Abstract
Obesity is a chronic metabolic disorder that is associated with numerous diseases including hyperlipidemia, diabetes mellitus, hypertension, atherosclerosis, cardiovascular disease, and cancer. Cinnamic acid is a phytochemical compound having many biological effects and could be considered for the management of obesity. This study is aimed to assess the possible anti-obesity and cardioprotective properties of cinnamic acid (CA) in high fat diet-fed rats (HFD). Male Wistar rats were divided into 4 groups. They received normal diet, HFD diet, HFD supplemented with fluvastatin (2 mg/kg/day) or cinnamic acid (30 mg/kg/day) for 7 weeks. The results showed an increase in body weight of HFD rats by ~27 % as compared to control group. Moreover, serum lipase activity underwent a significant rise by 103 % which led to an increase in the levels of total cholesterol (T-Ch), triglycerides (TG), LDL-cholesterol in serum of untreated HFD-fed rats. Furthermore, the concentration of leptin and angiotensin-converting enzyme (ACE) activity exhibited remarkable increases in serum of HFD-fed rats as compared to controls. Whereas, the administration of CA to HFD-fed rats improved the body weight gain and serum lipid profile and reverted back near to normal the activities of lipase and ACE. In addition, the echocardiography evidenced that CA is able to protect the aorta and aortic arch and avoided vasoconstriction by increasing their diameters and improved liver steatosis and kidney indices of toxicity. Overall, these results suggest that cinnamic acid exerts anti-obesity and antihypertensive effects through inhibition of lipid digestive enzymes and ACE.
Keywords: ACE, HFD, Lipase, Leptin
Introduction
During the last few decades, the incidence of obesity, in both developed nations and poor countries, has witnessed a considerable increase. Currently, more than one billion adults worldwide are suffering from overweight and at least 300 million of them are obese (Rich et al. 2012). It is due to an excess in consumed energy which leads to accumulation of fat in the body. This excess of energy source is frequently derived from the fat contained in the food itself. Notably, obesity is often associated with a variety of chronic diseases such as hyperlipidemia, diabetes mellitus, hypertension coronary artery diseases and certain cancers ( Lei et al. 2007). Thus, to reduce fat accumulation, the digestion and absorption of fat into the body should be prevented (Hanefeld and Sachse 2002). However, owing to the adverse side effects associated with many antiobesity drugs, more trials have focused on screening natural sources that have been reported to reduce body weight with minimum side effects (Rahul et al. 2007). One of the most important agents to study obesity and its side effects is the leptin level, adipose-derived hormone which reduces appetite. Leptin production is increased in obese animals and humans and correlates positively with body mass index (Brennan and Mantzoros 2006). Obese generally reveal an unusual high circulating concentration of leptin (Considine et al. 1996). The significant secretion leptin is accompanied by an increased angiotensin converting enzyme (ACE) level, a potent vasoconstrictor. The inhibition of ACE results in the reduced formation of angiotensin II and reduced metabolism of bradykinin, leading to a systematic dilation of the arteries and veins and a decrease in arterial blood pressure. Therefore, pancreatic lipase inhibitors are considered a valuable therapeutic reagent for treating diet promoted obesity in humans (Sharma and Henderson 2008; Mnafgui et al. 2012). Synthetic available inhibitors drugs of pancreatic lipase cause serious adverse effects, including constipation, insomnia, stomachache, and myocardial infarction (Bray 2001). In this respect several investigations focused in the research of phytochemicals more safe with limited side effects in the treatment of obesity and its complications. Many epidemiological studies have found that the consumption of foods and drinks with high phenolic content is associated with the prevention of obesity (Choi et al. 2007). In this respect, hydroxycinnamic acids which constitute a major class of phenolic acids that are widely available in seeds, fruits and vegetables, consumed as dietary phenolic compounds that play a vital role in the formation of commercially important intermediate molecules. Many studies discovered biological activities of cinnamic acid and its derivatives such as antioxidant, hepatoprotective, anxiolytic, insect repellent and antidiabetic (Kasetti et al. 2012) etc. Different substitutions on basic moiety lead to various pharmacological activities. The m-hydroxy or the p-methoxy residue on cinnamic acid is important functional groups as an effective insulin releasing agent. While, 3, 4-dihydroxycinnamic acid (caffeic acid) shows hepatoprotective activity (Sharma 2011). Cinnamic acid derivatives prove a variety of pharmacological activities without side effects. This research was carried out to evaluate the in vivo antiobesity and cardioprotective activities of cinnamic acid.
Material and methods
Chemicals
Lipase kit was purchased from Biolabo reagents France, DMSO (dimethylsulfoxide) was purchased from Merck (Germany) and porcine pancreatic lipase, cinnamic acid powder were obtained from Sigma-Aldrich, St. Louis, USA. ACE kit was purchased from Trinity, UK. Fluvastatin (trade name, lescol) tablets were purchased from local pharmacy. The remaining chemicals used were of analytical grade.
Determination of lipase activity in vitro
The method was modified from the assay reported by Nakai et al. (2005). The pancreatic lipase activity was measured using 4-methylumbelliferyl oleate (4-MU oleate) as a substrate. 25 μL of a sample solution dissolved in DMSO and 50 μL of a 0.1 mM 4-MU solution dissolved in a buffer consisting of 13 mM Tris–HCl, 150 mM NaCl, and 1.3 mM CaCl2 (pH 8.0) were mixed in the well of a microtiter plate, and 25 μL of the lipase solution (50 U/mL) in the above buffer was then added to start the enzyme reaction. After incubation at 25 °C for 30 min, 0.1 mL of 0.1 M sodium citrate (pH 4.2) was added to stop the reaction. The amount of 4-methylumbelliferone released by lipase was measured with a fluorometrical microplate reader (Fluoroskan Ascent C LabSystems, Inc.) at an excitation wavelength of 360 nm with a tolerance of ±40 nm and an emission wavelength of 460 nm with a tolerance of ± 20 nm. The lipase inhibition activity was determined by measuring the effect on the enzyme reaction rate after adding samples, compared with the control, Fluvastatin is used as positive control (Mnafgui et al. 2012 ). PI: percentage of enzyme activity inhibition.
Animals and treatments
The essays of this study were conducted on total 40 adult male Wistar rats, weighting 180 ± 10 g, which were obtained from the local Central Pharmacy, Tunisia. All rats were kept in an environmentally controlled breeding room (temperature: 20 ± 2 °C; humidity: 60 ± 5 %; 12 h dark/light cycle) where they had standard diets and free access to tap water. The experimental protocols were conducted in accordance with the guide for the care and use of laboratory animals issued by the University of Sfax, Tunisia, and approved by the Committee of Animal Ethics. The rats were randomly divided in four groups of eight animals each:
-
Group I:
(Control) normal male rats were fed with normal chow diet.
-
Group II:
(HFD) male rats received high fat diet (10 % sheep fat + 5 % carbohydrates + 0.1 % cholic acid / kg chow diet) to persuade hyperlipidemia for 7 weeks. Cholic acid is a bile acid involved to facilitate the formation of micelles, which promotes processing of dietary fat (Mnafgui et al. 2012).
-
Group III:
(Fluv) male rats received HFD and fluvastatin (2 mg / kg, body weight / daily) by gastric gavage route for 7 weeks.
-
Group IV:
(CA) male rats received HFD and treated with cinnamic acid by gastric gavage route (30 mg / kg of body weight / daily) for 7 weeks.
After the 7 weeks induction, the animals were weighted and sacrificed by decapitation in order to minimize the handling stress, and the trunk blood collected. The serum was prepared by centrifugation (1500 × g, 15 min, 4 °C), frozen and stored at −20 °C until analysis.
Toxicity study
The toxicity of cinnamic acid was tested using four doses (50, 100, 500, 1000 mg/kg body weight) (six rats for each dose). Six control rats were kept under the same conditions without any treatments. The animals were observed continuously during the first hour, and then every hour for 6 h, then after12 and 24 h, and finally after every 24 h, up to 3 weeks for any physical signs of toxicity such as writhing, gasping, salivation, diarrhea, cyanosis, any nervous manifestations, or mortality.
Diagnostic of abdominal sonography
Before the abdominal sonography, the experimental animals were anesthetized with ether and injected by chloral hydrochloride intraperitoneally. The liver, thoracic aorta and aortic arch diameters of the experimental animals were assessed by Echograph 4D (GE VOLUSON 730 EXPERT BT 05).
Biochemical analysis
The leptin level was determined by a commercially available Enzyme-Immunoassay (Alpco Diagnostics, Salem NH) which utilizes two specific polyclonal antibodies for mouse and rat leptin. The analyses of serum lipase and serum lipids level of triglycerides (TG), total-cholesterol (T-Ch), high density lipoprotein-cholesterol (HDL- c), were measured using the corresponding commercial kits (Biolabo, France) on an automatic biochemistry analyzer (BS 300, China) at the pathological laboratory of Sidi Bouzid Hospital. Serum LDL-cholesterol concentration was determined according to the following formula of Friedewal et al. (1972): LDL_cholesterol = Total cholesterol – (Triglycerides / 5) – (HDL_cholesterol). The serum activity of Angiotensin converting enzyme (ACE) was measured using the corresponding commercial kit (Trinity Biotech, United Kingdom). Serum levels of lactate deshydrogenase (LDH), Creatine phosphokinase (CPK), Aspartate aminotransferase (AST), alanine aminotransferase (ALT), activities and creatinine, uric acid and urea rates were measured in frozen aliquots of serum by standardized enzymatic procedures using commercial kits from (Biolabo, France) on an automatic biochemistry analyzer (Vitalab Flexor E, USA) at the clinic pathological laboratory of Sidi Bouzid Hospital.
Statistical analysis
Data are presented as means ± standard deviation (SD). Determinations were performed from eight animals per group and differences were examined by a one-way analysis of variance (ANOVA) followed by the Fisher test (Stat View). *P < 0.05 was considered statistically significant.
Results
Mortality and clinical observations
No deaths were observed either in HFD rats treated with cinnamic acid (30 mg / kg of body weight) and either at doses up to 1 g / kg or in the other groups over the administration period. Compared with the control group of rats, the experimental groups showed no treatment related changes in clinical signs such as external appearance, behavior and motility.
Effect of cinnamic acid (CA) on body weight of experimental rats
As shown in Fig. 1, rats were fed with the HFD for 7 weeks and showed a remarkable increase in body weight by 26.8 % as compared to control animals. The average body weight of HFD-fed rats which received orally cinnamic acid and fluvastatin underwent a significant decrease by 9 % and 10 % respectively (P < 0.05), as compared to HFD fed group.
Fig. 1.
Effect of CA on body weight of experimental rats. Values are given as mean ± SD for group of 8 rats each. Statistically, values are presented as follows: *P < 0.05 significant differences compared to controls. # P < 0.05 significant differences compared to HFD
The improvement of hyperleptinemia by cinnamic acid in HFD -fed rats
The HFD-fed rats showed a significant rise in serum leptin level by 126 % as compared to the level observed in control animals (Fig. 2). However, in response to fluvastatin and cinnamic acid, the HFD-fed rats showed significant decrease in serum leptin levels by 37 and 30 % respectively (P < 0.05) as compared to HFD group.
Fig. 2.
Effect of CA on serum leptin level of experimental rats. Values are given as mean ± SD for group of 8 rats each. Statistically, values are presented as follows: *P < 0.05 significant differences compared to controls. # P < 0.05 significant differences compared to HFD
Effect of cinnamic acid on lipase activity in vitro and in serum of experimental rats
As revealed in Table 1, the cinnamic acid exhibited good inhibition activity of pancreatic lipase (IC50 = 39.17 μg/mL) in a dose dependent pattern. In vivo, the HFD group proved a remarkable increase in serum lipase activity by 103 % as compared to control animals (Fig. 3). However, administration of cinnamic acid to HFD-fed rats decreased significantly the activity of serum lipase by 18 % (P <0.05) as compared to HFD untreated group.
Table 1.
In vitro, pancreatic lipase inhibition assays of cinnamic acid
| Sample | Concentration (μg/ml) | % Inhibition | IC 50 (μg/ml) |
|---|---|---|---|
| Cinnamic acid | 50 | 63.82 ± 2.37 | 39.17 |
| 100 | 74.46 ± 2.54 | ||
| 200 | 85.81 ± 2.18 | ||
| Fluvastatin | 25 | 74.58 ± 1.13 | 16.76 |
| 50 | 86.78 ± 1.27 | ||
| 100 | 93.35 ± 1.25 |
The data are expressed in mean ± S.E.M. n = 3 in each group
Fig. 3.
Effect of CA on lipase activity in serum of control and HFD rats. Values are given as mean ± SD for group of 8 animals each. Statistically, values are presented as follows: *P < 0.05 significant differences compared to controls. # P < 0.05 significant differences compared to HFD
Serum biochemical levels
Table 2 indicated a significant increase in serum T-Ch, TG, LDL-c by 77, 73 and 250 % respectively observed in HFD group accompanied with a remarked decrease in HDL-c level by 10 % as compared to control rats. However, treatment with cinnamic acid showed a significant reduction (P <0.05) in serum cholesterol, triglycerides and LDL-c by 23, 48 and 39 % respectively, and significant increase (P <0.05) in HDL-c as compared to HFD-fed rats. To evaluate the effect of cinnamic acid on the liver and kidney functions, serum toxicological markers indicating liver or kidney injury were measured at the end of the experimental period. Actually, the cinnamic acid treatment of HFD-fed rats significantly inhibited the HFD induced increase in serum AST, ALT, LDH and CPK activities (Table 3). The serum levels of urea, uric acid and creatinine were significantly increased in HFD group as compared to control one. However, CA-treated rats reverted back near to normal levels these serum biomarkers. Similar results to those observed in the CA-treated group were noted in HFD fed rats treated with fluvastatin. These data demonstrated that administration of 30 mg/kg/day cinnamic acid for 7 weeks induced no detectable adverse toxic effects on the rat.
Table 2.
Effect of cinnamic acid (CA) serum lipid profile
| Groups | Control | HFD | Fluv | CA |
|---|---|---|---|---|
| T-Ch | 1.4 ± 0.18 | 2.47 ± 0.17 * | 1.78 ± 0.43 *# | 1.91 ± 0.21 *# |
| TG | 1.37 ± 0.15 | 2.37 ± 0.35 * | 1.17 ± 0.31 # | 1.23 ± 0.26 # |
| LDL-c | 0.34 ± 0.11 | 1.22 ± 0.17 * | 0.68 ± 0.14 *# | 0.74 ± 0.06 *# |
| HDL-c | 0.87 ± 0.04 | 0.78 ± 0.05 * | 0.84 ± 0.06 # | 0.82 ± 0.05*# |
Total cholesterol (T-ch), triglycerides (TG), low density lipoprotein cholesterol (LDL-c), and high density lipoprotein-cholesterol (HDL-c) in serum. Values are given as mean ± SD for group of 8 rats. Statistically, values are represented as follows: *P < 0.05 significant differences compared to controls. # P < 0.05 significant differences compared to HFD
Table 3.
Effect of cinnamic acid (CA) on liver profile indices
| Groups | Control | HFD | Fluv | CA |
|---|---|---|---|---|
| Liver fonction | ||||
| AST (UI/L) | 115.16 ± 6.85 | 166.5 ± 22.13* | 131.75 ± 16.23*# | 128 ± 9.3*# |
| ALT (UI/L) | 57.83 ± 6.64 | 81.16 ± 4.35* | 72.5 ± 3.88*# | 54.33 ± 7.11#@ |
| LDH (UI/L) | 681.5 ± 91.88 | 1049.4 ± 87.63* | 862.25 ± 48.34*# | 631.16 ± 94.42#@ |
| CPK (UI/L) | 2047.25 ± 136.11 | 3703.33 ± 286.73* | 3212 ± 165.81*# | 3075 ± 115.62*# |
| Kidney fonction | ||||
| Urea (mmol/L) | 6.36 ± 0.66 | 7.98 ± 0.29* | 6.07 ± 0.89# | 6.03 ± 0.81# |
| Uric acid (μmol/l) | 29.16 ± 3.97 | 52 ± 5.21* | 24.75 ± 6.23# | 26 ± 2.91*# |
| Creatinine (μmol/l) | 41.5 ± 2.5 | 47.5 ± 2.07* | 42.4 ± 4.21# | 40.33 ± 4.45# |
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate deshydrogenase (LDH) and creatine phosphokinase (CPK) and kidney parameters (urea, uric acid and creatinine) of experimental groups of rats. Values are given as mean ± SD for groups of 8 rats. Statistically, values are presented as follows: P < 0.05 significant differences compared to controls. # P < 0.05 significant differences compared to HFD (high fat diet). @ P < 0.05 significant differences compared to fluvastatin (Fluv)
Effect of cinnamic acid on ACE activity
Figure 4 shows that the ACE activity in the serum of HFD-fed rats underwent a potent rise of 78 % (P <0.05) as compared to controls. However, the administration of cinnamic acid or fluvastatin to the HFD rats reverted back the activity of ACE in serum back by 42 and 39 %, respectively.
Fig. 4.
Effect of CA on serum ACE level of experimental rats. Values are given as mean ± SD for group of 8 rats each. Statistically, values are presented as follows: *P < 0.05 significant differences compared to controls. # P < 0.05 significant differences compared to HFD
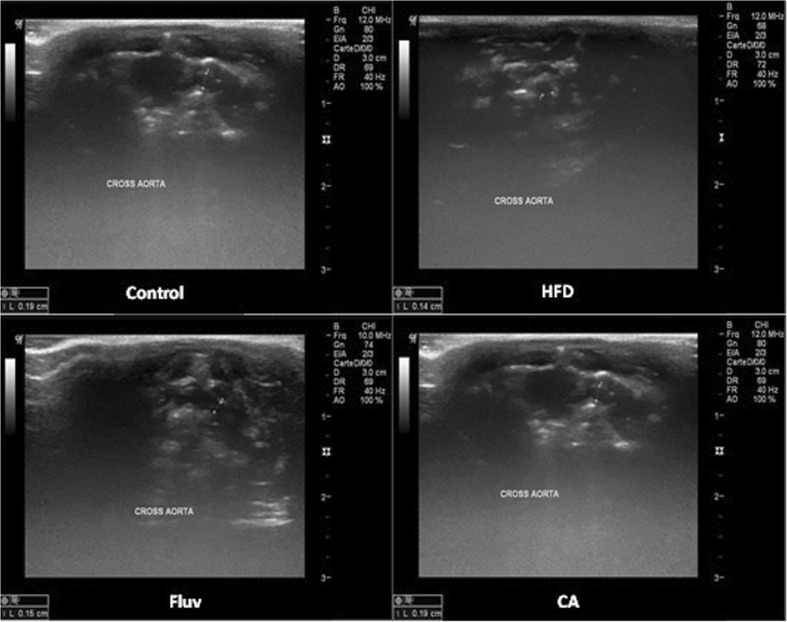
Sonography diagnosis and ultrasound measurements of the thoracic aorta and aortic arch diameter of rats
The diameter of the vascular structures was measured transversally and longitudinally. In cross section, the measure was adopted when the spherical structure appeared well. As shown in Figs. 5, 6 and Table 4, after 7 weeks, the HFD induced a significant decrease in the vascular diameters of the HFD-untreated rats with response to a reduce in thoracic aorta by 17.6 % and in aortic arch by 24 % as compared to control group. However, the administration of cinnamic acid and Fluvastatin restored the diameter of thoracic aorta by 12 and 10 % respectively and increased the aortic arch diameter by 23 % and 18 % respectively (P <0.05).
Fig. 5.
Thoracic sonography and measurement of aortic arch diameter of control and HFD groups of rats
Fig. 6.
Sonography and measurement of thoracic artery diameter of experimental animals
Table 4.
Effect of CA on thoracic aorta and aortic arch diameters of control and experimental groups of rats
| Groups | Control | HFD | Fluv | CA |
|---|---|---|---|---|
| Thoracic aorta diameter (cm) | 0.21 ± 0.008 | 0.17 ± 0.007* | 0.19 ± 0.008*# | 0.20 ± 0.008*# |
| Aortic arch diameter (cm) | 0.19 ± 0.007 | 0.14 ± 0.010* | 0.17 ± 0.010*# | 0.17 ± 0.009*# |
Values are given as mean ± SD for groups of 8 rats. Statistically, values are presented as follows: *P < 0.05 significant differences compared to controls. # P < 0.05 significant differences compared to HFD.
Interestingly, the echocardiography showed an heterogenic aspect in the liver of HFD-fed rats as compared to control, Fluv and CA groups. This heterogeneity indicated hepatic steatosis caused by the high fat diet (Fig. 7).
Fig. 7.
Liver sonography of treated animals one of each group
Furthermore, the sonography revealed a remarkable case of situs inversus called dextrocardia, a congenital condition in which the heart is reversed or mirrored from its normal position and being located on the right side of the thorax. This case was observed in a HFD-fed rat with no clinical signs of disease.
Discussion
Obesity is one of the most widespread metabolic disorders in contemporary society. It is closely related to hypertension, type 2 diabetes, coronary heart disease, cancer, respiratory complications, and osteoarthritis (Kopelman 2000). Considerable efforts have been devoted to the discovery of anti-obesity drugs worldwide. The focus has been shifted to treat the various ailments through plant-derived drugs due to their safety, efficacy, cultural acceptability and lesser side effects (Rahul et al. 2007). Cinnamic acid is found both in free form, and especially in the form of esters (ethyl, cinnamyl, benzyl). The latters are widely spread in the plants and possess wide range of activities. This research was carried out to evaluate the effects of cinnamic acid on obese rats induced by a HFD to assess its potential for the treatment of obesity and hypertension. Interestingly, our results demonstrated that cinnamic acid has a beneficial effect on the body weight of obese rats without changes in clinical signs such as external appearance, behavior, mental state, and daily activities. The increase in body weight was accompanied by an increase in leptin serum level. Leptin has dramatic impact on the understanding of milti-function polypeptide. It may be associated with the regulation of appetite, consumed energy and adiposity. Leptin is produced almost exclusively by adipose tissue and acts in the central nervous system through a specific receptor and multiple neuropeptide pathways to decrease appetite and increase energy expenditure (Zhang et al. 1994; Friedman and Halas 1998). A similar relationship between leptin levels and body weight has also been observed in a large number of clinical investigations (Li et al. 2011; Mnafgui et al. 2012). An important finding of this work was the lowered level of circulating leptin after the treatment of HFD fed animals with cinnamic acid which leads to decrease of their body weight. Moreover, our investigation showed an increase of pancreatic lipase activity in serum of HFD rats. The pancreatic lipase is responsible for the digestion of lipids by cleaving dietary triglycerides into monoacylglycerides and free fatty acids, both absorbable by enterocytes (Chakrabarti 2009). Dietary fat is not directly absorbed from the intestine unless it has been subjected to the action of pancreatic lipase (McDougall et al. 2009). Thus, pancreatic lipase is important to the digestion and absorption of fat. Its inhibition could therefore result in a reduced fat absorption, and thereby energy uptake, which is one of the key targets to mediate obesity. This study evidenced that cinnamic acid inhibited lipase activity in vitro with IC50 = 39.17 μg/ml. This was interestingly confirmed in vivo. The important inhibitory action is explained by the fact that cinnamic acid derivatives are naturally occurring substances found in fruits, vegetables, and flowers. They are consumed as dietary phenolic compounds (Adisakwattana et al. 2008). As well, polyphenols have some potential efficacy for preventing obesity through inhibition of key-enzymes related to fat metabolism including pancreatic lipase (Rahul et al. 2007; Mnafgui et al. 2012). In addition, the inhibitory action of lipase activity restricted the hydrolysis of dietary triglycerides nonabsorbable into monoglycerides and free fatty acids absorbable by the intestine. This effect leads to a decrease in T-Ch, LDL-c and TG levels in serum of CA-treated group of rats. Consequently, it showed a reduction of body weight as antiobesity action (Subramaniam et al. 2011). In previous studies, the chronic increases in circulating leptin caused sustained increases in arterial pressure and heart rate and was consistent with a possible role for leptin in obesity hypertension (Shek et al. 1998; Kohlstedt et al. 2009 ) demonstrated that; it also could increase heart rate by increasing cardiac sympathetic activity or by withdrawal of parasympathetic tone.
The sonography also revealed that HFD might contribute to hepatic steatosis, compared to Fluv and CA groups, and clearly appeared in Fig. 7. As a consequence, cinnamic acid might have an hepatoprotective effect. Our results showed a significant reduce of angiotensin-converting enzyme (ACE) activity in serum of CA treated HFD-fed rats which could explain the reduced lipogenic effect resulting in lower body weight gain observed in our study. In fact, ACE is a metallo-glycoprotein linked to the membrane that catalyses the hydrolysis of the decapeptide angiotensin I by cleaving off the C-terminal dipeptide, producing the octapeptide angiotensin II. This latter is responsible for increasing blood pressure (Loizzo et al. 2007). ACE, a key component of the renin-angiotensin system, is involved in the generation of angiotensin II, a potent vasoconstrictor peptide, and in the degradation of bradykinin, a potent vasoconstrictor (Erdös and Skidgel 1987). The components of the renin-angiotensin system, involved in blood pressure regulation and vascular smooth muscle cell proliferation, are important determinants of vasomotor tone (Amant et al. 1997). The obtained results (Figs. 5 and 6) interestingly demonstrated that cinnamic acid avoided vasoconstriction appeared in HFD group. ACE inhibitory activity is one of the therapeutic methods to treat hypertension (Mnafgui et al. 2012). The sonography of thoracic artery and aortic arch showed a decrease in diameters in HFD group as compared to control group. Though, in CA and Fluv groups, the diameters had not significantly change as compared to control group. Thereby, it can be deduced that cinnamic acid and fluvastatin prevent from hypertension by avoiding vasoconstriction.
This study confirmed, for the first time, that cinnamic acid has a cardioprotective property assessed by its anti-obesity and antihypertension effects. Furthermore, cinnamic acid decreased liver and kidney indices of toxicity such as ALT, AST, LDH, CPK activities and urea, uric acid, and creatinine levels.
Conclusion
In conclusion, we managed to prove that cinnamic acid can significantly reduce the body weight of obese rats and treat hyperlipidemia induced by the HFD without apparent illness effects. These effects are mediated through reducing the serum concentration of leptin and pancreatic lipase activity. In addition, cinnamic acid inhibited also the ACE activity in serum and improved a cardioprotective effect by defending animals against vasoconstriction and hypertension complications. Overall, CA is endowed with a powerful effect in the management of obesity and its related metabolic disorders such as hypertension.
Acknowledgments
This research was supported by the Tunisian Ministry of Higher Education and Scientific Research and the Tunisian Ministry of Public Health. Authors wish to thank Dr. Ahmad Laifi for his assistance in the realization of the echography.
Competing Interests
The authors declare no conflict of interest
Footnotes
Kais Mnafgui and Amal Derbali contributed equally to this work
References
- Adisakwattana S, Moonsan P, Yibchok-anun S. Insulin-releasing properties of a series of cinnamic acid derivatives in vitro and in vivo. J Agric Food Chem. 2008;56:7838–7844. doi: 10.1021/jf801208t. [DOI] [PubMed] [Google Scholar]
- Amant C, Hamon M, Bauters C, Richard F, Helbecque N, McFadden EP, Escudero X, Lablanche JM, Amouyel P, Bertrand ME. The angiotensin II type 1 receptor gene polymorphism is associated with coronary artery vasoconstriction. J Am Coll Cardiol. 1997;29:486–490. doi: 10.1016/S0735-1097(96)00535-9. [DOI] [PubMed] [Google Scholar]
- Bray GA. Drug treatment of obesity. Rev Endocr Metab Disord. 2001;2:403–418. doi: 10.1023/A:1011808701117. [DOI] [PubMed] [Google Scholar]
- Brennan AM, Mantzoros CS. Drug insight: the role of leptin in human physiology and pathophysiology-emerging clinical applications. Nat Clin Pract Endocrinol Metab. 2006;2:318–327. doi: 10.1038/ncpendmet0196. [DOI] [PubMed] [Google Scholar]
- Chakrabarti B. Pharmacotherapy of obesity: emerging drugs and targets. Expert Opin Ther Targets. 2009;13:195–207. doi: 10.1517/14728220802637063. [DOI] [PubMed] [Google Scholar]
- Choi H, Eo H, Park K, Jin M, Park EJ, Kim SH, Park JE, Kim S. A water-soluble extract from Cucurbita moschata shows anti-obesity effects by controlling lipid metabolism in a high fat diet-induced obesity mouse model. Biochem Biophys Res Commun. 2007;359:419–425. doi: 10.1016/j.bbrc.2007.05.107. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Erdös E, Skidgel RA. The angiotensin I-converting enzyme. Lab Investig. 1987;56:345–348. [PubMed] [Google Scholar]
- Friedewal WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Friedman JM, Halas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Hanefeld M, Sachse G. The effect of orlistat on body weight and glycemic control in overweight patients with type 2 diabetes: A randomized, placebo-controlled trial. Diabetes Obes Metab. 2002;4:415–423. doi: 10.1046/j.1463-1326.2002.00237.x. [DOI] [PubMed] [Google Scholar]
- Kasetti RB, Nabi SA, Swapna S, Apparao C. Cinnamic acid as one of the antidiabetic active principle (s) from the seeds of Syzygium alternifolium. Food ChemToxicol. 2012;50:1425–1431. doi: 10.1016/j.fct.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Kohlstedt K, Gershome C, Trouvain C, Hofmann WK, Fichtlscherer S, Fleming I. Angiotensin-converting enzyme (ACE) inhibitors modulate cellular retinolbinding protein 1 and adiponectin expression in adipocytes via the ACE dependent signaling cascade. Mol Pharmacol. 2009;75:685–692. doi: 10.1124/mol.108.051631. [DOI] [PubMed] [Google Scholar]
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- Lei F, Zhang XN, Wang W, Xing DM, Xie WD, Su H, Du LJ. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int J Obes. 2007;31:1023–1029. doi: 10.1038/sj.ijo.0803502. [DOI] [PubMed] [Google Scholar]
- LI J, Ma W, Wang S. Slower gastric emptying in high-fat diet induced obese rats is associated with attenuated plasma ghrelin and elevated plasma leptin and cholecystokinin concentrations. Regul Peptides. 2011;171:53–57. doi: 10.1016/j.regpep.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Loizzo MR, Tundis R, Statti GA, Passalacquai NG, Peruzzi L, Menichini F. In vitro angiotensin converting enzyme inhibiting activity of Salsola oppositifolia Desf., Salsola soda L. and Salsola tragus L. Nat Prod Res. 2007;21:846–851. doi: 10.1080/14786410701482582. [DOI] [PubMed] [Google Scholar]
- McDougall GJ, Kulkarni NN, Stewart D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem. 2009;115:193–199. doi: 10.1016/j.foodchem.2008.11.093. [DOI] [Google Scholar]
- Mnafgui K, Hamden K, Ben Salah H, Kchaou M, Nasri M, Slama S, Derbali F, Allouche N, Elfeki A (2012) Inhibitory activities of Zygophyllum album: A natural weight-lowering plant on key enzymes in high-fat diet-fed rats. Evid Based Complement Alternat Med, 2012, Article ID 620384. doi:10.1155/2012/620384 [DOI] [PMC free article] [PubMed]
- Nakai M, Fukui Y, Asami S, Yoshiko O, Takashi I, Hiroshi S, Tohru M, Fumio H, Yoshinobu K. Inhibitory effects of oolong tea polyphenols on pancreatic lipase in vitro. J Agric Food Chem. 2005;53:4593–4598. doi: 10.1021/jf047814+. [DOI] [PubMed] [Google Scholar]
- Rahul B, Birari RB, Bhutani KK. Pancreatic lipase inhibitors from natural sources unexplored potential,”. Drug Discov Today. 2007;12:879–889. doi: 10.1016/j.drudis.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Rich BS, Keel R, Ho VP. Turbendian H. Cofepime dosing in the morbidly obese patient population. Obes Surg. 2012;22:465–471. doi: 10.1007/s11695-011-0586-8. [DOI] [PubMed] [Google Scholar]
- Sharma P. Cinnamic acid derivatives: A new chapter of various pharmacological activities. J Chem Pharm Res. 2011;3:403–423. [Google Scholar]
- Shek EW, Brands M, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31:409–414. doi: 10.1161/01.HYP.31.1.409. [DOI] [PubMed] [Google Scholar]
- Subramaniam S, Subramaniam R, Rajapandian S, Uthrapathi S, Gnanamanickam VR, Dubey GP (2011) Antiatherogenic activity of ethanolic fraction of Terminalia arjuna bark on hypercholeterolemic rabbits. Evid Based Complement Alternat Med doi: 487916 [DOI] [PMC free article] [PubMed]
- Zhang Y, Proenca R, Maffel M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]