Abstract
Starches separated from three black gram cultivars were modified by acetylation and compared to their native starches. Acetylation was carried out by treating starches with 0.04 and 0.08 g of acetic anhydride/g of starch dry weight basis (db) at 25 °C. The extent of acetylation increased proportionally with the concentration of acetic anhydride used. Retrogradation of acetylated starch pastes decreased significantly (p ≤ 0.05) as revealed by significant decrease in syneresis, increased freeze thaw stability and increased light transmittance. The pasting curves of 10.7 % starch slurries showed that acetylation decreased the setback viscosity values by 51.2–82.8 % and pasting temperature by 3.1–5.6 °C than respective native starches. Differential scanning calorimetry observations also revealed significant decrease in gelatinisation temperature of acetylated starches than native starches. Hardness and adhesiveness of starch gels varied between 10.3 and 32.6 g and 4.6–82.3gs, respectively which were significantly lower than corresponding native starch gels.
Keywords: Black gram starch, Acetylation, Physico-chemical, Morphological, Pasting, Texture
Introduction
The black gram (Phaseolus mungo L.) is a legume which is a good source of protein and carbohydrate (Girish et al. 2012). Starch is the most abundant carbohydrate (40–47 %) in the black gram seeds (Gopalan et al. 1995; Zia-ur-Rehman 2007). It is deposited in partially crystalline granules which differ in molecular structure and morphology between and within varieties of plant species. Starch is made up of two polymers of D-glucose: amylose, an essentially unbranched α [1→4] linked glucan, and amylopectin, which has chains of α[1→4] linked glucoses arranged in a highly branched structure with α[1→6] branching links (Copeland et al. 2009). Approximately, 60 million tonnes of starch are extracted annually worldwide from various cereal, tuber and root crops, of which roughly 60 % is used in foods and 40 % in pharmaceuticals and non-edible purposes. Starches with a wide range of functional properties are needed for such a diverse range of end uses (Copeland et al. 2009; Burrell 2003).
Starch is present as a macro-constituent in many foods and its properties and interactions with other constituents, particularly water and lipids are of interest to the food industry. Therefore, starch is an important industrial material. Native starches have certain limitations like low shear resistance, high retrogradation and syneresis thus, limiting their industrial use (Betancur et al. 1997). Therefore, chemical modification of starch is often performed in order to extend the range of specific physical properties available for certain uses (Liu et al. 1997). Chemically modified starches have considerably altered physicochemical properties with better flexibility than un-acetylated starches for use in food products. Chemical modification involving acetylation is widely used method for starch modification (Wang and Wang 2002). In acetylated starch, part of hydroxyl groups in anhydroglucose units have been converted to acetyl groups. The introduction of acetyl groups reduces the bond strength between starch molecules thereby increases the swelling power and solubility of the starch granule, decreases the retrogradation of the starch and gives improved clarity and freeze thaw stability (Chi et al. 2008; Gonzalez and Perez 2002).
The objective of the present investigation was to evaluate the effect of acetylation on physico-chemical properties of starch separated from locally available cultivars of black gram.
Material & methods
Materials
Certified seeds of two black gram cultivars (Phaseolus mungo L. cv. PU-19 and T-9) were procured from National Seed Corporation Pusa, New Delhi, India whereas cultivar Mash 1–1 was procured from Department of Plant Breeding & Genetics, Punjab Agriculture University, Ludhiana, Punjab, India. These seeds were then raised at Kulgam, Jammu and Kashmir, India on three separate plots and harvested. Seeds were cleaned from the dirt, foreign material etc. and stored until further use at 20 °C. All the reagents used in the study were of analytical grade.
Methods
Starch isolation
Starch was isolated according the method of Wani et al. (2010).
Composition
Moisture (925.10), protein (984.13), fat (920.85) and ash (923.03) contents were determined according to the methods of AOAC (1990) procedures.
Acetylation of starch
Acetylation of native starch was carried out following the method of Wang and Wang (2002) with slight modifications. Starch (100 g db) was dissolved in distilled water (185 mL) to make 35 % slurry. pH of the slurry was adjusted with 1 M NaOH to 8.0–8.5 and then mechanically stirred for 30 min. Acetic anhydride (4 g for 4 % acetylation and 8 g for 8 % acetylation ) was added drop wise to the slurry while maintaining at pH 8.0–8.5. The reaction was continued for 60 min before acidifying to pH 5.5 with 1 M HCl. The slurry was then washed with three fold distilled water three times and dried at 40 °C.
Physico-chemical properties
Acetyl (%) and degree of substitution (DS)
The percent acetylation (% acetyl) and degree of substitution (DS) were determined titrimetrically following the method of Wurzburg (1978) as described by Wani et al. (2012).
Amylose content
The amylose content of starches isolated from the different cultivars was estimated using the method of Hoover and Ratnayake (2002).
Swelling & solubility index
Swelling power and solubility of the starches were determined using 2 % dry basis (db, w/v)aqueous suspension of starch at 90 °C according to the method of Leach et al. (1959).
Water absorption capacity
Water absorption capacity (WAC) of starch was described according to the method of Sofi et al. (2013) .
Freeze thaw stability
Freeze thaw stability, (% w/w) was determined by the method of Hoover and Ratnayake (2002).
Light transmittance (%)
Light transmittance of starch paste (1 %) was determined according to the method of Wani et al. (2012).
Syneresis (%)
It was determined according to the method of Wani et al.(2010).
Scanning electron microscopy
The starch granules were placed on an adhesive tape attached to a circular aluminum specimen stub. After coating vertically with gold–palladium, the samples were photographed using a scanning electron microscope (Hitachi S-3000H, Hitachi Corporation, Tokyo Japan).
Thermal properties
Thermal properties of native and acetylated starches were analyzed using differential scanning calorimetry -DSC (200 PC-Phox Phoenix, Netzsch., Burlington, Germany) equipped with a thermal analysis data station. Starch (3.5 mg, db) was weighed into a 40 μl capacity aluminum pan and distilled water was added with the help of Hamilton microsyringe to achieve a starch–water suspension containing 70 % water. Pans were hermetically sealed and allowed to stand for 1 h at room temperature before heating in DSC. The DSC was calibrated using indium and an empty aluminium pan was used as reference. Sample pans were heated at a rate of 10 °C/min from 20 to 100 °C and gelatinization parameters viz. Onset temperature, peak temperature, conclusion temperature and enthalpy of gelatinization were calculated from the DSC curves.
Pasting properties
The pasting properties of the starches were measured using a Rapid Visco Analyzer (RVA-4, Newport Scientific Pty Ltd, Warriewood, Australia) according to approved method of AACC 2000 (76–21). Starch (3 g, 14 % moisture basis) was added to 25 ml deionized water in a RVA canister. The starch slurries were held at 50 °C for 1 min before heating from 50 to 95 °C at a rate of 12 °C /min and held at 95 °C for 2 min. The slurry was then cooled at rate of 12 °C /min to 50 °C and held for 2 min.
Starch gel texture
The textural properties of starch gels were evaluated according to the methods of Singh et al. (2006).
Statistical analysis
The data reported are averages of triplicate observations. An analysis of variance with a significance level of 5 % was done, and Duncan’s test was applied to determine differences between means using the commercial statistical package (SPSS Inc, Chicago, IL, USA).
Result and discussion
Yield and composition of native black gramstarch
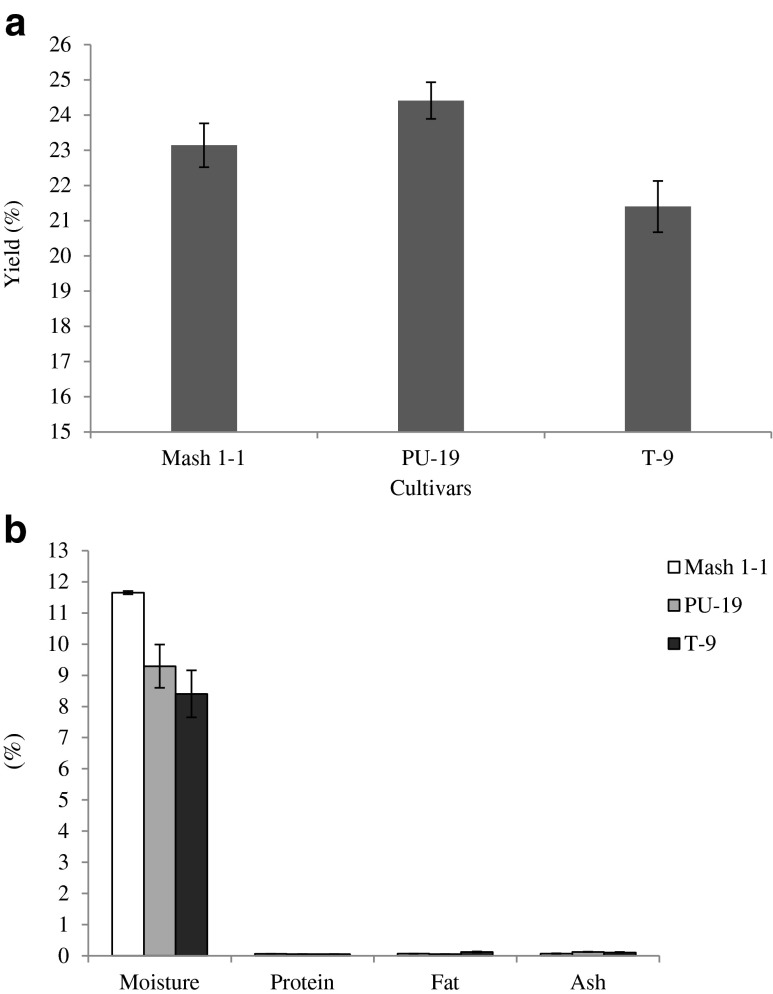
The yield of isolated starches from black gram cultivars varied from 21.4 to 24.4 % on dry basis (Fig. 1a). Literature has reported 45 % yield of starch from black gram flour (Sathe et al. 1982), and 18–45 % for most of the pulse seeds (Hoover and Ratnayake 2002). The starch yield values obtained in the present study were lower than values reported for black gram but were within the general range of pulses.
Fig. 1.
a Yield of isolated starches from black gram cultivars, b Composition of native black gram starches
Composition of native black gram starches is presented in Fig. 1b. Moisture content of native black gram starches varied from 8.4 to 11.7 %, wherein Mash 1–1 showed significantly
(p ≤ 0.05) higher value than rest of the cultivars. The ash, protein and fat contents were in the range of 0.05–0.19, 0.05–0.06 and 0.06–0.13 %, respectively. These parameters showed significant (p ≤ 0.05) differences among the cultivars. These values were well in agreement with the values previously reported for kidney bean. Kidney beans are reported to have 9.6–14.5 % moisture, 0.06–0.18 % ash, 0.1–0.6 % fat and 0.03–0.35 % protein (Wani et al. 2010; Hoover and Manuel 1996) and lentil starches (Ratnayake et al. 2001). However, the values of protein and fat were lower than reported for other pulse starches (Sirivongpaisal 2008; Hoover and Ratnayake 2002; Betancur-Ancona et al. 2001) implying that starches were pure.
Physico-chemical properties of black gram starches
Percent Acetylation (PAcet) and Degree of Substitution(DS)
Acetylation of black gram starch was also carried in similar way as in kidney bean using 0.04 and 0.08 g acetic anhydride /g of starch (Table 1). Results showed that PAcet and DS increased with increase in the concentration of acetic anhydride in the reaction medium. PAcet ranged from 0.80 to 2.09 while DS varied from 0.03 to 0.08 among the cultivars at different levels of acetylation. Cultivar Mash1-1 showed the lowest value of acetylation and DS whereas PU-19 revealed the highest. Similar results have been reported for kidney bean (PAcet 1.31–4.4 %, DS 0.05–0.17) and Canna starches (PAcet 1.41–2.55 %, DS 0.05–0.10), respectively (Saartrat et al. 2005; Hoover and Sosulski 1985). The differences in PAcet and DS may be due to differences in amylose content and intra-granular packing of starch (Islam et al. 1974). PAcet and DS values were significantly (p ≤ 0.05) different among the cultivars at different levels of acetylation.
Table 1.
Physico-chemical properties of some native and acetylated black gram starches (n = 3)
| Cultivar | Acetic anhydride g/g starch | Parameter | ||||||
|---|---|---|---|---|---|---|---|---|
| Acetylation (%) | DS | Amylose (%) | Swe. index (g/g) | Sol. index (%) | WAC (g/g) | FTS (%) | ||
| 0.00 | – | – | 40.6 ± 0.80f | 21.5 ± 0.17b | 17.5 ± 0.50de | 1.9 ± 0.04a | 61.0 ± 0.90c | |
| Mash 1–1 | 0.04 | 0.80 ± 0.01a | 0.03 ± 0.01a | 33.9 ± 0.16d | 27.0 ± 0.65d | 18.4 ± 0.01f | 1.9 ± 0.04a | 0.14 ± 0.04a |
| 0.08 | 1.59 ± 0.01d | 0.06 ± 0.01d | 33.1 ± 0.64d | 33. 9 ± 0.59f | 16.9 ± 0.43cde | 2.1 ± 0.02d | 0.11 ± 0.03a | |
| 0.00 | – | – | 30.3 ± 0.16c | 19.8 ± 0.35a | 15.3 ± 0.50a | 1.9 ± 0.06a | 58.11 ± 0.78b | |
| PU-19 | 0.04 | 1.09 ± 0.01c | 0.04 ± 0.01c | 29.6 ± 0.12c | 28.2 ± 0.54e | 17.3 ± 1.20de | 2.0 ± 0.03c | 0.14 ± 0.03a |
| 0.08 | 2.09 ± 0.02f | 0.08 ± 0.01e | 26.1 ± 0.16a | 37.9 ± 0.39g | 15.7 ± 0.33bc | 2.1 ± 0.01d | 0.07 ± 0.03a | |
| 0.00 | – | – | 39.4 ± 0.16e | 23.9 ± 0.01c | 16.3 ± 0.30bcd | 1.9 ± 0.01a | 60.9 ± 0.4c | |
| T-9 | 0.04 | 0.87 ± 0.10b | 0.33 ± 0.04b | 33.8 ± 0.67d | 34.6 ± 0.03f | 18.2 ± 0.43ef | 1.9 ± 0.03a | 0.10 ± 0.05a |
| 0.08 | 1.99 ± 0.02e | 0.08 ± 0.01e | 27.8 ± 0.83b | 42.9 ± 0..76h | 17.8 ± 0.50de | 2.0 ± 0.01c | 0.07 ± 0.02a | |
DS, Degree of substitution; Swe. Index, swelling index; Sol. Index, solubility index; FTS, Freeze thaw stability; WAC, Water absorption capacity
Values expressed are mean ± standard deviation
Means in the Column with different superscript were significantly different at p ≤ 0.05
Amylose
Apparent amylose content of native black gram starches varied from 30.33 to 40.64 % (Table 1). The highest amylose was found in Mash 1–1 while as the lowest was found in PU-19. These values were relatively lower than those reported for Pinto beans (52.4 %) and chickpeas (46.5 %) by Yanez-Farias Grelda et al. (1997) and field peas (42.9–43.7 %) by Ratnayake et al. (2001). The differences among amylose contents may be attributed to differences in enzyme activity involved in the biosynthesis of linear and branched components within the starch granules (Krossmann and Lloyd 2000). The amylose content of the starch granules has also been reported to be affected by climatic conditions and soil type during growth and granule size distribution (Singh et al. 2006). Acetylated starches had significantly (p ≤ 0.05) lower amylose content than native starches (Table 1). It might be due to introduction of acetyl groups in starch chains which impeded the formation of the helical structure of amylose in some areas by steric hindrance resulting in underestimation of amylose content (Gonzalez and Perez 2002).
Swelling and solubility index
The swelling and solubility index of native black gram starches varied with the cultivar (Table 1). Swelling index of native black gram starches varied from 19.8 to 23.9 g/g. Starch from T-9 revealed the highest swelling index and the lowest was observed for PU-19. It has been reported that swelling of starch is influenced by the amylose–lipid complexes, amylose content, interaction between starch chains within the amorphous and crystalline region of the granule, and the molecular structure of amylopectin (Zhou et al. 2004). Acetylated starches had significantly (p ≤ 0.05) higher swelling index in the range of 27.0–42.9 g/g compared to native starches. This may be due to the introduction of hydrophilic substituent groups that retained water molecules to form hydrogen bonds in the starch granules (Betancur et al. 1997). The introduction of acetyl groups in black gram starch could facilitate the access of water to amorphous areas due to intragranular structural disorganization caused by steric effects and disruption of hydrogen bonds in the starch granules. Increase in swelling of maize starch (Liu et al. 1997) and rice starch (Shon and Yoo 2006) after acetylation has been reported.
Solubility index of native black gram starches was in the range of 15.3–17.5 % (Table 1). The highest solubility index was observed for Mash1-1 and the lowest for PU-19 starch. This corresponds to the highest and the lowest amylose contents found in these cultivars as solubility is contributed by extent of amylose in the starch. Solubility index of acetylated starches was significantly (p ≤ 0.05) higher than native starches and varied from15.7 to 18.4 % among the cultivars. An increase in solubility can be attributed to the increased hydrophilicity of the starch. The increase in solubility index of maize starch (Liu et al. 1997) and rice starch (Shon and Yoo 2006) has been reported after acetylation.
Water absorption capacity
WAC of native and acetylated black gram starches were 1.9–2.0 and 1.9–2.1 g/g, respectively for cultivars studied (Table 1). Acetylated starches showed significantly (p ≤ 0.05) higher water absorption capacity than corresponding native starches in all the cultivars except Mash 1–1. Adebowale et al. (2006) reported WAC of sword bean starch increased significantly from 2.2 g/g to 2.5 g/g after acetylation. The lower water absorption capacity of native starches than acetylated starches might be related to a close association of native polymers. In acetylated starches there was weakening of intermolecular hydrogen bonds in starch with the introduction of acyl groups resulting in increased water binding.
Freeze thaw stability
Freeze thaw stability of native black gram starch gels were in the range of 58.1–61.0 % (Table 1). Results revealed significant (p ≤ 0.05) higher freeze thaw stability in PU-19 and the lower in Mash 1–1. It might be attributed to the lowest and the highest amylose content respectively, found in these two cultivars since amylose is largely responsible for retrogradation behaviour of starches. The amount of water released could be due to increased intermolecular and intramolecular hydrogen bonding due to interaction between starch chains (amylose-amylose, amylose-amylopectin and amylopectin-amylopectin) during frozen storage. Hoover and Ratnayake (2002) reported freeze thaw stability (64–72 %) for different black bean cultivars. Freeze thaw stability of acetylated black gram starches was significantly (p ≤ 0.05) lower than native black gram starches and varied in the range of 0.06–0.14 % syneresis (Table 1). This suggests that during frozen storage, interactions between starch chains occur either slowly or of very low magnitude in acetylated starches (Liu et al. 1999; Perera and Hoover 1999). The high freeze thaw stability shown by acetylated black gram starches makes them suitable for use in foods requiring frozen storage.
Light transmittance
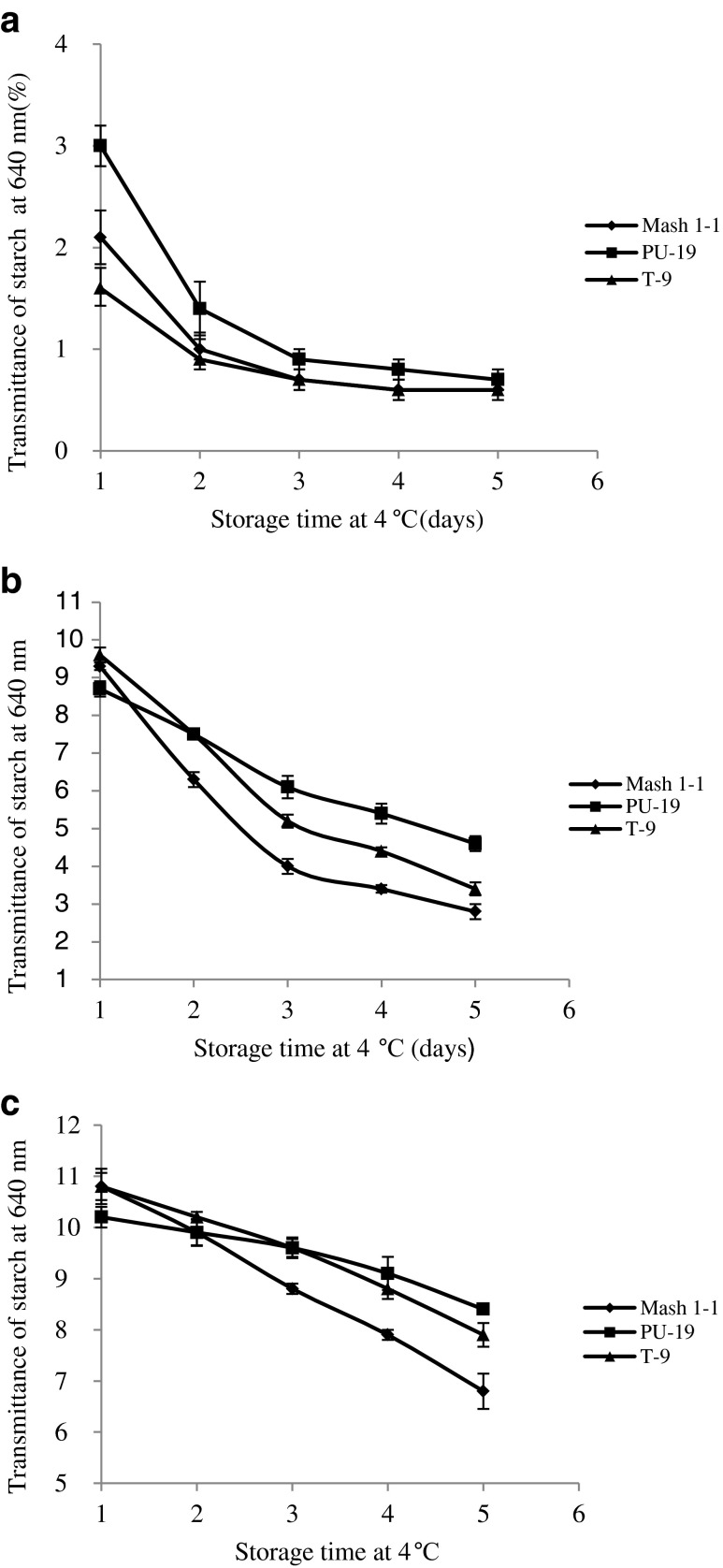
The light transmittance of native and acetylated starch gels decreased during refrigerated storage of 120 h (Fig. 2). The decrease in light transmittance may be attributed to retrogradation of starch gels. The light transmittance of native starch gels decreased sharply from 1.6–3.0 to 0.6–0.7 % during 120 h of refrigerated storage. Mash 1–1 and T-9 starches displayed significant decrease in light transmittance upto third day of storage and then remained constant while starch from PU-19 showed decrease even upto fifth day. Also PU-19 had the highest transmittance among the cultivars which is attributed to its lowest amylose content. Factors, like granule swelling, granule remnants, leached amylose and amylopectin, amylose and amylopectin chain lengths, intra or intermolecular bonding, lipids and substitution have been reported to be responsible for turbidity development in starches during storage (Jacobson et al. 1997). Jane et al. (1996) reported that legume starches contained varying amounts of phosphate monoester substituents, which might also have contributed to variation in light transmittance. Light transmittance of acetylated starches decreased from 8.5 to 10.8 % after 24 h to 2.8–8.4 % after 120 h of refrigerated storage. Acetylated black gram starches had significantly (p ≤ 0.05) higher light transmittance than native starch. Increase in light transmittance after acetylation could be due to chemical substitution of the hydroxyl groups in starch molecules by the acetyl moiety. This causes repulsion between adjacent starch molecules and apparently reduces interchain association, which facilitates improved transmittance. Garg and Jana (2011) also reported increase in transmittance of corn starches after acetylation.
Fig. 2.
Light transmittance of starch pastes (1 %) from some acetylated black gram starch, a 0.0 g acetic anhydride/ g starch, b 0.04 g acetic anhydride/ g starch, c 0.08 g acetic anhydride/g starch
Syneresis
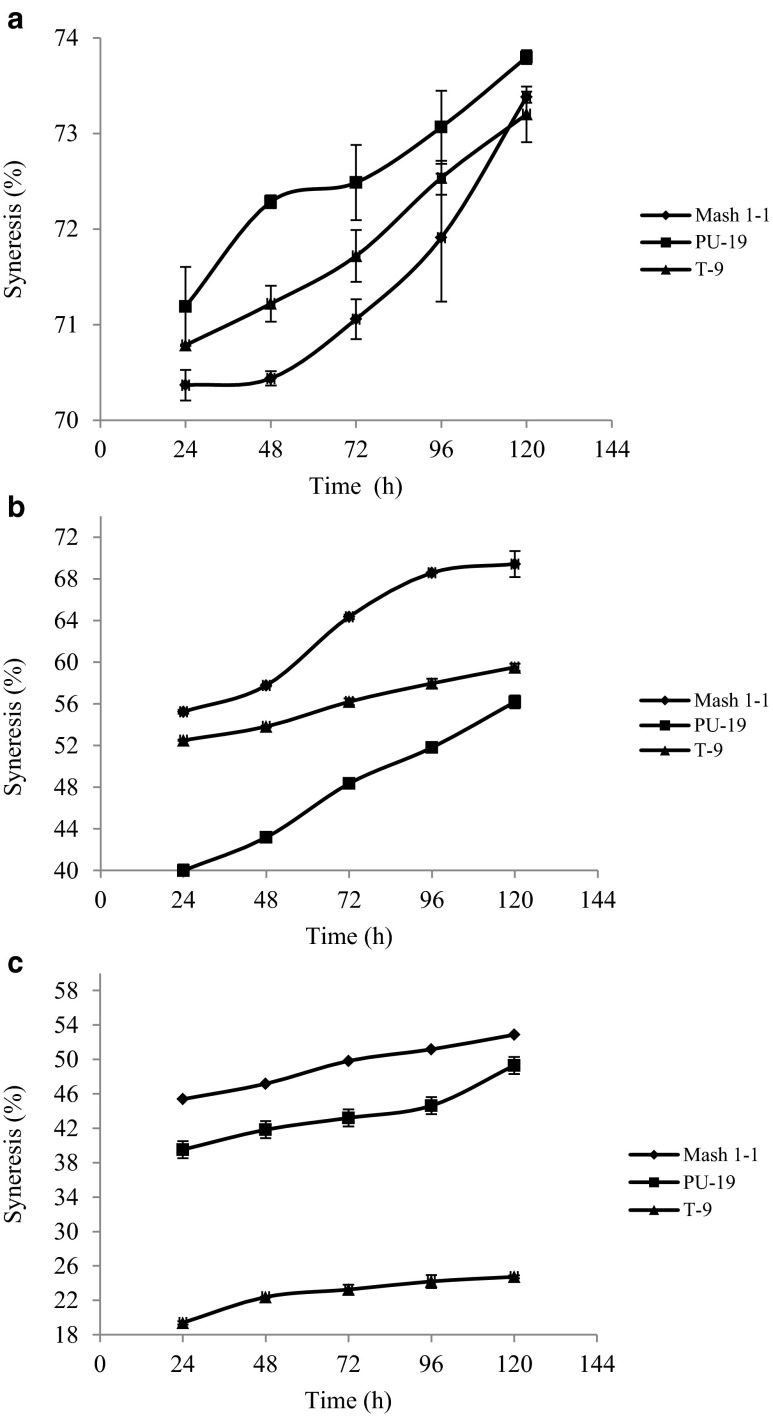
Syneresis (%) of native and acetylated starch gels increased with increase in storage up to 120 h (Fig. 3). The increase in syneresis during storage could be the result of increased intra and inter molecular hydrogen bonding leading to aggregation of the starch chains, with increased release of water from starch gels. In native starch gels the highest syneresis (71.9 %) was observed in PU-19 while as the lowest syneresis (70.4 %) was displayed by Mash 1–1 during first 24 h of storage. Further storage upto 120 h increased syneresis upto 73.2–73.8 %. High syneresis in black gram cultivars might be due to high amylose content, the interaction between leached out amylose and amylopectin chains leading to gel shrinkage (Hermansson and Svegmark 1996). Syneresis of acetylated starch gels increased from 19.4 to 55.3 % after 24 h of storage to 24.7–69.4 % after 120 h of storage. It was significantly (p ≤ 0.05) lower than native starch gels. This may be attributed to the presence of acetyl groups which may cause steric hindrances in re-association of amylose, resulting in increased water retention capacity of the starch molecules during refrigerated storage. Significant (p ≤ 0.05) differences in syneresis (%) were observed in native and acetylated starch gels. Hoover and Sosulski (1985) also observed decreasing trend in syneresis of acetylated bean starch gels.
Fig. 3.
Syneresis (%) of starch pastes (2 %) from some acetylated black gram starch, a 0.0 g acetic anhydride/ g starch, b 0.04 g acetic anhydride/ g starch, c 0.08 g acetic anhydride/g starch
Granular structure
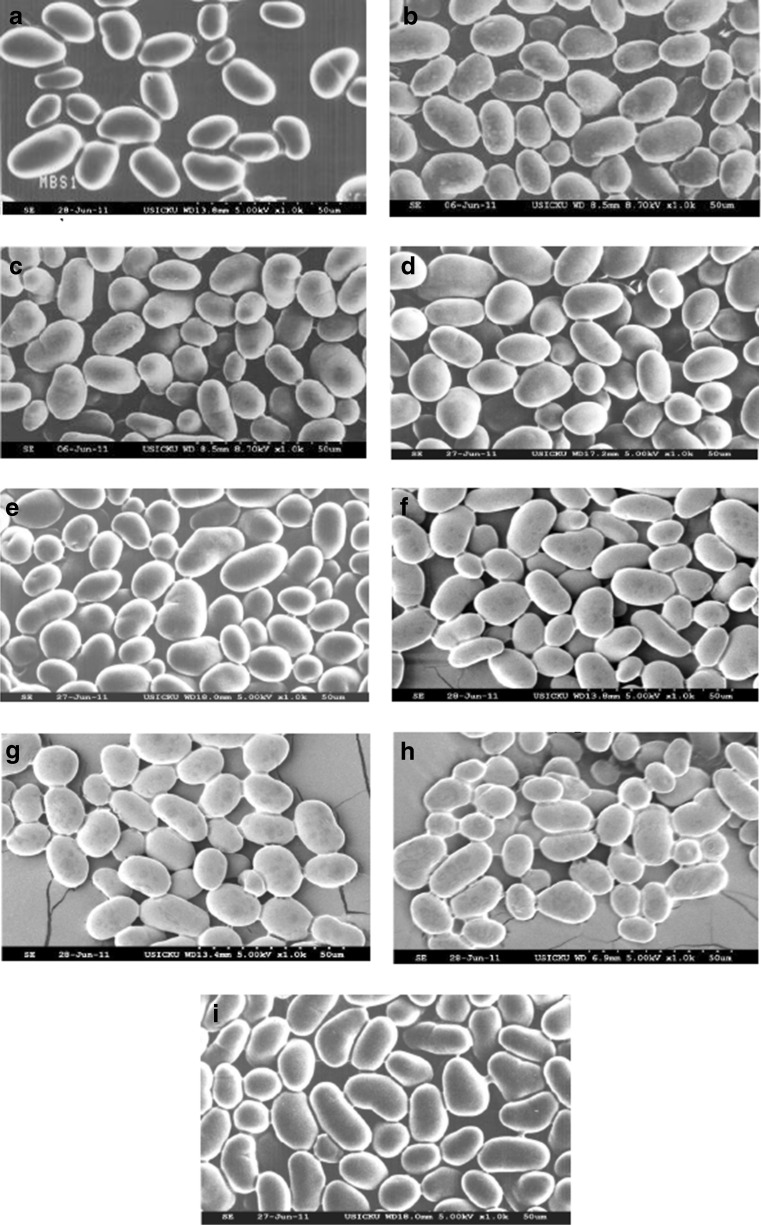
The scanning electron micrographs of native and acetylated black gram starches showed starch granules were round, elliptical and oval shaped with smooth surfaces (Fig. 4). Mean granule length, length range, mean granule width and width range of native and acetylated black gram starches were between 17.0–18.6, 9.1–26.4, 10.0–12.7 and 6.4–15.5 μm, respectively (Table 2). Mash 1–1 starch has the largest mean granule length (17.6 μm), while the lowest (17.0 μm) was observed in T-9 cultivar. PU-19 has the largest (11.8 μm) while T-9 has the lowest (10.7 μm) width of starch granules. Significant (p ≤ 0.05) differences were neither observed in size of native starch granules among the cultivars nor between native and acetylated starch granules. Sathe et al. (1982) reported length in range of 7.5–28.5 μm and width in the range of 7.5–27.0 μm for black gram starch. So, the results are in agreement with the range reported by different authors. It has been reported that size and shape starch granules influence the thermal properties (Wang et al. 2005; Noda et al. 1998).
Fig. 4.
Scanning electron micrographs (SEM) of native and acetylated mash bean starch; a Mash1-1 (0.00 g acetic anhydride /g starch), b PU-19 (0.00 g acetic anhydride /g starch), c T-9 (0.00 g acetic anhydride /g starch), d Mash1-1 (0.04 g acetic anhydride /g starch), e PU-19 (0.04 g acetic anhydride /g starch), f T-9 (0.04 g acetic anhydride /g starch), g Mash1-1 (0.04 g acetic anhydride /g starch), h PU-19 (0.04 g acetic anhydride /g starch), i T-9 (0.04 g acetic anhydride /g starch)
Table 2.
Morphological properties of some native and acetylated black gram starches (n = 10)
| Parameter | Acetic anhydride g/g starch | Cultivars | ||
|---|---|---|---|---|
| Mash 1–1 | P U-19 | T-9 | ||
| 0.00 | 17.6a | 17.3a | 17.0 a | |
| Mean granule length (μm) | 0.04 | 17.3 a | 18.6 a | 17.5 a |
| 0.08 | 17.1a | 17.1a | 17.7a | |
| 0.00 | 10.9–26.4a | 9.1–22.7a | 11.8–23.6a | |
| Length range (μm) | 0.04 | 10.0–26.4a | 7.3–25.5a | 9.1–25.5a |
| 0.08 | 11.8–22.7a | 10.0–23.6a | 13.6–27.3a | |
| 0.00 | 11.6a | 11.8a | 10.7a | |
| Mean granule width (μm) | 0.04 | 10.0a | 11.7a | 11.4a |
| 0.08 | 12.7a | 11.4a | 11.5a | |
| 0.00 | 9.1–15.5a | 6.4–15.5a | 7.3–14.6a | |
| Width range (μm) | 0.04 | 10.0–14.6a | 6.4–15.5a | 8.2–14.6a |
| 0.08 | 9.1–19.1a | 7.3–14.6a | 9.1–11.8a | |
Values expressed are mean ± standard deviation
Thermal properties
Thermal properties of native and acetylated black gram starches are presented in Table 3. The gelatinization temperatures of native starches varied from 67.8 to 77.3 °C and for acetylated starches it was in the range of 62.3–74.6 °C. Significant (p ≤ 0.05) differences were observed in To, Tp, Tc and ∆Hgel value of native and acetylated black gram starches. Acetylated starches displayed significantly (p ≤ 0.05) lower gelatinisation temperatures than the corresponding native starches. The decrease was highest in PU-19 with maximum degree of substitution and lowest for Mash 1–1 with minimum degree of substitution among the cultivars. This is attributed to the introduction of acetyl groups which interrupts the ordered structure of native starch, leading to decreased gelatinization temperature (Wang and Wang 2002). To of native black gram starches was in the range of 67.8–69.8 °C wherein cultivar Mash 1–1 displayed highest value. Acetylated starches had To of 66.3–66.7 °C. It has been reported that To represent the melting of the weakest crystallites (Nakazawa and Wang 2003; Wang et al. 1997). Su et al. (1997) reported To of 69.9 °C for Adzuki bean while Deshpande et al. (1982) reported To of 71.5 °C and 63.0 °C correspondingly for native and acetylated black gram starch. Our results are comparable to the results reported by these authors. Tp of native starches was in the range of 70.6–73.2 °C while acetylated starches had 63.0–71.1 °C. Native and acetylated black gram starch reported Tp of 73.0 °C and 66.0 °C, respectively (Deshpande et al. 1982). Tc of native black gram starches was observed in the range of 73.5–77.9 °C while acetylated starches had lower Tc (69.8–74.6 °C). Tc represents melting of crystallites of high stability (Jacobs and Delcour 1998). Su et al. (1997) reported Tc of 79.9 °C and 76.6 °C for kidney and Azduki bean respectively. Starch from PU-19 had lowest transition (To, Tp and Tc) temperature among the cultivars under study. This might be attributed to its lowest amylose content. To, Tp, and Tc have been reported to be influenced by amylose content (Protserov et al. 2000; Visser et al. 1997) distribution of amylopectin chains (Noda et al. 1998), and lipid complexed amylose chains (Hoover and Ratnayake 2002; Jayakody et al. 2005). ∆Hgel. of native black gram starch varied from 8.9 to 9.9 J/g while acetylated starches had 6.1–9.2 J/g. The enthalpy of gelatinization was observed to be highest for T-9 starch, whereas it was lowest for Mash 1–1. The ∆Hgel gives an overall measure of crystallinity and is an indicator of the loss of molecular order within the granule during gelatinisation (Hoover and Vasanthan 1994; Cooke and Gidley 1992). A lower ∆Hgel suggests a lower degree of organization in, or a lower stability of the crystals (Chiotelli and Meste 2002).
Table 3.
Thermal properties of of some native and acetylated black gram starches (n = 2)
| Starch source | Acetic anhydride g/g starch | To (°C) | Tp(°C) | Tc(°C) | ΔHgel(J/g) |
|---|---|---|---|---|---|
| 0.00 | 69.8 ± 0.8f | 73.2 ± 0.5c | 76.6 ± 0.4e | 9.8 ± 0.4c | |
| Mash 1–1 | 0.04 | 66.3 ± 0.8d | 69.2 ± 0.7c | 71.8 ± 0.5b | 8.9 ± 0.5bc |
| 0.08 | 65.2 ± 0.8c | 68.7 ± 0.5bc | 70.7 ± 0.8a | 8.2 ± 0.5b | |
| 0.00 | 67.8 ± 0.2e | 70.6 ± 0.6d | 73.5 ± 0.3c | 8.9 ± 0.6bc | |
| PU-19 | 0.04 | 63.7 ± 0.2b | 67.7 ± 0.9b | 71.7 ± 0.5b | 8.1 ± 0.8b |
| 0.08 | 62.3 ± 0.9a | 63.0 ± 0.6a | 69.8 ± 0.5a | 8.7 ± 0.6bc | |
| 0.00 | 68.4 ± 0.3e | 71.6 ± 0.4d | 77.3 ± 0.6e | 9.9 ± 0.4c | |
| T-9 | 0.04 | 66.5 ± 0.3d | 70.5 ± 0.7d | 74.6 ± 0.5d | 9.2 ± 0.7bc |
| 0.08 | 66.7 ± 0.6d | 71.1 ± 0.7d | 72.4 ± 0.5b | 6.1 ± 0.9a |
To, onset temperature; Tp, peak temperature, Tc, conclusion temperature: ∆Hgel, enthalpy of gelatinization (d.b)
Values expressed are mean ± standard deviation
Means in the column with different superscripts are significantly (p ≤ 0.05) different from one another
Pasting properties
Pasting properties of native and acetylated black gram starches are given in Table 4. Peak viscosity of native black gram starches varied from 5109.3 to 5685.0 cP. The highest peak viscosity was observed for PU-19 and the lowest for Mash 1–1. Peak viscosity has been reported to be influenced by the extent of amylose leaching, amylose-lipid complex formation, friction between swollen granules, granule swelling, and competition between leached amylose and remaining un-gelatinized granules for free water (Chung et al. 2008; Liu et al. 1997). Peak viscosity of acetylated black gram starches was significantly (p ≤ 0.05) lower (4039.0–4600.3 cP) than native starches. Decrease in peak viscosity of Canna starches after acetylation has been reported (Saartrat et al. 2005). However, these results were contrary to some other investigators (Wani et al. 2012; Liu et al. 1999; Betancur et al. 1997) who reported increase in viscosity when the acetyl groups were introduced into starch granules. Decrease in peak viscosities of acetylated black gram starches might be due to their chemical composition as well as the hydrophobic behaviour of acetyl group. Legume starches are reported to contain varying amount of phosphate groups (Jane et al. 1996). Phosphate groups are reported to have profound influence on pasting properties of potato starches (Jane et al. 1996; Galliard and Bowler 1987). Trough viscosity of native starches (Hot paste viscosity) ranged from 3426.3 to 3675.3 cP, wherein PU-19 starch showed the highest value (3675.3 cP) and T-9 starch displayed the lowest value (3426.3 cP). Trough viscosity of cultivar Mash 1–1 and T-9 did not vary significantly. Trough viscosity of acetylated black gram starches was observed from 2401.0 to 2832.3 cP and was significantly (p ≤ 0.05) lower than respective native starches. Trough viscosity has been reported to be influenced by the extent of amylose leaching, amylose-lipid complex formation, friction between swollen granules, granule swelling and competition between leached amylose and remaining granules for free water (Olkku and Rha 1978). Breakdown viscosity of native starches ranged from 1682.3 to 2009.7 cP. Lowest breakdown viscosity was observed for Mash 1–1 (1682.3 cP) and the highest presented by PU-19 (2009.7 cP). Breakdown viscosity of acetylated starches varied from 1389.7 to 1914.3 cP and it was significantly lower than native starches. Breakdown viscosity is the measure of the susceptibility of cooked starch to disintegration. This indicates that acetylated starches have more resistance to shear and the acetylated starch granules have less susceptibility to disintegration than native black gram starches. Final viscosity (Cool paste viscosity) of native black gram starches varied significantly from 4796.0 to 5277.0 cP. It is the measure of the ability of starch to form a viscous paste. Final viscosity of acetylated starches varied from1389.7 to 1914.3 cP. It was significantly (p ≤ 0.05) lower than native starches. Setback viscosity of native black gram starches decreased significantly from 1239.3 to 1850.7 cP to 318.0–604.7 cP on acetylation. Among the native black gram starches PU-19 showed the lowest value (1239.3 cP) and T-9 displayed highest value (1850.7 cP). Significantly lower setback viscosities of acetylated starches than their native counterparts might be due to the introduction of acetyl groups that restricted the tendency of the starch molecules to realign after cooling.
Table 4.
Pasting properties of some native and acetylated black gram starches (n = 3)
| Cultivars | Acetic anhydride g/g starch | Peak viscosity (cP) | Trough viscosity (cP) | Breakdown viscosity (cP) | Final viscosity (cP) | Setback viscosity (cP) | Pasting temperature (°C) |
|---|---|---|---|---|---|---|---|
| 0.00 | 5109.3 ± 31.5f | 3427.0 ± 25.0f | 1682.3 ± 56.5cd | 4769.0 ± 23.0f | 1342.0 ± 2.0f | 75.1 ± 0.1g | |
| Mash 1–1 | 0.04 | 4121.0 ± 28.0b | 2731.3 ± 13.5d | 1389.7 ± 14.5a | 3254.7 ± 12.5d | 523.3 ± 26.0c | 73.0 ± 0.4e |
| 0.08 | 4052.0 ± 36.0a | 2401.0 ± 25.0a | 1651.0 ± 11.0c | 2826.7 ± 2.5a | 425.7 ± 22.5b | 70.7 ± 0.5c | |
| 0.00 | 5685.0 ± 22.0h | 3675.3 ± 43.5g | 2009.7 ± 65.5f | 4915.0 ± 48.5f | 1239.3 ± 5.0e | 73.5 ± 0.1f | |
| PU-19 | 0.04 | 4039.0 ± 1.5a | 2576.3 ± 23.5b | 1463.0 ± 25.0b | 3181.0 ± 12.0c | 604.7 ± 35.5d | 69.8 ± 0.4b |
| 0.08 | 4308.3 ± 19.5d | 2600.3 ± 0.6b | 1708.0 ± 19.0cd | 3008.7 ± 25.5b | 408.3 ± 25.0b | 67.9 ± 0.1a | |
| 0.00 | 5162.0 ± 59.0g | 3426.3 ± 8.5f | 1735.7 ± 50.5d | 5277.0 ± 49.0g | 1850.7 ± 57.5g | 77.5 ± 0.0h | |
| T-9 | 0.04 | 4229.0 ± 9.0c | 2832.3 ± 10.5e | 1390.7 ± 9.3a | 3328.3 ± 49.7e | 496.0 ± 39.7c | 73.5 ± 0.4f |
| 0.08 | 4600.3 ± 16.5e | 2686.0 ± 10.0c | 1914.3 ± 26.5e | 3004.0 ± 46.9b | 318.0 ± 56.2a | 71.1 ± 0.0d |
Values expressed are mean ± standard deviation
Means in the with different superscript were significantly different at p ≤ 0.05
Pasting temperature of native black gram starches varied from 73.5 to 77.5 °C. PU-19 presented the lowest value and T-9 the highest value. Pasting temperature showed consistency with DSC values. Pasting temperature of the acetylated starches were significantly (p ≤ 0.05) lower (67.9–73.5 °C) than respective native starches (73.5–77.5 °C). Lowering of the pasting temperature of acetylated starches might be due to the insertion of acetyl groups into the starch molecules, especially into the amorphous region (Rutenberg MW Solarek 1984). Therefore, swelling of the acetylated starches was easier than the native starches. Similar results for pasting temperature of acetylated starches have been reported in earlier studies (Gonzalez and Perez 2002; Betancur et al. 1997; Liu et al. 1997). This characteristic is advantageous where a thickening agent must gel at lower temperatures, or simply to reduce energy costs during the manufacture of products where these starches are used (Betancur et al. 1997).
Starch gel texture
Texture profile analysis of native and acetylated starch gels are presented in Table 5. Texture profile analysis revealed native starch gels varied in hardness from 65.2 to 84.7 g. Gels prepared from T-9 had the highest hardness, while gels from PU-19 displayed the lowest hardness among the cultivars. van Luyten et al. (1992) reported that extent of the amylose gel network and deformability of swollen granules are the main factors contributing to gel strength. Hardness of acetylated starch gels was significantly (p ≤ 0.05) lower (10.3–32.6 g) than native starches. Starches with higher degrees of substitution showed higher percent decrease in hardness of their gels. This can be attributed to the inhibition of amylose chain interactions reducing the formation of junction zones leading to the formation of a weaker gel.
Table 5.
Texture analysis of of some native and acetylated black gram starches (n = 3)
| Cultivars | Acetic anhydride g/g starch | Hardness (g) | Cohesiveness | Gumminess (g) | Springiness | Chewiness (g) | Adhesiveness (g.s) |
|---|---|---|---|---|---|---|---|
| 0.00 | 68.1 ± 7.3a | 0.4 ± 0.1bc | 23.9 ± 5.8de | 0.9 ± 0.1a | 2 1.6 ± 6.6cd | 185.3 ± 12.3e | |
| Mash 1–1 | 0.04 | 31.4 ± 0.7de | 0.5 ± 0.1bc | 15.0 ± 3.0bc | 0.9 ± 0.0a | 13.8 ± 2.9ab | 82.3 ± 2.6c |
| 0.08 | 16.7 ± 0.6ab | 0.6 ± 0.1c | 10.2 ± 1.5ab | 0.9 ± 0.0a | 9.0 ± 1.6b | 7.7 ± 0.5a | |
| 0.00 | 65.2 ± 0.6f | 0.3 ± 0.1a | 21.5 ± 6.5cd | 0.9 ± 0.1a | 19.0 ± 8.3bcd | 108.0 ± 26.3d | |
| PU-19 | 0.04 | 24.6 ± 2.0cd | 0.6 ± 0.1c | 14.7 ± 1.3bc | 0.9 ± 0.1a | 13.2 ± 1.8ab | 44.2 ± 1.2b |
| 0.08 | 10.3 ± 0.4a | 0.7 ± 0.0de | 7.6 ± 0.6a | 0.9 ± 0.0a | 6.6 ± 0.7a | 9.8 ± 1.7 a | |
| 0.00 | 84.7 ± 9.5g | 0.3 ± 0.1a | 28.7 ± 5.2de | 0.9 ± 0.1a | 26.3 ± 5.4c | 169.6 ± 14.2e | |
| T-9 | 0.04 | 32.6 ± 3.6e | 0.6 ± 0.0b | 20.0 ± 2.1cd | 0.9 ± 0.1a | 19.1 ± 1.6bcd | 92.8 ± 4.5cd |
| 0.08 | 20.4 ± 2.0 bc | 0.8 ± 0.1e | 16.0 ± 0.5 bc | 0.9 ± 0.1a | 14.1 ± 0.8abc | 4.6 ± 0.4a |
Values expressed are mean ± standard deviation
Means in the column with different superscripts are significantly (p ≤ 0.05) different from one another
Cohesiveness, gumminess and chewiness of native starches gels of different black gramcultivars ranged from 0.3 to 0.4, 21.5–28.7 g and 19.0–26.3 g, respectively. Acetylated starch gels had cohesiveness, gumminess and chewiness of 0.5–0.8, 7.6–20.0 g and 6.6–19.1 g, respectively. Significant (p ≤ 0.05) differences were found in cohesiveness, gumminess and chewiness between native and acetylated starch gels. Adhesiveness of black gram starches decreased significantly from 107.95 to 169.61 gs to 4.6–92.8 gs. The variations in textural properties of starch gels are mainly influenced by variations in the rheological characteristics of the amylose matrix, the volume fraction and rigidity of gelatinized starch granules, and the phosphorus content, as well as the interactions between the dispersed and continuous phases of the gel (Biliaderis 1998). These factors, in turn, have been reported to be dependent on the amylose content and the structure of amylopectin (Yamin et al. 1999). The results obtained from texture analysis revealed a great difference between textures of native and acetylated black gram starches and confirmed that the retrogradation of black gram starches could be extensively reduced by acetylation.
Conclusion
The study on properties of acetylated black gram starches has shown that acetylation (in a range of 0.80–2.09 % acetyl group) induces gelatinization temperature reduction (3.1–5.6 οC). Retrogradation of black gram starch pastes and gels revealed significant decrease as evidenced by setback viscosities, syneresis, freeze thaw stability and hardness of gels by texture analysis. Hence, the starches can be used as thickening and stabilizing agents in food products such as sauces, ice creams, fruit jellies, and baked products as the acetyl % is within permissible limits (maximum FDA permissible level of acetyl in foods has been set at 2.5 %).
References
- AACC (2000) Approved methods of the American association of cereal chemists (10th ed., Vols. I & II)
- Adebowale KO, Afolabi TA, Olu-Owolabi BI. Functional, physicochemical and retrogradation properties of sword bean (Canavalia gladiata) acetylated and oxidized starch. Carbohydr Polym. 2006;65:93–102. doi: 10.1016/j.carbpol.2005.12.032. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 15. Washington, DC: Association of Official Analytical Chemists; 1990. [Google Scholar]
- Betancur AD, Chel GL, Canizares HE. Acetylation and characterization of Canavalia ensiformis starch. J Agric Food Chem. 1997;45:378–382. doi: 10.1021/jf960272e. [DOI] [Google Scholar]
- Betancur-Ancona DA, Guerrero LAC, Matos RC, Ortiz GD. Physicochemical and functional characterization of Baby Lima bean (Phaseolus lunatus) starch. Starch-Starke. 2001;53:219–226. doi: 10.1002/1521-379X(200105)53:5<219::AID-STAR219>3.0.CO;2-R. [DOI] [Google Scholar]
- Biliaderis CG. Structures and phase transitions of starch polymers. In: Walter RH, editor. Polysaccharide association structures in food. New York: Marcel Dekker; 1998. pp. 57–168. [Google Scholar]
- Burrell M M (2003) Starch: the need for improved quality & quantity: an overview. Journal of Experimental Botany 451–456 [DOI] [PubMed]
- Chi H, Xu K, Wu X, Chen Q, Xue D, Song C, Zhang W, Wang P. Effect of acetylation on the properties of corn starch. Food Chem. 2008;106:923–928. doi: 10.1016/j.foodchem.2007.07.002. [DOI] [Google Scholar]
- Chiotelli E, Meste ML. Effect of small and large wheat starch granules on thermo mechanical behaviour of starch. Cereal Chem. 2002;79:286–293. doi: 10.1094/CCHEM.2002.79.2.286. [DOI] [Google Scholar]
- Chung HJ, Liu Q, Donner E, Hoover R, Warkentin TD, Vandenberg B. Composition, molecular structure, properties, and in vitro digestibility of starches from newly released Canadian pulse cultivars. Cereal Chem. 2008;85(4):471–479. doi: 10.1094/CCHEM-85-4-0471. [DOI] [Google Scholar]
- Cooke D, Gidley MJ. Loss of crystalline and molecular order during starch gelatinization: origin of the enthalpic transition. Carbohydr Res. 1992;227:103–112. doi: 10.1016/0008-6215(92)85063-6. [DOI] [Google Scholar]
- Copeland L, Blazek J, Selman H, Tang CM. Form and functionality of starch. Food Hydrocoll. 2009;23(6):1527–1534. doi: 10.1016/j.foodhyd.2008.09.016. [DOI] [Google Scholar]
- Deshpande SS, Sathe SK, Rangnekar PD, Salunkhe DK. Functional properties of modified black gram (Phaseolus mungo L.) starch. J Food Sci. 1982;47:1528–1533. doi: 10.1111/j.1365-2621.1982.tb04975.x. [DOI] [Google Scholar]
- Galliard T, Bowler P. Morphology and composition of starch. In: Galliard T, editor. Starch: properties and potential; critical reports on applied chemistry. New York: Wiley; 1987. pp. 55–78. [Google Scholar]
- Garg S, Jana AK. Characterization and evaluation of acetylated starch with different acyl groups and degrees of substitution. Carbohydr Polym. 2011;83:1623–1630. doi: 10.1016/j.carbpol.2010.10.015. [DOI] [Google Scholar]
- Girish TK, Pratape VM, Prasada Rao UJS. Nutrient distribution, phenolic acid composition, antioxidant and alpha-glucosidase inhibitory potentials of black gram (Vigna mungo L.) and its milled by-products. Food Res Int. 2012;42:627–635. [Google Scholar]
- Gonzalez Z, Perez E. Effect of acetylation on some properties of rice starch. Starch. 2002;54:148–154. doi: 10.1002/1521-379X(200204)54:3/4<148::AID-STAR148>3.0.CO;2-N. [DOI] [Google Scholar]
- Gopalan C, Ramasastri BV, Balasubramanian SC. Nutritive value of Indian foods. Hyderabad: National Institute of Nutrition, ICMR; 1995. [Google Scholar]
- Hermansson AM, Svegmark K. Developments in the understanding of starch functionality. Trends Food Sci Technol. 1996;7:345–353. doi: 10.1016/S0924-2244(96)10036-4. [DOI] [Google Scholar]
- Hoover R, Manuel H. A comparative study of the physicochemical properties of starches from two lentil cultivars. Food Chem. 1996;53:275–284. doi: 10.1016/0308-8146(95)93933-I. [DOI] [Google Scholar]
- Hoover R, Ratnayake WS. Starch characteristics of black bean, chick pea, lentil, navy bean and pinto bean cultivars grown in Canada. Food Chem. 2002;78:489–498. doi: 10.1016/S0308-8146(02)00163-2. [DOI] [Google Scholar]
- Hoover R, Sosulski F. Studies on the functional characteristics and digestibility of starches from phaseolus vulgaris biotypes. Starch. 1985;37:181–191. doi: 10.1002/star.19850370602. [DOI] [Google Scholar]
- Hoover R, Vasanthan T. The effect of annealing on the physicochemical properties of wheat, oat, potato and lentil starches. J Food Biochem. 1994;17:303–325. doi: 10.1111/j.1745-4514.1993.tb00476.x. [DOI] [Google Scholar]
- Islam MN, Rutledge JE, James WH. Influence of rice crystallinity on cross-linking. Cereal Chem. 1974;51:51–56. [Google Scholar]
- Jacobs H, Delcour JA. Hydrothermal Modifications of granular starch, with retention of the granular structure. Review J Agric Food Chem. 1998;46(8):2895–2905. doi: 10.1021/jf980169k. [DOI] [Google Scholar]
- Jacobson MR, Obanni M, BeMiller JN. Retrogradation of starches from different botanical sources. Cereal Chem. 1997;74:571–578. doi: 10.1094/CCHEM.1997.74.5.511. [DOI] [Google Scholar]
- Jane J, Kasemsuwan T, Chen JF. Phosphorus in rice and other starches. Cereal Foods World. 1996;41:827–838. [Google Scholar]
- Jayakody L, Hoover R, Liu Q, Weber E. Studies on tuber starches. I. Structure and physicochemical properties of Innala (Solenostemon rotundifolius) starches grown in Sri Lanka. Food Res Int. 2005;38:615–629. doi: 10.1016/j.foodres.2004.11.015. [DOI] [Google Scholar]
- Krossmann J, Lloyd J. Understanding and influencing starch biochemistry. Crit Rev Biochem Mol Biol. 2000;35:141–196. [PubMed] [Google Scholar]
- Leach HW, McCown LD, Schoch TJ. Structure of the starch granule I. Swelling and solubility patterns of various starches and flours. Cereal Chem. 1959;36:535–545. [Google Scholar]
- Liu H, Ramsden L, Corke H. Physical properties and enzymatic digestibility of acetylated waxy and normal corn starch. Carbohydr Polym. 1997;34:283–289. doi: 10.1016/S0144-8617(97)00130-6. [DOI] [Google Scholar]
- Liu H, Ramsden L, Corke H. Physical properties and enzymatic digestibility of hydroxypropylated ae, wx and normal maize starch. Carbohydr Polym. 1999;40:175–182. doi: 10.1016/S0144-8617(99)00052-1. [DOI] [PubMed] [Google Scholar]
- Nakazawa Y, Wang YJ. Acid hydrolysis of native and annealed starches and branched structures of their Naegeli dextrins. Carbohydr Res. 2003;338:2871–2882. doi: 10.1016/j.carres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Noda T, Takahata Y, Sato T, Suda I, Morishita T, Ishiguro K, Yamakawa O. Relationships between chain length distribution of amylopectin and gelatinization properties within the same botanical origin for sweet potato and buck wheat. Carbohydr Polym. 1998;37:153–158. doi: 10.1016/S0144-8617(98)00047-2. [DOI] [Google Scholar]
- Olkku J, Rha CK. Gelatinization of starch and wheat flour starch-a review. Food Chem. 1978;3:293–317. doi: 10.1016/0308-8146(78)90037-7. [DOI] [Google Scholar]
- Perera C, Hoover R. Influence of hydroxyl-propylation on retrogradation properties of native, defatted and heat-moisture treated potato starches. Food Chem. 1999;64:361–375. doi: 10.1016/S0308-8146(98)00130-7. [DOI] [Google Scholar]
- Protserov VA, Karpov VG, Kozhevnikov GO, Wasserman LA, Yuryev VP. Changes of thermodynamic and structural properties of potato starches (Udacha and Acrosil) varieties during biosynthesis. Starch. 2000;52:461–466. doi: 10.1002/1521-379X(200012)52:12<461::AID-STAR461>3.0.CO;2-Y. [DOI] [Google Scholar]
- Ratnayake WS, Hoover R, Shahidi F, Perera C, Jane J. Composition, molecular structure and physicochemical properties of starches from four field pea (Pisum sativum L.) cultivars. Food Chem. 2001;74:189–202. doi: 10.1016/S0308-8146(01)00124-8. [DOI] [Google Scholar]
- Rutenberg MW Solarek D. Starch derivatives: production and uses. In: Whistler RL, BeMiller JN, Paschall EF, editors. Starch: chemistry and technology. London: Academic; 1984. pp. 311–388. [Google Scholar]
- Saartrat S, Puttanlek C, Rungsardthong V, Uttapap D. Paste and gel properties of low-substituted acetylated canna starches. Carbohydr Polym. 2005;61:211–221. doi: 10.1016/j.carbpol.2005.05.024. [DOI] [Google Scholar]
- Sathe SK, Rangnekar PD, Deshpande SS, Salunkhe DK. Isolation and partial characterization of black gram (Phaseolus mungo L.) Starch. J Food Sci. 1982;47:1524–1527. doi: 10.1111/j.1365-2621.1982.tb04974.x. [DOI] [Google Scholar]
- Shon KJ, Yoo B. Effect of acetylation on rheological properties of rice starch. Starch-Starke. 2006;58:177–185. doi: 10.1002/star.200500456. [DOI] [Google Scholar]
- Singh J, Mc-Carthy OJ, Singh H. Physico-chemical and morphological characteristics of New Zealand Taewa (Maori potato) starches. Carbohydr Polym. 2006;64:569–581. doi: 10.1016/j.carbpol.2005.11.013. [DOI] [Google Scholar]
- Sirivongpaisal P. Structure and functional properties of starch and flour from bambarra groundnut. Songklanakarin J Sci Technol. 2008;30(1):51–56. [Google Scholar]
- Sofi BA, Wani IA, Masoodi FA, Saba I, Muzaffar S. Effect of gamma irradiation on physicochemical properties of broad bean (Vicia faba L.) starch. LWT Food Sci Technol. 2013;54:63–72. doi: 10.1016/j.lwt.2013.05.021. [DOI] [Google Scholar]
- Su HS, Lu W, Chang KC. Microstructure and physicochemical characteristics of starches in six bean varieties and their bean paste products. Lebensm Wiss Technol. 1997;31:265–273. doi: 10.1006/fstl.1997.0350. [DOI] [Google Scholar]
- van Luyten H, Vliet T, Walstra P. Comparison of various methods to evaluate fracture phenomena in food materials. J Texture Stud. 1992;23(3):245–266. doi: 10.1111/j.1745-4603.1992.tb00524.x. [DOI] [Google Scholar]
- Visser RGF Suurs LCJM Steeneken PAMQ Jacobsen E Some physicochemical properties of amylose-free potato starch. Starch. 1997;49:443–448. doi: 10.1002/star.19970491104. [DOI] [Google Scholar]
- Wang YJ, Wang L. Characterisation of acetylated waxy maize starches prepared under catalysis by different alkali and alkaline earth hydroxides. Starch-Starke. 2002;54:25–30. doi: 10.1002/1521-379X(200201)54:1<25::AID-STAR25>3.0.CO;2-T. [DOI] [Google Scholar]
- Wang WJ, Powell AD, Oates CG. Effect of annealing on the hydrolysis of sago starch granules. Carbohydr Polym. 1997;33:195–202. doi: 10.1016/S0144-8617(96)00170-1. [DOI] [Google Scholar]
- Wang S, Gao W, Chen H, Xiao P. New starches from Fritillaria species medicinal plants. Carbohydr Polym. 2005;61(1):111–114. doi: 10.1016/j.carbpol.2005.03.017. [DOI] [Google Scholar]
- Wani IA, Sogi DS, Wani AA, Gill BS, Shivhare US. Physico-chemical properties of starches from Indian kidney bean (Phaseolus vulgaris) cultivars. Int J Food Sci Technol. 2010;45:2176–2185. doi: 10.1111/j.1365-2621.2010.02379.x. [DOI] [Google Scholar]
- Wani IA, Sogi DS, Gill BS. Physicochemical properties of acetylated starches from some Indian kidney bean cultivars. Int J Food Sci Technol. 2012;47:1993–1999. doi: 10.1111/j.1365-2621.2012.03062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurzburg OB. Starch, modified starch and dextrin. Products of the corn refining industry: seminar proceedings. Washington, DC: Corn Refiners Association, Inc; 1978. pp. 23–32. [Google Scholar]
- Yamin FF, Lee M, Pollak LM, White PJ. Thermal properties of starch in corn variants isolated after chemical mutagenesis of inbred line B73. Cereal Chem. 1999;76:175–181. doi: 10.1094/CCHEM.1999.76.2.175. [DOI] [Google Scholar]
- Yanez-Farias Grelda A, Moreno-Valencia JG, Falcon-Villa Madel Refugio Barron-Hoyos JM. Isolation and partial characterisation of starches from dry beans (Phaseolus vulgaris) and chickpeas (Cicer arietinum) Starch-Starke. 1997;49:341–345. doi: 10.1002/star.19970490904. [DOI] [Google Scholar]
- Zhou Y, Hoover R, Liu Q. Relationship between alpha-amylase degradation and the structure and physicochemical properties of legume starches. Carbohydr Polym. 2004;57:299–317. doi: 10.1016/j.carbpol.2004.05.010. [DOI] [Google Scholar]
- Zia-ur-Rehman Domestic processing effects on available carbohydrate content and starch digestibility of black grams (Vigna mungo) and chick peas (Cicer arietium) Food Chem. 2007;100(2):764–767. doi: 10.1016/j.foodchem.2005.10.041. [DOI] [Google Scholar]