Abstract
Efficiency of different methods for disruption of Streptococcus thermophilus cells, isolated from different dairy products, to release β-galactosidase and synthesis of GOS by extracted enzyme using whey supplemented with different concentrations of lactose as a substrate was studied. Unlike most other studies on GOS synthesis which used only one method of cell disruption and only few microbial strains, we compared five different cell disruption methods and used 30 strains of S. thermophilus in order to find out the most effective method and efficient strain for production of β-galactosidase. Appreciable amount of GOS (53.45 gL−1) was synthesized at a lactose concentration of 30 %, using enzyme (10 U mL−1 of reaction medium), extracted from S. thermophilus within a very short incubation time of 5 h at a temperature of 40 °C and pH 6.8. S. thermophilus is heavily employed in the preparation of fermented dairy products but this study extends the use of this organism for the production of GOS, a potential prebiotic.
Keywords: Galactooligosaccharides, Streptococcus thermophilus, β-galactosidase, Prebiotics
Introduction
The microbiota of humans is a consortium of numerous eukarya, bacteria, archaea, and viruses and therefore human beings are now being considered as a ‘superorganism’. The gut microbiota plays a fundamental role in the human health and disease. The healthy gut microbiota works symbiotically with host metabolic system for maintenance of proper health. Any stress on the gut microbiota due to biotic and abiotic factors lead to microecological disorders resulting in to enhanced risk of disease development (Shenderov 2013). Currently, use of prebiotics as a therapeutic approach for the restoration of gut microbial community is on constant rise. Much of the research in the field of prebiotics has used inulin and fructo-oligosaccharides, but nowadays galactooligosaccharides (GOS), because of their health promoting effects and stability over a wide range of temperature and pH, are drawing the interest of research groups and health professionals. GOS are well documented to be as effective prebiotic ingredients which modulate intestinal microbiota, barrier functions, and provide other beneficial health effects such as stool improvement, mineral absorption, weight management, carcinogenesis, and allergy alleviation (Figueroa-Gonzalez et al. 2011). Specifically, GOS supplements have been shown to exert positive impacts on intestinal Bifidobacterium and Lactobacillus populations in infants, to mitigate irritable bowel syndrome, and to reduce the severity and duration of travelers diarrhea (Silk et al. 2009; Drakoularakou et al. 2010). GOS has also been shown to inhibit pathogenic Vibrio cholerae and Cronobacter sakazakii binding to cell surface receptors of epithelial cells (Quintero et al. 2011; Sinclair et al. 2009) and prevent adhesion of Salmonella enterica serovar Typhimurium to murine enterocytes (Searle et al. 2010). Potential use of GOS in food products has been thoroughly described (Sangwan et al. 2011). GOS are synthesized using the enzyme β-galactosidase in a reaction known as transgalactosylation. Although β-galactosidase has been found in numerous biological systems, microorganisms such as yeasts, mold and bacteria still remain the only sources for commercial purposes. Data gleaned from the literature show that initial lactose concentration largely influenced the GOS yield (Torres et al.2010). Higher the concentration higher is the GOS yield. Because of relatively less solubility of lactose at lower temperature, higher temperature is desirable which increase the solubility of lactose as well as the GOS yield. Some studies have been focused on sourcing thermostable glycoside hydrolases. Glycoside hydrolases from Sulfolobus solfataricus (Park et al. 2008), Pyrococcus furiosus (Hansson et al.2001), Thermus sp. (Akiyama et al. 2001), Thermus caldophilus (Choi et al. 2003) and Thermus maritima (Ji et al. 2005; Kim et al. 2004) are examples of enzymes from hyperthermophilic microorganisms used at higher temperature.
Thermophilic lactic acid bacteria (LAB) are of great interest for the enzyme production because of their GRAS status. Amongst lactic acid bacteria, yogurt bacteria (Lactobacillus delbrueckii subsp.bulgaricus and Streptococcus thermophilus) are considered as the highest β-galactosidase producers. The β-galactosidase of these cultures has been characterized, and is observed as stable and active at high temperatures (Kreft et al. 2001). Such conditions, besides enhancing the rate of GOS production, prevent the growth of undesirable microorganisms in the reaction mixture as well. β-galactosidase of S. thermophilus has long been used for lactose hydrolysis but its transgalactosylation activity is poorly studied. Further, S. thermophilus is having GRAS status so the enzyme extracted can be directly used in reaction medium without any purification step. Besides, β-galactosidase from probiotic organisms was observed to produce a GOS mixture which confers selectivity on those probiotics when fermented by colonic microflora (Depeint et al. 2008). This study was designed to assess the efficiency of five different mechanical and chemical methods to disrupt the cells of S. thermophilus for release of β-galactosidase and transgalactosylation activity of the released enzyme. Crude cell free extract was used for the production in order to reduce the processing cost. Whey was used as a substrate for GOS production, which itself is an industrial waste and its use as a substrate reduce the cost as well as the environmental pollution.
Materials and method
Isolation and characterization of S. thermophilus
S. thermophilus was isolated from various dairy products including raw milk, dahi, cheese, lassi and yoghurt collected from the Institute’s experimental dairy plant, local market and rural and urban areas in Karnal and also from other places (Saharanpur (U.P), Sarsawa (U.P), Yamunanagar (Haryana), and Jalgaon (Maharashtra). M17 agar and broth supplemented with sodium β-glycerophosphate, supplemented with additional 1 % lactose were used for the isolation. For tentative identification of cultures they were examined microscopically for purity and morphology by Gram’s staining. Thereafter, an array of physiological and biochemical tests was performed as per standard methods (Botina et al. 2007) to identify the morphologically selected S. thermophilus isolates. These tests include catalase test; growth at 10 °C, 45 °C, growth at pH 9.6; resistance to heating at 65 °C for 30 min, growth in 2 % and 4 % NaCl, 0.04 % K-tellurite and in 0.1 % methylene blue; arginine hydrolysis; esculin hydrolysis test and carbohydrate fermentation (glucose, fructose, sucrose, mannose, mannitol, sorbitol, cellibiose, arabinose, lactose, mellibiose, galactose, xylose, rhamnose, maltose and inulin). All the biochemically identified S. thermophilus isolates were subjected to molecular characterization for definite confirmation of species using PCR by targeting strongly conserved sequences of S. thermophilus lacZ gene (Lick et al. 1996). The primers were custom synthesized from Integrated DNA technologies, U.S.A. (Table 1). The PCR programme comprised of one cycle of initial denaturation (4 min at 94 °C), followed by 30 cycles each of denaturation (30 s at 94 °C), primer annealing (45 s at 54 °C) and extension (45 s at 72 °C) followed by one cycle of final extension (7 min at 72 °C) (Table 1).
Table 1.
Primer pair used for the identification of S. thermophilus
| Species | Target gene | Primer | Sequence | Expected size |
|---|---|---|---|---|
| S. thermophilus | lacZ | lacZ (F) | 5′ CACTATGCTCAGAATACA3′ | 968 bp |
| lacZ (R) | 5′CGAACAGCATTGATGTTA3′ |
Extraction of β-galactosidase
Disruption of microbial biomass
Since β-galactosidase from lactic acid bacteria is an intracellular enzyme, five different disruption methods such as lysozyme treatment, SDS (sodium dodecyl sulphate)-chloroform method, glass bead extraction, sonication and microfluidizer, were applied to evaluate and compare the efficacy of these methods in order to find out the appropriate method for cell disruption.
Enzyme extraction by lysozyme
Ten ml of fermentation broth was harvested by centrifugation at 10,000 × g for 15 min at 4 °C. Supernatant was discarded and the pellet thus obtained was washed twice with 0.05 M Na- phosphate buffer (pH 6.8) and centrifuged at 10,000 × g for 15 min. Pellet was resuspended in 5.0 ml of 0.05 M Na- phosphate buffer followed by vigorous vortexing. To this solution, lysozyme (Sigma) at a concentration of 10 mg mL−1 was added and incubated at 37 °C for 15 min followed by addition of 0.5 ml of 4 M NaCl solution and incubation 37 °C for another 50 min. The cell suspension was then centrifuged at 10,000 × g for 15 min. Resultant supernatant was used for the enzyme assay and protein determination (Tari et al. 2010).
Enzyme extraction by glass beads
Pellet was obtained as above and resuspended in 5.0 mL of the same buffer followed by vigorous vortexing to disperse the cells homogeneously. To this mixture, 5.0 g of glass beads (Sigma) were added and vortexed using 10 operating cycles (1 operating cycle =1 min operation +30 sec cooling on ice). At the end of 8 cycles, the solution was centrifuged at 10,000 × g for 15 min at 4 °C and the supernatant was collected (Tari et al. 2010; Bury et al. 2001).
Enzyme extraction by SDS-choloroform
Five ml of fermentation broth was harvested and pellet was obtained as explained above. After washing, pellet was resuspended in 1 mL of 0.05 M Na-phosphate buffer followed by vigorous vortexing. Cell suspension was mixed with 0.9 ml Z buffer (0.06 M Na2HPO4, 0.04 M NaH2PO4, 0.01 M KCl 0.001 M MgSO4), chloroform (100 μL) and 0.1 % SDS solution (50 μL) and incubated of 5 min at 37 °C. Cell free extract was obtained as explained earlier (Oberoi et al. 2008).
Enzyme extraction by sonication
Fermentation broth (20 mL) was harvested by centrifugation and after washing the pellet was resuspended in 10 mL of the same buffer and vortexed. Cell suspension was sonicated using 10 operating cycles (1 operating cycle =1 min operation +30 sec cooling on ice). Cooling was achieved by placing the polypropylene tube in an ice water bath. Resulting suspension of ruptured cells was then centrifuged at 10,000 × g for 15 min at 4 °C to obtain supernatant (Feliu and Villaverde 1994).
Enzyme extraction by microfluidizer
Two hundred ml of the fermentation broth was harvested by centrifugation and the pellet was resuspended in 100 mL after washing. Cell disruption was performed on this solution using the microfluidizer (Microfluidics M-110P, Newton, USA) by applying 3 passes through the microfluidizer at 15,000 Pa. Afterwards the solution was centrifuged at 10,000 × g and 4 °C for 15 min; the supernatant was used for the enzyme assay (Choi et al. 1997).
Measurement of β-galactosidase and protein quantification
The activity assay was carried out as described by Splechtna et al. (2007) and Nguyen et al. (2006) with some modifications. The chromogenic substrate o-nitrophenol-β-D-galactopyranoside (ONPG) (4 mg mL−1) was dissolved in 50 mM sodium phosphate buffer (pH 6.8). Twenty microliter of the cell free extract was mixed with 480 μL of ONPG solution and incubated for 20 min at 42 °C. After 20 min reaction was stopped by the addition of 750 μL of 1 M Na2CO3 (sodium carbonate) to the reaction mixture. Absorbance was measured at 420 nm. One unit was defined as the quantity of enzyme that would liberate 1 μmol of o-nitrophenol (ONP) from ONPG per minute under the assay conditions. Protein concentration was determined by the method of Bradford (1976) using Bovine Serum Albumin as standard.
Production of GOS
Deproteinization of whey
Cheese whey was obtained from Experimental Dairy Plant National Dairy Research Institute, Karnal, and deproteinized by heating at 100 °C for 30 min. After settling of the whey proteins, the supernatant was filtered (Whatmann No. 1 filter paper) (Anvari and Khayati 2011). The whey was neutralized with 1 M NaOH solution. The pH of whey was adjusted to 6.8.
GOS Production
GOS was produced using whey supplemented with lactose as a substrate. Lactose concentration in deproteinized whey was estimated by using Lane and Eynon (1923) method and supplemented with the required amount of lactose to reach a final concentration ranging from 5 to 35 %. After supplementation the whey was distributed in test tubes (5.0 mL each) and autoclaved. Then the tubes were inoculated with cell free extract containing enzyme corresponding to 10 U mL−1. This mixture was then incubated for 5 h at a temperature of 40 °C. After the incubation was over, the reaction mixture was heated at a temperature of 100 °C for 15 min in order to deactivate the enzyme. This mixture was then filtered through 0.22 μm filter (Millipore) and analyzed for GOS.
Detection of GOS by high performance liquid chromatography (HPLC)
High Performance Liquid Chromatography (HPLC) analysis was used to accurately quantify GOS synthesis products. The HPLC system (Shimadzu, Kyoto, Japan) consisted of a manual injector (20 μL), a pump (CTO-20A) refractive index detector (Shimadzu RID-10A) and carbohydrate column (Phenomenax Luna Amino (NH2) column, 250 mm x 5 mm I.D.). The column temperature was maintained at 40 °C using a column heater (Shimadzu CTO-20A oven). The mobile phase was acetonitrile (75 % v/v) and distilled water (25 % v/v) filtered though a sterile micro-filter (0.45 μL) and deaerated for 20 min in ultrasonic equipment before use. Flow rate was kept at 1 mL min−1 (Neri et al. 2009; Martinez-Villaluenga et al. 2008; Splechtna et al. 2007).
Results
Isolation and characterization of streptococcus thermophilus
A total of 200 randomly selected colonies were activated in M17 broth and the activated cultures were transferred to sterile skim milk tubes and incubated at 42 °C for 18 h for curdling. Out of 200, one hundred sixty isolates showing positive clean lactic fermentation were presumptively screened out as lactic streptococcal isolates. Out of these 160 isolates, 62 were confirmed as S. thermophilus after microscopic, biochemical and molecular characterization. Isolates were examined microscopically for shape and arrangement of cells by Gram staining and negative staining. Gram-positive cocci with cells arranged in pairs and chains were presumptively identified as S. thermophilus. Fifty eight isolates showing undesirable morphological characteristics were discarded and remaining 102 were tested for catalase test, growth at 10 and 45 °C, resistance to heat treatment at 65 °C for 30 min, growth in 4 % NaCl, growth at 9.6 pH, growth in 0.1 % methylene blue, production of ammonia from arginine, growth on 0.04 % potassium tellurite and aesculin hydrolysis (Results not shown). After biochemical characterization, 76 isolates were selected for molecular characterization using PCR. Finally from a total of 200 isolates 62 were confirmed as S. thermophilus and used for extraction of β-galactosidase.
Extraction of β-galactosidase
Initially β-galactosidase was extracted from all the 62 isolates using SDS-chloroform method (Table 2) and 30 high enzyme strains were the used to extraxt enzyme using diffrent cell disruption techniques. Five different microbial cell disruption methods such as SDS-Chloroform, lysozyme treatment, glass bead extraction, microfluidizer and sonication were applied for the extraction of β-galactosidase from isolated strains of S. thermophilus in order to find an efficient method and potential strain for extraction of the enzyme of interest to produce GOS. Out of 62 isolates, 30, high enzyme producing strains (screened by SDS-chloroform method) were selected for extraction of enzyme using all the five techniques. Units of enzyme obtained by using five different methods from 30 selected strains were given in Table 3. The treatment with SDS-chloroform resulted in 1.31, 1.61, 1.99, and 3.82 times more β-galactosidase activity than lysozyme, glass beads, microfluidizer and sonication respectively (In case of highest enzyme producing strain ST61). Under the same conditions enzyme extraction by lysozyme resulted into second higher enzyme activity after SDS-chloroform. In case of mechanical methods glass beads resulted in to highest enzyme activity as compared to microfluidizer and sonication. Enzyme extraction using glass beads resulted in 1.23 and 2.37 times more β-galactosidase activity than microfluidizer and sonication respectively (In case of highest enzyme producing strain ST61). The lowest activity was obtained in the extraction method using sonication. Among all the strains tested for β-galactosidase production ST61 was found to produce the highest amount of enzyme (78.85, 60, 39.44, 48.81 and 20.59 U mg−1 of protein in case of treatment with SDS-chloroform, lysozyme, microfluidizer, glass beads and sonication respectively).
Table 2.
Extraction of β-galactosidase using SDS-chloroform method
| Isoaltes | β-galactosidase units (U/mg of protein) |
|---|---|
| ST1 | 14.91 ± 2.07 |
| ST2 | 38.41 ± 1.88 |
| ST3 | 7.29 ± 0.4 |
| ST5 | 46.94 ± 2.16 |
| ST6 | 15.22 ± 2.41 |
| ST8 | 40.36 ± 2.08 |
| ST9 | 13.38 ± 2.57 |
| ST10 | 32.27 ± 2.19 |
| ST11 | 47.7 ± 2.04 |
| ST14 | 30.18 ± 0.94 |
| ST17 | 5.82 ± 0.66 |
| ST18 | 15.98 ± 0.83 |
| ST19 | 18.89 ± 0.14 |
| ST20 | 33.12 ± 0.25 |
| ST21 | 46.45 ± 1.34 |
| ST22 | 36.35 ± 2.13 |
| ST24 | 43.05 ± 3.35 |
| ST25 | 17.05 ± 0.17 |
| ST28 | 57.75 ± 1.84 |
| ST29 | 6.03 ± 1.07 |
| ST32 | 38.28 ± 1.62 |
| ST33 | 37.33 ± 0.58 |
| ST36 | 45.07 ± 2.42 |
| ST37 | 42.38 ± 4.28 |
| ST39 | 47.25 ± 1.05 |
| ST40 | 35.49 ± 1.43 |
| ST41 | 16.13 ± 0.36 |
| ST43 | 21.92 ± 1.25 |
| ST44 | 19.59 ± 1.25 |
| ST46 | 11.36 ± 0.66 |
| ST48 | 35.11 ± 1.85 |
| ST50 | 37.25 ± 1.98 |
| ST51 | 40.85 ± 2.4 |
| ST52 | 48.43 ± 4.05 |
| ST53 | 37.93 ± 2.7 |
| ST54 | 9.57 ± 3.04 |
| ST57 | 9.86 ± 1.56 |
| ST58 | 22.39 ± 1.75 |
| ST60 | 23.07 ± 0.65 |
| ST61 | 78.85 ± 7.12 |
| ST62 | 23.1 ± 0.84 |
| ST64 | 8.22 ± 0.24 |
| ST66 | 11.92 ± 0.22 |
| ST67 | 12.21 ± 1.29 |
| ST71 | 20.5 ± 0.93 |
| ST72 | 13.3 ± 1.88 |
| ST74 | 38.08 ± 3.03 |
| ST75 | 38.22 ± 3.24 |
| ST77 | 46.26 ± 1.35 |
| ST79 | 47.71 ± 1.87 |
| ST82 | 44.09 ± 2.62 |
| ST84 | 46.79 ± 1.51 |
| ST86 | 21.99 ± 0.48 |
| ST87 | 16.46 ± 1.37 |
| ST88 | 20.13 ± 3.1 |
| ST90 | 50.91 ± 4.28 |
| ST91 | 14.41 ± 2.73 |
| ST93 | 23.55 ± 2.66 |
| ST95 | 16.95 ± 2.49 |
| ST96 | 25.51 ± 2.23 |
| ST99 | 20.93 ± 0.55 |
| ST100 | 6.72 ± 0.28 |
Level of significance at P < 0.05, ± standard error in triplicates
Table 3.
Extraction of β-galactosidase from selected strains of S. thermophilus using five methods of cell disruption
| Isolates | SDS-Chloroform (U mg−1 of protein) | Lysozyme (U mg−1 of protein) | Glass beads (U mg−1 of protein) | Microfluidizer (U mg−1 of protein) | Sonication (U mg−1 of protein) |
|---|---|---|---|---|---|
| ST2 | 38.41 ± 1.88 | 30.49 ± 1.05 | 28.11 ± 2.21 | 22.7 ± 0.55 | 10.35 ± 0.44 |
| ST5 | 46.94 ± 2.16 | 41.06 ± 2.12 | 36.41 ± 1.43 | 30.4 ± 0.67 | 13.66 ± 1.89 |
| ST8 | 40.36 ± 2.08 | 30.46 ± 1 | 24.25 ± 2.18 | 19.77 ± 1.91 | 9.14 ± 0.54 |
| ST10 | 32.27 ± 2.19 | 27.06 ± 2.85 | 21.88 ± 0.97 | 17.8 ± 1.32 | 9.6 ± 1.86 |
| ST11 | 47.7 ± 2.04 | 35.36 ± 2.29 | 28.09 ± 1.45 | 23.95 ± 2.01 | 13.46 ± 2.26 |
| ST14 | 30.18 ± 0.94 | 23.12 ± 2.03 | 18.81 ± 1.34 | 13.94 ± 2.1 | 6.67 ± 0.34 |
| ST20 | 33.12 ± 0.25 | 27.12 ± 1.42 | 24.15 ± 1.76 | 20.14 ± 1.52 | 13.66 ± 1.65 |
| ST21 | 46.45 ± 1.34 | 37.89 ± 1.75 | 30.06 ± 2.24 | 24.26 ± 3.05 | 16.37 ± 1.16 |
| ST22 | 36.35 ± 2.13 | 30.06 ± 0.65 | 23.38 ± 2.37 | 16.8 ± 1.28 | 10.35 ± 1.85 |
| ST24 | 43.05 ± 3.35 | 35.4 ± 2.09 | 27.92 ± 1.62 | 23.86 ± 1.65 | 13.23 ± 1.43 |
| ST28 | 57.75 ± 1.84 | 45.92 ± 2.31 | 37.11 ± 2.79 | 31.57 ± 2.48 | 17.53 ± 2.68 |
| ST32 | 38.28 ± 1.62 | 25.57 ± 2.32 | 17.32 ± 1.06 | 12.89 ± 1.83 | 5.14 ± 0.93 |
| ST33 | 37.33 ± 0.58 | 29.67 ± 1.42 | 22.44 ± 1.21 | 17.4 ± 2.62 | 10.13 ± 1.21 |
| ST36 | 45.07 ± 2.42 | 36.51 ± 2.19 | 28.53 ± 2.4 | 23.89 ± 3.61 | 13.32 ± 2.62 |
| ST37 | 42.38 ± 4.28 | 37.91 ± 2.9 | 31.33 ± 1.51 | 24.79 ± 2.58 | 15.21 ± 1.58 |
| ST39 | 47.25 ± 1.05 | 40.22 ± 2.8 | 30.04 ± 2.6 | 27.13 ± 2.84 | 18.56 ± 2.19 |
| ST40 | 35.49 ± 1.43 | 25.38 ± 2.91 | 18.01 ± 0.7 | 11.89 ± 1.94 | 5.87 ± 1.2 |
| ST48 | 35.11 ± 1.85 | 29.31 ± 2.65 | 18.68 ± 1.89 | 14.3 ± 1.05 | 6.16 ± 0.58 |
| ST50 | 37.25 ± 1.98 | 30.32 ± 3.95 | 21.66 ± 2.89 | 18.29 ± 1.34 | 10.03 ± 0.93 |
| ST51 | 40.85 ± 2.4 | 32.53 ± 2.37 | 26.97 ± 0.9 | 22.83 ± 1.3 | 12.4 ± 2.26 |
| ST52 | 48.43 ± 4.05 | 38.11 ± 2.96 | 28.52 ± 2.69 | 25.02 ± 2.01 | 15.07 ± 2.48 |
| ST53 | 37.93 ± 2.7 | 25.35 ± 0.94 | 17.69 ± 2.84 | 13.13 ± 2.28 | 8.03 ± 1.9 |
| ST61 | 78.85 ± 7.12 | 60 ± 4.05 | 48.81 ± 2.99 | 39.44 ± 3.34 | 20.59 ± 3.16 |
| ST74 | 38.08 ± 3.03 | 33.32 ± 2.08 | 25.69 ± 2.99 | 20.9 ± 1.09 | 11.75 ± 2.02 |
| ST75 | 38.22 ± 3.24 | 29.75 ± 1.34 | 23.48 ± 2.34 | 19.07 ± 1.28 | 7.18 ± 1.44 |
| ST77 | 46.26 ± 1.35 | 37.52 ± 0.99 | 35.15 ± 3.51 | 29.38 ± 1.35 | 14.97 ± 2.15 |
| ST79 | 47.71 ± 1.87 | 40.47 ± 1.31 | 33.02 ± 2.2 | 29.27 ± 0.84 | 21.19 ± 0.64 |
| ST82 | 44.09 ± 2.62 | 35.04 ± 3.03 | 28.11 ± 3.55 | 25.22 ± 0.94 | 15.91 ± 1.6 |
| ST84 | 46.79 ± 1.51 | 37.37 ± 1.3 | 31.16 ± 3.58 | 27.99 ± 0.68 | 9.95 ± 1.02 |
| ST90 | 50.91 ± 4.28 | 41.98 ± 3.78 | 33.02 ± 2.45 | 28.36 ± 2.31 | 15.54 ± 0.56 |
Level of significance at P < 0.05, ± standard error in triplicates
GOS production
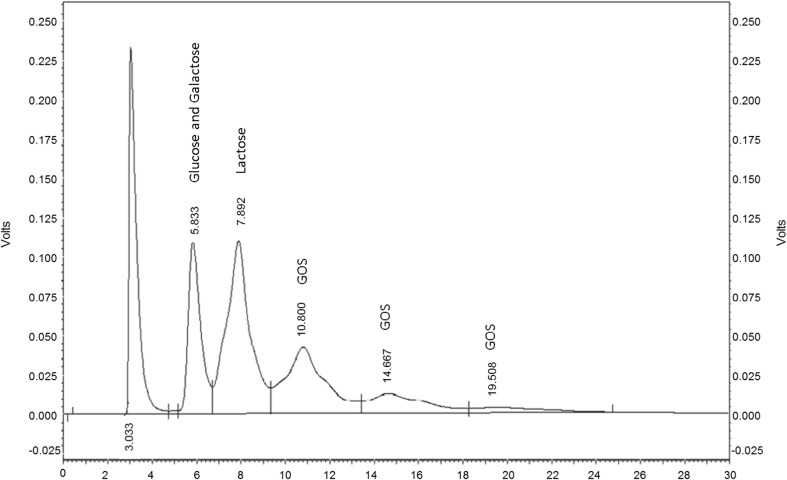
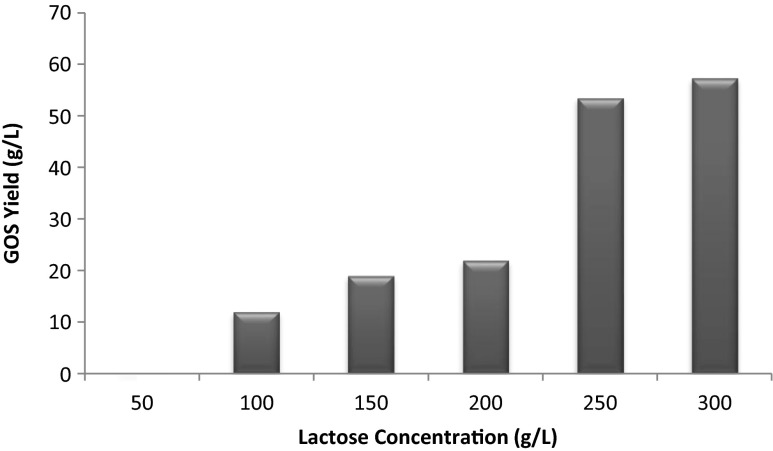
Initially, enzyme extracted from all the 30 selected strains of S. thermophilus was used for GOS production. It was observed that the amount of GOS produced using enzyme extracted from different strains was almost similar. Therefore the strain ST61 (high enzyme producer) was selected for enzyme extraction. GOS production was detected by using HPLC and was found to be 19 g L−1 of the reaction medium at 15 % lactose concentration; incubation temperature of 40 °C, time of incubation 5 h, using 10 U of enzymes per ml of lactose solution and at a pH of 6.8. During the reaction apart from GOS, glucose, galactose, and lactose were also observed (Fig. 1). An increment in GOS production was observed with increasing lactose concentration. For lactose concentrations of 50, 100, 150, 200, 250, 300 and 350 g L−1 the maximum yields were 0, 12, 19, 22, 42, 53.45 and 57.27 g L−1 respectively after 5 h (Fig. 2).
Fig. 1.
HPLC chromatogram representing GOS production along with mono and disaccharides
Fig. 2.
Effect of lactose concentration on final GOS yield
Discussion
Lactic acid bacteria (LAB) are widely used as starter cultures in the manufacture of dairy products owing to their capability of efficient utilization of milk constituents. S. thermophilus, one of the most important industrial dairy starter cultures, is a thermophilic LAB and is a promising microorganism for the production of β-galactosidase. For the isolation of some prolific, β-galactosidase producing S. thermophilus strains, a total number of 40 different samples of milk and milk products (raw milk (12), dahi (14), lassi (12) and yoghurt (2)) were collected. In the present study, M17 media (used for isolation of S. thermophilus) was modified by additional 1 % lactose as addition of lactose to the growth medium was reported to significantly improve the β-galactosidase production and competitively exclude interfering organisms because of its faster utilization by S. thermophilus (Sriphannam et al. 2012). A total number of 200 randomly selected colonies (60 isolates from raw milk, 70 isolates from dahi, 60 from lassi and 10 from yoghurt) were activated in M17 broth and the activated cultures were transferred to sterile skim milk tubes and incubated at 42 °C for 18 h for curdling. Out of 200, one hundred sixty isolates showing positive clean lactic fermentation were presumptively screened out as lactic streptococcal isolates.
All the 160 lactic streptococci isolates selected were tested for purity and morphology by microscopic examination. The isolates which showed typical morphological characteristics on microscopic observations were further subjected to biochemical and molecular characterization. The observations were interpreted as per the criteria followed by Botina et al. (2007). Twenty six cultures were discarded on the basis of biochemical tests and remaining 76 were subjected to molecular characterization for definite confirmation. Amplification of species specific lacZ gene of S. thermophilus was carried out using universal primer pairs (ST1/ST2) devised by Lick et al. (1996). On the basis of species specific PCR, out of 76 biochemically identified isolates 62 were confirmed as S. thermophilus (26 from raw milk, 18 from dahi, 14 from lassi and 4 from yoghurt). Since β-galactosidase activity represents an essential function for S. thermophilus to grow in its natural environment, milk, where the only fermentable sugar is lactose, there is chromosomal stability of this target sequence, hence, lac Z primer is a universal primer for S. thermophilus.
The enzyme β-galactosidase can be obtained from a wide variety of sources such as microorganisms, plants, and animals. Microorganisms offer various advantages over other available sources such as easy handling, higher multiplication rate, and high production yield. As a result of commercial interest in β-galactosidase, a large number of microorganisms have been assessed as potential sources for its production (Panesar et al. 2010). S. thermophilus is a promising microorganism for the production of β-galactosidase enzyme, since their β-galactosidase is active at neutral pH and is more heat stable than the widely used Kluyveromyces lactis β-galactosidase (Tari et al. 2010). Moreover, hydrolysis of lactose can be achieved rapidly without growth of undesirable microorganisms at these conditions (neutral pH and high temperatures). In the present study the transgalactosylation activity of β-galactosidase was utilized for the production of GOS and transgalctosylation reaction was reported to be increased with increasing temperature. Therefore keeping these points in view we selected S. thermophilus as a source organism for extraction of β-galactosidase.
However, the β-galactosidase from thermophilic LAB is an intracellular enzyme. Its release from microbial cells is obtained either by mechanical disruption or by chemical permeabilization of the cell membrane. The effectiveness of the various disruption methods differs for different microbial genera and strains (Tari et al. 2010). In this study five different disruption methods such as SDS-Chloroform, lysozyme treatment, glass bead extraction, microfluidizer and sonication were applied. Initially all the 62 characterized strains were used for the enzyme extraction by the use of SDS-chloroform method which was reported as the most effective detergent for disruption of S. thermophilus cells (Somkuti and Dominiecki 1998). Thirty higher enzyme producing strains were selected and further subjected to remaining four techniques for cell disruption. Among the methods used for cell disruption, sonication (ultrasonics) is one of the most widely used cell disruption technique at laboratory scale (Wang 1997). This technique of cell disruption requires neither sophisticated devices nor extensive technical training at laboratory but hardly suitable for the industrial purpose. All the methods of cell disruption were optimized to obtain maximum disruption. In case of lysozyme the concentration of lysozyme used for the cell disruption was standardized and use of 10 mg of lysozyme per ml of the cell suspension was found to be suitable for cell disruption. In some other studies, 22 mg of lysozyme per ml of the cell suspension was used for cell disruption (Tari et al. 2010; Ustok et al. 2010). In glass beads and sonication, the time required to disrupt the cells was standardized. In both cases 10 operating cycles (1 operating cycle =1 min operation +30 sec cooling on ice) were found to be appropriate for disruption. In case of microfluidizer, pressure and number of passes required for cell disruption were optimized, three passes through the microfluidizer at 15,000 Pa. were found to be suitable for disruption.
Enzymatic (Lysozyme) cell lyses can be carried out on any scale but it is not suitable for large-scale preparations because of the higher cost of lysozyme. Two methods that are most commonly used for large scale cell disruption are high speed bead mill and high pressure homogenizer (Bury et al. 2001). Most of the studies are focused mainly in the comparison of chemical and mechanical treatment (Geciova et al. 2002; Bury et al. 2001). Therefore this study will be one of the limited studies highlighting a comparison of chemical, mechanical methods and enzymatic methods. In the present study the highest β-galactosidase enzyme activity was achieved when extraction was performed by using SDS-chloroform method followed by lysozyme, glass beads, microfluidizer and sonication. The lowest activity was obtained in the extraction method using sonication. This might be because of the incomplete disruption of cell wall since β-galactosidase is a large enzyme which makes it necessary to disrupt the microbial cell completely to liberate the enzyme. Another reason for obtaining low enzyme activity units in sonication might be the emergence of intense heat from the absorption of sonication energy into suspensions, probably leading to enzyme inactivation (Ismail et al. 2010). As the GOS aimed to be produced in this study would have its designated use as food ingredient, the use of food grade enzyme was an obvious choice. Therefore SDS-chloroform method cannot be used for the study. On one hand, enzymatic (lysozyme) lysis has the advantage of being specific and gentle, but on the other hand it is an expensive method. Among remaining three mechanical methods viz. glass beads, microfluidizer and sonication; glass beads were yielding highest amount of β-galactosidase but this method was very time consuming. Under same processing time microfluidizer was able to process 40 times more cell suspension as compared to glass beads. Therefore microfluidizer was selected as a method of cell disruption for the rest of the study.
In a study by Kara (2004), lysozyme method, sonication method, and liquid nitrogen method were evaluated for protein release from the cells of Lactobacillus plantarum and sonication was found to be the most effective method whereas Bury et al. (2001) found sonication as least effective method of cell disruption in coparison to high-pressure homogenization and bead milling. Among all the strains tested for β-galactosidase production ST61 was found to produce the highest amount of enzyme (78.85, 60, 39.44, 48.81 and 20.59 U mg−1 of protein in case of treatment with SDS-chloroform, lysozyme, microfluidizer, glass beads and sonication respectively).
To investigate whether production costs can be reduced by avoiding laborious and expensive chromatographic steps for the purification of the β-galactosidase, experiments were performed with the crude β-galactosidase extract from S. thermophilus directly obtained after cell disruption and separation of cell debris by centrifugation. It was reported that there is no obvious difference in the obtained GOS yields using either pure or crude β-galactosidase at 37 °C (Splechtna et al. 2007). Because of the GRAS status of S. thermophilus it is also safe to use the crude extract in food and feed applications. For the production of GOS whey was used as a cost effective substrate. Initially, enzyme extracted from all the 30 selected strains of S. thermophilus was used for the GOS production. As, the amount of GOS produced using enzyme extracted from different strains was almost similar. Therefore the strain ST61 (high enzyme producer was selected for further studies).
Data gleaned from the literature showed that maximum GOS yield is largely influenced by initial lactose concentration (Cho et al.2003; Splechtna et al. 2007; Valero 2009). This dependence on initial lactose concentration has at least two contributing factors: increased availability of saccharide galactosyl acceptors, and decreased availability of water. In this work, to determine the influence of substrate concentration on the GOS production, assays were performed at 40 °C, 10 U/ml of enzyme, pH 6.8 using 50–350 g lactose L−1. For initial lactose concentrations of 50, 100, 150, 200, 250, 300 and 350 g L−1, the maximum yields were 0, 12, 19, 22, 42, 53.45 and 57.27 g L−1 respectively after 5 h. Transgalactosylation significantly increased with lactose concentrations from 50 to 300 g L−1 due to the fact that, in a diluted lactose solution, water can be more competitive to be an acceptor for the β-galactosyl groups, releasing galactose from the active site. On the other hand, in a high lactose concentration solution, lactose has more chances to act as the acceptor for the β-galactosyl groups, binding with the enzyme galactose complex and forming GOS. However, further increases in initial lactose concentrations from 300 g L−1 to 350 g L−1 do not led to a considerable increase in the yield of GOS. Therefore 300 g of lactose L−1 of reaction medium was taken as optimum substrate concentration. These observations are consistent with results obtained by Zhou and Chen (2001); Nakkharat et al. (2006) and Park et al. (2008).
Compared to lactose concentration and lactose conversion, other process parameters, such as enzyme concentration, pH and temperature, have minimal effects on GOS production (Iwasaki et al. 1996), although they affect the reaction rates. The results of the study led us to the conclusion that cell free extract of S. thermophilus is a food grade source of β-galactosidase, having appreciable transgalactosylation activity. Enzyme from this organism has the potential to be used for GOS production even in its crude form. Further it was observed that enzyme extracted from different strains of the same organism resulted in similar GOS yield showing that enzyme from different strains of same species possess similar transgalactosylation activity. Most importantly, increasing lactose concentration to certain level led to a proportionate increasing in GOS production and this elevation in GOS yield steadied thereafter. S. thermophilus is a thermophilic organism and β-galactosidase from this organism can withstand higher temperature than other lactic acid bacteria. Therefore there is a scope to optimize the GOS production by carrying out the reaction at higher temperature which is known to increase the GOS yield. The synthesis of GOS using exogenous β-galactosidase from the organisms having GRAS status is a promising venture for the cost effective production of novel functional ingredients important for the health consumers around the world.
Acknowledgments
The authors thank National Agriculture Innovative Project (NAIP), New Delhi, for funding the project and FrieslandCampina Domo for making the GOS available for this study. The authors thank the Director of NDRI for supporting the work.
References
- Akiyama K, Takase M, Horikoshi K, Okonogi S. Production of galactooligosaccharides from lactose using a β-glucosidase from Thermus sp. Z-1. Biosci Biotechnol Biochem. 2001;65:438–441. doi: 10.1271/bbb.65.438. [DOI] [PubMed] [Google Scholar]
- Anvari M, Khayati G. Submerged yeast fermentation of cheese whey for protein production and nutritional profile analysis. Int J Food Sci Technol. 2011;3:122–126. [Google Scholar]
- Botina SG, Trenina MA, Tsygankov YD, Sukhodolets VV. Comparison of genotypic and biochemical characteristics of streptococcus thermophilus strains isolated from sour milk products. Appl Biochem Microbiol. 2007;43:598–603. doi: 10.1134/S0003683807060051. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bury D, Jelen P, Kalab M. Disruption of Lactobacillus delbrueckii ssp. bulgaricus 11842 cells for lactose hydrolysis in dairy products: a comparison of sonication, high-pressure homogenization and bead milling. Innov Food Sci Emerg Technol. 2001;2:23–29. doi: 10.1016/S1466-8564(00)00039-4. [DOI] [Google Scholar]
- Cho YJ, Shin HJ, Bucke C. Purification and biochemical properties of a galactooligosaccharide producing β-galactosidase from Bullera singularis. Biotechnol Lett. 2003;25:2107–2111. doi: 10.1023/B:BILE.0000007077.58019.bb. [DOI] [PubMed] [Google Scholar]
- Choi H, Laleye L, Amantea GF, Simard RE. Release of aminopeptidase from Lactobacillus casei sp. casei by cell disruption in a microfluidizer. Biotechnol Tech. 1997;11:451–453. doi: 10.1023/A:1018489327675. [DOI] [Google Scholar]
- Choi JJ, Oh EJ, Lee YJ, Suh DS, Lee JH, Lee SW, Shin HT, Kwon ST. Enhanced expression of the gene for beta-glycosidase of Thermus caldophilus GK24 and synthesis of galacto-oligosaccharides by the enzyme. Biotechnol Appl Biochem. 2003;38:131–6. doi: 10.1042/BA20020119. [DOI] [PubMed] [Google Scholar]
- Depeint F, Tzortzis G, Vulevic J, I’Anson K, Gibson GR. Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of bifidobacterium bifidum NCIMB 41171, in healthy humans, a randomized, double-blind, crossover, placebo-controlled intervention study. Am J Clin Nutr. 2008;87:785–791. doi: 10.1093/ajcn/87.3.785. [DOI] [PubMed] [Google Scholar]
- Drakoularakou A, Tzortzis G, Rastall RA, Gibson GR. A double-blind, placebo controlled, randomized human study assessing the capacity of a novel galacto-oligosaccharide mixture in reducing travellers diarrhoea. Eur J Clin Nutr. 2010;64:146–152. doi: 10.1038/ejcn.2009.120. [DOI] [PubMed] [Google Scholar]
- Feliu JX, Villaverde A. An optimized ultrasonication protocol for bacterial cell disruption and recovery of β-galactosidase fusion proteins. Biotechnol Tech. 1994;8:509–514. doi: 10.1007/BF00222845. [DOI] [Google Scholar]
- Figueroa-Gonzalez I, Quijano G, Ramirez G, Cruz-Guerrero A. Probiotics and prebiotics: perspectives and challenges. J Sci Food Agric. 2011;9:1341–1348. doi: 10.1002/jsfa.4367. [DOI] [PubMed] [Google Scholar]
- Geciova J, Giesova M, Jelen P, Plockova M. Disruption of streptococcus thermophilus 143 culture by three mechanical methods for increased β-galactosidase activity. Milchwiss. 2002;57:509–511. [Google Scholar]
- Hansson T, Kaper T, van der Oost J, de Vos WM, Adlercreutz P. Improved oligosaccharide synthesis by protein engineering of beta-glucosidase CelB from hyperthermophilic Pyrococcus furiosus. Biotechnol Bioengr. 2001;73:203–210. doi: 10.1002/bit.1052. [DOI] [PubMed] [Google Scholar]
- Ismail SA, El-Mohamady Y, Helmy WA, Abou-Romia R, Hashem AM. Cultural condition affecting the growth and production of β-galactosidase by Lactobacillus acidophilus NRRL 4495. Aust J Basic Appl Scib. 2010;4:5051–5058. [Google Scholar]
- Iwasaki K, Nakajima M, Nakao S. Galacto-oligosaccharide production from lactose by an enzymatic batch reaction using β-galactosidase. Process Biochem. 1996;31:69–76. doi: 10.1016/0032-9592(94)00067-0. [DOI] [Google Scholar]
- Ji ES, Park NH, Oh DK. Galacto-oligosaccharide production by a thermostable recombinant beta-galactosidase from Thermotoga maritima. World J Microb Biot. 2005;21:759–764. doi: 10.1007/s11274-004-5487-8. [DOI] [Google Scholar]
- Kara F (2004) Release and characterization of beta-galactosidase from Lactobacillus plantarum. Dissertation, The graduate school of natural and applied sciences, Middle East Technical University.
- Kim CS, Ji ES, Oh DK. Characterization of a thermostable recombinant beta-galactosidase from Thermotoga maritima. J Appl Microbiol. 2004;97:1006–1014. doi: 10.1111/j.1365-2672.2004.02377.x. [DOI] [PubMed] [Google Scholar]
- Kreft ME, Roth L, Jelen P. Lactose hydrolyzing ability of sonicated cultures of Lactobacillus delburickii ssp. bulgaricus 11842. Lait. 2001;81:355–364. doi: 10.1051/lait:2001137. [DOI] [Google Scholar]
- Lane JH, Eynon L (1923) Volumetric determination of reducing sugars by means of Fehling’s solution, with methylene blue as internal indicator. IS1 XXV:143–149
- Lick S, Keller M, Bockelmann W, Heller K. Rapid identification of Streptococcus thermophilus by primer-specific PCR amplification based on its lacZ gene. System Appl Microbiol. 1996;19:74–77. doi: 10.1016/S0723-2020(96)80012-9. [DOI] [Google Scholar]
- Martinez-Villaluenga C, Cardelle-Cobas A, Corzo N, Olano A, Villamiel M. Optimization of conditions for galactooligosaccharides synthesis during lactose hydrolysis by β-galactosidase from Kluyveromyces lactis (Lactozym 3000 L HP G) Food Chem. 2008;107:258–264. doi: 10.1016/j.foodchem.2007.08.011. [DOI] [Google Scholar]
- Nakkharat P, Kulbe KD, Yamabhai M, Haltrich D. Formation of galacto oligosaccharides during lactose hydrolysis by a novel β-galactosidase from the moderately thermophilic fungus Talaromyces thermophilus. Biotechnol J. 2006;1:633–638. doi: 10.1002/biot.200600013. [DOI] [PubMed] [Google Scholar]
- Neri DFM, Balcao VM, Costa RS, Rocha I, Ferreira E, Torres DPM. Galacto-oligosaccharides production during lactose hydrolysis by free Aspergillus oryzae beta galactosidase and immobilized on magnetic polysiloxane-polyvinyl alcohol. Food Chem. 2009;115:92–99. doi: 10.1016/j.foodchem.2008.11.068. [DOI] [Google Scholar]
- Nguyen TH, Splechtna B, Steinbock M, Kneifel W, Lettner HP, Kulbe KD, Haltrich D. Purification and characterization of two novel β-galactosidases from Lactobacillus reuteri. J Agric Food Chem. 2006;54:4989–98. doi: 10.1021/jf053126u. [DOI] [PubMed] [Google Scholar]
- Oberoi HS, Bansal S, Dhillon GS. Enhanced β-galactosidase production by supplementing whey with cauliflower waste. Int J Food Sc Technol. 2008;43:1499–1504. doi: 10.1111/j.1365-2621.2008.01738.x. [DOI] [Google Scholar]
- Panesar PS, Kumari S, Panesar R (2010) Potential applications of immobilized β-galactosidase in food processing industries. Enzyme Res 1–16 [DOI] [PMC free article] [PubMed]
- Park HY, Kim HJ, Lee JK, Kim D, Oh DK. Galactooligosaccharide production by a thermostable beta-galactosidase from Sulfolobus solfataricus. World J Microb Biot. 2008;24:1553–1558. doi: 10.1007/s11274-007-9642-x. [DOI] [Google Scholar]
- Quintero M, Maldonado M, Perez-Munoz M, Jimenez R, Fangman T, Rupnow J, Wittke A, Russell M, Hutkins R (2011) Adherence inhibition of Cronobacter sakazakii to intestinal epithelial cells by prebiotic oligosaccharides. Curr Microbiol 62:1448–1454 [DOI] [PubMed]
- Sangwan V, Tomar SK, Singh RRB, Ali B. Galactooligosaccharides: novel components of designer foods. J Food Sci. 2011;76:R103–R111. doi: 10.1111/j.1750-3841.2011.02131.x. [DOI] [PubMed] [Google Scholar]
- Searle LE, Cooley WA, Jones G, Nunez A, Crudgington B, Weyer U, Dugdale AH, Tzortzis G, Collins JW, Woodward MJ, La Ragione RM. Purified galactooligosaccharide, derived from a mixture produced by the enzymic activity of Bifidobacterium bifidum, reduces Salmonella enterica serovar Typhimurium adhesion and invasion in vitro and in vivo. J Med Microbiol. 2010;59:1428–1439. doi: 10.1099/jmm.0.022780-0. [DOI] [PubMed] [Google Scholar]
- Shenderov BA. Metabiotics: novel idea or natural development of probiotic conception. Microb Ecol Health Dis. 2013;24:20399. doi: 10.3402/mehd.v24i0.20399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk DB, Davis A, Vulevic J, Tzortzis G, Gibson GR. Clinical trial: The effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2009;29:508–518. doi: 10.1111/j.1365-2036.2008.03911.x. [DOI] [PubMed] [Google Scholar]
- Sinclair HR, de Slegte J, Gibson GR, Rastall RA (2009) Galactooligosaccharides (GOS) inhibit Vibrio cholerae toxin binding to its GM1 receptor. J Agric Food Chem 57:3113–3119 [DOI] [PubMed]
- Somkuti GA, Dominiecki ME, Steinberg DH. Permeabilization of Streptococcus thermophilus and Lactobacillus delbureckii subsp. bulgaricus with ethanol. Curr Microbiol. 1998;36:202–206. doi: 10.1007/s002849900294. [DOI] [PubMed] [Google Scholar]
- Splechtna B, Nguyen T, Zehetner R, Lettner HP, Lorenz W, Haltrich D. Process development for the production of prebiotic galacto-oligosaccharides from lactose using β-galactosidase from Lactobacillus sp. Biotechnol J. 2007;2:480–485. doi: 10.1002/biot.200600230. [DOI] [PubMed] [Google Scholar]
- Sriphannam W, Unban K, Ashida H, Yamamoto K, Khanongnuch C. Medium component improvement for β-galactosidase production by a probiotic strain Lactobacillus fermentum CM33. Afr J Biotechnol. 2012;11:11242–11251. [Google Scholar]
- Tari C, Ustok FI, Harsa S. Production of food grade β-Galactosidase from artisanal yogurt strains. Food Biotechnol. 2010;24:78–94. doi: 10.1080/08905430903562807. [DOI] [Google Scholar]
- Torres D, Goncalves M, Teixeira J, Rodrigues L. Galacto-Oligosaccharides: Production, Properties, Applications, and Significance as Prebiotics. Compr Rev Food Sci Food Saf. 2010;9:438–454. doi: 10.1111/j.1541-4337.2010.00119.x. [DOI] [PubMed] [Google Scholar]
- Ustok FI, Tari C, Harsa S. Biochemical and thermal properties of β-galactosidase enzymes produced by artisanal yoghurt cultures. Food Chem. 2010;119:1114–1120. doi: 10.1016/j.foodchem.2009.08.022. [DOI] [Google Scholar]
- Valero JIS. Production of galacto-oligosaccharides from lactose by immobilized β-galactosidase and posterior chromatographic separation. Disseration: Graduate school of The Ohio State University; 2009. [Google Scholar]
- Wang D, Sakakibara M (1997) Lactose hydrolysis and β-galactosidase activity in sonicated fermentation with Lactobacillus strains Ultrasonics. Sonochemistry 4:255–261 [DOI] [PubMed]
- Zhou QZK, Chen XD. Effects of temperature and pH on the catalytic activity of the immobilized β-galactosidase from Kluyveromyces lactis. Bioch Eng J. 2001;9:33–40. doi: 10.1016/S1369-703X(01)00118-8. [DOI] [Google Scholar]