Abstract
The collagen of skin, scales and fins of Catla catla and Cirrhinus mrigala were isolated and characterised. Nine fishes of each fish species of three weight groups were collected from a commercial fish farm. Collagen characterisation using SDS-PAGE revealed the molecular weights (kDa) of the C. catla skin, scales, and fins which ranged from 120 to 210, 70 to 201, and 68 to 137 kDa, respectively. The size of the collagen of C. mrigala skin, scales and fins ranged from 114 to 201, 77 to 210, and 70 to 147 kDa, respectively. Glycine and alanine were the most abundant amino acid, whereas tryptophan was totally absent in all selected tissues. Thus, significant variation exists in type of collagen and amino acid profile within the weight groups of the two fish species. The imino acid (proline and hydroxyproline) contents estimated in C. catla and C. mrigala skin (161–165 and 160–168), scales (155–159 and 152–161) and fins (162–171 and (152–155) residues/1,000 residues, respectively. The proximate analysis was also performed for skin, scales and fins. The maximum protein content of the skin was determined as 26.10 % and 22.90 % in the C. catla and C. mrigala, respectively, from the W3 weight group. The scales of the W3 weight group exhibited maximum protein contents of 25.90 and 21.77 % for C. catla and C. mrigala, respectively. The maximum protein contents (19.04 % and 18.12 %) were recorded for C. catla and C. mrigala, respectively in the fins.
Keywords: Skin, Fins, Scales, Characterisation, Collagen, Fish, Amino acid
Introduction
Collagen is a fibrous protein that contributes as a major structural protein in the connective tissue of animal skin and bone. Generally, collagen has been applied in cosmetic, biomedical and pharmaceutical industries (Jongjareonrak et al. 2005; Nalinanon et al. 2007). Fish skin can be used as an alternative source for collagen since mammalian collagens are associated with several problems such as the outbreak of mad cow disease and the constraint for some religions, mainly Islam and Judaism (Nalinanon et al. 2008). Therefore, skin collagens from several fish species such as bigeye snapper (Kittiphattanabawon et al. 2005; Nalinanon et al. 2007), black drum (Ogawa et al. 2003), brown stripe red snapper (Jongjareonrak et al. 2005), carp (Duan et al. 2009), channel catfish (Liu et al. 2007), Nile perch (Muyonga et al. 2004), oscillate puffer fish (Nagai et al. 2002), sheephead seabream (Ogawa et al. 2003), threadfin bream (Nalinanon et al. 2008) and yellowfin tuna (Woo et al. 2008) have been extracted and characterized. Although there are many reports about collagen from skin of marine organisms, there are few studies of fish scales like the studies of Kimura et al. (1991) and those of Shirai (Nomura et al. 1996).
Consequently, much attention has been paid to the alternative sources of collagen, especially from fish skin and fish bone generated as the by-products from fish processing plants. Fish collagens have been extracted from fish skin (Nalinanon et al. 2007; Skierka and Sadowska 2007; Wang et al. 2007). Fish offal, such as the skin, bones, scales, and fins, have garnered increasing attention as an alternative collagen resource (Gomez-Guillen et al. 2002). Fish collagen can be produced from the discarded portion of the fish offal waste, such as the skin, scales, and fins, which are rich collagen sources (Dun et al. 2008). The skins, bones, cartilage, tendons, ligaments, blood vessels, teeth, cornea, and all other vertebrate organs harbor nineteen collagen variants, types I-XIX (Senaratne et al. 2006). Particularly, type I collagen can be observed in all connective tissues, such as the skin and bones. The objective of this study was to isolate and characterise the collagen from the skin, fins and scales, which are the by-products of Catla catla and Cirrhinus mrigala, at a low or no cost.
Materials and methods
Materials
Fifty-four fish samples of the three C. catla and C. mrigala weight groups W1 (501–600 g), W2 (601–900 g) and W3 (901–1,100 g) were obtained from the Fish Seed Hatchery, Faisalabad, Pakistan. The average weights and lengths of the three C. catla weight groups were 506.44 ± 1.16, 806.8 ± 2.44, and 1028.4 ± 3.22 g and 38.1 ± 0.44, 46.4 ± 0.88, and 49.5 ± 0.64 cm for W1, W2 and W3, respectively. The average weight and length of C. mrigala were 515.9 ± 1.22, 818.4 ± 1.44, and 1016.3 ± 1.88 g and 39.22 ± 0.61, 46.33 ± 0.70, and 50.44 ± 1.1 cm for W1, W2 and W3, respectively (Table 1). The fish were transported to the Fisheries Research Laboratory under chilled conditions and stored at −20 °C. The fish samples (skin, scales, and fins) were lyophilised at −50 °C by using a manifold freeze drier by Christ, Alpha 1–4 LD, Germany, Lypholyzer to extract the water to make the samples suitable for storage.
Table 1.
Details of weight and Length parameters of Catla catla and Cirrhinus mrigala
| Category | Catla catla | Cirrhinus mrigala | ||
|---|---|---|---|---|
| Weight (g) | Length (cm) | Weight (g) | Length (cm) | |
| W1 | 506.7 ± 1.16 | 38.1 ± 0.44 | 515.9 ± 1.22 | 39.22 ± 0.61 |
| W2 | 806.8 ± 2.44 | 46.4 ± 0.88 | 818.4 ± 1.44 | 46.33 ± 0.70 |
| W3 | 1028.4 ± 3.22 | 49.5 ± 0.64 | 1016.3 ± 1.88 | 50.44 ± 1.1 |
To remove non-collagenous proteins, the prepared fish skin was mixed with 0.1 MNaOH at a skin/alkali solution ratio of 1:10 (w/v). The mixture was continuously stirred for 6 h at 4 °C and the alkali solution was changed every 2 h. The treated skin was then washed with cold water to a neutral or faintly basic pH of wash water was reached. The pH of wash water was determined using a digital pH meter (Sartorious North America, Edgewood, NY, USA).
Chemical reagents
All the chemicals used were of analytical grade. Pepsin (EC 3.4.23.1; 3,260 U/mg) from porcine stomach mucosa and papain (EC 3.4.22.2; 30000 USP-U/mg) were obtained from Merck (Darmstadt, Germany). ß-Mercaptoethanol (b-ME), V8 protease from Staphylococcus aureus (EC3.4.21.19, 800 U/mg powder); and type I collagen from calf skin were purchased from Sigma Chemical Co. (St. Louis, MO, USA). HMW-SDS markers (MW range of 53 kDa to 250 kDa) were obtained from GE Healthcare UK (Buckinghamshire, UK) with following constituent proteins Myosin: 25 μg, 220000 (Mr), rabbit muscle (source), a-2-Macroglobulin: 100 μg, 170000 (Mr), bovine plasma (source), b-Galactosidase: 16 μg, 116000 (Mr), E. coli (source), Transferrin.. Sodium dodecyl sulphate (SDS), trichloroacetic acid, Folin–Ciocalteu’s phenol reagent, acetic acid and tris(hydroxylmethyl) aminomethane were obtained from Merck (Darmstadt, Germany).
Proximate analysis
The proximate composition of all three weight groups of C. catla and C. mrigala was assessed for the skin, scales and fins by following the methods described in the Association of Official Analytical Chemists (1990).
Isolation of skin, fin and scale collagens
The collagens were isolated using the method described by Nagai and Suzuki (1999) with modifications. All of the samples were prepared at ambient temperature (22–23 °C) with the exception of centrifugation, which was performed at a temperature not higher than 4 °C. Following isolation, the collagen was characterised by the method described by Laemmli (1970).
Extraction of acid soluble collagen (ASC)
ASC was extracted as per the method of Nagai and Suzuki (1999). All processes were carried out at the 4 °C with continuous stirring. The pre-treated samples were defatted with 10 % butyl alcohol with a solid/solvent ratio of 1:10 (w/v) for 48 h and the solvent was changed every 8 h. Defatted samples (skin, scales and fins) were washed with cold water (5–8 °C), followed by soaking in 0.5 M acetic acid with a solid/solvent ratio of 1:15 (w/v) for 24 h. The mixture was filtered through two layers of cheese cloth and the residue was re-extracted under the same conditions. Both filtrates were combined. The collagen was precipitated by adding NaCl (powder) to a final concentration of 2.6 M in the presence of 0.05 M tris (hydroxymethyl) aminomethane, pH 7.0. The resultant precipitate was collected by centrifuging at 20,000 g for 60 min, using a refrigerated centrifuge Avanti J-E (Beckman Coulter, Inc., Palo Alto, CA, USA). The pellet was dissolved in a minimum volume of 0.5 M acetic acid, and dialysed against 50 volumes of 0.1 M acetic acid for 24 h, followed by the dialysis in the same volume of distilled water for another 24 h. The dialysate was freeze dried and was referred to as “acid soluble collagen; ASC”. The yield of ASC was calculated from the percentage of the dry weight of collagen extracted in comparison with the wet weight of the initial skin, scales and fins used.
Extraction of pepsin soluble collagen (PSC)
The undissolved residue obtained after ASC extraction was used for PSC extraction as described by Singh et al. (2011). The residue was soaked in 0.5 M acetic acid with a solid/solvent ratio of 1:15 (w/v) and porcine pepsin (20 U/g residue) was added. The mixtures were continuously stirred at 4 °C for 48 h, followed by filtration with two layers of cheesecloth. The filtrate was subjected to precipitation and the pellet was dialysed in the same manner as those for ASC previously described. The dialysate was freeze dried and was referred to as “pepsin soluble collagen; PSC”. The yield of PSC was also calculated in the same manner as for ASC. Additionally, the accumulated yield of collagen was calculated from the yields of both ASC and PSC.
Quantification/estimation of extracted protein
The amount of protein in the sample solution was assessed using the method described by Bradford (1976).
Qualitative estimation of extracted collagen by SDS-PAGE
The vertical slab gel (Scie-Plas, Model No. TV 50) in the discontinuous buffer system was used for SDS-PAGE (7.5 % polyacrylamide gel concentration) to analyse the extracted collagen as described by Jamilah et al. (2013). The samples were dissolved in 0.02 M sodium phosphate containing 1 % sodium dodecyl sulphate and 3.5 M urea as a denaturing agent (pH 7.2). The mixtures were then centrifuged at 8,500 g for 5 min at room temperature to remove undissolved debris. Solubilised samples containing 20 μg/μl of protein were mixed at 1:1 (v/v) ratio with the sample buffer (0.5 M Tris–HCl, pH 6.8, containing 4 % SDS, 20 % glycerol). Gels were casted in Mini Protein unit (Bio-Rad Laboratories Inc., Richmond, CA, USA) and samples were loaded on the gel and a constant current of 15 mA/gel was passed through for 75 min. Gels were then stained with 0.05 % (w/v) Coomassie Blue R-250 and destained overnight. For the determination of molecular weights of proteins gel doc apparatus (VILBER LOURMAT, France) and software of photocapt (Version 12.5) was used.
Amino acid analysis
The freeze-dried samples of extracted collagen from the skin, scales, and fins were hydrolysed in the presence of 1 % phenol (v/v) in an inert atmosphere with 6 N HCl at 105 °C for 24 h. The hydrolysates were then vacuum-dried (Vacuum drying oven VT 6025, Heraeus Instruments GmbH, Hanau, Germany) at 60 °C until brittle sheets were formed. The derivatisation was carried out using AccQ-Fluor Reagent kit (Waters Co., Milford, USA). The collagens samples were hydrolyzed under reduced pressure in 4.0 mol/L methanesulphonic acid containing 2 mL/L 3-2(2-aminoethyl) indole at 115 °C for 24 h. The hydrolysates were neutralized with 3.5 mol/L NaOH and diluted with 0.2 mol/L citrate buffer (pH 2.2). Amino acids were analysed from 20 μl of sample, which was performed using the Aracus Amino Acid Analyzer (MembraPure, Germany).
Statistical analysis
ANOVA was performed using the Minitab version 15 software package, and the means were compared by Tukey’s test.
Results and Discussion
Proximate composition of the fish skin, scales and fins
The moisture content of the C. catla and C. mrigala skin was recorded and is presented in Table 2. The moisture content of fishes of the W3 weight group differed significantly from those of the W1 and W2 groups (P < 0.05). The comparison of the mean moisture content demonstrated non-significant differences in the skin of both fish species. The skin protein contents in the W1, W2, and W3 weight groups of C. catla and C. mrigala were 24.35 ± 0.95, 25.09 ± 1.09, and 26.10 ± 1.24 % and 22.09 ± 0.77, 22.60 ± 0.88, and 22.90 ± 0.55 %, respectively. No significant difference (P > 0.05) in the skin protein content among the three weight groups was observed (Table 2). The total skin, fat contents of the three C. catla weight groups were 2.11 ± 0.20, 2.45 ± 0.31, and 2.60 ± 0.38 %, respectively and those in the three C. mrigala weight groups were 2.03 ± 0.28, 2.25 ± 0.30, and 2.70 ± 0.40 %, respectively (Table 2). The total fat contents differed significantly (P < 0.05) for both fish species. For the three weight groups, the C. catla skin ash contents were 0.66 ± 0.14, 1.06 ± 0.22, and 1.48 ± 0.36 %, respectively and those of C. mrigala were 1.35 ± 0.20, 1.70 ± 0.23, and 2.13 ± 0.44 %, respectively. The moisture contents in the scales of C. catla and C. mrigala were 67.36 ± 1.31, 65.71 ± 1.40, and 63.20 ± 1.80 % and 70.40 ± 0.90, 68.61 ± 0.81, and 67.76 ± 0.95 % for the W1, W2, and W3 weight groups, respectively (Table 3). A significant difference (P < 0.05) in the scale moisture contents between the three weight groups was observed. The protein contents of the C. catla scales were 22.26 ± 0.78, 23.04 ± 0.71, and 23.90 ± 0.95 % in the three weight groups, respectively. The protein contents in the C. mrigala scales were 20.36 ± 0.67, 21.46 ± 0.55, and 21.77 ± 0.66 % in the three weight groups, respectively, and these contents differed significantly between each weight group (Table 3). The total fat contents of the C. catla and C. mrigala scales were 2.88 ± 0.27, 2.98 ± 0.12, and 3.18 ± 0.44 % and 1.68 ± 0.33, 2.88 ± 0.41, and 2.95 ± 0.33 % in the W1, W2, and W3 weight groups, respectively. The total ash contents of the C. catla scales were 5.11 ± 0.66, 6.55 ± 0.46, and 7.16 ± 0.51 % in the three weight groups, respectively. The total ash contents of the C. mrigala scales were 4.51 ± 0.28, 4.95 ± 0.47, and 5.15 ± 0.40 % in the three weight groups, respectively. The ash content in W3 differed significantly from the W1 and W2 contents (Table 3). The proximate analysis of the total moisture content of C. catla fins revealed 63.22 ± 1.12, 61.26 ± 0.95, and 59.76 ± 1.28 % in the W1, W2, and W3 groups, respectively. The total moisture contents in C. mrigala fins were 64.80 ± 1.08, 62.30 ± 1.21, and 60.72 ± 1.41 % in the W1, W2, and W3 weight groups, respectively (Table 4). The moisture contents in the three weight groups differed significantly among each other (P < 0.05). The protein contents of the C. catla and C. mrigala fins were 17.44 ± 0.57, 18.37 ± 0.71, and 19.04 ± 0.75 % and 17.05 ± 0.80, 17.90 ± 0.91, and 18.12 ± 0.94 % in the W1, W2, and W3 weight groups, respectively. A significant difference (P < 0.05) in the protein contents in the W1, W2, and W3 weight groups was observed. The total fat contents in C. catla fins were 4.98 ± 0.21, 6.71 ± 0.47, and 7.14 ± 0.51 % in the W1, W2, and W3 weight groups, respectively (Table 4). The total fat contents of the C. mrigala fins were 5.21 ± 0.46, 5.75 ± 0.57, and 5.97 ± 0.68 % in the W1, W2, and W3 weight groups, respectively. The total ash contents of the C. catla and C. mrigala fins were 10.67 ± 1.10, 11.57 ± 1.27 and 12.05 ± 1.31 % and 11.35 ± 0.31, 11.67 ± 0.40, and 11.81 ± 0.50 % in the W1, W2, and W3 weight groups, respectively. The statistical analysis revealed that the weight groups differed significantly (P < 0.05) from each other. The maximum protein content was recorded for the C. catla weight group W3. The two fish species revealed a non-significant difference (P > 0.05) with respect to their protein contents. The protein and fat content increased with increasing weight and size. These findings were consistent with the findings of Jabeen and Shakoor (2011) and with those of Javed (1998) and Mahboob et al. (1996) who reported a positive, highly significant correlation between the protein and fat contents in carp. Isolating collagen from fish offal, such as the skin, may represent an efficient, alternative collagen source, which is similar to the findings of Gomes- Guillen et al. (2002) and Shao et al. (2009), who reported the presence of collagen in tissues such as the skin and scales.
Table 2.
Proximate analysis of skin of Catla catla and Cirrhinus mrigala of three weight groupsMeans in the same column for each species followed by different letters are significantly different according to Ducan’s test (P ≤ 0.05); ± Standard Error (S.E)
| Species | Weight categories | Moisture | Protein | Fat | Ash | Carbohydrate |
|---|---|---|---|---|---|---|
| C. catla | W1 | 72.44 ± 0.88a | 24.35 ± 0.95a | 2.11 ± 0.20a | 0.66 ± 0.14a | 0.44 ± 0.02a |
| W2 | 70.88 ± 1.10a | 25.09 ± 1.05a | 2.45 ± 0.31b | 1.06 ± 0.22b | 0.60 ± 0.01b | |
| W3 | 69.15 ± 0.66a | 26.10 ± 1.24a | 2.60 ± 0.38c | 1.48 ± 0.36b | 0.67 ± 0.06a | |
| C.mrigala | W1 | 73.44 ± 1.05a | 22.09 ± 0.77a | 2.03 ± 0.28a | 1.35 ± 0.20a | 1.09 ± 0.11a |
| W2 | 71.70 ± 1.33a | 22.60 ± 0.88a | 2.25 ± 0.30b | 1.70 ± 0.23a | 1.75 ± 0.38b | |
| W3 | 70.37 ± 1.66b | 22.90 ± 0.55b | 2.70 ± 0.40c | 2.13 ± 0.44b | 1.90 ± 0.21a |
Table 3.
Proximate analysis of scales of Catla catla and Cirrhinus mrigala of three weight groups
| Species | Weight categories | Moisture | Protein | Fat | Ash | Carbohydrate |
|---|---|---|---|---|---|---|
| C.catla | W1 | 67.36 ± 1.31a | 22.26 ± 0.78a | 2.88 ± 0.27a | 5.11 ± 0.66a | 2.39 ± 0.45a |
| W2 | 65.71 ± 1.40b | 23.04 ± 0.71b | 2.98 ± 0.12a | 6.55 ± 0.46b | 1.72 ± 0.41b | |
| W3 | 63.20 ± 1.80c | 23.90 ± 0.95c | 3.18 ± 0.44b | 7.16 ± 0.51c | 2.56 ± 0.28b | |
| C.mrigala | W1 | 70.40 ± 0.90a | 20.36 ± 0.67a | 1.68 ± 0.33a | 4.51 ± 0.28a | 3.05 ± 0.41a |
| W2 | 68.61 ± 0.81b | 21.46 ± 0.55b | 2.88 ± 0.41b | 4.95 ± 0.47a | 2.10 ± 0.47b | |
| W3 | 67.76 ± 0.95c | 21.77 ± 0.66c | 2.95 ± 0.33b | 5.15 ± 0.40b | 2.37 ± 0.44b |
Means in the same column for each species followed by different letters are significantly different according to Ducan’s test (P ≤ 0.05); ± Standard Error (S.E)
Table 4.
Proximate composition of fins of Catla catla and Cirrhinus mrigala of three weight groups
| Species | Weight categories | Moisture | Protein | Fat | Ash | Carbohydrate |
|---|---|---|---|---|---|---|
| C.catla | W1 | 63.22 ± 1.12a | 17.44 ± 0.57a | 4.98 ± 0.21a | 10.67 ± 1.10a | 3.69 ± 0.28a |
| W2 | 61.26 ± 0.95b | 18.37 ± 0.71b | 6.71 ± 0.47b | 11.57 ± 1.27b | 2.09 ± 0.34b | |
| W3 | 59.76 ± 1.28c | 19.04 ± 0.75c | 7.14 ± 0.51b | 12.05 ± 1.31c | 2.01 ± 0.26a | |
| C.mrigala | W1 | 64.80 ± 1.08a | 17.05 ± 0.80a | 5.21 ± 0.46a | 11.35 ± 0.31a | 1.59 ± 0.38a |
| W2 | 62.30 ± 1.21b | 17.90 ± 0.91b | 5.75 ± 0.57b | 11.67 ± 0.40b | 2.38 ± 0.41a | |
| W3 | 60.72 ± 1.41c | 18.12 ± 0.94c | 5.97 ± 0.68c | 11.81 ± 0.50c | 3.38 ± 0.57b |
Means in the same column for each species followed by different letters are significantly different according to Ducan’s test (P ≤ 0.05); ± Standard Error (S.E)
In the present study, a proximate analysis of the scales and fins of both fish species revealed a significant difference in the protein contents within the three weight groups. The protein contents appeared to increase in the scales and fins with increasing fish weight. The presence of collagen in the fish scales, which are considered waste, was also supported by the findings of Zhang et al. (2007), who reported a high amount of collagen in fish scales and bones. These results that identify collagen from a waste source, such as fish processing by-products, have also been reported by Nagai and Suzuki (1999), who revealed that type I collagen could be isolated from fish skin, bones, and fins. We observed the most protein content was in the skin, whereas the scales and fins displayed relatively low amounts of protein. These findings were corroborated by the findings of Iqbal (2002). The variation in the proximate analysis of both fish species may be attributed to the inherent potential of each fish species. Nalinanon et al. (2007) reported that fish protein contents depend on the fishing period and genetic potential. When food is lacking, albumin and globulin are degraded, and the amount of collagen in the skin increases. The differences between fish species and the protein isolation method may contribute to the differences between the results.
Collagen profiling in the skin, scales, and fins by SDS-PAGE
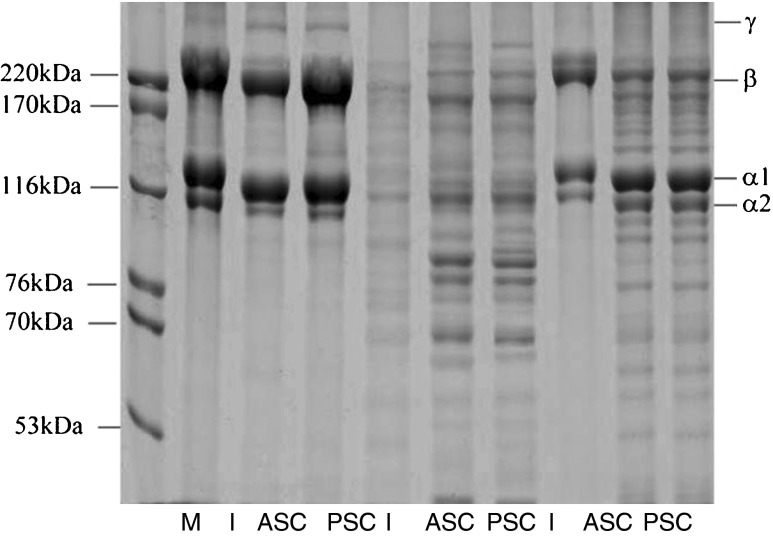
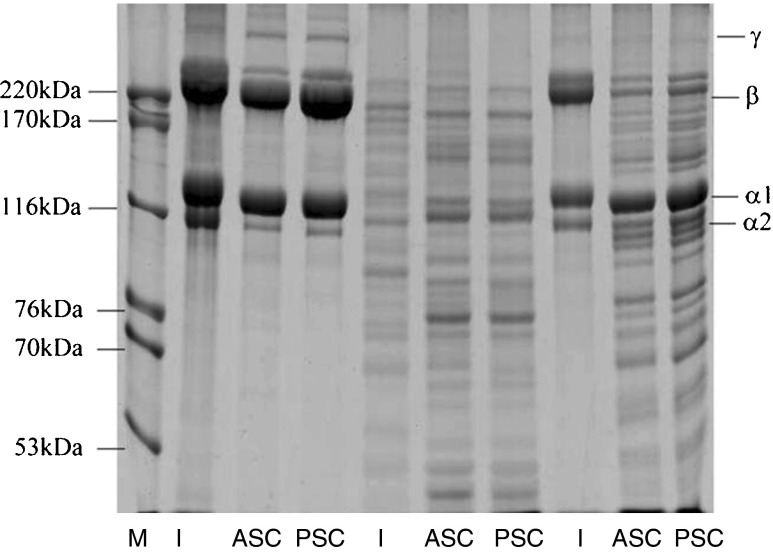
Collagen profiling of the skin of both fish species was performed using SDS-PAGE. The gel photographs of collagen extracted from C. catla skin demonstrated that a marker with known molecular weights ranging from 53 to 220 kDa purchased from HMW-SDS by GE Healthcare was run in the first well. Protein characterisation using SDS-PAGE revealed the molecular weights (kDa) of the C. catla skin, scales, and fins ranged from 112 to 220, 55 to 172, and 70 to 201 kDa, respectively. The size of the collagen proteins of the C. mrigala skin, scales and fins ranged from 114 to 210, 67 to 180, and 68 to 210 kDa, respectively. There were no differences in the skin collagen profiles among the three weight categories when characterised by SDS-PAGE (Fig. 2). For the same collagen, ASC or PSC, similar protein patterns was observed between collagens from the skin, scales and fins of Catla catla and C. mrigala (Figs. 1 and 2). All collagens comprised α- and β-chains as the major components. The result suggested that no disulfide bonds were found in those collagens. This was reconfirmed from the amino acid profile as cysteine was not detected in those collagens (Tables 5, 6 and 7). The molecular weight of PSC was found slightly lower than that of ASC. Peptide maps of skin, scales and fins collagens were compared with the selected fish species (Figs. 1 and 2). The similar peptide maps were observed for collagens for peptide maps of ASC and PSC from the same waste source (skin, scales and fins). However, slight differences were noticeable between the same collagen from skin, scales and fins in Catla catla and C. mrigala. These findings are consistent with the findings of Duan et al. (2009), who reported that skin collagen from fish of various ages was readily soluble in dilute acetic acid and was characterised using SDS-PAGE. This characterisation is also consistent with the findings of Nomura et al. (1996), who adopted the same method. In the present study, three distinct bands of molecular weights ranging from 112 to 220 kDa in C. catla and from 114 to 210 kDa in C. mrigala were observed; these findings were similar to those reported by Ogawa et al. (2003) and Yung et al. (2005). The isolated collagen may be type I, which was previously observed by Kimura et al. (1993) and Muyonga et al. (2004), who reported that the carp skin, scale, and bone collagen was the type I based on its electrophoretic mobility.
Fig. 2.
Gel photograph demonstrating the protein molecular weight marker (M and I) and the collagen patterns of the skin (wells 3 and 4), scales (6 and 7) and fins (9 and 10) of Cirrhinus mrigala
Fig. 1.
Gel photograph demonstrating the protein molecular weight marker (M and I) and the collagen patterns of the skin wells (3 and 4), scales (6 and 7) and fins (9 and 10) of Catla catla
Table 5.
Amino acid composition of collagen isolated from skin of Catla catla and Cirrhinus mrigala in three weight groups
| Amino Acid | Catla catla | Cirrhinus mrigala | SE and Main effects | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 1st | 2nd | 3rd | Sp | W | Sp x W | |
| Aspartic acid | 31 | 35 | 37 | 29 | 30 | 31 | 0.16*** | 0.20 | 0.26** |
| Threonine | 20 | 21 | 21 | 22 | 23 | 23 | 0.18*** | 0.31* | 0.36 |
| Serine | 38 | 39 | 38 | 36 | 35 | 37 | 0.20* | 0.35 | 0.36 |
| Glutamic acid | 111 | 112 | 114 | 99 | 100 | 101 | 0.17*** | 0.23** | 0.30* |
| Glycine | 164 | 164 | 165 | 180 | 181 | 182 | 0.15*** | 0.25** | 0.26** |
| Alanine | 171 | 172 | 173 | 190 | 191 | 190 | 0.23* | 0.15** | 0.17** |
| Valine | 30 | 30 | 31 | 29 | 27 | 30 | 0.17** | 0.35 | 0.36 |
| Methionine | 16 | 18 | 19 | 18 | 18 | 20 | 0.21* | 0.22* | 0.22* |
| Isoleucine | 14 | 14 | 16 | 8 | 9 | 9 | 0.17*** | 0.26 | 0.27 |
| Leucine | 88 | 87 | 89 | 92 | 93 | 93 | 0.16*** | 0.27* | 0.28* |
| Tyrosine | 6 | 5 | 6 | 4 | 5 | 4 | 0.13*** | 0.20* | 0.21* |
| Phenylalanine | 18 | 19 | 19 | 16 | 15 | 15 | 0.15** | 0.17* | 0.17* |
| Hydroxylysine | 5 | 5 | 6 | 4 | 5 | 5 | 0.16** | 0.19* | 0.19* |
| Lysine | 46 | 47 | 49 | 45 | 47 | 46 | 0.24* | 0.27** | 0.30 |
| Histidine | 3 | 4 | 4 | 3 | 2 | 3 | 0.16** | 0.23* | 0.24** |
| Arginine | 67 | 67 | 65 | 47 | 47 | 46 | 0.49 | 0.50 | 0.50 |
| Hydroxyproline | 95 | 97 | 105 | 85 | 90 | 97 | 0.14** | 0.17* | 0.22 |
| Proline | 66 | 67 | 60 | 83 | 70 | 69 | 0.17** | 0.20** | 0.22* |
| Imino acid | 161 | 164 | 165 | 168 | 160 | 163 | 0.18** | 0.19** | 0.20** |
1st = weight group; 2nd = weight group 2; 3rd = weight group 3; w = Weight; Sp× w= Species× Weight interaction
Table 6.
Amino acid composition of collagen isolated from scales of Catla catla and Cirrhinus mrigala in three weight groups
| Amino Acids | Catla catla | Crrhinus mrigala | SE and Main effects | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 1st | 2nd | 3rd | Sp | W | Sp X W | |
| Aspartic acid | 35 | 37 | 38 | 39 | 40 | 38 | 0.15*** | 0.26 | 0.25*** |
| Threonine | 29 | 25 | 25 | 20 | 22 | 23 | 0.17*** | 0.25*** | 0.29** |
| Serine | 35 | 37 | 38 | 32 | 31 | 33 | 0.15*** | 0.18** | 0.25 |
| Glutamic acid | 70 | 71 | 73 | 68 | 69 | 68 | 0.17*** | 0.20*** | 0.34 |
| Glycine | 185 | 188 | 189 | 181 | 179 | 186 | 0.35*** | 0.40* | 0.41* |
| Alanine | 192 | 193 | 195 | 171 | 173 | 174 | 0.40*** | 0.50** | 0.52** |
| Valine | 16 | 16 | 18 | 18 | 17 | 19 | 0.16* | 0.24 | 0.25* |
| Methionine | 14 | 17 | 18 | 12 | 13 | 14 | 0.10*** | 0.18* | 0.20* |
| Isoleucine | 13 | 15 | 16 | 14 | 15 | 14 | 0.21 | 0.22** | 0.25* |
| Leucine | 17 | 18 | 16 | 14 | 16 | 17 | 0.22 | 0.24** | 0.33 |
| Tyreonine | 3 | 2 | 3 | 1 | 2 | 2 | 0.11*** | 0.22* | 0.18** |
| Phenylalanine | 11 | 12 | 13 | 9 | 7 | 8 | 0.13* | 0.22* | 0.26 |
| Hydroxylysine | 6 | 5 | 7 | 10 | 11 | 11 | 0.11*** | 0.22* | 0.22* |
| Lysine | 44 | 45 | 46 | 35 | 33 | 36 | 0.18* | 0.25** | 0.27* |
| Histidine | 5 | 6 | 5 | 6 | 6 | 5 | 0.16** | 0.27 | 0.20* |
| Arginine | 50 | 51 | 53 | 49 | 48 | 45 | 0.28* | 0.30** | 0.55 |
| Hydroxyproline | 75 | 75 | 76 | 77 | 78 | 79 | 0.14** | 0.25* | 0.28** |
| Proline | 80 | 81 | 83 | 85 | 86 | 82 | 0.20* | 0.30* | 0.35** |
| Imino acid | 155 | 156 | 159 | 152 | 154 | 161 | 0.22* | 0.23** | 0.43* |
1st = weight group; 2nd = weight group 2; 3rd = weight group 3; w = Weight; Sp× w = Species × Weight interaction
Table 7.
Amino acid composition of collagen isolated from fins of Cala catla and Cirrhinus migala under three weight groups
| Amino Acids | Catla catla | Cirrhinus mrigala | SE and main effects | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 1st | 2nd | 3rd | Sp | W | Sp X W | |
| Aspartic acid | 30 | 32 | 33 | 35 | 36 | 38 | 0.15*** | 0.25 | 0.17*** |
| Threonine | 29 | 27 | 27 | 24 | 25 | 25 | 0.14*** | 0.19** | 0.28** |
| Serine | 39 | 41 | 43 | 44 | 44 | 46 | 0.16*** | 0.22* | 0.29 |
| Glutamic acid | 71 | 73 | 75 | 60 | 61 | 60 | 0.13*** | 0.28* | 0.32 |
| Glycine | 180 | 182 | 183 | 191 | 189 | 190 | 0.26** | 0.60** | 0.62** |
| Alanine | 199 | 200 | 203 | 180 | 181 | 183 | 0.31** | 0.61** | 0.62** |
| Valine | 30 | 31 | 32 | 29 | 29 | 28 | 0.16** | 0.27 | 0.20** |
| Methionine | 21 | 22 | 20 | 23 | 24 | 25 | 0.13*** | 0.21* | 0.16** |
| Isoleucine | 12 | 13 | 15 | 16 | 18 | 19 | 0.16** | 0.19** | 0.26* |
| Leucine | 25 | 27 | 27 | 20 | 21 | 22 | 0.21* | 0.30*** | 0.40 |
| Tyrosine | 3 | 2 | 3 | 5 | 6 | 7 | 0.12*** | 0.20* | 0.23* |
| Phenylalanine | 7 | 11 | 14 | 9 | 11 | 12 | 0.18* | 0.22*** | 0.29 |
| Hydroxylysine | 10 | 13 | 15 | 11 | 10 | 8 | 0.15*** | 0.26* | 0.25* |
| Lysine | 86 | 84 | 87 | 75 | 73 | 73 | 0.16** | 0.25** | 0.29* |
| Histidine | 5 | 7 | 6 | 3 | 4 | 2 | 0.11*** | 0.29 | 0.22* |
| Arginine | 49 | 45 | 48 | 45 | 40 | 46 | 0.24* | 0.40** | 0.60 |
| Hydroxyproline | 85 | 89 | 88 | 70 | 72 | 75 | 0.13** | 0.17** | 0.25** |
| Proline | 77 | 81 | 83 | 85 | 81 | 87 | 0.16** | 0.30** | 0.32* |
| Imino acid | 162 | 170 | 171 | 155 | 153 | 152 | 0.14** | 0.43** | 0.41* |
1st = weight group 1; 2nd = weight group 2; 3rd = weight group 3; w = Weight; Sp× w = Species × Weight interaction
The acid-soluble collagen extracted from C. mrigala scales were run in wells 6 and 7 of weight group W3, which revealed five fractions of ASC and four fractions of PSC in well with molecular weights of 67, 88, 114, 160 and 180 kDa and 65, 70, 100 and 145 kDa . The gel photograph revealed differences in ASC and PSC collagen extracted from the scales in C. mrigala (Fig. 2). The acid-soluble collagen extracted from C. catla scales were run in wells 6 and 7, and revealed seven fractions in each well with molecular weights of 55, 67, 74, 90, 97, 121 and 172 kDa. The gel photograph demonstrated that there were differences in ASC and PSC collagens extracted from the scales of the Catla catla (Fig. 1). These results are consistent with the findings of Ogawa et al. (2003) and Zhang et al. (2007), who also reported lack of a significant difference between the subunit molecular masses of the collagen from fins and scales. The C. catla fin collagen was run in wells 9 and 10 and revealed six fractions in each well with molecular weights of 70, 82, 111, 120, 180 and 201 kDa (Fig. 1). The extracted collagen from C. mrigala fins were run in wells 9 and 10, and revealed seven factions in each well with molecular weights of 68, 74, 90, 102, 110, 120 and 210 kDa. The gel photograph demonstrated the difference in the fin collagen profiles extracted from fish (Fig. 2). Nagai and Suzuki (2000); Nagai et al. (2004); Rodziewicz-Motowidlo et al. (2008) and Nagai et al. (2008) reported that collagen is the primary protein in preparing 75 % of the protein contents. The remaining 25 % is composed of smaller fragments that appear under the α bands corresponding to the molecular weight ranging from 73 to 155 kDa, including the probable elastin bands (8 %) and the remaining unidentified proteins and peptides (17 %).
ASC and PSC were isolated from the skin, scales and fins with a yield of 5.8 and 7.2; 3.9 and 5.6 and 6.7 and 8.8 %, respectively based on wet weight of skin, scales and fins. The yield of ASC and PSC isolated from the skin, scales and fins of C. mrigala was estimated at 4.7 and 6.5; 3.2 and 5.1 and 5.7 and 7.7 %, respectively based on their wet weight. ASC and PSC yield obtained from the skin, scales and fins of C. catla were significantly higher compared to C. mrigala. The skin, scales and fins were not completely solubilised by 0.5 M acetic acid, as shown by the low yield of ASC. These findings were in line with Jongjareonrak et al. (2005) who reported the incomplete solubilisation of bigeye snapper skin in 0.5 M acetic acid. The present findings were substantiated by the fact that collagen molecules in striped catfish skin were most likely cross-linked by covalent bonds through the condensation of aldehyde groups at the telopeptide region as well as the inter-molecular cross-linking, leading to a decrease in the solubility of collagen (Zhang et al. 2007). The yields of ASC and PSC from a brown banded bamboo shark (Chiloscyllium punctatum) were 9.38 % and 8.86 % (wet weight basis), respectively (Kittiphattanabawon et al. 2010b). With further limited pepsin digestion, the cross-linked molecules in the telopeptide region were cleaved, resulting in further extraction. Pepsin was able to cleave specifically at the telopeptide region of collagen from the skin of bigeye snapper (Nalinanon et al. 2007). The cumulative yield of ASC and PSC in skin, scales and fins of C. catla and C. mrigala was 13, 9.5 and 13; 11.2, 8.3 and 13.1 %, respectively. We are of the view that pepsin could be used as an aid for increasing the extraction yield of collagen from the skin, scales and fins of both the fish species.
Amino acid analysis
Amino acids were profiled from C. catla and C. mrigala skin, scales, and fins from three weight groups (Table 5, 6 and 7). The differences remained significant for a skin amino acid profile for both fish species in the three weight groups (Tables 5, 6 and 7). Few amino acids displayed non-significant differences. Glycine was the most abundant amino acid identified in the skin, followed by alanine in both the fish species. Alanine was recorded as the most abundant amino acid in the scales and fins of C. catla and C. mrigala. Also, the collagen consisted of proline and hydroxyproline, which are unique amino acids found in collagen. Duan et al. (2009) reported that glycine is a major amino acid in carp collagen. The results of the present study are also supported by the findings of Zhang et al. (2007) and Kui and Mendis (2008), who demonstrated that glycine is the major amino acid in each collagen type. In the skin, scales and fins of C. catla and C. mrigala the alanine and leucine were found slightly in higher content. These are not commonly present in higher contents in many other proteins (Kittiphattanabawon et al. 2010a). The amino acid composition of collagen from these two fish species was similar to common carp, channel catfish and silver carp (Duan et al. 2009). Tyrosine, phenylalanine and histidine were found in a low quantity in skin, scales and fins of both the fish species. The imino acid (proline and hydroxyproline) contents of the C. catla and C. mrigala skin, scales and fins were (Skin; 161–165 and 160–168), (Scales; 155–159 and 152–161) and (fins; 162-171and 152–155) residues/1,000 residues, respectively, which was similar to those of grass carp skin collagen (186 residues/1,000 residues) (Zhang et al. 2007). The imino acid contents in fins of Catla catla (171 residues/1,000 residues) in this study are relatively slightly higher than those of skin collagens from cold-water fish, such as cod (154 residues/1,000 residues) (Giraud-Guille et al. 2000) and oscillate puffer fish (170 residues/1,000 residues) (Nagai et al. 2002). The hydroxyl group of the hydroxyproline plays a part in the stability of the helix by interchain hydrogen bonding via a bridging water molecule as well as direct hydrogen bonding with a carbonyl group (Wang et al. 2007). However, imino acid contents of both the fish species from unicorn leather jacket skin were lower than those reported for collagen from the skin of black drum and sheep’s head (Ogawa et al. 2003), brown stripe red snapper (Jongjareonrak et al. 2005) and bigeye snapper (Kittiphattanabawon et al. 2005), which contained imino acids ranging from 190 to 221 residues/1,000 residues. Proline and hydroxyproline contents vary with species and their living habitat (Foegeding et al. 1996; Jongjareonrak et al. 2005; Kittiphattanabawon et al. 2005). Imino acids contribute to the stability of the helix structure of collagen (Ikoma et al. 2003; Jongjareonrak et al. 2005). The differences between the two fish species and three weight groups for amino acid composition of collagen isolated from skin, scales and fins were statistically highly significant (p < 0.01), thereby, indicating a qualitative difference in the collagen of cattle cattle and Cirrhinus mrigala (Table 5, 6 and 7).
Pyrrolidine rings of proline and hydroxyproline impose restrictions on the conformation of the polypeptide chain and help to strengthen the triple helix (Bae et al. 2008). Hydroxylysine of 4–6 residues/1,000 residues was found in both collagens, suggesting the partial cross-linking of collagen via covalent bond (Mechanic et al. 1987). Therefore, collagen from C. catla and C. mrigala might have the different molecular properties from that of other species due to the difference in imino acid content and cross-linking. The results suggest that the two collagen types may possess similar thermal stabilities due to analogous living conditions. The hydroxyproline and proline contents in C. catla and C. mrigala were extremely similar. Collagen contents are closely correlated with thermo stability, as reported by Shao et al. (2009). The present study suggests that C. mrigala collagen exhibits a higher thermal stability compared to C. catla . However, it is lower than mammalian collagen, and these findings are consistent with the observations of Rodziewicz-Motowidlo et al. (2008), who reported that the hydroxyproline and proline contents of silver carp are slightly higher than those of carp. The amino acid contents of both fish types vary among the three tissues selected (skin, scales, and fins). Similar results have been reported by Nagai et al. (2004), who observed that the collagen amino acid contents correlated with the water temperature of their normal habitat. Methionine and cysteine were negligible in the C. catla and C. mrigala skin, scales, and fins from the three weight groups. Collagen from the outer C. mrigala skin is typically considered to be type I collagen. Similar findings have also been reported by Rodziewicz-Motowidlo et al. (2008), who demonstrated that C. mrigala skin harbours extremely low methionine content; the authors also considered collagen from the silver carp skin as type I. Tryptophan was not recorded in the collagen isolated from the skin, scales, and fins of both fish species. Tyrosine and phenylalanine were identified in extremely small quantities, which may be attributed to the presence of type I collagen. Similar findings have also been reported by Chen et al. (2004). The largest consumer of amino acids is the food flavoring industry, which uses monosodium glutamate, alanine, aspartate and arginine to improve the flavour of food. The second largest consumer of amino acids is the animal feed industry, which uses lysine, methionine, threonine, tryptophan and others to improve the nutritional quality of animal feed.
Conclusions
An adequate amount of both ASC and PSC collagen in the skin, scales, and fins of C. catla and C. mrigala were successfully isolated and classified as type 1 collagen. It can be successfully utilised in cosmetic, biomedical and pharmaceutical industries, at a low or no cost. The proximate analysis indicated that the nutritional values of fish skin, scales, and fins are fairly high, and these useful resources may be wasted with the exception of their occasional use in fish meal manufacturing. Alanine was the most abundant amino acid closely followed by glycine in the skin, scales, and fins, whereas tryptophan was completely absent in the skin, scales and fins of both the fish species.
Acknowledgments
The authors would like to their sincere appreciation to the Deanship of Scientific at King Saud University for its funding of this research through the Research Group Project No. RGP-1435-012.
References
- Association of Official Analytical Chemists (AOAC) (1990) In: Helrich K (ed) Official methods of analysis 14th edn. Washington, DC
- Bradford MM. A rapid and sensitive method for the quantitation of microgram of protein utilizing principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bae I, Osatomi K, Yoshida A, Osako K, Yamaguchi A, Hara K. Biochemical properties of acid-soluble collagens extracted from the skins of underutilized fishes. Food Chem. 2008;108:49–54. doi: 10.1016/j.foodchem.2007.10.039. [DOI] [Google Scholar]
- Chen HL, Li H, Liu B, Gao LZ. Studies on bullfrog skin collagen. Food Chem. 2004;84:65–69. doi: 10.1016/S0308-8146(03)00277-2. [DOI] [Google Scholar]
- Dun R, Jackson HT, Smith Y. Methods for processing and utilization of low cost fishes: a critical appraisal. J Food Sci Technol. 2008;32:1–12. [Google Scholar]
- Duan R, Zhang J, Dua X, Yao X, Konno K. Properties of collagen from skin, scale and bone of carp (Cyprinus carpio) Food Chem. 2009;112:702–706. doi: 10.1016/j.foodchem.2008.06.020. [DOI] [Google Scholar]
- Foegeding EA, Laneir TC, Hultin HO. Characteristics of edible muscle tissues. In: Fennema OR, editor. Food chemistry. New York: Marcel Dekker; 1996. pp. 902–906. [Google Scholar]
- Giraud-Guille MM, Besseau L, Chopin C, Durand P, Herbage D. Structural aspects of fish skin collagen which forms ordered arrays via liquid crystalline states. Biomaterials. 2000;21:899–906. doi: 10.1016/S0142-9612(99)00244-6. [DOI] [PubMed] [Google Scholar]
- Gomez-Guillen MC, Turnay F, Fernández-Diaz MD, Ulmo N, Lizarbe MA, Montero P. Structural and physical properties of gelatin extracted from different marine species: a comparative study. Food Hydrocoll. 2002;16:25–34. doi: 10.1016/S0268-005X(01)00035-2. [DOI] [Google Scholar]
- Ikoma T, Kobayashi H, Tanaka J, Walsh D, Mann S. Physical properties of type I collagen extracted from fish scales of Pagrus major and Oreochromis niloticus. Int J Biol Macromol. 2003;32:199–204. doi: 10.1016/S0141-8130(03)00054-0. [DOI] [PubMed] [Google Scholar]
- Iqbal Z (2002) Proximate composition of skin, scales and fins of Catla catla, Labeo rohita and Cirrhina mrigala. M. Sc. Thesis, University of Panjab, Pakistan, 92p
- Jabeen F, Shakoor A. Chemical composition and fatty acid profile of three freshwater fish species. Food Chem. 2011;125:691–696. doi: 10.1016/j.foodchem.2010.09.103. [DOI] [Google Scholar]
- Jamilah B, Umi Hartina MR, Mat Hashim D, Sazili AQ. Properties of collagen from barramundi (Lates calcarifer) skin. Int Food Res J. 2013;20:835–842. [Google Scholar]
- Javed M (1998) Growth performance and meat quality of major carps as influenced by pond fertilization and feed supplementation. Ph.D Thesis, Agriculture University, Faisalabad
- Jongjareonrak A, Benjakul S, Visessanguan W, Nagai T, Tanaka M. Isolation and characterisation of acid and pepsin-solubilised collagens from the skin of brown stripe red snapper (Lutjanus vitta) Food Chem. 2005;93:475–484. doi: 10.1016/j.foodchem.2004.10.026. [DOI] [Google Scholar]
- Kimura S, Miyauchi Y, Uchida N. Scale and bone type I collagens of carp (Cyprinus carpio) Comp Biochem Physiol. 1991;99B:473–476. doi: 10.1016/0305-0491(91)90073-m. [DOI] [PubMed] [Google Scholar]
- Kimura S, Omura Y, Ishida M, Shirai H. Molecular characterization of fibrollar collagen from the body wall of starfish Asterias amurensis. Comp Biochem Physiol Part B Comp Biochem. 1993;104:663–668. doi: 10.1016/0305-0491(93)90194-A. [DOI] [Google Scholar]
- Kittiphattanabawon P, Benjakul S, Visessanguan W, Nagai T, Tanaka M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus) Food Chem. 2005;89:363–372. doi: 10.1016/j.foodchem.2004.02.042. [DOI] [Google Scholar]
- Kittiphattanabawon P, Benjakul S, Visessanguan W, Kishimura H, Shahidi F (2010a) Isolation and characterisation of collagen from the skin of brownbanded bamboo shark (Chiloscyllium punctatum). Food Chem 119:1519–1526
- Kittiphattanabawon P, Benjakul S, Visessanguan W, Shahidi F. Isolation and characterization of collagen from the cartilages of brownbanded bamboo shark (Chiloscyllium punctatum) and blacktip shark (Carcharhinus limbatus) Food Sci Technol. 2010;43:792–800. [Google Scholar]
- Kui SK, Mendis E. Bioactive compounds from marine processing byproducts- a review. Food Res Int. 2008;39:383–393. [Google Scholar]
- Liu H, Li D, Guo S. Studies on collagen from the skin of channel catfish (Ictalurus punctaus) Food Chem. 2007;101:621–625. doi: 10.1016/j.foodchem.2006.01.059. [DOI] [Google Scholar]
- Mechanic GL, Katz EP, Henmi M, Noyes C, Yamauchi M. Locus of a histidine-based, stable trifunctional, helix to helix collagen cross-link: stereospecific collagen structure of type I skin fibrils. Biochemistry. 1987;26:3500–3509. doi: 10.1021/bi00386a038. [DOI] [PubMed] [Google Scholar]
- Mahboob S, Sheri AN, Raza SH. Proximate comoposition of major common and some chinese carp as influenced by pond fertilizer and feed supplementation in composite culture system. J Aquac Trop. 1996;11:227–284. [Google Scholar]
- Muyonga JH, Cole CGB, Duodu KG. Characterisation of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus) Food Chem. 2004;85:81–89. doi: 10.1016/j.foodchem.2003.06.006. [DOI] [Google Scholar]
- Nagai T, Suzuki N. Isolation of collagen from fish waste material – skin, bone, and fins. Food Chem. 1999;68:277–281. doi: 10.1016/S0308-8146(99)00188-0. [DOI] [Google Scholar]
- Nagai T, Suzuki N. Preparation and characterization of several fish bone collagen. J Food Biochem. 2000;24:427–436. doi: 10.1111/j.1745-4514.2000.tb00711.x. [DOI] [Google Scholar]
- Nagai T, Araki Y, Suzuki N. Collagen of the skin of oscillate puffer fish (Takifugu rubripes) Food Chem. 2002;78:173–177. doi: 10.1016/S0308-8146(01)00396-X. [DOI] [Google Scholar]
- Nagai T, Izumi M, Ishii M. Fish scale collagen. Preparation and partial characterization. Int J Food Sci Technol. 2004;104:239–244. doi: 10.1111/j.1365-2621.2004.00777.x. [DOI] [Google Scholar]
- Nagai T, Suzuki N, Nagashima T. Collagen from common minke whale (Balaenoptera acutorostrata) unesu. Food Chem. 2008;111:296–301. doi: 10.1016/j.foodchem.2008.03.087. [DOI] [PubMed] [Google Scholar]
- Nalinanon S, Benjakul S, Visessanguan W, Kishimura H. Use of pepsin for collagen extraction from the skin of bigeye snapper (Priacanthus tayenus) Food Chem. 2007;104:593–601. doi: 10.1016/j.foodchem.2006.12.035. [DOI] [Google Scholar]
- Nalinanon S, Benjakul S, Visessanguan W, Kishimura H. Tuna Pepsin: characteristics and its use for collagen extraction from the skin of threadfin bream (Nemipterus spp.) J Food Sci. 2008;73:C413–C419. doi: 10.1111/j.1750-3841.2008.00777.x. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Sakai H, Ishi Y, Shirai K. Preparation and some properties of type I collagen from fish scale. Biosci Biotech Biochem. 1996;60:2092–2094. doi: 10.1271/bbb.60.2092. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Moody MW, Portier RJ, Bell J, Schexnayder M, Losso JN. Biochemical properties of black drum and Sheepshead sea bream skin collagen. J Agri Food Chem. 2003;51:8088–8092. doi: 10.1021/jf034350r. [DOI] [PubMed] [Google Scholar]
- Rodziewicz-Motowidlo S, Śladewska A, Mulkiewicz E, Kolodziejczyk A, Aleksandowicz A, Miszkiewicz J, Stepnowski P. Isolation and characterization of a thermally stable collagen preparation from the outer skin of the silver carp Hypophthalmichthys molitrix. Aquaculture. 2008;285:130–134. doi: 10.1016/j.aquaculture.2008.08.026. [DOI] [Google Scholar]
- Senaratne LS, Park P, Kim S. Isolation and characterization of collagen from brown backed toadfish (Lagocephalus gloveri) Skin. Bioresou Technol. 2006;97:191–197. doi: 10.1016/j.biortech.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Shao K, Terashima M, Hozan D, Shirai K. Preparation and dynamic viscoelasticity characterization of alkali solubilized collagen from shark skin. J Agric Food Chem. 2009;48:685–690. doi: 10.1021/jf990389d. [DOI] [PubMed] [Google Scholar]
- Singh P, Soottawat B, Sajid M, Hideki K. Isolation and characterisation of collagen extracted from the skin of striped catfish (Pangasianodon hypophthalmus) Food Chem. 2011;124:97–105. doi: 10.1016/j.foodchem.2010.05.111. [DOI] [Google Scholar]
- Skierka E, Sadowska M. The influence of different acids and pepsin on the extractability of collagen from the skin of Baltic cod (Gadus morhua) Food Chem. 2007;105(3):1302–1306. doi: 10.1016/j.foodchem.2007.04.030. [DOI] [Google Scholar]
- Wang L, An X, Xin Z, Zhao L, Hu Q (2007) Isolation and characterization of collagen from the skin of deep-sea redfish (Sebastes mentella). J Food Sci 72(8):E450–E455 [DOI] [PubMed]
- Woo JW, Yu SJ, Cho SM, Lee YB, Kim SB. Extraction optimization and properties of collagen from yellowfin tuna (Thunnus albacares) dorsal skin. Food Hydrocoll. 2008;22:879–887. doi: 10.1016/j.foodhyd.2007.04.015. [DOI] [Google Scholar]
- Yung M, Yoshida C, Fujisawa S, Mizuta S, Yoshinaka R. Identification and characterization of molecular species of collagen in fish skin. Food Sci. 2005;66:247–251. [Google Scholar]
- Zhang Y, Liu W, Li G, Shi B, Miao Y, Wu X. Isolation and partial characterization of pepsin-soluble collagen from the skin of grass carp (Centopharyngodon idella) J Food Chem. 2007;103:906–912. doi: 10.1016/j.foodchem.2006.09.053. [DOI] [Google Scholar]