Abstract
The physicochemical and emulsifying properties of legume protein isolates prepared from chickpea (CPI), faba bean (FPI), lentil (LPI) and soy (SPI) were investigated in the presence and absence of genipin. Solubility was highest for CPI (~94 %), followed by LPI (~90 %), FPI (~85 %) and SPI (~50 %). Surface characteristics revealed similar zeta potentials (~ − 47 mV) for CPI, LPI and FPI, but lower for SPI (~ − 44 mV). Contrastingly, surface hydrophobicity was greatest for CPI (~137 arbitrary units, AU), followed by SPI/LPI (~70 AU) and FPI (~24 AU). A significant (from 16.73 to ~8.42 mN/m) reduction in interfacial tension was observed in canola oil–water mixtures in the presence of non-crosslinked legume protein isolates. The extent of legume protein isolate-genipin crosslinking was found to be similar for all isolates. Overall, creaming stability increased in the presence of genipin, with maximum stability observed for SPI (65 %), followed by FPI (61 %), LPI (56 %) and finally CPI (50 %).
Keywords: Legume, Protein, Emulsion, Genipin, Crosslinking
Introduction
Food emulsions maybe oil-in-water or water-in-oil systems, whereby one phase is dispersed as micron-sized droplets within the other (Friberg and Larsson 1997; Damodaran 2005; McClements 2007). Emulsions can be stabilized through the addition of low molecular weight emulsifiers or high molecular weight proteins, which act to inhibit separation into a more thermodynamically stable state (McClements 2007). Emulsifiers are comprised of both hydrophobic and hydrophilic moieties that become integrated into the oil–water interface to lower the interfacial tension (Bos and van Vliet 2001). The effectiveness of protein-based emulsifiers is dependent on properties of the protein (e.g., source, size, solubility, concentration, conformation and surface characteristics) (Schwenke 2001; McClements 2007); emulsification conditions (e.g., level and duration of shear, oil/water ratio, and type of homogenizer) and solvent effects (e.g., temperature, pH and the presence of salts) (McClements 2004; Can Karaca et al. 2011). Proteins are ideal materials for improving emulsion stability due to their amphiphilic nature (i.e., having both hydrophilic and hydrophobic moieties), enabling them to align at, or unfold at the oil–water interface to lower interfacial tension and form a cohesive viscoelastic coating/film around the droplets (Tcholakova et al. 2006). Electrostatic repulsive forces and steric interactions between neighboring droplets, and increases in continuous phase viscosity in the presence of proteins, all play a role in stabilizing oil-in-water emulsions (Lam and Nickerson 2013). Since emulsions are thermodynamically unstable, breakdown over time will occur based on several established mechanisms including: creaming, flocculation, coalescence and Ostwald ripening (Damodaran 2005; McClements 2007).
The use of plant proteins as emulsifiers has been underutilized by the food industry due to insufficient physicochemical information relating to their structure, and to their functionality in food matrices (McClements 2004). While soy is the current market standard for vegetable proteins, several legume proteins are becoming of interest as ingredients due to their nutritional value, renewability, availability, low cost and functionality. Can Karaca et al. (2011) investigated the emulsifying properties of chickpea, faba bean, lentil and pea protein isolates prepared by isoelectric precipitation and salt extraction, to find both the legume source and production method to influence their performance. Emulsion capacities for the various protein solutions at pH 7.0 ranged between 476 and 542 g oil/g protein, with the lentil proteins giving the highest measured values. The authors also reported emulsions formed using isoelectric-precipitated chickpea and lentil protein isolates produced the smallest droplets with relatively high surface charge leading to comparable creaming behavior and emulsification activity/stability indices as soy. The emulsifying properties of other legume proteins have also been explored, including pea (Ducel et al. 2004), lupin (Jayasena et al. 2010), broad bean and pea (Tsoukala et al. 2006).
The role of cross linking in improving emulsification has largely been unexplored as it relates to possible food applications. Tang et al. (2013) investigated the use of transglutaminase to set a soy protein stabilized emulsion to form a gel for increased stability. Herrero et al. (2011) also saw improved emulsion stability with the addition of transglutaminase to soy protein isolates. However the choice of fixatives for use in the food industry is quite restricted. Genipin is a novel naturally derived crosslinking agent obtained from the Gardenia fruit via enzymatic hydrolysis from its parent compound, geniposide, by β-glucosidase (Butler et al. 2003; Nickerson et al. 2006b). Historically, Gardenia fruits have been used in traditional Chinese medicine for their anti-inflammatory, diuretic and haemostatic properties (Butler et al. 2003). Although genipin has not received food-safe status in North America it is currently approved for use in food products in Japan, Korea and Taiwan (Nickerson et al. 2006c). Genipin forms inter- and intra-molecular covalent bonds with primary amine groups (e.g., lysine) so as to modify protein structure (Butler et al. 2003). Genipin has been extensively used to crosslink a variety of proteins including but not limited to: gelatin, bovine serum albumin (BSA), whey and gelatin-carrageenan mixtures (Butler et al. 2003; Annan et al. 2008; Huang et al. 2009; Devi and Maji 2010).
To the best of our knowledge, limited published information is available on the crosslinking potential of genipin as a means to improve protein-stabilized emulsions, and further on its affinity to various legume proteins (e.g., derived from chickpea, faba bean, lentil and soy). Hence, the overall goals of this research were to characterize the physicochemical properties of the aforementioned legume protein isolates, investigate their crosslinking ability with genipin, and determine the potential of genipin induced legume protein crosslinking as a means for improving the stability of protein-stabilized emulsions.
Materials and methods
Raw material
Chickpea (CDC Frontier), faba bean (SSNS) and green lentil (CDC Grandora) seeds used in this project were donated by the Crop Development Centre (Saskatoon, SK). Commercially defatted soy flour (Cargill: Prolia 200/70) was purchased from Cargill Limited (Winnipeg, MB). Genipin powder (98 % by HPLC) was purchased from Challenge Bioproducts Co., Ltd (Yun-Lin Hsien, Taiwan R.O.C.), whereas canola oil was purchased from Loblaw Companies Ltd. (Brampton, ON, Canada). All chemicals used in this study were purchased from VWR International (Mississauga, ON, Canada). All water used in this research, labeled as MQW, was produced from a Millipore Milli-Q™ water purification system (Millipore Corporation, Milford, MA, USA).
Legume isolate production and proximate analysis
Legume seeds were initially ground employing a bowl grinder (Cuisinart Mini-Prep Plus), followed by a fine grind (IKA A11 basic. IKA Works Inc., Wilmington, NC) to give flour. In the case of soy, commercially available defatted flour was used as the starting material. Legume flours from seed were defatted in hexane (L’Hocine et al. 2006) and then concentrated utilizing a modified isoelectric precipitation procedure (Mondor et al. 2009; Boye et al. 2010; Papalamprou et al. 2010). In brief, the defatted legume flour was dispersed in MQW at a 1 to 10 (w:v; flour:MQW) ratio, followed by pH adjustment to 9.0 with 1.0 M NaOH so as to facilitate protein dissolution. The resulting mixutre was stirred at 1,000 rpm (Ikamag Ret-G, IKA Labortechnik, Germany) for 1 h, and then centrifuged at 5,000 × g for 20 min at 4 °C (Sorvall RC6+; Thermo Fisher Scientific, Waltham, MA, USA). The supernatant was collected for later use, and the process was repeated with a 1 to 5 (w:v) pellet:MQW ratio. Supernatants from both extractions were pooled and adjusted to pH 4.6 with 1.0 M HCl so as to facilitate protein precipitation. The precipitate was collected following centrifugation (5,000 × g, 20 min, 4 °C); washed with 25 mL of MQW, frozen (−30 °C), and then freeze dried (Labconco FreeZone, Kansas City, MO) to yield a free flowing powder. Protein isolates were stored at 4 °C in sealed tubes for later use. The crude ash, lipid, moisture and protein (%N × 6.25 for chickpea, faba bean and lentil (Makri et al. 2006; Lee et al. 2007; Papalamprou et al. 2010); ×5.70 for soy (Kolakowski 2001) contents for each isolate were determined according to the Association of Official Analytical Chemists (AOAC 2003) methods: 923.03, 920.85, 925.10, and 920.87, respectively. The carbohydrate content was determined on the basis of percent differential from 100 %. All proximate analysis results were performed in triplicate for each protein isolate preparation.
Amino acid composition
The amino acid composition of each protein isolate was determined employing AOAC Official Methods 985.2 and 988.15 (Landry and Delhaye 1993; White et al. 1986; AOAC 2003). This work was conducted by POS Bio-Sciences Corp. (Saskatoon, SK). Briefly, to individual 20 × 150 mm screw cap Pyrex tubes was added 20 mg of legume protein isolate. To each tube was added 15.00 mL of 6 N HCl for total amino acids, or 10 M NaOH for tryptophan, followed by sample flushing with N2. Tubes were then capped and placed into an oven at 110 °C ± 0.5 °C for 20 h. Following acid digestion, the individual amino acids were quantified using high pressure liquid chromatography employing the pico-tag amino acid analysis system (Waters Corporation, Milford, MA). Sample amino acid concentration was normalized for each isolate based on its crude protein content.
Physicochemical properties
All protein isolates were prepared in MQW and were adjusted to pH 7.0 using 0.1 M NaOH and/or 0.1 M HCl (Accumet pH meter, Fisher Scientific, Waltham, MA, USA) followed by mechanical stirring at 1,000 rpm for 2 h at room temperature (22–23 °C) prior to testing, except where noted. All experiments were conducted with adjusted (based on the crude protein results for each isolate) protein concentrations on a weight basis. All results are reported as the mean ± one standard deviation (n = 3).
Protein solubility
Protein solubility was determined using the following modified (Morr et al. 1985) micro-Kjeldahl analysis protocol. To a protein content weight of 0.20 g for each protein isolate was added 18.00 g of MQW and the resulting suspension was adjusted to pH 7.0 with 0.1 N HCl and/or 0.1 N NaOH. Sample pH was monitored and maintained throughout a 1 h stirring (1,000 rpm) period at room temperature (22–23 °C). The total weight of the sample solution was brought to 20.00 g with MQW to give a final protein concentration of 1.00 % (w/w). The sample solution was then allowed to remain static for 10 min before being transferred to a 50 mL tube and centrifuged for 10 min at 7,200 rpm (Morr et al. 1985). A 5.00 g aliquot of the supernatant was taken for micro-Kjeldahl analysis (Labconco Micro Digester and Labconco Rapid Distillation Apparatus; Labconco Co., Kansas City, MO, USA). Protein solubility was determined by dividing the nitrogen content of the supernatant by the total nitrogen in the sample (×100 %).
Zeta potential
Overall surface charge of each protein isolate was determined by measuring electrophoretic mobility (UE) of prepared protein solutions at pH 7.0 using a Zetasizer Nano-ZS90 (Malvern Instruments, Westborough, MA, USA). The zeta potential (ζ) was determined from UE values employing the Henry equation:
| 1 |
where: ε is the permittivity, f(κα) is a function related to the ratio of particle radius (α) and the Debye length (κ), and η is the dispersion viscosity. For this work, the Smoluchowski approximation f(κα) of 1.5 was used. Protein solutions (0.05 %, w/v) were prepared for each legume isolate. A 1 mL syringe was used to inject an aliquot of the sample into the zetasizer sample cell. A refractive index (RI) of 1.450 was used for each protein sample, and water was used as the dispersant with a viscosity of 0.8872 cP; the RI was 1.330 and the dielectric constant was 78.5. An equilibrium time of 120 s was used for each analysis followed by 10–100 measurements until an acceptable standard deviation was reached, typically 10 measurements were required.
Surface hydrophobicity
Surface hydrophobicity for each legume isolate was determined using the fluorescent probe, 8-anilino-1-naphthalenesulfonic acid (ANS) (Kato and Nakai 1980) with modifications developed by Wang et al. (2005). Protein solutions (0.10 %, w/v) were prepared by dispersing the powder in 10 mM sodium phosphate buffer (pH 7.0) for 2 h using a magnetic stirrer (1,000 rpm). Each solution was subsequently diluted to obtain protein concentrations of 0.02 %, 0.04 %, 0.06 %, 0.08 % and 0.10 % (w/v). To 4 mL of each protein solution (0.02 %–0.10 %; w/v) was added 20 μL of 8 mM ANS solution (in 10 mM sodium phosphate buffer at pH 7.0) and the resulting solution was vortexed (Baxter Diagnostics Inc., Deerfield, IL, USA) at setting 10 for 10 s. Samples were then placed in the dark for 15 min. Fluorescent intensity (FI) was measured using a FluoroMax-4 Spectrofluorometer (HoribaJobin Yvon, Kyoto, Japan) with an excitation wavelength and slit width of 390 nm and 1 nm, respectively and an emission wavelength and slit width of 470 nm and 1 nm, respectively. FI measurements were also obtained for an ANS blank and protein blanks (without ANS) at each concentration. The FI values of these controls were both subtracted from the FI values of the ANS-protein samples. The initial slope of the plot of FI against % protein concentration was calculated by linear regression analysis and used as an index of average sample surface hydrophobicity.
Interfacial tension
The interfacial tensions between prepared protein isolate solutions (0.10 %; w/w) and canola oil was determined according to the Du Noüy ring method using a semi-automatic tensiometer (Lauda TD2, GmbH & Co., Lauda-Königshofen, Germany). This value was then compared to the interfacial tension between MQW and canola oil (without protein isolates). In this procedure, 40 mL of a prepared protein isolate solution was stirred overnight (16–18 h) at room temperature. To this solution was added 30 mL of canola oil and the interfacial tension between the two discontinuous phases was determined. Interfacial tension was calculated from the maximum force (Fmax) exerted on the ring as it was pulled through the interface using the following equation:
| 2 |
where, γ is the interfacial tension, R is the radius of the ring (9.55 mm), and β is a correction factor that is dependent on the dimensions of the ring and the density difference of the liquids used (in these experiments β = 0.1 g/cm3).
Protein crosslinking with genipin
Individual legume protein isolate solutions at a concentration of 0.10 % (w/w) were prepared in MQW. After stirring, genipin powder was added to each solution set to achieve final concentrations of: 2.5, 5.0, 7.5, and 10.0 mM. The resulting solutions were stirred (1,000 rpm) for 1 h at room temperature and then allowed to crosslink statically for 24 h. An aliquot of each solution was removed and sample absorbance at 288 nm was measured using a UV/Vis Spectrophotometer (Optizen 2120UV. Mecasys Co. Ltd, Korea.). Blanks consisting of each legume protein isolate solution (0.10 % w/v) without added genipin were run in conjunction with all sample sets. The initial slope of the plot of absorbance at 288 nm versus genipin concentration was calculated by linear regression analysis and was used as an index of the average genipin induced crosslinking of the legume protein isolates.
Creaming stability
Legume protein isolate solutions were prepared with (10.0 mM) and without genipin to determine the impact of crosslinking on creaming stability. Oil in water emulsions (10.0 mL) were prepared by homogenizing 5.0 mL of prepared protein solution (0.50 % w/w) with 5.0 mL of canola oil at 13,000 rpm for 5 min using a homogenizer (Polytron® MR PT 2100, Kinematica Inc. Bohemia, NY, USA). Immediately after preparation, emulsions were transferred to a 10 mL sealed graduated glass cylinder and subsequent sample separation into an opaque cream layer (top) and a turbid aqueous layer (bottom) after 24 h of static treatment at room temperature was determined. Percent creaming stability (CS) was calculated using the following equation:
| 3 |
where, VB is the volume of the aqueous protein solution (5.0 mL) before emulsification and VA is the volume of the turbid aqueous layer that has ‘fallen out’ of the emulsion after 24 h.
Statistical analysis
All sample data are reported as the mean ± one standard deviation. A one way analysis of variance (ANOVA) with a Scheffe post-hoc test was used to determine statistical differences between the various protein-types, as it related to protein levels within the isolate (proximate analysis) and physicochemical properties (solubility, surface charge, surface hydrophobicity and interfacial tension). For creaming stability, a two-way analysis of variance was performed to test the effect of protein-type and genipin. A Pearson comparison and a general linear model with backwise stepwise regression were used to determine the relationship between the physicochemical properties of legume protein isolates (without genipin) and creaming stability. All statistical analyses were performed using Systat 10.0 software (Systat Software, Inc. Chicago, IL).
Results and discussion
Composition of legume protein isolates
Protein isolates were prepared from raw chickpeas, faba beans and lentils as well as defatted soy flour using isoelectric precipitation. Proximate compositions of the resulting isolates are shown in Table 1. Protein content (on a wet weight basis (w.b.)) was determined to be ~85.8 %, ~86.3 %, ~83.8 % and ~90.9 % for CPI, FPI, LPI and SPI, respectively, however differences between isolates were not significant (p > 0.05). As there is no universal scheme for classifying legume protein products, all materials were deemed to be an ‘isolate’ rather than a concentrate in the present study. In the case of soy, Pearson (1983) developed criteria requiring a minimum protein content of 85 % on a dry weight basis (6.25 nitrogen conversion factor) to be classified as an isolate. When protein levels were converted from a wet to dry basis in the present study, the legume protein levels were ~87.6 %, ~89.8 %, ~91.6 %, and ~97.1 % (dry weight basis) for CPI, FPI, LPI and SPI, respectively. The isoelectric precipitation method for protein extraction typically involves first hydrating defatted flour at alkali pH (9.0) to solubilize the proteins, followed by centrifugation to remove insoluble matter (e.g., fibre, carbohydrates), followed by pH adjustment to near the legume protein’s isoelectric point (pI) to induce precipitation. At the pI (~4.5–5.0), legume proteins assume a net neutral charge and tend to aggregate and fall out of solution. Isoelectric precipitation typically yields mainly globulin proteins (Papalamprou et al. 2010), whereas other extraction methods, such as salt extraction, yield isolates comprised of a mixture of globulins and albumins (Liu et al. 2008). Protein levels in the present study were comparable to others found in literature. For example, Can Karaca et al. (2011) using similar legumes and a similar extraction method reported protein levels of ~85.4 %, ~84.1 %, ~81.9 %, and ~87.6 % for CPI, FPI, LPI and SPI respectively. In addition, protein levels on a dry weight basis of ~90.2 % and ~78.0 % have been reported for LPI (Joshi et al. 2012) and CPI (Sánchez-Vioque et al. 1999) respectively, when prepared using similar isoelectric precipitation extraction procedures.
Table 1.
Proximate composition of chickpea (CPI), faba bean (FPI), lentil (LPI) and soy (SPI) protein isolates. Carbohydrate levels were determined based on the percent difference from 100 %. Data represents the mean ± one standard deviation (n = 3)
| Sample | Protein (%, w.b.) |
Moisture (%) |
Lipid (%, w.b.) |
Ash (%, w.b.) |
Carbohydrate (%, w.b.) |
|---|---|---|---|---|---|
| CPI | 85.76 ± 0.26 | 2.39 ± 0.00 | 0.83 ± 0.04 | 4.41 ± 3.64 | 6.89 |
| FPI | 86.30 ± 1.26 | 3.85 ± 0.05 | 0.00 ± 0.00 | 3.89 ± 1.35 | 5.96 |
| LPI | 83.81 ± 1.32 | 8.48 ± 0.05 | 0.77 ± 0.01 | 3.83 ± 1.27 | 3.11 |
| SPI | 90.86 ± 5.20 | 6.41 ± 0.01 | 0.00 ± 0.08 | 2.19 ± 0.07 | 0.54 |
Proximate analysis revealed very low lipid levels (≤0.83 %) within the isolates due to the defatting procedure. These low lipid levels are not expected to hinder the dispersion of isolates in solution (MQW) during physicochemical testing. Removal of lipids prior to the extraction process helps reduce protein-lipid interactions from occurring, which would inhibit dissolution of proteins and therefore limit isolation (Leyva-Lopez et al. 1995). Moisture levels for isolates were found to be ~2.4 %, ~3.9 %, ~6.4 % and ~8.5 % for CPI, FPI, SPI and LPI, respectively, reflecting either the efficiency of the freeze drying process or the relative strength of protein-water interactions (Table 1). Ash contents of ~2.2 %, ~3.8 %, ~3.9 % and ~4.4 % (w.b.) for SPI, LPI, FPI and CPI respectively and carbohydrate levels of ~0.5 %, ~3.1 %, ~6.0 %, and ~6.9 % (w.b.), by differentiation from 100 %, for SPI, LPI, FPI and CPI respectively were determined for these materials (Table 1). Select proximate analysis literature values for legume protein isolates produced by similar isoelectric precipitation procedure are: ~78.0 %, ~3.3 %, 3.5 %, ~2.9 % and ~11.8 % for protein, moisture, lipid, ash and carbohydrate (by difference), respectively on a dry weight basis for CPI (Sánchez-Vioque et al. 1999); ~90 %, ~4.0 %, ~0.0 %, ~6.0 % and ~4.2 % for protein (%N × 6.25), moisture, crude fat, ash and carbohydrate, respectively for a winged bean protein isolate; and ~97.0 %, ~4.7 %, ~0.0 %, ~3.4 % and ~0.0 %, for protein, moisture, crude fat, ash and carbohydrate, respectively for an industrially produced SPI.
Amino acid profiles for each protein isolate (normalized to 100 % based on the protein content of each sample) are given in Table 2. Lysine content is of particular importance because of its reactivity with the genipin, the natural crosslinking agent used in this study (Butler et al. 2003; Nickerson et al. 2006a, b; Maji and Hussain 2009). Lysine contents for the CPI, FPI, LPI and SPI products were found to be ~6.3 %, ~6.0 %, ~6.8 % and ~5.7 % respectively. Similar lysine contents should correspond to similar crosslinking potential with genipin, however, within a MQW in oil emulsion setting, lysine residue exposure to genipin (within the aqueous phase) may be altered as proteins unfold and re-orientate at the oil–water interface (McClements 2004; Damodaran 2005). Lysine levels are comparable to those reported for protein isolates in literature. For example, Vioque et al. (2012) reported a ~7.0 % lysine level for a FPI prepared by isoelectric precipitation, and Okezie and Bello (1988) reported that an industrially produced SPI had a lysine content of 6.1 %. In addition, Iqbal et al. (2006) report lysine contents (adjusted on the basis of protein content) for four legumes with values of: ~7.2 % (chickpea), ~7.5 % (cowpea), ~7.0 % (lentil) and ~8.1 % (green pea).
Table 2.
Normalized amino acid profiles (%) of chickpea (CPI), faba bean (FPI), lentil (LPI) and soy (SPI) protein isolates
| Amino acid | CPI | FPI | LPI | SPI |
|---|---|---|---|---|
| Phenylalanine | 6.40 | 4.42 | 5.70 | 5.02 |
| Isoleucine | 4.28 | 4.38 | 4.82 | 4.20 |
| Tryptophan | 0.83 | 0.91 | 0.83 | 1.29 |
| Leucine | 7.76 | 7.82 | 8.19 | 7.11 |
| Valine | 4.22 | 4.64 | 4.96 | 3.93 |
| Methionine | 1.50 | 0.80 | 0.95 | 1.31 |
| Tyrosine | 2.99 | 3.88 | 3.79 | 3.54 |
| Cysteine | 1.17 | 0.96 | 0.77 | 1.31 |
| Alanine | 3.85 | 3.99 | 4.03 | 3.23 |
| Threonine | 3.34 | 3.81 | 3.77 | 3.57 |
| Histidine | 3.04 | 3.09 | 2.90 | 2.95 |
| Glycine | 3.63 | 4.12 | 3.84 | 3.76 |
| Serine | 6.88 | 6.77 | 6.97 | 6.46 |
| Arginine | 9.51 | 9.72 | 8.76 | 7.81 |
| Lysine | 6.31 | 5.95 | 6.75 | 5.68 |
| (Glutamic acid + Glutamine) | 16.68 | 17.59 | 16.45 | 20.82 |
| Proline | 4.27 | 4.52 | 4.24 | 4.98 |
| (Aspartic acid + Aspargine) | 13.34 | 12.63 | 12.28 | 13.02 |
| 100 | 100 | 100 | 100 |
Physicochemical properties of legume protein isolates
Surface characteristics
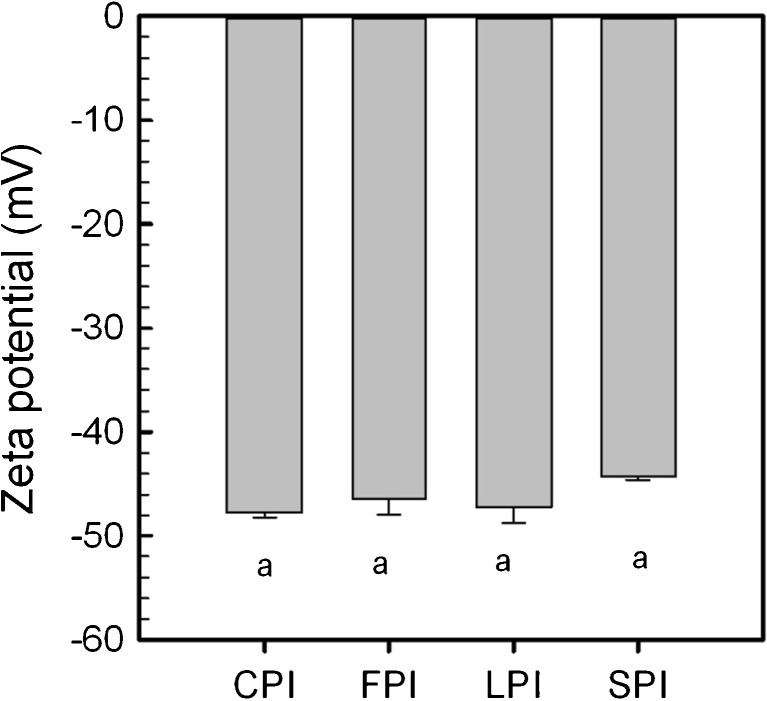
The surface charge or zeta potential values for CPI, FPI, LPI and SPI products at pH 7.0 are shown in Fig. 1. An analysis of variance showed that all isolates were statistically similar (p > 0.05), at −47.7, −46.4, −47.2 and −44.3 mV for CPI, FPI, LPI and SPI, respectively. Proteins carry a net negative charge at pH 7.0, as all are above their isoelectric point (where zeta potential is 0 mV). The net negative charge at pH 7.0 arises primarily from the negatively charged R groups found on the aspartate (pKR = 3.65) and glutamate (pKR = 4.25) amino acids spatially located on the protein surface (Nelson and Cox 2005). Can Karaca et al. (2011) and Tang and Sun (2011) reported the isoelectric point of legume globulin proteins to be approximately at pH 4.5. Surface charge values from this study were similar to those reported in literature. For example, Joshi et al. (2012) reported the surface charge of LPI at pH 7.0 to be −43.3 mV compared to values of ~ −55 mV for WPI and BSA. In addition, Tang and Sun (2011) reported zeta potential values at pH 7.0 of ~ −40 mV for legume vicilin proteins isolated from kidney, red and mung beans. Having a high protein surface charge is important during the formation of emulsions, as it promotes protein solubility (caused by electrostatic repulsion between negatively charged proteins), promotes greater hydration of proteins in solution (or protein-water interactions) and migration to the oil–water interface (Schwenke 2001; McClements 2004; Damodaran 2005). High surface charges also play a role in maintaining emulsion stability, as they induce an electric charge (dependant on pH) to the viscoelastic film surrounding the discontinuous droplets. A charged emulsion droplet surface repels others, with a similar charge, to inhibit coalescence and flocculation (mechanisms for instability) (McClements 2004; Damodaran 2005).
Fig. 1.
Zeta potential (mV) of chickpea (CPI), faba bean (FPI), lentil (LPI) and soy (SPI) protein isolates at pH 7.0. Data represent the mean ± one standard deviation (n = 3). Data with same letters signifies no statistical differences (p > 0.05)
Fluorescence spectroscopy can be employed as a sensitive tool for protein analysis (e.g., structural changes, folding, aggregation, surface hydrophobicity) based on the intrinsic protein fluorescence from the amino acid tryptophan (and tyrosine to a lesser extent), and through the extrinsic fluorescence from fluorescent dyes such as ANS. The interactions of extrinsic fluorescent dyes with proteins leads to changes in fluorescence after excitation, which is the basis of protein characterisation by this method (Hawe et al. 2008). ANS has very low fluorescence in aqueous solutions, but becomes highly fluorescent when adsorbed onto hydrophobic binding sites spatially distributed on protein surfaces. Ion pairing between the negatively charged sulfonate groups of ANS and positively charged amino acids (histidine, lysine and arginine) also plays a role in dye adsorption to the protein surface. When absorption of light excites electrons of the dye molecule, there are several mechanisms including, but not limited to: vibrational relaxation, solvent relaxation and fluorescence to return electrons to their ground state. In the case of fluorescence, emission occurs when electrons fall from the lowest vibrational state to the ground state (Hawe et al. 2008). The magnitude of fluorescence can be influenced by solvent polarity, viscosity and temperature, or processing factors that impact the proteins conformation and exposure of buried hydrophobic groups.
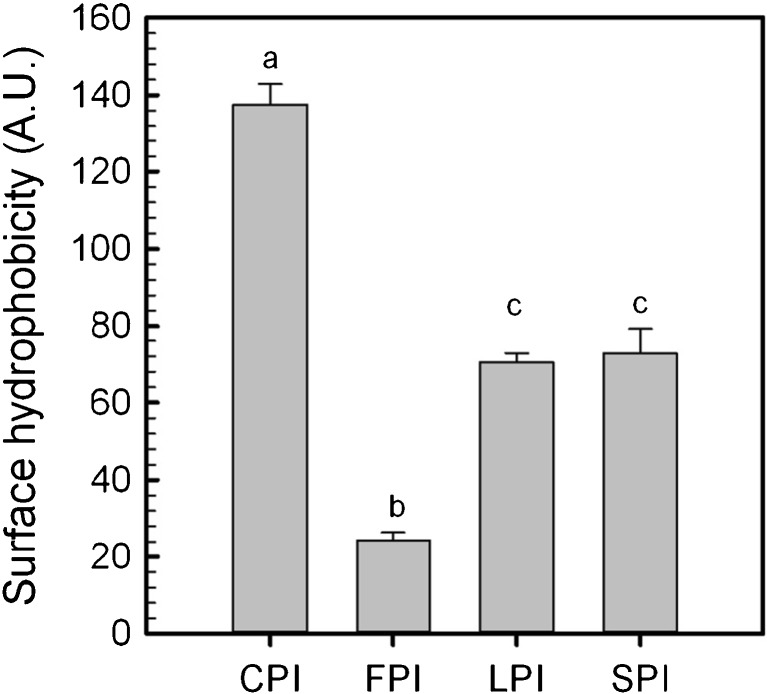
The average surface hydrophobicity was determined by the ANS fluorescent probe binding method for CPI, FPI, LPI and SPI products at pH 7.0 and is given in Fig. 2. An analysis of variance indicated that CPI was significantly higher (~137.5 arbitrary units, A.U.) (p < 0.05) than the other isolates, followed by SPI (~72.8 A.U.) and LPI (~70.4 A.U.) which were similar in magnitude (p < 0.05), and then FPI (~24.4 A.U.) (p < 0.05). Surface hydrophobicity values were different from those reported in literature for these particular legume proteins (potentially due to the use of different protein concentrations in generating the slope), but Can Karaca et al. (2011) reported the same pattern of decreasing hydrophobicity with: CPI > LPI = SPI > FPI. However, Tang and Sun (2011) report a similar surface hydrophobicity magnitude, as determined in this study, for vicilin isolates produced from kidney, red and mung beans of between ~149 and ~259 AU. The low hydrophobicity of FPI found in the present study relative to the other legume proteins examined is thought to reflect both protein conformation in solution and their aggregation behavior, resulting in less of the hydrophobic moieties being exposed.
Fig. 2.
Average surface hydrophobicity (arbitrary units, A.U.) of chickpea (CPI), faba bean (FPI), lentil (LPI) and soy (SPI) protein isolates at pH 7.0. Data represent the mean ± one standard deviation (n = 3). Data with same letters signifies no statistical differences (p > 0.05)
A protein with high surface hydrophobicity can readily align at the oil–water interface, and re-orient itself such that its hydrophobic moieties position themselves towards the oil phase and the hydrophilic moieties towards the aqueous phase (Schwenke 2001; Damodaran 2005). Depending on its amino acid sequence and the level of folding/unfolding of a protein at the interface, various loops or tails can develop in which sections of the protein extend from the surface of the droplet into the continuous phase creating a steric hindrance which reduces the likelihood of aggregation, flocculation and coalescence between neighboring droplets. (Schwenke 2001; McClements 2004; Damodaran 2005). The surface characteristics of a protein, within an emulsion, are reliant on the extent of protein interaction with both its dispersive solvent/phase, and with the interface. Accessible surface area, unfolding and re-orientation at the interface may explain how protein isolates with different physicochemical properties may exhibit similar emulsification properties and vice versa (Schwenke 2001).
Protein solubility
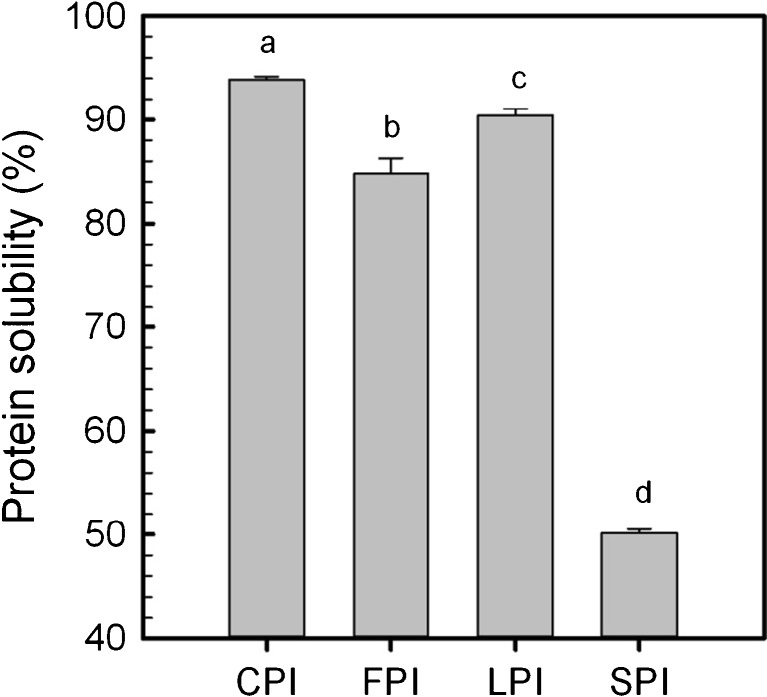
Percent protein solubility at pH 7.0 was determined for all legume protein isolates (Fig. 3). An analysis of variance revealed that all isolates displayed significantly different (p < 0.05) solubility values, which were found to be the highest for CPI (~94 %), followed by LPI (~90 %), FPI (~85 %) and SPI (~50 %). Similar solubility for legume protein isolates prepared by isoelectric precipitation has been reported in literature for CPI (>80 %) by Sánchez-Vioque et al. (1999), and for CPI, FPI and LPI (all >80 %) by Carbonaro et al. (1997). Solubility is mediated by the balance of protein-protein and protein-solvent (aqueous phase) interactions, the latter promoting solubility, which can further be influenced by environmental factors (e.g., temperature, pH, ionic strength) (McClements 2007; Can Karaca et al. 2011) and by processing (e.g., extraction or post-extraction treatments) (Kinsella 1979). High surface charges are important for fostering sufficient electrostatic repulsion between proteins, such that they can overcome electrostatic and van der Waals attractive forces, to remain dispersed in solution. In the present study, SPI displayed the lowest surface charge (~ − 44 mV) relative to the other three isolates (Fig. 1), which may have contributed to its reduced solubility. However, solubility of proteins is influenced by other factors, such as salts, conformation, pH, level of association/disassociation and hydrophobicity (Carbonaro et al. 1997; McClements 2004; Damodaran 2005). In general, proteins that have higher surface hydrophobicity tend to be negatively correlated to solubility (Can Karaca et al. 2011), however in the present study, CPI showed both the highest solubility and surface hydrophobicity which reflects the complexity behind fully understanding the structure-function mechanism related to solubility. This could be a factor not of the average surface characteristics (e.g., charge vs. hydrophobicity), but rather of the apparent surface characteristics, which are influenced by the frequency and distribution of charges on the folded protein surface (Schwenke 2001; Tang and Sun 2011).
Fig. 3.
Protein solubility (%) of chickpea (CPI), faba bean (FPI), lentil (LPI) and soy (SPI) protein isolates at pH 7.0. Data represent the mean ± one standard deviation (n = 3). Data with same letters signifies no statistical differences (p > 0.05)
Interfacial properties
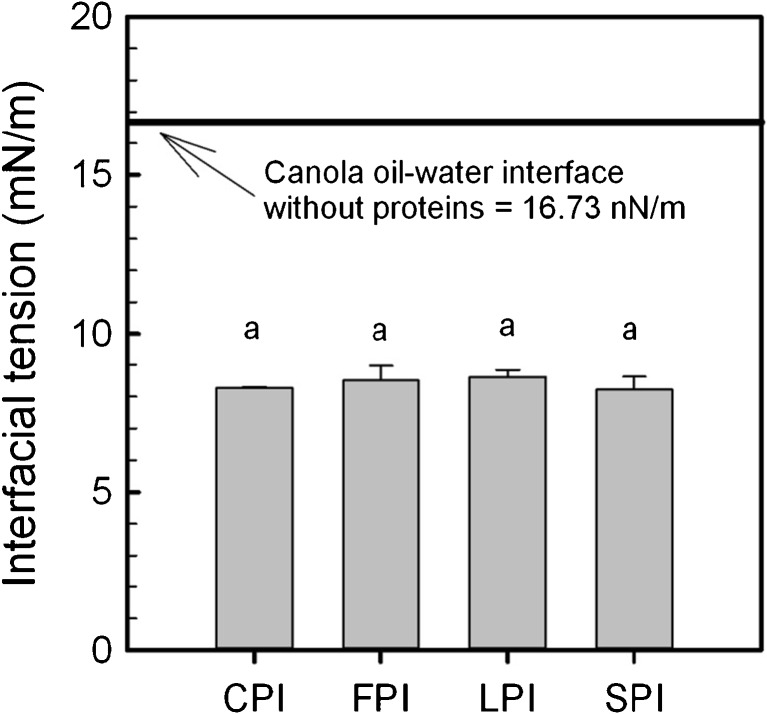
During emulsion formation, proteins migrate to the oil–water interface and re-align to allow positioning of hydrophobic groups towards the oil phase and hydrophilic groups towards the aqueous phase, followed by the formation of a viscoelastic film that resists flocculation or coalescence through electrostatic repulsive forces (depending on the pH) and steric stabilization (Schwenke 2001; McClements 2004; Damodaran 2005; Joshi et al. 2012). The ability of a protein to align at the interface can be described by its ability to reduce interfacial tension between oil and water phases. Interfacial tension is a measurement of the force (i.e. energy) required to move a probe (e.g., du Nöuy ring) through an interface (Can Karaca et al. 2011). Its ability to reduce this tension will enable smaller emulsion droplets to form, to give a more stable emulsion (Damodaran 2005). In the present study, interfacial tension was measured through a MQW-canola oil interface for CPI, FPI, LPI and SPI products at pH 7.0, and is shown in Fig. 4. Each of the four legume protein isolates, when added to the aqueous phase, were shown to reduce the interfacial tension of a MQW-canola oil interface from 16.73 mN/m to values ranging from 8.23 to 8.62 mN/m (Fig. 4), however no difference in interfacial reduction was found regardless of protein type (p > 0.05). Values were similar to those reported in the literature for a protein induced reduction of interfacial tension between water and oil, although materials and/or methods differed. For example, Joshi et al. (2012) report the interfacial tension, as measured by the pendant drop method for an olive oil–water mixture with LPI added at 10 mg/mL to be ~12 mN/m, reduced from ~22 mN/m without protein. This was reported to be typical for other non-legume globular proteins at an oil–water interface and the reduction of ~10 mN/m is comparable to the ~8.3 mN/m reduction caused by the addition of legume protein isolates in this study. Can Karaca et al. (2011) also reported a similar magnitude in the reduction of interfacial tension (~6.1 mN/m) at a flaxseed oil–water interface with the inclusion of CPI, FPI, LPI and SPI (0.25 % w/w), when compared to water–oil alone.
Fig. 4.
Interfacial tension (mN/m) of chickpea (CPI), faba bean (FPI), lentil (LPI) and soy (SPI) protein isolates at pH 7.0 for a MQW-canola oil interface. Data represent the mean ± one standard deviation (n = 3). Data with similar letters signifies no statistical differences (p > 0.05)
Affinity of genipin to the legume protein isolates
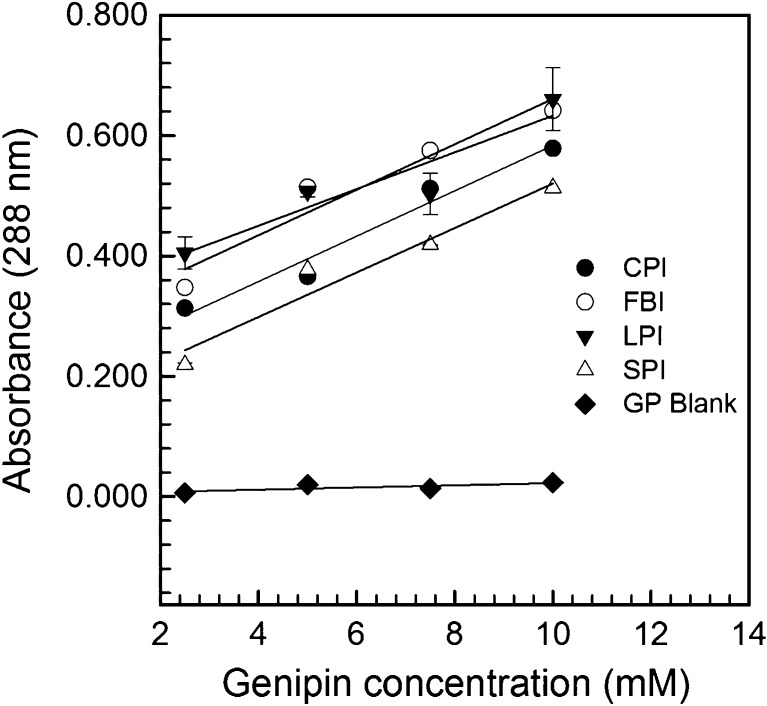
Genipin is considered a novel, non-toxic covalent crosslinking agent extracted from Gardenia fruit (Sung et al. 1999; Mi et al. 2000; Butler et al. 2003; Nickerson et al. 2006b). Researchers have used genipin to crosslink a variety of materials including, but not limited to: bovine serum albumin (BSA), chitosan, gelatin, gelatin-carrageenan mixtures, soy proteins and whey proteins for purposes including: wound dressings, hydrogels and micro/nanoparticles and films (Butler et al. 2003; Annan et al. 2008; Huang et al. 2009; Devi and Maji 2010). To our knowledge, there has been little work on its affinity to legume proteins and potential for use in stabilizing emulsions. To investigate the affinity of genipin to our legume protein isolates absorbance was read at 288 nm, using a spectrophotometer as a function of genipin concentration. A linear increase (R2 range of 0.870–0.967) was found as genipin concentrations increased from 2.5 to 10 mM when in the presence of the proteins (Fig. 5). As such, the slopes were taken as an index of legume protein isolate-genipin crosslinking affinity. Slopes for CPI, FPI, LPI and SPI-genipin reactions were determined to be similar at 0.0369, 0.0377, 0.0305 and 0.0378, respectively. The differences observed in the spectroscopic results for the four legume protein isolates may be explained by differences in the spatial arrangement and surface exposure of lysine groups, as well as protein flexibility. Spectrophotometric results have been used previously to measure polymer crosslinking with genipin. For example, Butler et al. (2003) found that an absorbance peak developed at 605 nm in a glucosamine-genipin mixture, which increased in intensity as a function of reaction time. The authors also reported the development of peaks at 240 and 280 nm in chitosan:genipin mixtures, which they related to polymer crosslinking.
Fig. 5.
Absorbance at 288 nm of mixtures of chickpea (CPI), faba bean (FPI), lentil (LPI) and soy (SPI) protein isolates, as well as a genipin (GP)-MQW blank, as a function of genipin concentration at pH 7.0. Data represent the mean ± one standard deviation (n = 3)
The proposed mechanism for protein-genipin crosslinking involves the following two reactions: (1) a nucleophilic substitution to the dihydropyran ring of genipin followed by a Schiff’s base reaction; and (2) a separate Schiff’s base reaction with the ester group of the genipin molecule. For both reactions, a primary amine group from the protein is required for crosslinking with genipin (Butler et al. 2003). The first reaction is initiated by nucleophilic attack at C3 of genipin by a primary amine group on the protein that results in dihydropyran ring opening and formation of an aldehyde group and secondary amine. The ring then closes as the secondary amine reacts with the aldehyde group, to form a heterocyclic ring bound to a protein molecule (Butler et al. 2003). The second reaction is a SN2 nucleophilic attack at the ester group on the genipin molecule by a primary amine on the protein so as to produce an amide linkage. The evidence for these two reaction mechanisms was based on 13C nuclear magnetic resonance (NMR), infrared spectroscopy (IR) and rheological data collected during genipin crosslinking experiments with chitosan, BSA, gelatin and glucosamine (Butler et al. 2003).
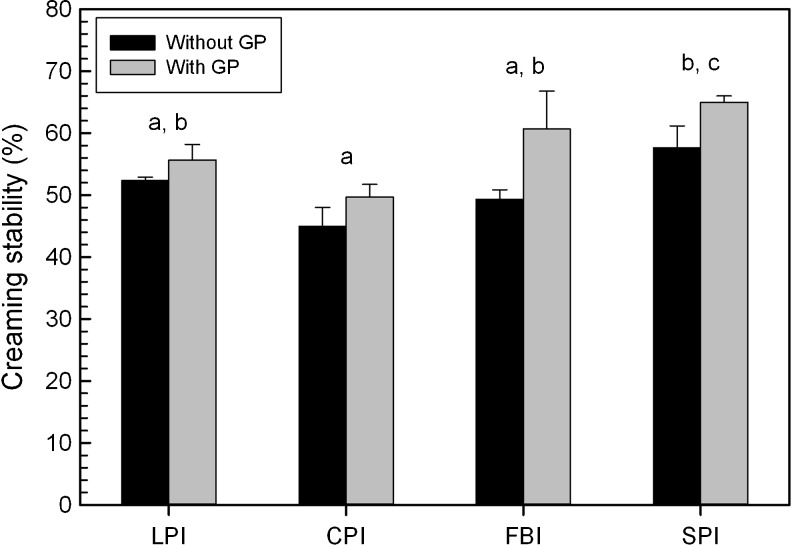
Creaming stability
The creaming stability of MQW–canola oil emulsions, stabilized with CPI, LPI, FPI and SPI were investigated over a 24 h period with and without genipin at pH 7.0, and are given in Fig. 6. A two-way analysis of variance revealed that overall only the main effects of protein-type and genipin (p < 0.001) were significant, whereas their interaction was not (p > 0.05). Overall, protein stabilized emulsions prepared with genipin showed increased stability (57.7 %) relative to those without (51.08 %). Emulsion stability was also found to be significantly higher for soy (~61.3 %), followed by FPI (~55.0 %) and LPI (~54.0 %), and then CPI (~47.3 %) (Fig. 6). When relating the physicochemical results to creaming stability (without genipin) using the Pearson correlation, it was found that only solubility was (negatively) correlated (r = −0.795, p < 0.01). A backward stepwise regression model, which was able to explain 63.2 % of data variability, found a similar conclusion where only solubility was a significant factor (F = 17.142, p < 0.01):
| 4 |
Fig. 6.
Creaming stability (%) of chickpea (CPI), faba bean (FPI), lentil (LPI) and soy (SPI) protein-stabilized canola oil-MQW emulsions at pH 7.0, with and without 10 mM genipin (GP) after 24 h. Data represent the mean ± one standard deviation (n = 3). Data with same letters signifies no statistical differences (p > 0.05) for legume proteins for only the main effect of protein type. The effect of genipin was also significant, however was not denoted by letters in the figure, whereas the interaction term was deemed not to be significant
Overall, only solubility was found to be significantly (p < 0.01) correlated with creaming stability. Unexplained variance may be due to protein source. Reduced solubility associated with the SPI may reflect the formation of a more cohesive viscoelastic film at the interface, and a more viscous continuous phase, which will inhibit the rate of creaming. In contrast, more soluble proteins may remain in the bulk solution, with less aggregation occurring at the interface. The effect on continuous phase viscosity will also be less if the protein remained in solution.
Creaming stability refers to the ability of a protein stabilized emulsion to resist creaming, where oil droplets flocculate and coalescence, then migrate upwards due to the density difference from MQW (Damodaran 2005; Liu et al. 2010; Can Karaca et al. 2011). The ability of an emulsion to resist creaming is largely dependent on droplet size and density contrast between phases (McClements 2007). In the present study, it was hypothesized that legume proteins acted to stabilize the emulsions by first migrating to, and then re-aligning at the canola oil-MQW interface to form a viscoelastic film during emulsion formation. This film maintains droplet size by resisting flocculation and coalescence through electrostatic repulsive forces (negative zeta potential) and steric hindrance, and in the presence of genipin, was presumed to become stronger and more resistance to punctures, etc. (Damodaran 2005; McClements 2007).
Conclusions
Overall, legume protein isolates were able to act as emulsifiers due to their amphiphilic nature (i.e., having both hydrophobic and hydrophilic moieties) to stabilize MQW-canola oil emulsions, which was enhanced in the presence of genipin, and had similar affinity to all of the legume proteins tested. However, a full understanding of the impact of the protein’s physicochemical properties on emulsion stability was difficult, reflecting the complexity of the system. To be an effective emulsifier, the protein needs to have sufficient surface charge to remain soluble in solution, such that it can migrate to the oil–water interface, and have enough surface hydrophobicity to align and re-orient once there (Schwenke 2001; Damodaran 2005). Based on these criteria, it would have been presumed that CPI would give the best emulsion stability based on its high solubility and surface hydrophobicity relative to the other proteins. However, CPI displayed the lowest stability (under the experimental conditions used) of the four legume protein isolates used in this study. In this study, genipin is thought to covalently crosslink the exposed lysine groups of proteins positioned at the oil–water interface so as to strengthen the formed viscoelastic film. In the case of genipin, crosslinking is not instantaneous and most likely occurs after the emulsions are protein stabilized. Further, it is hypothesized that genipin will induce crosslinking with neighboring proteins remaining in the bulk solution so as to increase the continuous phase viscosity that leads to enhanced emulsion stability. The latter is not thought to be substantial since bridging flocculation did not arise, which would cause emulsion instability relative to those without genipin. To better understand structure-function-mechanisms in legume protein stabilized emulsions, further studies on the role of protein characteristics (e.g., conformation, concentration and level of denaturation), solvent effects (e.g., pH, temperature, salts), processing factors (e.g., homogenization rates and duration), emulsion characteristics (e.g.., oil–water ratio, droplet size) and genipin-legume protein crosslinking studies are needed.
Acknowledgments
The authors gratefully acknowledge the financial support provided by the Saskatchewan Agriculture Development Fund.
Conflict of interest
None.
References
- Annan NT, Borza AD, Truelstrup Hansen L. Encapsulation in alginate-coated gelatin microspheres improves survival of the probiotic Bifidobacterium adolescentis 15703 T during exposure to simulated gastro-intestinal conditions. Food Res Int. 2008;41(2):184–193. doi: 10.1016/j.foodres.2007.11.001. [DOI] [Google Scholar]
- AOAC. (2003). Official Method of Analysis, 17th Edn. Washington, DC: Association of Official Analytical Chemists, Methods 920.85, 920.87, 923.03, 925.10, 985.2 and 988.15
- Bos MA, van Vliet T. Interfacial rheological properties of adsorbed protein layers and surfactants: a review. Adv Colloid Int Sci. 2001;91(3):437–471. doi: 10.1016/S0001-8686(00)00077-4. [DOI] [PubMed] [Google Scholar]
- Boye JI, Aksay S, Roufik S, Ribéreau S, Mondor M, Farnworth E, Rajamohamed SH. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res Int. 2010;43(10):537–546. doi: 10.1016/j.foodres.2009.07.021. [DOI] [Google Scholar]
- Butler MF, Ng YF, Pudney PDA. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J Polym Sci Part A: Polym Chem. 2003;41(24):3941–3953. doi: 10.1002/pola.10960. [DOI] [Google Scholar]
- Can Karaca A, Low N, Nickerson M. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res Int. 2011;44(9):2742–2750. doi: 10.1016/j.foodres.2011.06.012. [DOI] [Google Scholar]
- Carbonaro M, Cappelloni M, Nicoli S, Lucarini M, Carnovale E. Solubility-digestibility relationship of legume proteins. J Agric Food Chem. 1997;45(9):3387–3394. doi: 10.1021/jf970070y. [DOI] [Google Scholar]
- Damodaran S. Protein stabilization of emulsions and foams. J Food Sci. 2005;70(3):54–66. doi: 10.1111/j.1365-2621.2005.tb07150.x. [DOI] [Google Scholar]
- Devi N, Maji TK. Microencapsulation of isoniazid in genipin-crosslinked gelatin-A–k-carrageenan polyelectrolyte complex. Drug Dev Ind Pharm. 2010;36(1):56–63. doi: 10.3109/03639040903061355. [DOI] [PubMed] [Google Scholar]
- Ducel V, Richard J, Popineau Y, Boury F. Adsorption kinetics and rheological interfacial properties of plant proteins at the oil–water interface. Biomacromolecules. 2004;5(6):2088–2093. doi: 10.1021/bm049739h. [DOI] [PubMed] [Google Scholar]
- Friberg SE, Larsson K. Food emulsions. 3. New York: Marcel Dekker; 1997. [Google Scholar]
- Hawe A, Sutter M, Jiskoot W. Extrinsic fluorescent dyes as tools for protein characterization. Pharm Res. 2008;25(7):1487–1499. doi: 10.1007/s11095-007-9516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero AM, Carmona P, Pintado T, Jiménez-Colmenero F, Ruíz-Capillas C. Infrared spectroscopic analysis of structural features and interactions in olive oil-in-water emulsions stabilized with soy protein. Food Res Int. 2011;44(1):360–366. doi: 10.1016/j.foodres.2010.10.006. [DOI] [Google Scholar]
- Huang KS, Lu K, Yeh CS, Chung SR, Lin CH, Yang CH, Dong YS. Microfluidic controlling monodisperse microdroplet for 5-fluorouracil loaded genipin-gelatin microcapsules. J Control Release. 2009;137(1):15–19. doi: 10.1016/j.jconrel.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Iqbal A, Khalil IA, Ateeq N, Khan MS. Nutritional quality of important food legumes. Food Chem. 2006;97(2):331–335. doi: 10.1016/j.foodchem.2005.05.011. [DOI] [Google Scholar]
- Jayasena V, Chih HJ, Nasar-Abbas SM. Functional properties of sweet lupin protein isolated and tested at various pH levels. Res J Agric Biol Sci. 2010;6(2):130–137. [Google Scholar]
- Joshi M, Adhikari B, Aldred P, Panozzo JF, Kasapis S, Barrow CJ. Interfacial and emulsifying properties of lentil protein isolate. Food Chem. 2012;134(3):1343–1353. doi: 10.1016/j.foodchem.2012.03.029. [DOI] [PubMed] [Google Scholar]
- Kato A, Nakai S. Hydrophobicity determined by fluorescence probe methods and its correlation with surface properties of proteins. Biochim Biophys Acta. 1980;624(1):13–20. doi: 10.1016/0005-2795(80)90220-2. [DOI] [PubMed] [Google Scholar]
- Kinsella JE. Functional properties of soy proteins. J Am Oil Chem Soc. 1979;56(3):242–258. doi: 10.1007/BF02671468. [DOI] [Google Scholar]
- Kolakowski E. Protein determination and analysis in food systems. In: Sikorski ZE, editor. Chemical and functional properties of food proteins. Boca Raton: CRC Press; 2001. pp. 57–112. [Google Scholar]
- L’Hocine L, Boye JI, Arcand Y. Composition and functional properties of soy protein isolates prepared using alternative defatting and extraction procedures. J Food Sci C-Food Chem Toxicol. 2006;71(3):C137–C145. [Google Scholar]
- Lam RSH, Nickerson MT. Food proteins: a review on their emulsifying properties using a structure-function approach. Food Chem. 2013;141(2):975–984. doi: 10.1016/j.foodchem.2013.04.038. [DOI] [PubMed] [Google Scholar]
- Landry J, Delhaye S. Determination of tryptophan in feedstuffs: comparison of sodium hydroxide and barium hydroxide as hydrolysing agents. Food Chem. 1993;49(1):95–97. doi: 10.1016/0308-8146(94)90238-0. [DOI] [Google Scholar]
- Lee HC, Htoon AK, Paterson JL. Alkaline extraction of starch from Australian lentil cultivars Matilda and Digger optimised for starch yield and starch and protein quality. Food Chem. 2007;102(3):551–559. doi: 10.1016/j.foodchem.2006.03.042. [DOI] [Google Scholar]
- Leyva-Lopez NE, Vasco N, Barba de la Rosa AP, Paredes-Lopez O. Amaranth seed proteins: effect of defatting on extraction yield and on electrophoretic patterns. Plant Foods Hum Nutr. 1995;47(1):49–53. doi: 10.1007/BF01088166. [DOI] [PubMed] [Google Scholar]
- Liu LH, Hung TV, Bennett N. Extraction and characterization of chickpea (Cicer arietinum) albumin and globulin. J Food Sci. 2008;73(5):C299–C305. doi: 10.1111/j.1750-3841.2008.00773.x. [DOI] [PubMed] [Google Scholar]
- Liu S, Elmer C, Low NH, Nickerson MT. Effect of pH on the functional behavior of pea protein isolate-gum Arabic complexes. Food Res Int. 2010;43(2):489–495. doi: 10.1016/j.foodres.2009.07.022. [DOI] [Google Scholar]
- Maji TK, Hussain MR. Microencapsulation of Zanthoxylum limonella Oilv (ZLO) in genipin crosslinked chitosan–gelatin complex for mosquito repellent application. J Appl Polym Sci. 2009;111(2):779–785. [Google Scholar]
- Makri EA, Papalamprou EM, Doxastakis GI. Textural properties of legume protein isolate and polysaccharide gels. J Sci Food Agric. 2006;86(12):1855–1862. doi: 10.1002/jsfa.2531. [DOI] [Google Scholar]
- McClements DJ. Protein-stabilized emulsions. Curr Opin Colloid Interface Sci. 2004;9(5):305–313. doi: 10.1016/j.cocis.2004.09.003. [DOI] [Google Scholar]
- McClements DJ. Critical review of techniques and methodologies for characterization of emulsion stability. Crit Rev Food Sci Nutr. 2007;47(7):611–649. doi: 10.1080/10408390701289292. [DOI] [PubMed] [Google Scholar]
- Mi FL, Sung HW, Shyu SS. Synthesis and characterization of a novel chitosan-based network prepared using naturally occurring crosslinker. J Polym Sci Part A: Polym Chem. 2000;38(15):2804–2814. doi: 10.1002/1099-0518(20000801)38:15<2804::AID-POLA210>3.0.CO;2-Y. [DOI] [Google Scholar]
- Mondor M, Aksay S, Drolet H, Roufik S, Farnworth E, Boye JI. Influence of processing on composition and antinutritional factors of chickpea protein concentrates produced by isoelectric precipitation and ultrafiltration. Innov Food Sci Emerg Technol. 2009;10(3):342–347. doi: 10.1016/j.ifset.2009.01.007. [DOI] [Google Scholar]
- Morr CV, German B, Kinsella JE, Regenstein JM, Van Buren JP, Kilara A, Lewis BA, Mangino ME. A collaborative study to develop a standardized food protein solubility procedure. J Food Sci. 1985;50(6):1715–1718. doi: 10.1111/j.1365-2621.1985.tb10572.x. [DOI] [Google Scholar]
- Nelson DL, Cox MM. Lehninger principles of biochemistry. 4. New York: W.H. Freeman and Company; 2005. pp. 78–85. [Google Scholar]
- Nickerson MT, Paulson AT, Wagar E, Farnworth R, Hodge SM, Rousseau D. Some physical properties of crosslinked gelatin–maltodextrin hydrogels. Food Hydrocoll. 2006;20(7):1072–1079. doi: 10.1016/j.foodhyd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Nickerson MT, Patel J, Heyd DV, Rousseau D, Paulson AT. Kinetic and mechanistic considerations in the gelation of genipin-crosslinked gelatin. Int J Biol Macromol. 2006;39(4–5):298–302. doi: 10.1016/j.ijbiomac.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Nickerson MT, Farnworth R, Wagar E, Hodge SM, Rousseau D, Paulson AT. Some physical and microstructural properties of genipin-crosslinked gelatin–maltodextrin hydrogels. Int J Biol Macromol. 2006;38(1):40–44. doi: 10.1016/j.ijbiomac.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Okezie BO, Bello AB. Physicochemical and functional properties of winged bean flour and isolate compared with soy isolate. J Food Sci. 1988;53(2):450–454. doi: 10.1111/j.1365-2621.1988.tb07728.x. [DOI] [Google Scholar]
- Papalamprou EM, Doxastakis GI, Kiosseoglou V. Chickpea protein isolates obtained by wet extraction as emulsifying agents. J Sci Food Agric. 2010;90(2):304–313. doi: 10.1002/jsfa.3816. [DOI] [PubMed] [Google Scholar]
- Pearson AM. Soy proteins. In: Hudson BJF, editor. Developments in food proteins. London: Applied Science Publishers; 1983. pp. 67–108. [Google Scholar]
- Sánchez-Vioque R, Clemente A, Vioque J, Bautista J, Millán F. Protein isolates from chickpea (cicer arietinum L.): chemical composition, functional properties and protein characterization. Food Chem. 1999;64(2):237–243. doi: 10.1016/S0308-8146(98)00133-2. [DOI] [Google Scholar]
- Schwenke KD. Reflections about the functional potential of legume proteins. Rev Food Nahrung. 2001;45(6):377–381. doi: 10.1002/1521-3803(20011001)45:6<377::AID-FOOD377>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Sung HW, Huang RN, Huang LLH, Tsai CC. In vitro evaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation. J Biomater Sci Polym Ed. 1999;10(1):63–78. doi: 10.1163/156856299X00289. [DOI] [PubMed] [Google Scholar]
- Tang CH, Sun X. A comparative study of physicochemical and conformational properties in three vicilins from Phaseolus legumes: implications for the structure-function relationship. Food Hydrocoll. 2011;25(3):315–324. doi: 10.1016/j.foodhyd.2010.06.009. [DOI] [Google Scholar]
- Tang C-h, Yang M, Liu F, Chen Z. A novel process to efficiently form transglutaminase-set soy protein isolated-stabilized emulsion gels. LWT-Food Sci Technol. 2013;53(1):15–21. doi: 10.1016/j.lwt.2013.03.002. [DOI] [Google Scholar]
- Tcholakova S, Denkov ND, Sidzhakova D, Campbell B. Effect of thermal treatment, ionic strength, and pH on the short-term and long-term coalescence stability of β-lactoglobulin emulsions. Langmuir. 2006;22(14):6042–6052. doi: 10.1021/la0603626. [DOI] [PubMed] [Google Scholar]
- Tsoukala A, Papalamprou E, Makri E, Doxastakis G, Braudo EE. Adsorption at the air–water interface and emulsification properties of grain legume protein derivatives from pea and broad bean. Colloids Surf B. 2006;53(2):203–208. doi: 10.1016/j.colsurfb.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Vioque J, Alaiz M, Giron-Calle J. Nutritional and functional properties of Vicia faba protein isolates and related fractions. Food Chem. 2012;132(1):67–72. doi: 10.1016/j.foodchem.2011.10.033. [DOI] [PubMed] [Google Scholar]
- Wang H, Pato MD, Shand PJ. Biochemical properties of natural actomyosin extracted from normal and pale, soft, and exudative pork loin after frozen storage. J Food Sci. 2005;70(4):C313–C320. doi: 10.1111/j.1365-2621.2005.tb07179.x. [DOI] [Google Scholar]
- White JA, Hart RJ, Fry JC. An evaluation of the waters Pico-tag system for the amino-acid analysis of food materials. J Autom Chem. 1986;8(4):170–177. doi: 10.1155/S1463924686000330. [DOI] [PMC free article] [PubMed] [Google Scholar]