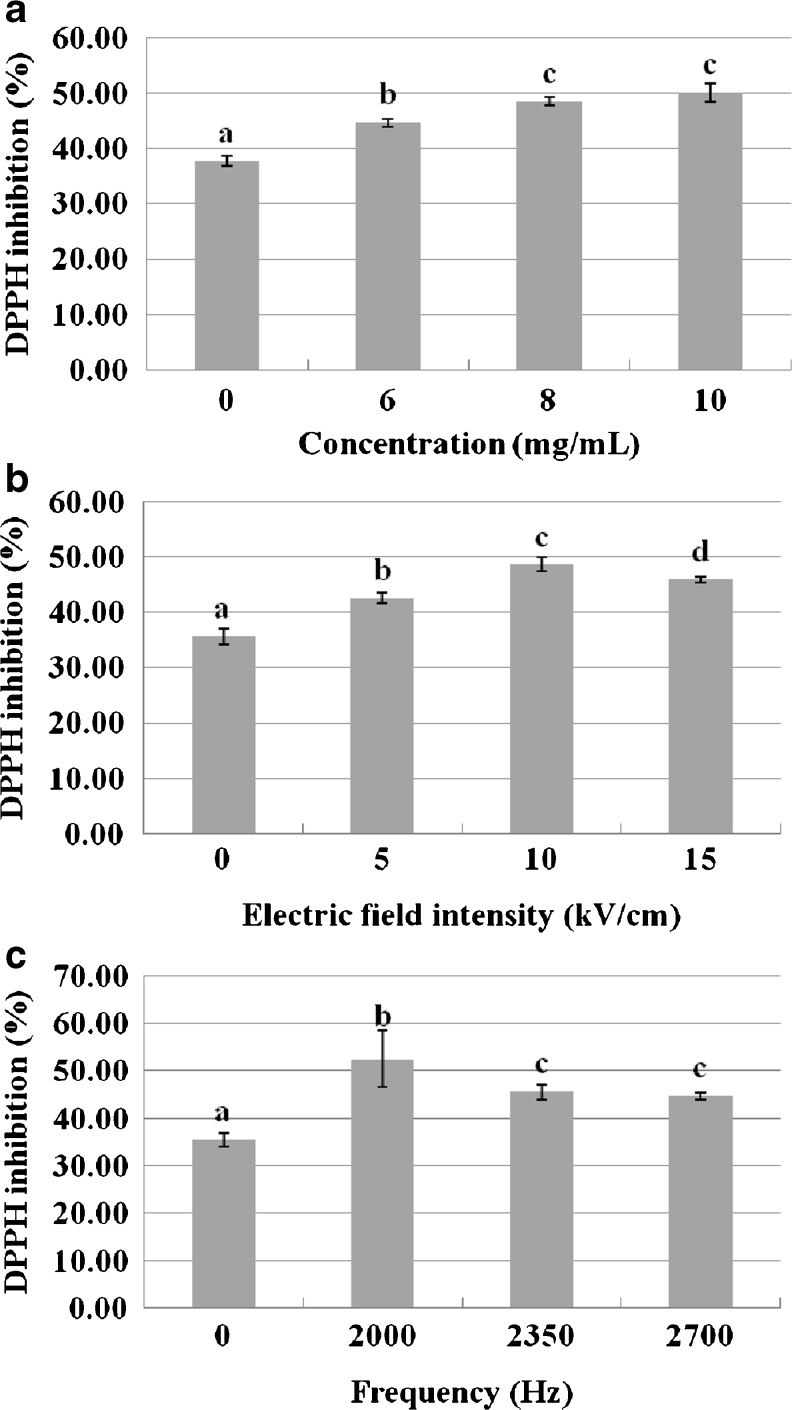
The effects of concentration of CPS on antioxidant activity were shown in Fig. 2a. The results showed that, when the concentration of CPS increased from 0 to 10 mg mL−1, the DPPH inhibition significantly increased (P < 0.05). The highest value was 50.05 ± 1.61 %, obtained from 10 mg mL−1 of concentration of CPS. Therefore, concentration of 10 mg mL−1 was chosen as the best ratio parameter for the other experiments. The results investigated that concentration of peptide solution had obvious influence on the antioxidant activity of polypeptides after treated by PEF technology. The reason might be that, different concentration lead to different liquid content of the material, and cause the dielectric constant changes. Eventually, the electrolysate produced by PEF processing are easier to penetrate cell membrane and combine with active peptides. Consequently, the concentration of peptides salution has an effect on the activity of peptides.
Fig. 2.
The effects of concentration of CPS solution, electric field intensity and frquency on DPPH inhibition. (a) Effects of concentration on the antioxidant activity of CPS. Electric field intensity is of 10 kV cm−1, Frequency is of 2,000 Hz; Concentration increased from 0 to 10 mg mL−1. (b) Effects of electric field intensity on the antioxidant activity of CPS. Concentration is of 10 mg mL−1, Frequency is of 2,000 Hz; Electric field intensity increased from 0 to 15 kV cm−1. (c) Effects of frequency on the antioxidant activity of CPS. Concentration is of 10 mg mL−1, Electric field intensity is of 10 kV cm−1; Frequency increased from 0 to 2,700 Hz. The same letter means that the variance of two samples is not significant (P > 0.05), and the different letters mean that variance of two samples are significant (P < 0.05). Values are means ± SD
Effects of electric field intensities on antioxidant activity
Figure 2b illustrates that the DPPH inhibition of CPS was influenced by electric field intensity. When the electric field intensity was increased from 0 to 10 kV cm−1, the DPPH inhibition increased from 35.50 ± 1.43 % to 48.54 ± 1.25 %. The results indicated that the highest value of antioxidant activity of DPPH inhibition was 48.54 ± 1.25 %, when the electric field intensity was 10 kV cm−1. However, when the electric field intensity increased from 10 to 15 kV cm−1, DPPH inhibition decreased from 48.54 ± 1.25 % to 45.83 ± 0.49 %. The similar tendency has been reported previously (Lin et al. 2011). These results may be from the effects of polarization of the protein molecule and polarized structures, which tend to attract each orther by electrostatic forces (Jin et al. 2011; Lin et al. 2013). Therefore, a PEF electric field intensity of 10 kV cm−1 was used in the following experiments.
Effects of frequency on antioxidant activity
Figure 2c shows that the antioxidant activity changed slightly when PEF pulse frequency was changed. According to the results, the DPPH inhibition was correlated with the frequency, and the highest value (30.50 ± 1.43 %) was obtained with frequency of 2,000 Hz. However, when the frequency was increased from 2,000 to 2,700 Hz, the DPPH inhibition significantly decreased (P < 0.05). Therefore, a frequency of 2,000 Hz was used in the following experiments. Based on previous studies of PEF processing on food preservation and extraction, an increase of pulse duration and frequency was shown to cause changes of the structure of cell membranes and protection of some nutrients in large molecules (Lin et al. 2011; Wang et al. 2012b). In this study, the lower level of frequency (2,000 Hz) could positively affect the DPPH inhibition of CPS.
Analysis and model fitting RSM
To visualize the effects of the independent variables on the dependent one, surface response and contour plots of the quadric polynomial models were generated by varying two of the independent variables within the experimental range (−1,1) while holding the other two constants at the central point. Based on the OFAT tests, the effects of the parameters were investigated to obtain an optimized, experimental condition of PEF processing. The optimal model generated was a second–order polynomial equation. Table 2 shows the experimental conditions and results of DPPH inhibition of CPS according to the factorial design. The results of ANOVA are shown in Table 3. The final mathematical model can be expressed by the following quadratic equation.
Table 3.
Regression coefficients estimate and significance test for the quadratic polynomial model
| Model term | Coefficient estimate |
Standard error |
Sum of square | Mean square | F value | Prob > F (P < 0.05) |
|---|---|---|---|---|---|---|
| Intercept | 69.39 | 1.69 | ||||
| X1 | 6.11 | 1.03 | 298.53 | 298.53 | 34.99 | 0.00* |
| X2 | 3.97 | 1.03 | 126.09 | 126.09 | 14.78 | 0.01* |
| X3 | −0.22 | 1.03 | 0.38 | 0.38 | 0.05 | 0.84 |
| X1 × X2 | −1.87 | 1.46 | 13.91 | 13.91 | 1.63 | 0.26 |
| X1 × X3 | −0.38 | 1.46 | 0.57 | 0.57 | 0.07 | 0.81 |
| X2 × X3 | −0.28 | 1.46 | 0.31 | 0.31 | 0.04 | 0.86 |
| X1 × X1 | −6.93 | 1.52 | 177.51 | 177.51 | 20.81 | 0.01* |
| X2 × X2 | 0.33 | 1.52 | 0.40 | 0.40 | 0.05 | 0.84 |
| X3 × X3 | −6.54 | 1.52 | 157.87 | 157.87 | 18.51 | 0.01* |
| Model | 757.49 | 84.17 | 9.87 | 0.01* | ||
| Residual | 42.65 | 8.53 | ||||
| Lack of fit | 39.88 | 13.29 | 9.58 | 0.10 | ||
| Pure error | 2.77 | 1.39 | ||||
| Cor. total | 800.15 |
R = 0.97; R2 = 0.95; adjusted.R2 = 0.85; predicated. R2 = 0.19; adequate. precision =8.95
Where Y is the absorbance value of the DPPH inhibition at 520 nm, and X1, X2, X3 are the variables for the concentration of corn peptide suspension (mg mL−1), electric field intensity (kV cm−1) and frequency (Hz), respectively.
The regression model of RSM was tested by ANOVA, as shown in Table 3. The P–value of the model was 0.01, which meant that the model was significant. And the P–value of lack of fit was 0.10, which meant that the model does not significantly lack fit (Wang et al. 2012b). The value of adjusted determination coefficient, R2 adj. was 0.95, which indicated a high degree of correlation between the observed and predicted values. The value of adequate precision was 8.95, which indicates an adequate signal. Therefore, the regression model could be used to navigate the design space.
The P–values were used as the tools to check the significance of each coefficient. The smaller the P–value, the more significant was the corresponding coefficient. According to the ANOVA for DPPH inhibition as shown in Table 3, concentration (X1), electric field intensity (X2), concentration squared (X1 × X1), and frequency squared (X3 × X3) exerted significant effects (P < 0.05). Meanwhile, the other coefficients: frequency (X3), the interactive effect of concentration and electric field intensity (X1 × X2), the interactive effect of concentration and frequency (X1 × X3), the interactive effect of electric field intensity and frequency (X2 × X3), and electric field intensity squared (X2× X2) were not significantly influential (P > 0.05).
Response surface and contour plots
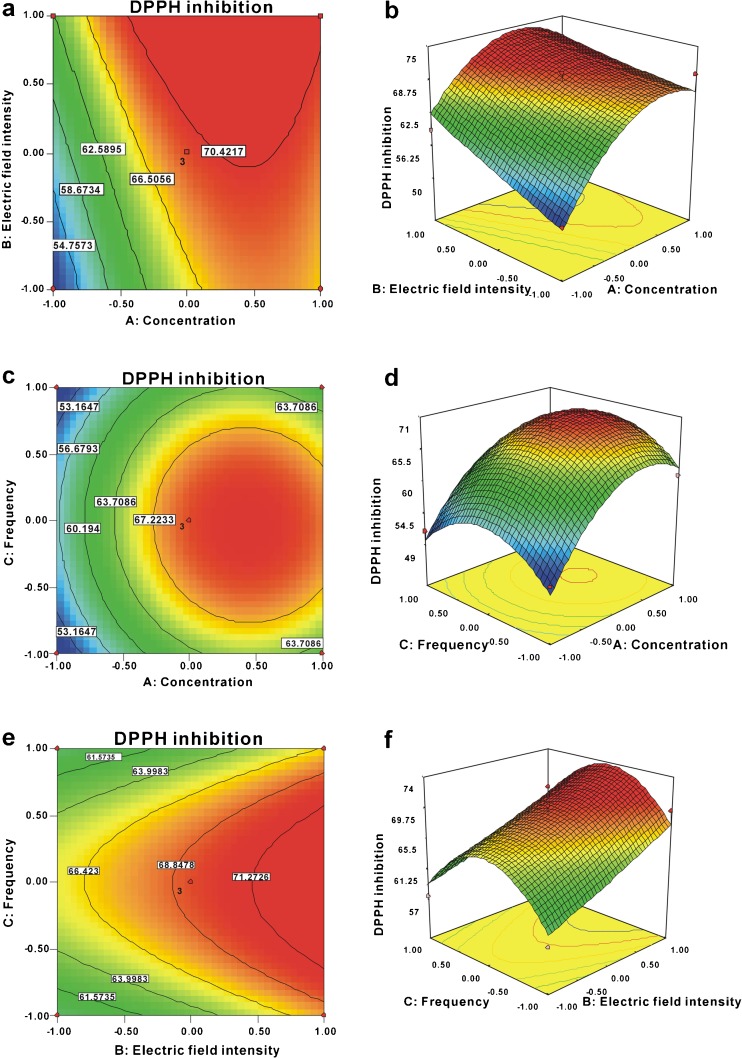
The results of response surface analysis generated by combining two of three independent variables versus the corresponding DPPH inhibition are profiled in Fig. 3. The contour plots at the bottom of the response surface presenting an elliptical shape means that the interaction effect was significant. As shown in Fig. 3a and b, for the electric field intensity lower than 10 kV cm−1, the positive effect of concentration on DPPH inhibition of CPS could be observed. And for the concentration higher than 7.50 mg mL−1, the positive effect of electric field intensity on DPPH inhibition of CPS could be observed. The effect of concentration and frequency on DPPH inhibition of CPS was illustrated in Fig. 3c and d, when the electric field intensity was fixed at 10 kV cm−1, concentration and frequency demonstrated quadratic effects on DPPH inhibition. When the frequency was kept from 2,175 Hz to 2,525 Hz, the significant quadric effect of DPPH inhibition was shown at the increase of concentration ranging from 7 to 9 mg mL−1, and DPPH inhibition obtained the highest value (69.07 %). The effect of electric field intensity and frequency on DPPH inhibition of CPS was illustrated in Fig. 3e and f, and the concentration was constant at 8 mg mL−1. The results indicated that the increase in electric field intensity led to a rise of DPPH inhibition of CPS when frequency was kept from 2,175 Hz to 2,525 Hz. By analyzing the plots, the optimal conditions for PEF treatment were 10 mg mL−1 concentration, 15 kV cm−1 electric field intensity, and 2,000 Hz frequency. In these optimal conditions, the validated experimental DPPH inhibition was 76.46 %, which matched the predicted value well.
Fig. 3.
Response surface plots and contour plots of DPPH inhibition of the CPS. The DPPH inhibition is shown as a function of the interactions between concentration and electric field intensity (a, b), concentration and frequency (c, d), or electric field intensity and frequency (e, f)
Analyzed by MIR spectroscopy
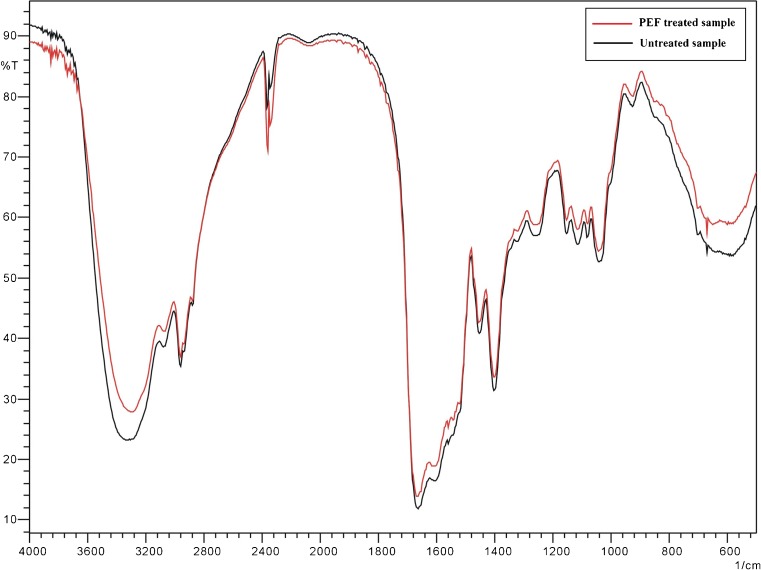
According to Fig. 4, —OH stretch was observed at 3,650 — 3,580 cm−1 and 3,400 — 3,200 cm−1, —NH2 and —NH stretch was observed at 3,500 — 3,300 and 3,400 — 3,200 cm−1, ≡CH stretch was observed at 3,300 cm−1, unsaturated C—H stretch was observed over 3,000 cm−1, saturated C—H stretch was observed at 3,000 — 2,800 cm−1, —CH3 stretch was observed at 2,960 ± 5 cm−1 and 2,870 ± 10 cm−1, —CH2 stretch was observed at 2,930 ± 5 cm−1, —C ≡ C— stretch was observed at 2,600 — 2,100 cm−1, —N ≡ N stretch was observed at 2,310 — 2,135 cm−1, =C = C— stretch was observed at 1,950 cm−1, —C = O stretch was observed at 1,850 — 1,600 cm−1, C = C stretch was observed at 1,680 — 1,620 cm−1, —NO2 stretch was observed at 1,600 — 1,500 cm−1 and 1,300 — 1,250 cm−1, C—O stretch was observed at 1,300 —1,000 cm−1, S = O stretch was observed at 1,220 — 1,040 cm−1, C —O—C stretch was observed at 1,150 — 900 cm−1. The fractions of peptide bonds in α–helical, β–sheet, and aperiodic conformations can be accurately estimated by analysis of the amide I band (1,600 — 1,700 cm−1) in the MIR region of CPS. The PEF treated sample had no change in above groups and had a slightly higher absorbance than the untreated sample from 3637.75 cm−1 to 3000.00 cm−1. It indicated that PEF processing could change the structure of peptides, so the results of MIR spectroscopy might be different with the untreated sample.
Fig. 4.
PEF treated sample and untreated sample. Mid–infrared spectroscopy was measured at a resolution of 4 cm−1, over the full mid–IR range (400 – 4,000 cm−1). The KBr sample pellets were prepared by mixing 1 mg of a sample with 200 mg of KBr. PEF treated sample and untreated sample were placed sample compartment with automatic accessory recognition at the scanning speed of 2.8 mm s−1. After background correction, all spectra were baseline corrected, and then samples spectra were obtained
Conclusions
In this paper, the effect of PEF technology on improving antioxidant activity of the CPS was observed. When evaluated using the DPPH inhibition assay, the antioxidant activity of peptides can be improved by PEF processing. Based upon the results of the OFAT experiments and optimization by RSM, it can be seen that concentration, electric field intensity, and pulse frequency had significant effects on the response values. In conclusion, a desirable quadratic polynomial mathematical model was obtained with the following optimal technical conditions for peptides: concentration of CPS 10 mg mL−1, electric field intensity 15 kV cm−1, and pulse frequency 2,000 Hz. Under these conditions, PEF technology has great influence on improving antioxidant activity of polypeptides (MW 10 to 30 kDa) from CPS. Optimized conditions showed an increase in DPPH inhibition of 32.10 %, compared to the antioxidant activity of the polypeptide without PEF treatment. It can be concluded that PEF technology could improve the antioxidant activity of antioxidant peptides from corn protein under the optimized conditions. Additionally, the change of structure and functional groups of samples were analyzed using MIR. In MIR, the PEF treated sample had a slightly higher absorbance than the untreated sample from 3637.75 cm−1 to 3000.00 cm−1.
Acknowledgments
The authors acknowledge the financial support provided by the Key Projects in the National Science & Technology Pillar Program during the Twelfth Five–Year Plan Period (2012BAD33B03) and the Youth Scientific Innovation Leading Talent and Team Building Project of Jilin Province (20140519014JH). The authors are grateful for Professor Yongguang Yin for the support PEF experiment.
Appendices
Corn peptides (CPS)
Zein peptides (ZPS)
2, 2–diphenyl–1–picrylhydrazyl (DPPH)
Pulsed electric field (PEF)
Response surface methodology (RSM)
Molecular weight (MW)
Ultra filtration (UF)
Mid–infrared spectroscopy (MIR)
Standard deviation (SD)
One–factor– at–a–time (OFAT)
Box–Behnken design (BBD)
Analysis of variance (ANOVA)
The coefficient of correlation (R2)
Footnotes
Ke Wang and Ying Wang is contributed equally to this study and should be regarded as first joint author
References
- Balasa, A., Heckelmann, A.K. & Knorr, D. (2008). Pulsed electric fields: Novel non thermal technology and its applications in food processing. Proceedings of 4th Central European Congress on Food; Cavtat/Croatia, 97–103.
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Dai YF, He ZY, Chen J, Tao GJ, Qin F. The separation, purification and anti-oxidative activity of corn peptide. Food Mach. 2008;24:5–8. [Google Scholar]
- Jin Y, Wang M, Lin SY, Guo Y, Liu JB, Yin YG. Optimization of extraction parameters for trehalose from beer waste brewing yeast treated by high-intensity pulsed electric fields (PEF) Afr J Biotechnol. 2011;82:19144–19152. [Google Scholar]
- Katalinic V, Milos M, Kulisic T, Jukic M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006;94:550–557. doi: 10.1016/j.foodchem.2004.12.004. [DOI] [Google Scholar]
- Li HM, Guo P, Hu X, Xu L, Zhang XZ. Preparation of corn (Zea mays) peptides and their protective effect against alcohol-induced acute hepatic injury in NH mice. Biotechnol Appl Biochem. 2007;47:169–174. doi: 10.1042/BA20060183. [DOI] [PubMed] [Google Scholar]
- Li HM, Hu X, Guo P, Fu P, Xu L, Zhang XZ. Antioxidant properties and possible mode of action of corn protein peptides and zein peptides. J Food Biochem. 2009;34:44–60. doi: 10.1111/j.1745-4514.2009.00292.x. [DOI] [Google Scholar]
- Lin SY, Guo Y, Liu JB, You Q, Yin YG, Cheng S. Optimized enzymatic hydrolysis and pulsed electric field treatment for production of antioxidant peptides from egg white protein. Afr J Biotechnol. 2011;55:11648–11657. [Google Scholar]
- Lin SY, Guo Y, You Q, Yin YG, Liu JB. Preparation of antioxidant peptide from egg white protein and improvement of its activities assisted by high-intensity pulsed electric field. J Sci Food Agric. 2012;92:1554–1561. doi: 10.1002/jsfa.4742. [DOI] [PubMed] [Google Scholar]
- Lin SY, Wang LY, Jones G, Trang H, Yin YG, Liu JB. Optimized extraction of calcium malate from eggshell treated by PEF and an absorption assessment in vitro. Int J Biol Macromol. 2012;50:1327–1333. doi: 10.1016/j.ijbiomac.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Lin SY, Jin Y, Liu MY, Yang YY, Zhang MS, Guo Y, Jones G, Liu JB, Yin YG. Research on the preparation of antioxidant peptides derived from egg white with assisting of high-intensity pulsed electric field. Food Chem. 2013;139:330–306. doi: 10.1016/j.foodchem.2013.01.048. [DOI] [PubMed] [Google Scholar]
- Miao FS, Yu WQ, Wang YG, Wang MJ, Liu XY, Li FL. Effects of corn peptides on exercise tolerance, free radical metabolism in liver and serum glutamic-pyruvic transaminase activity of mice. Afr J Pharm Pharmacol. 2010;4:178–183. [Google Scholar]
- Miliauskas G, Venskutonis PR, van Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–237. doi: 10.1016/j.foodchem.2003.05.007. [DOI] [Google Scholar]
- Prior RL, Hoang H, Gu LW, Wu XL, Bacchiocca M, Howard L, Hampsch-Woodill M, Huang D, Ou B, Jacob R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J Agric Food Chem. 2003;51:3273–3279. doi: 10.1021/jf0262256. [DOI] [PubMed] [Google Scholar]
- Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Byrne DH. Comparison of ABTS, DPPH, FRAP and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal. 2006;19:669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- Wang J, Wang K, Lin SY, Zhao P, Jones G, Trang H, Liu JB, Ye HQ. Improvement of antioxidant activity of peptides with molecular weights ranging from 1 to 10 kDa by PEF technology. Int J Biol Macromol. 2012;51:244–249. doi: 10.1016/j.ijbiomac.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Wang K, Wang J, Ping Z, Lin SY, Liu JB, Jones G, Huang HC. Optimized PEF treatment for antioxidant polypeptides with MW 10–30 kDa and preliminary analysis of structure change. Int J Biol Macromol. 2012;51:819–825. doi: 10.1016/j.ijbiomac.2012.07.020. [DOI] [PubMed] [Google Scholar]
- Wong SP, Leong LP, Koh JHW. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006;99:775–783. doi: 10.1016/j.foodchem.2005.07.058. [DOI] [Google Scholar]
- Xu L, Zhang XZ, Guo Y, Ren Q, Wu XX. Preparation and anti-oxidative effect of corn peptides. Chem Res Chin Univ. 2002;18:299–302. [Google Scholar]
- Yamaguchi M, Takada M, Nozaki O, Ito M, Furukawa Y. Preparation of corn peptide from corn gluten meal and its administration effect on alcohol metabolism in stroke-prone spontaneously hypertensive rats. J Nutr Sci Vitaminol. 1996;42:219–231. doi: 10.3177/jnsv.42.219. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Nishikiori F, Ito M, Furukawa Y. Effect of corn peptide on alcohol metabolism and plasma free amino acid concentrations in healthy men. Biosci Biotechnol Biochem. 1997;61:1474–1481. doi: 10.1271/bbb.61.1474. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Takeuchi M, Ebihara K. Inhibitory effect of peptide from corn gluten meal on 7,12-dimethylbenz [a] anthracene-induced mammary tumor progression in rats. Nutr Res. 1997;17:1121–1130. doi: 10.1016/S0271-5317(97)00083-3. [DOI] [Google Scholar]
- Zheng XQ, Li LT, Liu XL, Wang XJ, Lin J, Li D. Production of hydrolysate with antioxidative activity by enzymatic hydrolysis of extruded corn gluten. Appled Microbiol Biotechnol. 2006;73:763–770. doi: 10.1007/s00253-006-0537-9. [DOI] [PubMed] [Google Scholar]