Abstract
The purpose of this study was to evaluate fermentation of milk in the presence of green tea (Camellia sinensis) with respect to changes in antioxidant activity, phenolic compounds and the growth of lactic acid bacteria. Pasteurized full fat cow’s milk and starter culture were incubated at 41 °C in the presence of two different types of green tea extracts. The yogurts formed were refrigerated (4 °C) for further analysis. The total phenolic content was highest (p < 0.05) in air-dried green tea-yogurt (MGT) followed by steam-treated green tea (JGT) and plain yogurts. Four major compounds in MGTY and JGTY were detected. The highest concentration of major phenolic compounds in both samples was related to quercetin-rhamnosylgalactoside and quercetin-3-O-galactosyl-rhamnosyl-glucoside for MGTY and JGTY respectively during first 7 day of storage. Diphenyl picrylhydrazyl and ferric reducing antioxidant power methods showed highest antioxidant capacity in MGTY, JGTY and PY. Streptococcus thermophillus and Lactobacillus spp. were highest in MGTY followed by JGTY and PY. This paper evaluates the implementation of green tea yogurt as a new product with functional properties and valuable component to promote the growth of beneficial yogurt bacteria and prevention of oxidative stress by enhancing the antioxidant activity of yogurt.
Keywords: Yogurt fermentation, Antioxidant activity, Microbial growth, Green tea
Introduction
Fermented milk products are becoming increasingly popular because of the numerous health benefits associated with highly digestible nutrients especially for individuals with lactose maldigestion, high biochemical (Wu et al. 2008) and antioxidant activities (Kullisaar et al. 2003) and probiotic organism colonisation of intestine after ingestion (Korcznska et al. 2008). The addition of some materials, particularly fruits, into yogurt is practiced to increase the appealing taste of yogurt (Clark and Plotka 2004). This is commercially carried out after fermentation of milk to allow un-interrupted microbial fermentation of milk. However, the presence of additives during the fermentation of milk by starter culture may result in improved end-product (Amirdivani and Baba 2011) particularly when these materials contain phytochemicals that can positively affect the bacteria growth and metabolism (Ranadheera et al. 2012).
Consumer demand for natural foods has increased due to the health and wellness benefits associated with naturally grown foods. This results in the use of naturally derived compounds such as plant extracts as antimicrobials in food (Tiwari et al. 2009). Green tea, because of its high contents in polyphenols with myriad of biological activities such as anti-carcinogenic, anti-viral, anti-allergic, anti- inflammatory and immune-stimulating effects (Scalbert et al. 2005) can add certain functional properties to food. These biological activities are the outcome of the polyphenolic compounds ability to act as free radical scavengers due to their redox properties (reducing agents, hydrogen donors and singlet oxygen quenchers). Free radicals play important roles in many degenerative diseases like cancer, atherosclerosis and diabetes (Beckman and Ames 1998). Formation of free radicals, such as superoxide anion radical and hydroxyl radical, is an unavoidable consequence in aerobic organisms during respiration (Terahara et al. 2001). These radicals are very unstable and react rapidly with other groups or substances in body, leading to cell or tissue injury. The body has its own defence system against radical oxidation scavenging (ROS) based on antioxidant enzymes, as well as low molecular mass non-enzymatic antioxidant compounds. However, these are not effective enough to totally prevent the damage, and therefore, food supplements containing antioxidants can assist the human body to reduce oxidative damage (Kullisaar et al. 2003). Thus, the addition of green tea into yogurt before fermentation may impart unique enhancing properties associated with tea flavour, antioxidant properties and health-promoting benefits by virtue of phenolic compounds and subsequently improve growth and stability of probiotic bacteria in fermented milk and increased benefit of yogurt nutrition. Although there are several studies on increased polyphenolic compounds towards improving nutritional and biochemical properties of yogurt, their changes in food as a result of fermentation, and subsequent free radical-scavenging capacity of yogurt formulated with bioactive compounds are relatively scarce.
The principle aims of this study were i) to determine key phenolic compounds from green tea commonly consumed as beverage; ii) to determine the changes in phenolic compounds after the fermentation of milk by yogurt bacteria and refrigerated storage of yogurt; iii) to evaluate the effects of phenolic compounds on the growth of Lactobacillus spp. and S. thermophillus. during fermentation of milk; and iv) to determine the antioxidant activities (free radical-scavenging capacity) of green tea-yogurts.
Materials and methods
Raw materials
Commercially available green teas were purchased from local shops in the form of ground dried tea leaves in tea bags (BOH and OSK brands from Malaysia and Japan respectively). Both tea had similar method of preparation except that BOH green tea (MGT) was exposed to solar (1–2 h) and indoor (4 h) withering as opposed to steaming for OSK green tea (JGT), prior to rolling and heat (90 °C) drying.
Milk and yogurt bacteria
Fresh, pasteurized and homogenized cows’ milk (4 % full cream) was purchased from local grocery shops. Yogurt bacteria mixture (Chris Hansen, Denmark) consisted of the following bacteria: Lactobacillus acidophilus LA-5, Bifidobacterium Bb-12, Lactobacillus casei LC-01 and Streptococcus thermophilus Th-4 and Lactobacillus delbruekii ssp. bulgaricus at the ratio of 4:4:1:1:1.
Preparation of starter culture
Yogurt starter culture was prepared by inoculating 1 L pasteurized full cream (4 %) milk with yogurt bacteria (1 % w/v). The milk-yogurt bacteria mixture was incubated at 41 °C for 12 h and the yogurt formed was stored at 4 °C and used as starter culture within 3 days. We routinely found the pH of the starter culture to range between 4.1 and 4.3 and viable bacteria to range between 2.0 and 5.0 × 106 Log10 cfu mL−1 and 6.0–10.0 × 108 Log10 cfu mL−1 for Lactobacillus spp. and S. thermophilus respectively on the 14 day of storage, which were slightly lower than those reported by Buyong et al. (1998).
Preparation of tea infusions
Tea extracts were prepared essentially as described by Jaziri et al. (2009). Tea (2 g) from each tea bag was taken out and infused in 100 ml of hot water (87–90 °C, 10 min, 2.0 % (w/v) corresponding to the strength of a normal “cup of tea”). The infuscate was filtered through Whatman No. 4 paper and was used for further analyses.
Preparation of plain and tea-yogurts
Homogenized and pasteurized full fat (4 %) milk (1 L) was divided into three portions. The first two milk portions were subjected to ebullition with green tea (MGT or JGT) at 2.0 % (w/v). The teas were infused for 10 min and the milks were filtered through sterile cotton to strain off visible plant particles. The strained milks thus obtained were cooled to 45 °C and inoculated with 10 % (v/v) yogurt starter culture and divided equally into three sterile beakers. Yogurt fermentation was carried out by incubating at 42 °C until the pH reached 4.5 to yield MGT- and JGT-yogurts (MGTY and JGTY respectively). Plain yogurt (PY) was made by inoculating the third milk portion without green tea infusion with 10 % (v/v) yogurt starter culture followed by incubation at 42 °C until the pH reached 4.5.
Preparation of yogurt for fermentation assay
Samples of milk were taken during every 1 h of the milk fermentation until the pH of yogurt reached 4.5. Plain- and tea-yogurts were homogenized (polytron at maximum setting for 10 s) and these were directly used in the analysis of microbial growth. To find out the effect of fermentation on the antioxidant activity, non-hydrolysed casein was removed. Briefly, a portion (15 mL) of fermented milk was collected and the pH (Mettler-Toledo 320, Shanghai) was adjusted to 4.6 by adding 1 M HCl. The suspension was centrifuged (10,000 g for 20 min at 5 °C), and the supernatant was passed through a 0.45-μm filter. The filtrate was stored at −20 °C for further analysis.
Total phenolic analysis
Total phenolic content (TPC) were determined as described by Shetty et al. (2005). Briefly, 1 mL of tea yogurt extract was transferred into a test tube and followed by the addition of 1 mL of 95 % ethanol and 5 ml of dH2O. Folin-Ciocalteu reagent (diluted 1:1 with distilled water) was added to each sample followed by thorough mixing using a vortexer. Na2CO3 (5 %, 1 mL) was added to the reaction mixture and these were left to stand for 60 min at room temperature. The absorbance (725 nm; Spectronic-Genesys, USA) values were converted to total phenolics expressed in microgram equivalents of gallic acid (μgGAE) per ml of the sample. Standard curves were simultaneously established for each assay using various concentration of gallic acid (5–60 μg/mL) in methanol (Amirdivani and Baba 2011).
Determination of antioxidant activity
Two chemical tests were used to evaluate the antioxidant activity of yogurt. The DPPH radical inhibition assay evaluate antioxidant power from the inhibition of free radicals whereas the ferric reducing antioxidant power (FRAP) assay evaluated the antioxidant property from a redox reaction occurring between the substrate (electron donor) and Fe3+ ions (electron acceptor), producing Fe2+ ions.
1,1-diphenyl-2-picrylhydrazyl (DPPH) radical inhibition (DRI) assay
Yogurt extracts (250 mL) were added into 3 ml of 60 mmol/L DPPH in ethanol (Sigma Aldrich, Germany). The decrease in absorbance was monitored at 517 nm (Spectronic-Genesys, USA) until a constant reading was obtained. The constant reading for the yogurt extracts and control (consisting of 250 ml of water in place of extract) was used in calculating the % inhibition of DPPH oxidation (Apostolidis et al. 2007; Amirdivani and Salihin 2011) as follows:
Ferric reducing/antioxidant power (FRAP)
The ferric reducing antioxidant power (FRAP) was assessed according to methods described by Lucas et al. (2006). Briefly, after appropriate dilutions, 400 μl of yogurt extracts were mixed with 3.6 ml of the freshly prepared ferric-tripyridyltriazine (TPTZ) reagent. This buffer was prepared by mixing 300 mmol L−1 acetate buffer (pH 3.6), 8 mmol 2,4,6- tris (2-pyridyl)-s-triazine in 30 mmol L−1 HCl; 20 mmol L−1 FeCl3 in the ratio of 10:1:1. The mixtures were incubated at 37 °C for 10 min followed by brief centrifugation (1400 × g, 2 min) followed by absorbance reading (593 nm) of the blue TPTZ complex formed with reduced ferrous ions at against a blank sample. The results were calculated from a standard scale of FeSO4.7H2O and expressed as mmol Fe2+ E L−1.
Enumeration of viable cell count (VCC) in yogurt
Yogurt bacteria were enumerated using spread plate method and pour plate method for S.thermophillus and Lactobacillus spp. respectively. 1) Pour plate method using MRS (De Man, Rogosa and Sharp) agar medium was used to support the growth of Lactobacillus spp. under anaerobic condition and 2) Spread plate method using M17 agar medium was used to support the growth of S. thermophilus. Samples of yogurts were decimally diluted to 10−5,10−6 and 10−8 in sterile peptone water and 1.0 mL or 0.1 mL aliquots of the diluted yogurt were plated on individual MRS or M17 plates respectively and incubation was carried out at 37 ° C for 24–48 h. Colonies formed on agar were counted and CFU/mL was calculated as follows:
LC-MS analysis of phenolic compounds
The LC-MS/MS equipment consisted of a quarternary pump with vacuum degasser, an auto injector (Agilent Technologies, Santa Clara, California, USA) and a mass spectrometer (AB Sciex 3200QTrap, Toronto, Canada). Chromatogram was recorded with a full scan and MS/MS data collection and negative electrospray ionisation. Data collection and subsequent processing were performed using AB Sciex Analyst software. The analytical column (C18, 50 mm × 1.1 mm i.d; Phenomenex Aqua, USA) was operated at 35 °C. The mobile phase A was water with 5 mM ammonium formate and 0.1 % formic acid, while mobile phase B was acetonitrile with 5 mM ammonium formate and 0.1 % formic acid. The chromatographic program run consisted of a linear gradient step of 10 % B to 90 % B in 7.0 min followed by isocratic hold for 3.0 min and re-equilibration back to 10%B for 5.0 min. Total run time was 15.0 min.
Statistical analysis
A total of three separate experiments were carried out and assays were performed in triplicate. Data were expressed as mean ± standard deviation and the data were analyzed using SPSS 19.0 (Chicago, IL, USA) for Windows. General Linear Model procedures and Tukey test for means comparison were used for determining significant difference at p < 0.05.
Results and discussion
Effects of green tea on yogurt bacteria growth during fermentation
The viable cell counts (VCC) of Lactobacillus spp. in the yogurts ranged from 4.61 to 12.54 × 108 cfu /mL (Table 1). The increase in Lactobacillus spp. VCC in yogurt attributed to green tea (~12 × 108 Log10 cfu mL−1) was around 2 fold higher compared to plain-yogurt (6.17 × 108 Log10 cfu mL−1). The VCC S. thermophilus in inoculated milk increased from about 77 × 106 Log10 cfu mL−1 at the initial stage of fermentation to 83.52 ± 0.27 × 106 Log10 cfu mL−1 in PY and120 × 106 Log10 cfu mL−1 in green tea yogurts (Table 2). Higher microbial population throughout the fermentation may explain faster reduction in the fermentation time for green tea yogurts than for plain yogurt (300; 300 and 360 min respectively for MGTY, JGTY and PY). Slightly higher microbial growth in MGTY than in JGTY suggest that there could be substantial differences in the tea extract constituents prepared from MGT and JGT, possibly as a result of differences in the methods of preparation. It is possible that exposure to extreme heat (steaming process) in JGT could have resulted in the degradation of more phenolic compounds and thus making this tea less stimulating to microbial growth than MGT. Nevertheless the positive effect of the addition of tea on the yogurt starter microflora found in the present study can be attributed to selective effect of tea constituents toward LAB. The antimicrobial effect of green tea was shown detrimental towards pathogenic bacteria but not towards LAB. However, this effect may be absent in highly processed tea since Jaziri et al.2009 found no effects on the survival of the starter bacteria in yogurts as a result of adding black tea.
Table 1.
The number of Lactobacillus ssp. in yogurts as affected by the green tea supplementation during fermentation
| Time (min) | P-Y | MGT-Y | JGT-Y |
|---|---|---|---|
| 0 | 4.61 ± 0.07ax | 4.95 ± 0.67ax | 4.71 ± 0.53ay |
| 60 | 4.69 ± 0.06bx | 6.86 ± 1.00by | 5.97 ± 1.17by |
| 120 | 4.73 ± 0.04bx | 8.19 ± 0.76by | 8.91 ± 0.35by |
| 180 | 5.51 ± 0.10bx | 10.44 ± 1.43by | 9.97 ± 0.07by |
| 240 | 5.82 ± 0.04bx | 11.17 ± 1.30by | 11.17 ± 0.23by |
| 300 | 5.92 ± 0.06bx | 12.54 ± 0.70by | 11.06 ± 0.07by |
| 360 | 6.17 ± 0.08bx | – | – |
Bacteria counts in yogurts Lactobacillus spp. CFU (×108 cells/ml), Values are means ± SD (n = 3)
P-Y Plain yogurt, MGT-Y Malaysian green tea yogurt, JGT-Y Japanese green tea yogurt
abMeans in the same row with different alphabets are significantly different (p < 0.05) for each type of yogurt
xyMeans in the same column with different alphabets are significantly different (p < 0.05) for the same fermentation period
Table 2.
The number of S. thermophilus in yogurts as affected by the green tea supplementation during fermentation
| Time (min) | P-Y | MGT-Y | JGT-Y |
|---|---|---|---|
| 0 | 77.21 ± 1.64ax | 77.60 ± 0.75ay | 78.03 ± 0.74ay |
| 60 | 78.40 ± 1.35ay | 85.59 ± 1.22ax | 83.53 ± 1.11ax |
| 120 | 79.31 ± 0.34ax | 93.32 ± 1.20ax | 88.04 ± 0.16ax |
| 180 | 81.21 ± 0.28bx | 101.17 ± 1.07ay | 96.34 ± 1.36ay |
| 240 | 81.93 ± 0.41bx | 108.12 ± 1.49ay | 103.61 ± 0.75ay |
| 300 | 82.20 ± 0.41bx | 120.40 ± 1.03ay | 118.04 ± 0.17ay |
| 360 | 83.52 ± 0.27bx |
Bacteria counts in yogurt S. thermophilus CFU (×106 cells/ml). Values are means ± SD (n = 3)
P-Y Plain yogurt, MGT-Y Malaysian green tea yogurt, JGT-Y, Japanese green tea yogurt
abMeans in the same row with different alphabets are significantly different (p < 0.05) for each type of yogurt
xyMeans in the same column with different alphabets are significantly different (p < 0.05) for the same fermentation period
Quantitative analysis of major phenolic compounds in green tea
LC-MS/MS was used for the detection of phenolic acids, anthocyanidin and flavanoids in green tea. Phenolic acids such as chlorogenic acid, quinic acid, dicaffeoquinic acid, gallic acid, and anthocyanidin and flavanoids derivatives such as catechin, epicatechin, epigallocatechin gallate, quercetin, kampferol were present in both green teas (Table 3). Twenty four compounds were detected in MGT whereas only 16 compounds were present in JGT. Quercetin-rhamnosylgalactoside, epicatechin, quinic acid conjugate, epicatechingallate, epigallocatechin and gallocatechin were detected in both green teas. The major phenolic compounds in MGT were 1). epigallocatechin gallate (5.05 mg/mL), 2). epicatechin gallate (3.00 mg/ml), 3). quercetin-rhamnosylgalactoside (7.19 mg/mL), 4). epigallocatechin (3.60 mg/mL), 5). gallocatechin (7.48 mg/mL), 6). epicatechin (7.26 mg/mL) and 7). kaempferol-3-O-rutinoside (6.56 mg/mL), whereas major phenolic compounds in JGT were 1).quinic acid (3.66 mg/mL), 2). epigallocatechin (6.43 mg/mL), 3).quercetin-3-O-galactosyl-rhamnosyl (4.92 mg/mL), 4). gallocatechin (6.00 mg/mL) and 5). epicatechin (7.01 mg/mL). Other compounds occurred in much smaller quantities. Kaempferol 3 rutinoside and myricitin-3-O-glucoside and quercetin-rhamnosylgalactoside are the main flavonols detected in tea leaves in the present studies. These compounds make up 2–3 % of the tea water-soluble extract (Balentine 1997).
Table 3.
Major phenolic compounds in Malaysian and Japanese green tea infusion
| Phenolic compounds | MGT extract (mg/mL) | JGT extract (mg/mL) |
|---|---|---|
| Quinic Acid | 0.03 | 3.66 |
| Gallocatechin | 7.48 | 6 |
| Chlorogenic Acid | 0.003 | ND |
| Epicatechin | 7.26 | 7.01 |
| Dicaffeoquinic Acid | ND | 0.42 |
| 6-C-glucosyl-8-C-arabinosyl apigenin | 0.01 | 0.79 |
| myricitin-3-O-glucoside or galactoside | 0.94 | 0.01 |
| quercetin-rhamnosylgalactoside | 7.19 | 1.39 |
| Kaempferol −3-O- rutinoside | 6.56 | 0.31 |
| quercetin-3-O-galactosyl | 0.57 | 4.92 |
| kaempferol-3-O-glucoside | 0.002 | 0.84 |
| Catechin | 0.001 | 0.005 |
| Epicatechin Gallate | 3 | 0.01 |
| Epigallocatechin | 3.6 | 6.43 |
| Epigallocatechin Gallate | 5.05 | 0.004 |
| epigallocatechin-3-O-(4-O-methyl) gallate | 0.0003 | ND |
| Gallic Acid | 0.0009 | 0.0018 |
| Gallocatechin Gallate | 0.08 | 0.1 |
| Dichlorogenic Acid conjugate | 0.008 | ND |
| Gallocatechin Gallate Conjugate | 0.01 | ND |
| kaempferol-rhamnose-hexose-rhamnose | 0.05 | ND |
| Kampferol Rhamnoside | 0.27 | ND |
| Procyanidin B1 | 0 | ND |
| Quercetin | 0.004 | ND |
| quercetin-3-glucoside | 0.01 | ND |
| 6-C-arabinosyl-8-C-glucosyl apigenin | ND | 0.01 |
MGT extract Malaysian green tea extract, JGT extract Japanese green tea extract, ND Not Detected
The high value of phenolic derivatives in MGT compared to JGT may stem from different tea tree environment and cultivation, and tea leaves post-harvest processing methods. MGT and JGT had similar method of preparation and processing except that the former was exposed to solar (1–2 h) and indoor (4 h) withering as opposed to steaming for the latter, prior to rolling and heat (90 °C) drying. Epigallocatechin (EGC), epicatechin (EC) and gallocatechin (GC) were similarly detected as major catechins in both green teas. Catechins are colourless, water-soluble compounds which impart bitterness and astringency to green tea infusion (Heijnen et al.2000). Almost all of the characteristics of manufactured tea, including its taste, colour, and aroma, are associated directly or indirectly with modifications to the catechins.
Changes in green tea polyphenolic compounds after fermentation of milk
Differences in the type and quantity of green teas phenolic compounds were detected between the yogurts during storage. Four major compounds were detected in MGTY at the end of the fermentation (fresh yogurt, Table 4). Phenolic compounds with the highest concentration were represented by quercetin-rhamnosylgalactoside (8.90 mg/ml) followed by gallocatechin (7.34 mg/mL), epicatechin (6.43 mg/mL) and kaempferol-3–rutinoside (6.41 mg/mL) in first day of storage. Refrigerated storage increased quercetin-rhamnosylgalactoside to highest value in day 7 of storage (12.28 mg/mL). This detected compound is the typical phenolic compounds antioxidants in green tea (Del Rio et al.2004) and have been proposed to impart health-promoting properties (Ruiz et al. 2007). Interestingly the concentration of quercetin-rhamnosylgalactoside in MGT was lower (7.19 mg/mL; p < 0.05) than those observed in MGTY both in day 1 and 7 of storage. Extended refrigerated storage to day 28 decreased the concentrations of all phenolic compounds.
Table 4.
Concentration of bioactive compounds in Malaysian green tea yogurt (MGTY) extract during 28 days of refrigerated storage
| Phenolic compounds | Day1 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|
| Quinic Acid Conjugate | 1.15 | ND | ND | ND | ND |
| Gallocatechin | 7.34 | 5.14 | 5.21 | 1.15 | 1.09 |
| Chlorogenic Acid | 0.003 | ND | ND | ND | ND |
| Epicatechin | 6.43 | 5.52 | 6.61 | 6.05 | 5.44 |
| 6-C-glucosyl-8-C-arabinosyl apigenin | 0.034 | ND | 0.001 | 0.001 | 0.001 |
| myricitin-3-O-glucoside | 0.003 | ND | ND | ND | ND |
| quercetin-rhamnosylgalactoside | 8.90 | 12.28 | 9.63 | 8.79 | 5.73 |
| Kaempferol 3 rutinoside | 6.41 | 1.57 | 1.21 | 1.00 | 4.96 |
| kaempferol-3-O-glucoside | 0.003 | ND | ND | ND | ND |
| quercetin-3-O-galactosyl- rhamnosyl-glucoside | ND | 0.22 | 0.04 | ND | ND |
ND Not Detected
Four major compounds were also observed in JGTY (Table 5), with the highest represented by gallocatechin (5.81 mg/mL) followed by quercetin-3-O-galactosyl- rhamnosyl-glucoside (5.92 mg/mL), epicatechin (4.96 mg/mL) and quinic acid conjugate (1.58 mg/mL). Although refrigerated storage increased (p < 0.05) concentration of phenolic compounds on day 7 for epicatechin and quercetin-3-O-galactosyl-rhamnosyl-glucoside (an increase of 6.71 and 9.21 mg/mL, respectively compared to day 1 yogurt), the concentration of most compounds decreased by day 28 day of storage. Gallocatechin decreased by 10.54 %, epicatechin by 18.20 %, quercetin-rhamnosylgalactoside by 55.32 %, and kaempferol −3–rutinoside by 29.23 %. Surprisingly the concentration of quercetin-3-O-galactosyl- rhamnosyl-glucoside in JGTY (5.92, 9.21 and 6.60 mg/mL) was higher in day 1, 7 and 14 of storage than those observed in JGT (4.92 mg/ml; p < 0.05). This suggests that the phenolic compounds in both green teas might be broken down into related compound during the fermentation of milk. The bacterial fermentation of milk appeared to have high impact on quercetin-3-O-galactosyl-rhamnosyl-glucoside present in both green tea yogurts leading to increased concentration of quercetin-3-O-galactosyl-rhamnosyl-glucoside than those observed in JGT. The differences in the declining of phenolic compounds in both yogurts towards day 28 of refrigerated storage may be contributed by various chemical and physical factors such as protection by the gel structure of yogurt (Rozman and Gasperlin 2007), binding to amphipathic yoghurt peptides (Papadopoulou and Frazier 2004), decrease in pH (Kalt et al. 1999) or complexation with proteins and polysaccharides (Rawel et al. 2003). The detected insignificant peaks (p > 0.05) in plain yogurt (data not shown) may resulted from the phenolic compounds originally present in milk such as estrogens (Pape-Zambito et al. 2010).
Table 5.
Concentration of bioactive compounds in Japanese green tea yogurt (JGTY) extract during 28 days of refrigerated storage
| Phenolic compounds | Day 1 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|
| Quinic Acid Conjugate | 1.58 | 1.06 | 1.78 | 1.01 | 0.9 |
| Gallocatechin | 5.81 | ND | 5.30 | 4.96 | 4.74 |
| Epicatechin | 4.96 | 6.71 | 5.86 | 3.83 | 3.96 |
| Dicaffeoquinic Acid Conjugate | 0.17 | 0.18 | ND | ND | ND |
| 6-C-glucosyl-8-C-arabinosyl apigenin | 0.75 | 1.11 | 0.20 | 0.31 | 0.28 |
| quercetin-rhamnosylgalactoside | 1.30 | ND | ND | ND | ND |
| Kaempferol 3 rutinoside | 0.29 | 0.26 | ND | ND | ND |
| quercetin-3-O-galactosyl- rhamnosyl-glucoside | 5.92 | 9.21 | 6.60 | 4.00 | 3.82 |
ND Not Detected
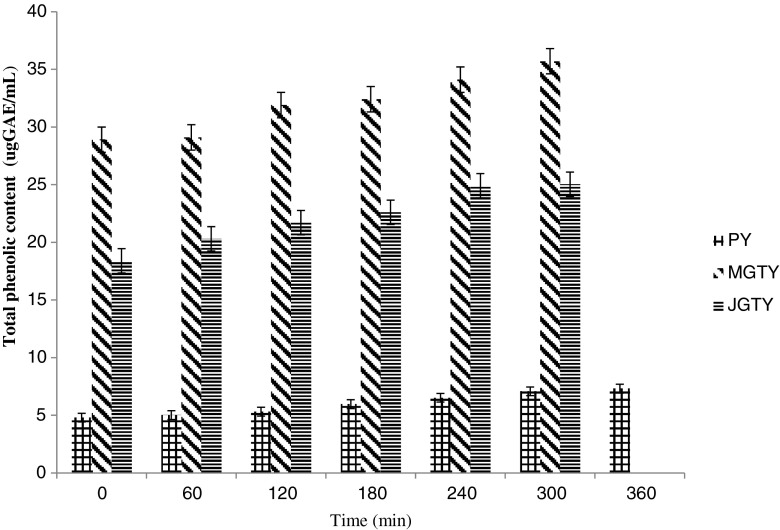
Total phenolic content (TPC) of yogurts during fermentation
MGTY and JGTY had higher (p < 0.05) TPC than plain yogurt during fermentation (Fig. 1). Lowest TPC were present in plain yogurt at the beginning of fermentation (28.9, 18.4 and 4.8 ugGAE/mL respectively for MGTY, JGTY and PY). The addition of green tea into milk showed an increase in TPC at the end of fermentation (35.7 and 25.03ugGAE/mL, respectively for MGTY and JGTY). Fermentation also affected the bioactive constituents in green tea. Since TPC measures phenolic compounds in milk and green tea extracts, it is reasonable to assume that the increase in TPC values were contributed by further breakdown of phenolic compounds from these two sources during fermentation as a result of microbial metabolic activities. For instance, during fermentation, enzymes such as amylases, and proteases derived from the peptides or microbes contribute to the modification of sample composition (Loponen et al. 2004). In addition bound phenolics may also be released by enzymatic treatment (Bartolome and Gomez-Cordoves 1999) or the microbial enzyme activity (present studies) prior to extraction. Since plain-yogurt contains no plant extracts, the TPC values in plain-yogurt reflect phenolic compounds related to milk protein breakdown (Damin et al. 2009). The amino acid tyrosin for instance has a phenolic side chain suggested (Shah 2000) to give rise to the reading in TPC. Another possibility is that microbial utilization of phenolic acids such as ferulic and p-coumaric acid during fermentation process and post acidification lead to the production of other phenolic acids such as vanillic and p-hydroxybenzoic acids before the aromatic ring structure is broken down (Blum 1998).
Fig. 1.
Total phenolic content (ugGAE/mL) in plain- and green tea-yogurts during fermentation. Values are means ± SD (n = 3), Total phenols were expressed as ug gallic acid equivalent (ugGAE)/mL. PY = Plain yogurt; MGTY = Malaysian green tea yogurt; JGTY = Japanese green tea yogurt
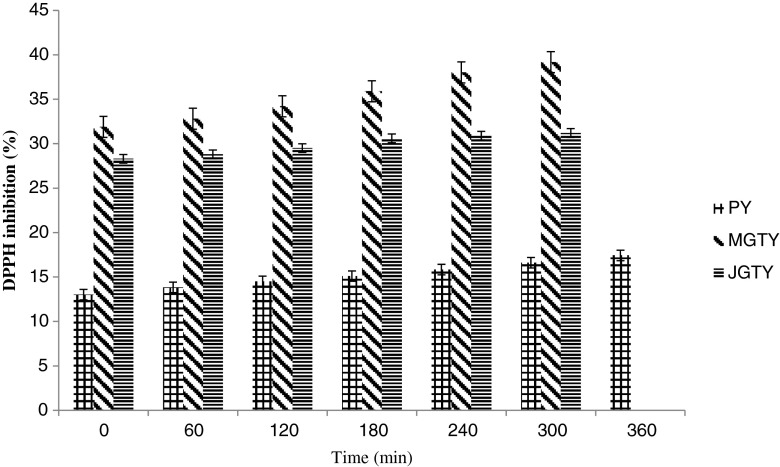
Effect of green tea on the changes of antioxidant activity during fermentation
The eventual benefits of tea on yogurt bacteria growth must be viewed from the potential differences in the antioxidants capacity present in the tea used. This is because lactic acid bacteria used in the production of yogurt are known to have antioxidant properties (Lin and Yen 1999) attributed to reactive oxygen species scavenging, metal ion chelation, enzyme inhibition, and the reduction activity and inhibition of ascorbate autoxidation (Lin and Yen 1999). Adding green tea infusion to milk resulted higher antioxidant activity (p < 0.05) in green tea-milk than in plain milk at the beginning of the fermentation (31.9 ± 0.06, 28.3 ± 0.27 and 13.01 ± 0.4 % for MGTY, JGTY and PY respectively; Fig. 2). This is in line with increased DPPH inhibition in milk following the addition of plant and fruits (Apostolidis et al. 2007). Inhibition of DPPH oxidation by yogurts at the end of fermentation increased (p < 0.05) to 39 ± 0.77 % for MGTY followed by JGTY (31.2 ± 0.14 %) and PY (17.43 ± 0.21 %). The present studies therefore suggest the presence of green tea increased the growth of LAB bacteria and these microbes subsequently increased the antioxidant activity of yogurt during fermentation. It is not clear from the present study the source of increased antioxidant activity in green tea-yogurts. One plausible explanation is that the green tea-induced increase in microbial growth resulted in increased formation of metabolic compounds and/or degradation products of milk proteins (Papadimitriou et al. 2007) with antioxidant activity. While this may be true for MGTY, it is not for JGTY since the mean absolute increase in antioxidant activity during fermentation (2.9 %) was lower than PY (4.4 %), in contrast to 7.9 % for MGTY.
Fig. 2.
Antioxidant capacity (% inhibition of DPPH oxidation) by plain- and green tea-yogurts during fermentation. Values are means ± SD (n = 3). PY = Plain yogurt; MGTY = Malaysian green tea yogurt; JGTY = Japanese green tea yogurt
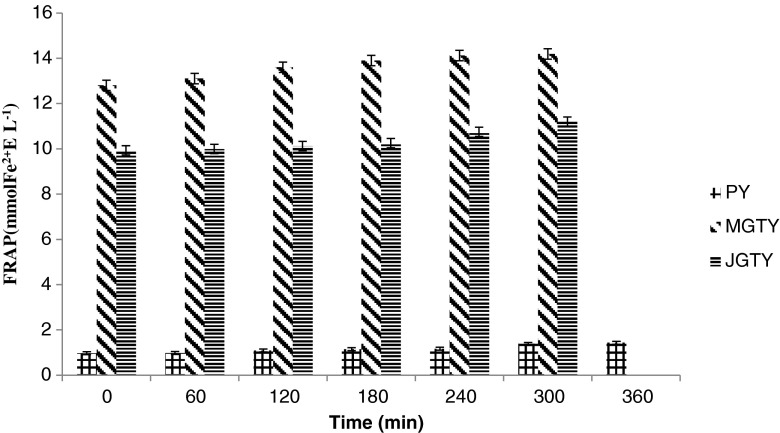
The highest FRAP value was found in MGTY (12.8 mmol Fe2+/L), followed by JGTY and PY (9.93 and 0.97 to mmol Fe2+/L, respectively) at the first hour of incubation at 41 °C (Fig. 3). There was little increase in FRAP attributed to fermentation milk. Comparative data on changes in FRAP value due to fermentation is limited. The present studies showed sustained FRAP values during the incubation period of 5–6 h, which is in agreement with Hubert et al. (2008) which also reported sustained initial ferric-reducing power for up to 6 h of incubation period followed by a significant decrease until 48 h of incubation. The lack of increase in FRAP values during fermentation indicate minimal contribution of increased yogurt bacteria growth in the presence of green tea on increasing the yogurt redox potential.
Fig. 3.
The FRAP (ferric reducing antioxidant power) values of yogurts in the absence or presence of green tea extracts. Values are means ± SD (n = 3). PY = Plain yogurt; MGTY = Malaysian green tea yogurt; JGTY = Japanese green tea yogurt
Conclusion
The present study addressed the changes in antioxidant capacity of yogurts as a result of fermenting milk in the presence of green tea infusions. Results of this study demonstrate that green tea enhanced the growth of Lactobacillus spp. and S. thermophilus during fermentation of milk. Furthermore, the increased microbial metabolic activities in green tea yogurts had encouraged the degradation of phenolic compounds. However, the marked changes in green tea phenolic compounds composition had little effects on antioxidant activities of green tea yogurt after milk fermentation and during refrigerated storage. Green tea is a promising new and valuable component that can promote the growth of yogurt bacteria and enhance the antioxidant activity of yogurt.
References
- Amirdivani S, Baba AS (2011) Changes in yogurt fermentation characteristics, and antioxidant potential and in vitro inhibition of angiotensin-1 converting enzyme upon the inclusion of peppermint, dill and basil. LWT - Food Sci Technol 44(6):1458–1464
- Apostolidis E, Kwon YI, Shetty K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov Food Sci Emerg Technol. 2007;8:46–54. doi: 10.1016/j.ifset.2006.06.001. [DOI] [Google Scholar]
- Balentine DA. Special issue: tea and health. Crit Rev Food Sci Nut. 1997;8:691–692. doi: 10.1080/10408399709527796. [DOI] [Google Scholar]
- Bartolome B, Gomez-Cordoves C. Barely spent grain: release of hydroxycinnamic acids (ferulic and p-coumaric acids) by commercial enzyme preparation. J Food Sci Agr. 1999;79:435–439. doi: 10.1002/(SICI)1097-0010(19990301)79:3<435::AID-JSFA272>3.0.CO;2-S. [DOI] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Blum U. Effects of microbial utilization of phenolic acids and their phenolic acid breakdown products on allelopathic interactions. J Chem Ecol. 1998;24:685–708. doi: 10.1023/A:1022394203540. [DOI] [Google Scholar]
- Buyong N, Kok J, Luchansky JB. Use of genetically enhanced, pediocin-producing starter-culture, Lactococcus lactis subsp. Lactis MM217, to control Listeria monocytogenes in cheddar cheese. Appl Environ Microbiol. 1998;64:4842–4845. doi: 10.1128/aem.64.12.4842-4845.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, S. and Plotka, V.C. (2004). Yogurt and sour cream: Operational Procedures and processing equipment in Hand book of food and beverage fermentation technology. N.Y.
- Damin MR, Alcântara MR, Nunesb AP, Oliveiraa MN. Effects of milk supplementation with skim milk powder, whey protein concentrate and sodium caseinate on acidification kinetics, rheological properties and structure of nonfat stirred yogurt”. LWT - Food Sci Technol. 2009;42(10):1744–1750. doi: 10.1016/j.lwt.2009.03.019. [DOI] [Google Scholar]
- Del Rio DD, Stewart AJ, Mullen WW, Burns JJ, Lean EJ, Brighenti FF, Crozier AA. HPLC-MSn analysis of phenolic compounds and purine alkaloids in green and black tea. J Agr Food Chem. 2004;52(10):2807–2815. doi: 10.1021/jf0354848. [DOI] [PubMed] [Google Scholar]
- Heijnen CGM, Haenen GRMM, Wiseman SA, Tijburg LBM, Bast A. “The interaction of tea flavonoids with the NO-system: discrimination between good and bad. Food Chem. 2000;70:365–370. doi: 10.1016/S0308-8146(00)00105-9. [DOI] [Google Scholar]
- Hubert J, Berger M, Nepveu F, Paul F, Daydé J. Effects of fermentation on the phytochemical composition and antioxidant properties of soy germ. Food Chem. 2008;109:709–721. doi: 10.1016/j.foodchem.2007.12.081. [DOI] [PubMed] [Google Scholar]
- Jaziri I, Slama MB, Mhadhbi H, Urdaci MC, Hamdi M. Effect of green and black teas (Camellia sinensis L.) on the characteristic microora of yogurt during fermentation and refrigerated storage. Food Chem. 2009;112:614–620. doi: 10.1016/j.foodchem.2008.06.017. [DOI] [Google Scholar]
- Kalt W, Forney CF, Martin A, Prior RL. Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of small fruits. J Agr and Food Chem. 1999;47:4638–4644. doi: 10.1021/jf990266t. [DOI] [PubMed] [Google Scholar]
- Korcznska M, Weinbreck F, Ouwehand AC. The gut microbiota and weight management Any role for probiotics?”. Agro Food Industry hi-tech. 2008;19(5):436–442. [Google Scholar]
- Kullisaar T, Songisepp E, Mikelsaar M, Zilmer K, Vihalemm T, Zilmer M. Antioxidative probiotic fermented goats’ milk decreases oxidative stress-mediated atherogenity in human subjects. Brit J Nutr. 2003;90:449–456. doi: 10.1079/BJN2003896. [DOI] [PubMed] [Google Scholar]
- Lin MY, Yen CL. Antioxidative ability of lactic acid bacteria. J Agr Food Chem. 1999;47:1460–1466. doi: 10.1021/jf981149l. [DOI] [PubMed] [Google Scholar]
- Loponen J, Mikola M, Katina K, Sontag-Strohm T, Salovaara H. Degradation of HMW glutenins during wheat sourdough fermentations. Cereal Chem. 2004;81:87–93. doi: 10.1094/CCHEM.2004.81.1.87. [DOI] [Google Scholar]
- Lucas A, Rock E, Chamba JF, Verdier-Metz I, Brachet P, Coulon JB. Respective effects of milk composition and the cheese-making process on cheese compositional variability in components of nutritional interest. Lait. 2006;86:21–41. doi: 10.1051/lait:2005042. [DOI] [Google Scholar]
- Papadimitriou CG, Mastrojiannaki AV, Silva AV, Gomes AM, Malcata FX, Alichanidis E. Identification of peptides in traditional and probiotic sheep milk yoghurt with angiotensin I-converting enzyme (ACE)-inhibitory activity. Food Chem. 2007;105:647–656. doi: 10.1016/j.foodchem.2007.04.028. [DOI] [Google Scholar]
- Papadopoulou A, Frazier RA. Characterization of protein–polyphenol interactions. Trends in Food Sci Technol. 2004;15:186–190. doi: 10.1016/j.tifs.2003.09.017. [DOI] [Google Scholar]
- Pape-Zambito DA, Roberts RF, Kensinger RS. Estrone and 17beta- stradiol concentrations in pasteurized-homogenized milk and commercial dairy products. J Dairy Sci. 2010;93(6):2533–2540. doi: 10.3168/jds.2009-2947. [DOI] [PubMed] [Google Scholar]
- Ranadheera CS, Evans CA, Adams MC, Baines SK. Probiotic viability and physico-chemical and sensory properties of plain and stirred fruit yogurts made from goat’s milk. Food Chem. 2012;135:1411–1418. doi: 10.1016/j.foodchem.2012.06.025. [DOI] [PubMed] [Google Scholar]
- Rawel HM, Rohn S, Kroll J. Influence of a sugar moiety (rhamnosylglucoside) at 3-O position on the reactivity of quercetin with whey proteins. Int J Biol Macromol. 2003;32:109–120. doi: 10.1016/S0141-8130(03)00044-8. [DOI] [PubMed] [Google Scholar]
- Rozman B, Gasperlin M. Stability of vitamins C and E in topical microemulsions for combined antioxidant therapy. Drug Deliv. 2007;14(4):235–245. doi: 10.1080/10717540601067786. [DOI] [PubMed] [Google Scholar]
- Ruiz B, Hölzlwimmer LQ, Haller M. Quercetin inhibits TNF-induced NF-kappaB transcription factor recruitment to proinflammatory gene promoters in murine intestinal epithelial cells’. J Nutr. 2007;137(5):1208–1215. doi: 10.1093/jn/137.5.1208. [DOI] [PubMed] [Google Scholar]
- Scalbert A, Manach C, Morand C, Rémésy C. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- Shah NP. Effect of milk-derived bioactives: an overview. Brit J Nutr. 2000;84(1):3–10. doi: 10.1017/s000711450000218x. [DOI] [PubMed] [Google Scholar]
- Shetty K, Vattem DA, Clydesdale FM. Clonal screening and sprout-based bioprocessing for phenolic phytochemicals for functional foods. In: Shetty K, Paliyath G, Pometto AL III, Levin RE, editors. Food biotechnology. 2. Boca Raton: CRC Press; 2005. [Google Scholar]
- Terahara M, Kurama S, Takemoto N. Prevention by lactic acid bacteria of the oxidation of human LDL. Biosci Biotech Biochem. 2001;65:1864–1868. doi: 10.1271/bbb.65.1864. [DOI] [PubMed] [Google Scholar]
- Tiwari BK, Valdramidis VP, O’ Donnell CP, Muthukumarappan K, Bourke P, Cullen PJ. Application of natural antimicrobials for food preservation. J Agr Food Chem. 2009;57:5987–6000. doi: 10.1021/jf900668n. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Chen HB, Chen WP. Fermentation parameter and partial biochemical characterisation of milk clotting enzyme from Chinese distiller’s yeast. Ann Microbiol. 2008;58(4):717–722. doi: 10.1007/BF03175580. [DOI] [Google Scholar]