Abstract
The survival and effect of free and encapsulated probiotic Lactobacillus plantarum LS5 on acidity, exopolysaccharide production, phase separation and influence on the sensory attributes of probiotic and synbiotic Doogh (typical Iranian drink based on fermented milk) supplemented with Helianthus tuberosus inulin were studied over 22 days storage. Results showed addition of L.plantarum LS5 (free or encapsulated) increased acid development (°D) in Doogh during storage. In addition, phase separation in Doogh with encapsulated probiotic bacteria was slower compared to Doogh with free probiotic bacteria. More exopolysaccharides were observed in Doogh with encapsulated culture compared to those without encapsulated culture. The results confirmed that there was an increased survival of L.plantarum LS5 due to protection of cells by microencapsulation. Also addition of inulin improved survival of free or encapsulated cells in Doogh during storage, but effect of inulin on acidity, exoploysaccharide content and phase separation of samples containing free or encapsulated cells was not significant (P > 0.05). Moreover, sensory evaluation results indicated addition of free or encapsulated probiotic cells and inulin did not significantly affect appearance and color, acidity, flavor and after taste of the Doogh samples over the storage period. Therefore, probiotic and synbiotic Doogh (supplemented with free or encapsulated L.plantarum LS5 and Helianthus tuberosus inulin) are potentially suitable for using as functional dairy foods.
Keywords: Doogh, Helianthus tuberosus inulin, Lactobacillus plantarum LS5, Probiotic, Synbiotic
Introduction
The presence of live microorganisms, in particular Lactobacillus strains, in food as probiotic bacteria has been traditionally associated with certain health benefits (Schrezenmeir and De Vrese 2001). Probiotic bacteria, defined as “living microorganisms, which upon ingestion in certain numbers, exert health benefits beyond inherent basic nutrition” (Guarner an Shaafsma 1998). Prebiotics are non-digestible but fermentable carbohydrates that pass through to the large intestine and selectively stimulate the proliferation and/or activity of populations of desirable bacteria or limited the growth of bacteria belonging to fusobacteria and clostridia in the colon, thereby improving the consumer’s health (Gibson and Roberfroid 1995; Kolida et al. 2002).
Inulin as oligosaccharide is a mixture of oligomer and polymer chains with a variable number of fructose molecules, joined by β bonds (2–1), which also generally contain a glucose molecule at the end of the chain. Inulin is a non-digestible fructan frequently used as a prebiotic ingredient that occurs naturally in numerous foods of vegetable origin, such as Helianthus tuberosus (Franck 2002). A food product containing both probiotics and prebiotics is called as synbiotic (Buriti et al. 2007; Ahmadi et al. 2012; Dhewa et al. 2012).
The physical protection of bacteria by encapsulation is a novel approach to improve the bacterial survival. As a result, encapsulation of probiotics could prevent decrease of viability in unfavorable and stressful conditions of a hostile environment (Homayouni et al. 2008). It has been reported that microencapsulation using calcium alginate have increased the survival and viability of probiotic bacteria in dairy product during storage (Godward and Kailasapathy 2003).
Probiotic bacteria have been incorporated into fermented dairy product which is a perfect vehicle for delivery of these organisms in the human diet (Donkor et al. 2007). Doogh (typical Iranian drink based on fermented milk) could possess synbiotic effects, due to the simultaneous incorporation of L. plantarum LS5 and inulin. Nonetheless, the possible degradation of Helianthus tuberosus inulin added to this dairy product, by the metabolism of free and encapsulated native probiotic, should be investigated. To the best of our knowledge, there is no report available on the application of L. plantarum strains as a probiotic and inulin as a prebiotic in Doogh.
The aim of the present study was to evaluate the prebiotic potential of a synbiotic Doogh manufactured with inulin and supplemented with a potentially probiotic L. plantarum LS5 culture, and to compare the physicochemical characteristics of the product with probiotic and control Doogh during storage at 4 ± 1 °C for up to 22 days.
Material and methods
Cultures
L. plantarum LS5 isolated from the traditional Kurdish cheese (Milani 2012) was obtained from strain collection of Department of Food Science and Technology, Ferdowsi University of Mashhad. Probiotic activity of L. plantarum LS5 was confirmed previously by Hashemi et al. (2014). Lactobacillus strain was reactivated in MRS broth and incubated under anaerobic conditions using an atmosphere generation system (GasPak system, Anaerobic system BR038B, Oxoid) at 37 °C.
Commercial lyophilized Y-type culture containing mixed culture of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus (commercially known as Yf-3331) were supplied by Chr-Hansen (Horsholm, Denmark). This starter culture is commonly used by dairy industry to produce fermented milk products.
Microencapsulation
All glasswares and solutions used in the procedures were sterilized at 121 °C for 15 min. Alginate beads were produced using a modified encapsulation technique firstly reported by Sheu et al. (1993) and Sultana et al. (2000). A 1 % (w/v) alginate (Sigma A 2033; High mannuronic acid) mixture in distilled water was prepared containing 0.1 % L.plantarum culture (18 h old culture grown in MRS broth). The mixture (20 mL) was added into 100 mL canola oil containing 0.5 % Tween 80 (Sigma P 8047). The mixture was stirred at 900 rpm for 20 min (Heydolph Stirrer, Germany) until it was fully emulsified. Then 100 mL of 0.1 M calcium chloride solution were added and stirred (100 rpm, 20 min). Finally, the mixture was allowed to stand 40 min to separate prepared calcium alginate beads in the bottom of beaker at the calcium chloride layer. The oil layer was drained and beads were collected in the 0.1 % peptone solution and stored at 4 °C.
Morphology and metabolic activity of microencapsulated bacteria
In this study, the morphology of the microcapsules was determined by scanning electron microscopy (SEM) and also by optical microscopy (BX-41, Japan). For SEM (LEO 1450VP, Germany) the beads were coated with gold-palladium (SC7620, UK) and examined at an accelerating voltage of 35.0 kV.
Metabolic activities of free and encapsulated cells were determined according to Sultana et al. (2000) and Homayouni et al. (2008).
Extraction of inulin from Helianthus tuberosus
Extraction of inulin was done according to Paseephol et al. (2007) with brief modifications. Three kg lots of peeled tubers were sliced and dried at 55 °C for 48 h. Then they were ground and mixed with water (1/12 (w/v)) at 65 °C. The resulting extract was filtered through muslin cloth and then concentrated to 50 % of the original volume using evaporator operating under a vacuum of 68 kPa (70 °C) with steam supply at 138 kPa. To remove impurities, the concentrate was mixed with 50 % slurry of calcium hydroxide 2 % at 55 °C for 30 min, resulting in the formation of a flocculent precipitate and a brighter yellow supernatant. By this technique, the pH of juice rose from 5 to 6 to 10 to 12. After filtration under vacuum condition by using a paper filter (Whatman No. 4), sixteen mL phosphoric acid 10 % was added to the filtrate with vigorous stirring to adjust its pH to ca. 8–9, causing the precipitation of excess calcium and coagulated organic material. The mixture was allowed to stand at 60 °C for 2–3 h before refiltering (Whatman No. 4). The clarification process was repeated twice. Activated carbon powder was added to the filtrates at 60 °C (20 % w/v) and mixed for 15–30 min in order to remove colored materials. The treated syrup was filtered (Whatman No. 1) and the clear syrup obtained was further concentrated by rotary evaporation (hahn shin, South Korea) at 67 °C, to obtain syrups with 32 °B. Then, inulin was precipitated in ethanol 99 % and was dried with freeze drying (Martin Christ Gefriertrocknungsanlagen GmbH).
Doogh-making procedure
Doogh milk with 6 % of milk solid nonfat was prepared using reconstituted skim milk powder and sterilized potable water. The milk was heat treated at 95 °C for 15 min and then was inoculated with starter bacteria (Yf-3331) following incubation at 45 °C ± 1 °C until acidity reached to 132 °D. Sample was cooled down to 20 °C and added with sterilized water (50 % v/v) containing 1 or 2 % inulin and 0.8 % (w/v) of sodium chloride (based on final product). Each sample was then pasteurized at 90 °C for 30 min and cooled to approximately 37 °C followed by inoculation with free and encapsulated L. plantarum LS5 (~1010 CFU/mL). Control sample was prepared without addition of inulin and probiotic. Doogh samples were then poured into 250 mL sterile plastic cups and stored at 4 °C for 22 days.
Enumeration
For routine enumeration, total viable counts of L. plantarum strain were determined on MRS agar (1.5 %, w/v,Oxoid), and incubation at 37 °C. To count the encapsulated bacteria incorporated into Doogh, 1 mL sample was re-suspended in 9 mL of 1 % (w/v) sodium citrate and vortexed up to 2 min to allow complete releasing of the bacteria from alginate capsules. The analyses of samples were done in triplicate and final results were expressed as average of data.
Acidity and spontaneous phase separation
Acidity was titrated by M/9 (1/9 normality) NaOH solution and expressed in Dornic degrees (°D) (Maragkoudakisa et al. 2006). Spontaneous phase separation in the stored Doogh was determined by the siphon method as described by Amatayakul et al. (2006).
Crude exoploysaccharide content
The quantity of crude exoploysaccharide (EPS) in Doogh samples was determined during the storage period of 22 days at 4 °C by the method described by Purwandari et al. (2007). The 0 h results of synbiotic Doogh were subtracted from all the samples (contain 1 and 2 % inulin) to rule out any differences due to co-precipitation of the added inulin. The results were expressed as mg of crude EPS per 100 g of Doogh.
Sensory evaluation
All Doogh samples were subjected to sensory evaluation to test their acceptability (Appearance and color, Acidity, Flavor, After test and Overall liking) using a five point hedonic scale, where 1 = dislike extremely, 2 = dislike moderately, 3 = neither like nor dislike, 4 = like moderately and 5 = like extremely. A total of 12 semi-trained panelists were used in this study. All ingredients are food grade and all tested products were approved for human subject sensory evaluation by Institute of Standards and Industrial Research of Iran (ISIRI 11324) and National Food and Drug Administration.
Statistical analysis
The results were analyzed using one-way ANOVA, and significant differences between groups were determined by the Duncan’s multiple range test. All statistical analyses were performed using the SPSS (SPSS 16.0 for Windows; SPSS Inc., Chicago, IL, USA). Differences were considered significant at P < 0.05.
Results and discussion
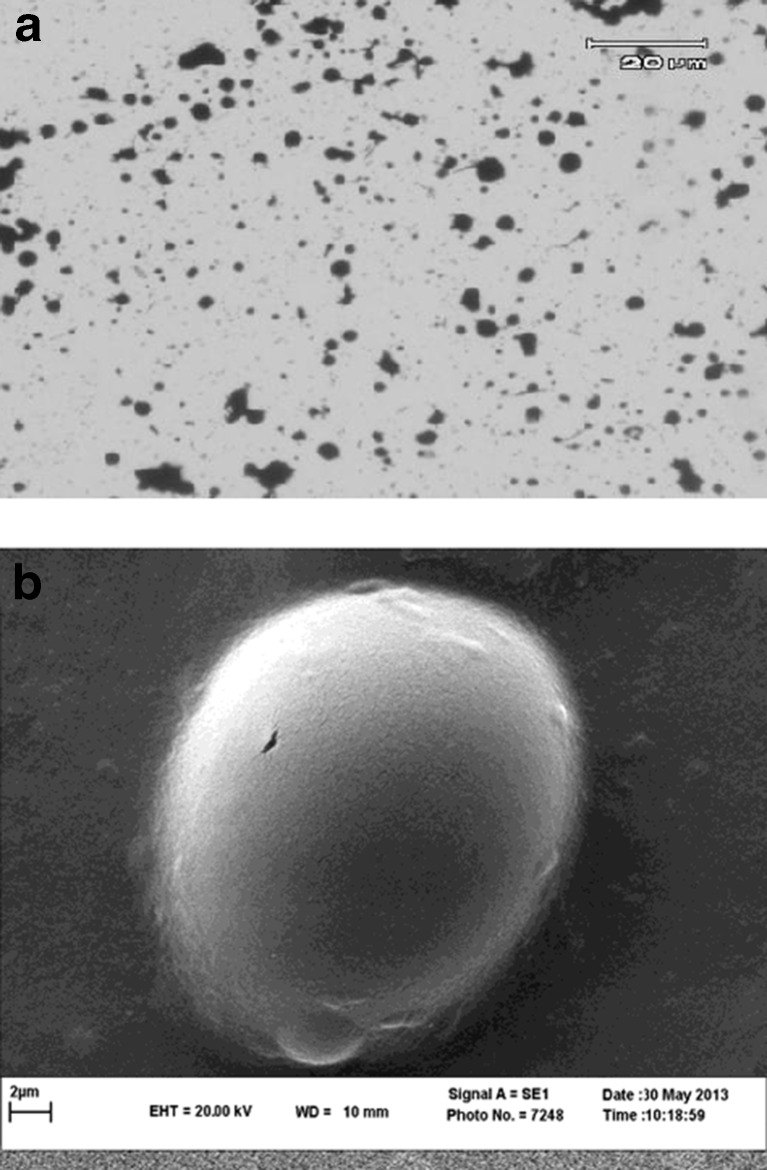
SEM and optical microscopy evaluations of the alginate beads were carried out to examine surface morphology. The photographs of beads which have been taken showed that the beads were spherical and had harsh surface characteristics (Fig. 1).
Fig. 1.
Optical microscopy (a) and SEM (b) of encapsulated Lactobacillus plantarum LS5
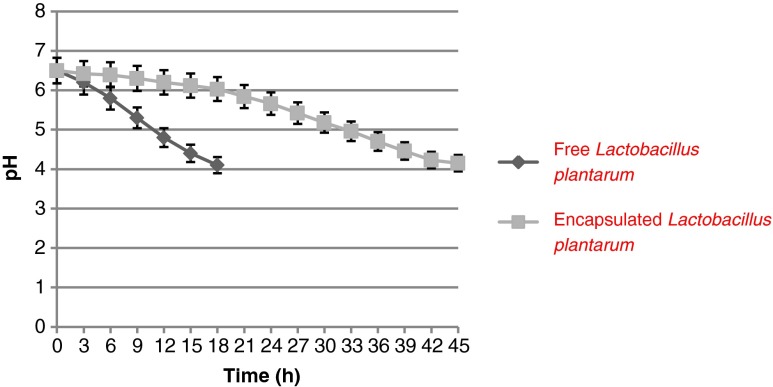
The metabolic activity of microencapsulated bacteria can be monitored by determination of pH (Sultana et al. 2000). Acidification kinetics over a time of 48 h in MRS broth medium containing free and encapsulated cells of L. plantarum LS5 were compared to show that if the encapsulated bacterial cells are active and could produce acid similar to free cells (Fig. 2). The results showed that free and encapsulated cells changed the pH value of MRS broth with time, indicating that bacterial cells were remained active in the calcium alginate beads. However, the time taken for the encapsulated cells to arrive at the same pH was longer than that taken by the free cells. The time taken for free cells to decrease the initial pH value of MRS broth to 4 was approximately 18 h whereas that of encapsulated cells was about 45 h. This could be due to the slow uptake of nutrients and slow release of the metabolites across the encapsulating alginate shell of beads (Homayouni et al. 2008).
Fig. 2.
Effect of free and encapsulated Lactobacillus plantarum LS5 on pH
Table 1 show the bacterial counts in Doogh samples. The results showed that there were significant losses in the cell numbers of both free and encapsulated L.plantarum LS5 over a period of 22 days. There were about 2.7 log losses in number of cells of free L.plantarum LS5. The encapsulated bacteria, however, showed only 1.71 log decrease in cell numbers. Nighswonger et al. (1996) found that, although the viable counts of some of the L. acidophilus and L. casei used as adjuncts in yoghurt production declined significantly after storage, most probiotic populations were between 5 and 7 log CFU/g at the end of the products shelf life. In another study, both the adjunct strain L. paracasei subsp. tolerans and the starter cultures of yoghurt were present in high levels, above 7 log CFU/g, after 2 weeks of storage (Maragkoudakisa et al. 2006). Elsewhere, however, probiotic fermented milks have been reported with low microbial loads below 5 log CFU/g (Vinderola and Reinheimer 2000). The application of microencapsulation methods in probiotics has been previously reported. Microencapsulation seems to be the most capable technology to protect bacterial cells from unfavorable environment (Kailasapathy 2002). There is increasing evidence that encapsulation is useful in protecting the probiotic cells designed to be added to acidic foods (Sultana et al. 2000). Krasaekoopt et al. (2003) indicated that microencapsulation facilitates the produce of fermented dairy products in which probiotic bacteria have consistent characteristics and higher stability during storage. In another study, the use of encapsulated cultures in mayonnaise improved the viability of the encapsulated bacteria compared to mayonnaise with free bacteria up to 6–8 weeks (Khalil and Mansour 1998).
Table 1.
Survival of L.plantarum LS5 (log CFU/mL) in Doogh samples
| sample | 0 | 1 | 11 | 22 |
|---|---|---|---|---|
| Time (Day) | ||||
| LP | 9.6 ± 0.29aA | 9.06 ± 0.1aB | 8.35 ± 0.02aC | 6.9 ± 0.05aD |
| LP + 1 % | 9.6 ± 0.21aA | 9.3 ± 0.05cB | 8.53 ± 0.02bC | 7.29 ± 0.01bD |
| LP + 2 % | 9.65 ± 0.17aA | 9.43 ± 0.02bB | 8.64 ± 0.02cC | 7.41 ± 0.02cD |
| CLP | 9.39 ± 0.05aA | 9.36 ± 0.05bcA | 8.8 ± 0.01dB | 7.68 ± 0.03dC |
| CLP + 1 % | 9.34 ± 0.08aA | 9.36 ± 0.04bcA | 8.92 ± 0.01eB | 7.97 ± 0.01eC |
| CLP + 2 % | 9.5 ± 0.19aA | 9.41 ± 0.03bB | 8.98 ± 0.01fC | 8.12 ± 0.01fD |
LP, Free L.plantarum; CLP, Encapsulated L.plantarum; 1 and 2: Inulin concentrations (%). Means within a column with the same lowercase letters are not significantly different at P < 0.05 and means within a row with the same uppercase letters are not significantly different at P < 0.05
Results also showed the addition of inulin powders significantly improved the viability of free and encapsulated L.plantarum LS5 in comparison to control sample. Inulin at 2 % concentration showed better ability in sustaining free and encapsulated cells than inulin at 1 % concentration up to 22 days of Doogh storage. These results are in good agreement with the findings of Paseephol and Sherkat (2009) who reported a marked increase in L. casei LC-01 counts with the addition of Jerusalem artichoke inulin powders. The utilization of inulin by different probiotic bacteria has been reported previously by Desai et al. (2004) and Donkor et al. (2007) who found that inulin powders were the favored carbon source for Lactobacillus strains. In addition, Makras et al. (2005) reported that inulins improve the viability of the probiotic bacteria during cold storage because they supply additional nutrients for increasing culture growth. However, inulin utilization could be attributed mostly to strain specific response of probiotic cells to prebiotic supplementation (Paseephol and Sherkat 2009; Rezaei et al. 2012).
Table 2 shows variations in acidity of experimental and control Doogh during refrigerated storage for up to 22 days. The addition of inulin powders at two concentrations and probiotic cells (free and encapsulated) affected the initial acidity of Doogh in comparison to control sample. There were no significant differences (P > 0.05) in the acid values of synbiotic and probiotic (free and encapsulated) Doogh samples throughout the storage period. However, all treatment Doogh samples were significantly different with control sample in acid value during storage. These observations were consistent with the findings of Paseephol and Sherkat (2009) and Ramchandran and Shah (2009) who reported probiotic and synbiotic yoghurts did not show significantly different in acid value during storage time.
Table 2.
Acid values in Doogh samples
| sample | 1 | 11 | 22 |
|---|---|---|---|
| Time (day) | |||
| Control | 66.67 ± 1.15aA | 67.3 ± 1.53aA | 67.3 ± 2.08aA |
| LP | 74 ± 1bA | 80.4 ± 1.1bB | 86.8 ± 1.2bC |
| LP + 1 % | 74. 7 ± 1.53bA | 81.1 ± 1.68bB | 87.6 ± 1.8bC |
| LP + 2 % | 73 ± 1bA | 79.3 ± 1.1bB | 85.6 ± 1.2bC |
| CLP | 73.3 ± 0.58bA | 79. 7 ± 0.63bB | 86 ± 0.69bC |
| CLP + 1 % | 72. 7 ± 1.15bA | 78.9 ± 1.3bB | 85.2 ± 1.4bC |
| CLP + 2 % | 74.3 ± 0.58bA | 80.8 ± 0.63bB | 87.2 ± 0.69bC |
LP, Free L.plantarum; CLP, Encapsulated L.plantarum; 1 and 2: Inulin concentrations (%). Means within a column with the same lowercase letters are not significantly different at P < 0.05 and means within a row with the same uppercase letters are not significantly different at P < 0.05
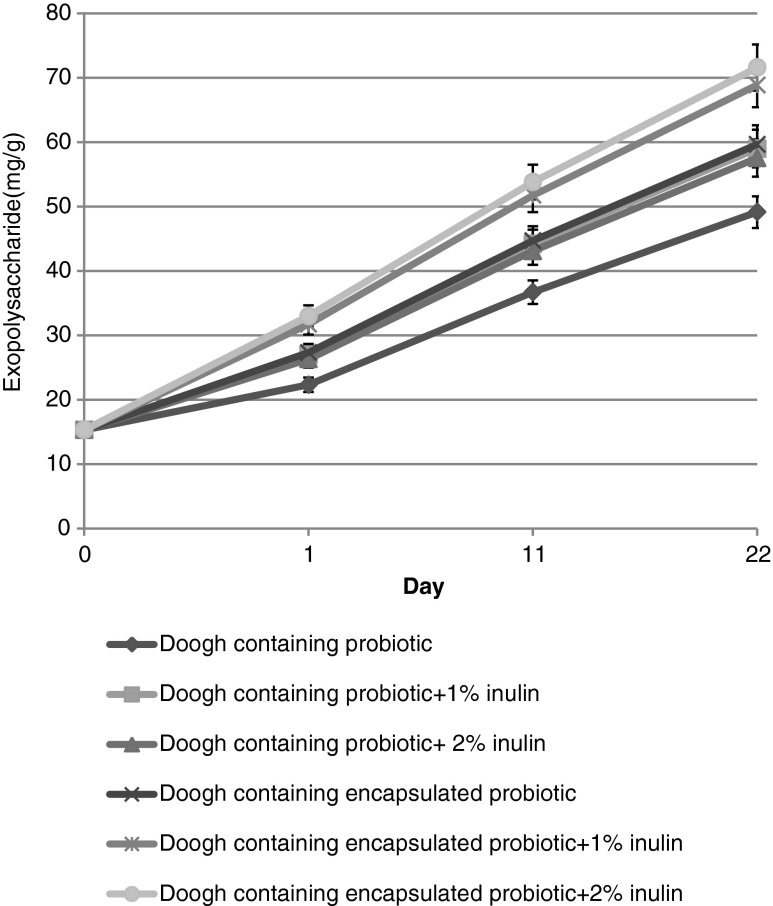
The yield of crude EPS in Doogh samples is shown in Fig. 3. Results indicated samples with encapsulated cells had more EPS content than samples with free cells. Maximum EPS was observed in all samples at the end of 22 days. Moreover, effect of inulin on EPS was not significant in samples with free or encapsulated cells. These results were in consistent with Kailasapathy (2006) who found encapsulation of probiotic cells in comparison to free cells increased EPS content of yoghurts during storage. Doleyres et al. (2005) reported the concentration of EPS in yoghurt remained stable during storage, whereas Amatayakul et al. (2006) indicated an increase in the EPS content in yoghurt made with ropy starter cultures during storage. As yet, there are no reports on the changes in EPS content of Doogh.
Fig. 3.
Exoplolysaccharide content of Doogh samples
The spontaneous percent whey separation exhibited by Doogh samples during storage at 4 °C for 22 days is presented in Table 3. Results showed all treated Doogh samples had lower phase separation than control sample during storage. In general, it was lower in Doogh samples supplemented with encapsulated bacteria than in samples with free cells. In addition, addition of inulin at two concentrations did not improve the prevention of phase separation in samples with encapsulated cells, but for samples with free bacteria, effect of inulin at 2 % was significant.
Table 3.
Phase separation in Doogh samples
| sample | 1 | 11 | 22 |
|---|---|---|---|
| Time (day) | |||
| Control | 20.2 ± 0.76aA | 62.8 ± 1aB | 65.1 ± 1.1aB |
| LP | 17.8 ± 0.76bA | 55.3 ± 0.57bB | 56.9 ± 0.63bB |
| LP + 1 % | 18.3 ± 0.76bcA | 54.5 ± 0.5bB | 55.9 ± 0.55bcB |
| LP + 2 % | 17.8 ± 0.76bcA | 53.2 ± 0.76cB | 54.5 ± 0.84cB |
| CLP | 17.3 ± 0.76bcA | 48.2 ± 0.29dB | 49 ± 0.3dB |
| CLP + 1 % | 16.8 ± 0.76cA | 47.5 ± 0.5dB | 48.2 ± 0.55dB |
| CLP + 2 % | 16.7 ± 0.58cA | 47 ± 0.0.5dB | 47.7 ± 0.55dB |
LP, Free L.plantaruml; CLP, Encapsulated L.plantarum; 1 and 2: Inulin concentrations (%). Means within a column with the same lowercase letters are not significantly different at P < 0.05 and means within a row with the same uppercase letters are not significantly different at P < 0.05
The alginate used in this study for encapsulation is usually used as food stabilizer in the manufacture of dairy products to prevent phase separation. For example, it enhances viscosity and binds water hence reduces phase separation in stirred yoghurts (Kailasapathy 1996). On the other hand, EPS produced by lactic acid bacteria are used in dairy products to improve body and texture of them. The shear induced microstructure in dairy products made with EPS producing bacteria has been shown to consist of compartmentalized protein aggregates between channels containing EPS, which hinders phase separation (Hassan et al. 1995). The improved water holding capacity of dairy containing EPS, due to the high water binding property of EPS, is another reason for decreased phase separation (Sodini et al. 2004). Therefore, by incorporating polymer encapsulated bacteria it is possible to prevent phase separation of Doogh.
The average sensory scores of all panelists are shown in Table 4. The results showed that there were no significant differences (P > 0.05) in the appearance and color of the Doogh samples. It was expected that addition of alginate capsules to Doogh samples could slightly color them; however, the panelist could not identify the differences in the appearance and color between Doogh samples with encapsulated cells from the other samples. There was no significant difference in the acceptability scores for acidity of Doogh samples. These results are in good agreement with the findings of Kailasapathy (2006) who reported there was no significant difference in the sensory scores for acidity of probiotic yoghurts (free and encapsulated) and control. In this study, although acid production increased in probiotic samples, the panelist could not identify the differences in the acidity between probiotic samples (free and encapsulated) and control. On the basis of sensory evaluation test, acid value changes in probiotic samples could not affect liking scores of the panelists. The panelists also could not differentiate the seven types of Doogh samples in flavor or after taste attributes. The mean overall liking scores were not significantly different between the control and treated samples, and treated samples were liked by panelists.
Table 4.
Sensory evaluation in Doogh samples
| Sample | Appearance and color | Flavor | Acidity | After test | Overall liking |
|---|---|---|---|---|---|
| Control | 3.4 ± 0.67a | 4.7 ± 0.78a | 4.1 ± 0.67a | 4.3 ± 0.78a | 4 ± 0.74a |
| LP | 3.6 ± 0.51a | 4.8 ± 0.39a | 4.3 ± 0.49a | 4.5 ± 0.67a | 4.25 ± 0.75a |
| LP + 1 % | 3.4 ± 0.67a | 4.9 ± 0.67a | 4.4 ± 0.51a | 4.25 ± 0.75a | 4 ± 0. 6a |
| LP + 2 % | 3.6 ± 0.66a | 4.9 ± 0.67a | 4.6 ± 0.51a | 4.25 ± 0.62a | 4 ± 0.6a |
| CLP | 3.6 ± 0.67a | 4.8 ± 0.58a | 4.4 ± 0.51a | 4.1 ± 0.67a | 4.2 ± 0.57a |
| CLP + 1 % | 3.6 ± 0.51a | 4.8 ± 0.39a | 4.6 ± 0.67a | 4.25 ± 0.75a | 4.1 ± 0.9a |
| CLP + 2 % | 3.75 ± 0.62a | 4.9 ± 0.67a | 4.7 ± 0.77a | 4.3 ± 0.65a | 4.25 ± 0.75a |
LP, Free L.plantarum; CLP, Encapsulated L.plantarum; 1 and 2: Inulin concentrations (%). Each column with same small letters are not significantly different at P < 0.05
Conclusions
This study showed that probiotic and synbiotic Doogh with free or encapsulated L.plantarum LS5 and Helianthus tuberosus inulin could be made without sensory modifications from the traditional Doogh making technology. Probiotic culture in free or encapsulated forms increased acidity, EPS content and decreased phase separation of Doogh samples. However, encapsulation cells were more active than free cells for increasing EPS and decreasing phase separation. Microencapsulation also enhanced the survival of probiotic culture compared to free cells in Doogh stored over 22 days. Although Inulin improved survival of free and encapsulated cells, but did not affect EPS, acidity and phase separation of samples. On the basis of all data were obtained in this study, probiotic and synbiotic Doogh (supplemented with free or encapsulated L.plantarum LS5 and Helianthus tuberosus inulin) could be used as functional dairy foods.
References
- Ahmadi A, Milani E, Madadlou A, Mortazavi SA, Rezaei Mokarram R, Salarbashi D. Synbiotic yogurt-ice cream produced via incorporation of microencapsulated lactobacillus acidophilus (la-5) and fructooligosaccharide. J Food Sci Technol. 2012 doi: 10.1007/s13197-012-0679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatayakul T, Halmos AL, Sherkat F, Shah NP. Physical characteristics of yogurts made using exopolysaccharide producing starter cultures and varying casein to whey protein ratios. Int Dairy J. 2006;16:40–51. doi: 10.1016/j.idairyj.2005.01.004. [DOI] [Google Scholar]
- Buriti FCA, Cardarelli HR, Filisetti TMCC, Saad SMI. Synbiotic potential of fresh cream cheese supplemented with inulin and Lactobacillus paracasei in co-culture with Streptococcus thermophilus. Food Chem. 2007;104:1605–1610. doi: 10.1016/j.foodchem.2007.03.001. [DOI] [Google Scholar]
- Desai AR, Powell IB, Shah NP. Survival and activity of probiotic lactobacilli in skim milk containing prebiotics. J Food Sci. 2004;69:FMS57–FMS60. [Google Scholar]
- Dhewa T, Pant S, Mishra V. Development of freeze dried symbiotic formulation using a probiotic strain of Lactobacillus plantarum. J Food Sci Technol. 2012 doi: 10.1007/s13197-011-0457-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doleyres Y, Schaub L, Lacroix C. Comparison of the functionality of exopolysaccharides produced in situ or added as bioingredients on yogurt properties. J Dairy Sci. 2005;88:4146–4156. doi: 10.3168/jds.S0022-0302(05)73100-3. [DOI] [PubMed] [Google Scholar]
- Donkor ON, Nilmini SLI, Stolic P, Vasiljevic T, Shah NP. Survival and activity of selected probiotic organisms in set-type yoghurt during cold storage. Int Dairy J. 2007;17:657–665. doi: 10.1016/j.idairyj.2006.08.006. [DOI] [Google Scholar]
- Franck A. Technological functionality of inulin and oligofructose. Br J Nutr. 2002;87:287–291. doi: 10.1079/BJNBJN/2002550. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Roberfroid MD. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Godward G, Kailasapathy K. Viability and survival of free, encapsulated and co-encapsulated probiotic bacteria in yoghurt. Milk Sci Int (Milchwissenschaft) 2003;58:396–399. [Google Scholar]
- Guarner F, Shaafsma GJ. Probiotics. Int J Food Microbiol. 1998;39:237–238. doi: 10.1016/S0168-1605(97)00136-0. [DOI] [PubMed] [Google Scholar]
- Hashemi SMB, Shahidi F, Mortazavi SA, Milani E, Eshaghi Z. Potentially probiotic Lactobacillus strains from traditional Kurdish cheese. Probiotics Antimicrob Proteins. 2014;6:22–31. doi: 10.1007/s12602-014-9155-5. [DOI] [PubMed] [Google Scholar]
- Hassan AN, Frank JF, Schmidt KA, Shalabi SI. Formation of yogurt microstructure and three-dimensional visualization as determined by confocal scanning laser microscopy. J Dairy Sci. 1995;78:2629–2636. doi: 10.3168/jds.S0022-0302(95)76892-8. [DOI] [PubMed] [Google Scholar]
- Homayouni A, Azizi A, Ehsani MR, Yarmand MS, Razavi SH. Effect of microencapsulation and resistant starch on the probiotic survival and sensory properties of synbiotic ice cream. Food Chem. 2008;111:50–55. doi: 10.1016/j.foodchem.2008.03.036. [DOI] [Google Scholar]
- Kailasapathy K. Polysaccharide ingredients in dairy product applications: increase in cheese yield. Food Aust. 1996;48:458–461. [Google Scholar]
- Kailasapathy K. Microencapsulation of probiotic bacteria: technology and potential applications. Curr Issues Intest Microbiol. 2002;3:39–48. [PubMed] [Google Scholar]
- Kailasapathy K. Survival of free and encapsulated probiotic bacteria and their effect on the sensory properties of yoghurt. LWT. 2006;39:1221–1227. doi: 10.1016/j.lwt.2005.07.013. [DOI] [Google Scholar]
- Khalil AH, Mansour EH. Alginate encapsulated bifidobacteria survival in mayonnaise. J Food Sci. 1998;63:702–705. doi: 10.1111/j.1365-2621.1998.tb15817.x. [DOI] [Google Scholar]
- Kolida S, Tuohy K, Gibson GR. The human gut flora in nutrition and approaches or its dietary modulation. BNF Nutr Bull. 2002;25:223–231. doi: 10.1046/j.1467-3010.2000.00050.x. [DOI] [Google Scholar]
- Krasaekoopt W, Bhandari B, Deeth H. Evaluation of encapsulation techniques of probiotics for yogurt. Int Dairy J. 2003;13:3–13. doi: 10.1016/S0958-6946(02)00155-3. [DOI] [Google Scholar]
- Makras L, Van Acker G, De Vuyst L. Lactobacillus casei subsp. casei 8700:2 degrades inulin-type fructans exhibiting different degrees of polymerisation. Appl Environ Microbiol. 2005;71:6531–6537. doi: 10.1128/AEM.71.11.6531-6537.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragkoudakisa PA, Miarisa C, Rojeza P, Manalisb N, Magkanari F, Kalantzopoulosa G, Tsakalidou E. Production of traditional Greek yoghurt using Lactobacillus strains with probiotic potential as starter adjuncts. Int Dairy J. 2006;16:52–60. doi: 10.1016/j.idairyj.2004.12.013. [DOI] [Google Scholar]
- Milani E (2012) Evaluation of synbiotic Kordish cheese production using isolated native strains from traditional Kordish cheese & other pure probiotic strains. Dissertation, University of Ferdowsi of Mashhad
- Nighswonger BD, Brashears MM, Gilliland SE. Viability of L. acidophilus and L. casei in fermented milk products during refrigerated storage. J Dairy Sci. 1996;79:212–219. doi: 10.3168/jds.S0022-0302(96)76353-1. [DOI] [PubMed] [Google Scholar]
- Paseephol T, Sherkat F. Probiotic stability of yoghurts containing Jerusalem artichoke inulins during refrigerated storage. J Funct Foods. 2009;131:1–318. [Google Scholar]
- Paseephol T, Small D, Sherkat F. Process optimisation for fractionating Jerusalem artichoke fructans with ethanol using response surface methodology. Food Chem. 2007;104:73–80. doi: 10.1016/j.foodchem.2006.10.078. [DOI] [Google Scholar]
- Purwandari U, Shah NP, Vasiljevic T. Effects of exopolysaccharide-producing strains of Streptococcus thermophilus on technological and rheological properties of set-type yogurt. Int Dairy J. 2007;17:1344–1352. doi: 10.1016/j.idairyj.2007.01.018. [DOI] [Google Scholar]
- Ramchandran L, Shah NP. Effect of exopolysaccharides and inulin on the proteolytic, angiotensin-I-converting enzyme- and α-glucosidase-inhibitory activities as well as on textural and rheological properties of low-fat yogurt during refrigerated storage. Dairy Sci Technol. 2009;89:583–600. doi: 10.1051/dst/2009039. [DOI] [PubMed] [Google Scholar]
- Rezaei R, Khomeiri M, Aalami M, Kashaninejad M. Effect of inulin on the physicochemical properties, flow behavior and probiotic survival of frozen yogurt. J Food Sci Technol. 2012 doi: 10.1007/s13197-012-0751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrezenmeir J, De Vrese M. Probiotics, prebiotics, and synbiotics: approaching a definition. Am J Clin Nutr. 2001;73:361S–364S. doi: 10.1093/ajcn/73.2.361s. [DOI] [PubMed] [Google Scholar]
- Sheu TY, Marshall RT, Heymann H. Improving survival of culture bacteria in frozen desserts by microentrapment. J Dairy Sci. 1993;76:1902–1907. doi: 10.3168/jds.S0022-0302(93)77523-2. [DOI] [PubMed] [Google Scholar]
- Sodini I, Remeuf F, Haddad S, Corrieu G. The relative effect of milk base starter, and process on yogurt texture. Crit Rev Food Sci Technol. 2004;44:113–137. doi: 10.1080/10408690490424793. [DOI] [PubMed] [Google Scholar]
- Sultana K, Godward G, Reynolds N, Arumugaswamy R, Peiris P, Kailasapathy K. Encapsulation of probiotic bacteria with alginate-starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. Int J Food Microbiol. 2000;62:47–55. doi: 10.1016/S0168-1605(00)00380-9. [DOI] [PubMed] [Google Scholar]
- Vinderola CG, Reinheimer JA. Enumeration of Lactobacillus casei in the presence of L. acidophilus, bifidobacteria and lactic acid starter bacteria in fermented dairy products. Int Dairy J. 2000;10:271–275. doi: 10.1016/S0958-6946(00)00045-5. [DOI] [Google Scholar]