Abstract
A multiplex real-time isothermal amplification assay was developed using molecular beacons for the detection of Bacillus cereus and Staphylococcus aureus by targeting four important virulence genes. A correlation between targeting highly accessible DNA sequences and isothermal amplification based molecular beacon efficiency and sensitivity was demonstrated using phi(Φ)29 DNA polymerase at a constant isothermal temperature of 30 °C. It was very selective and consistently detected down to 101 copies of DNA. The specificity and sensitivity of this assay, when tested with pure culture were high, surpassing those of currently used PCR assays for the detection of these organisms. The molecular beacon based real-time isothermal amplification (MBRTIA) assay could be carried out entirely in 96 well plates or well strips, enabling a rapid and high-throughput detection of food borne pathogens.
Keywords: Detection, Food borne pathogens, B. cereus, S. aureus, Molecular beacon
Introduction
Foodborne pathogen contamination is a global problem. The U.S. Department of Agriculture (USDA) estimates that the economic burden of foodborne illness ranges from $10 to $83 billion annually (Nyachuba 2010). Although the symptoms of foodborne illness are often mild and self-limiting, severe cases can account to hospitalisation and even death (CDC 2011). With the continual emergence of new pathogens, the differential diagnosis or identification of etiological agents is the important first step to control the spread of food pathogens. The illness may become life-threatening if appropriate early diagnosis and therapy is not undertaken quickly. Hence, fast, accurate, and sensitive detection of these organism is of foremost importance. The detection of pathogenic microbial species by regulatory agencies is still primarily based on traditional microbiological culture methods that may take several days to complete. That is the current culture-based methods are time-consuming and may require long incubation times to obtain results. Determining the precise source and quantity of microbial contamination is crucial when devising strategies to reduce future outbreaks. Only 2 of the 27 outbreak surveys on fresh produce clearly identified a point of contamination. This emphasized the significance and requirement for rapid and accurate pathogen identification methods (NACMCF National Advisory Committee on Microbiological Criteria for Foods 1999). Antibody methods depend on the recognition of surface antigens. These methods have numerous drawbacks including cross-reactivity and possible interference of antigen expression by environmental conditions (Harry et al. 1995). DNA hybridization techniques are also being increasingly used in the detection and identification of food borne pathogens (Hill et al. 1998) mainly with the extensive use of polymerase chain reaction (PCR). The assays involve both amplification of single DNA targets and multiple targets (multiplex PCR) for specific detection of pathogens. Most PCR assays require visualization of the amplification product by ethidium bromide staining of agarose gels. The specificity of PCR detection assays has been increased by, utilizing either scoring of the target DNA or post-PCR hybridization-capture methods (Chen and Griffiths 2001). Nevertheless, these approaches have met with limited success, because these modifications formulate the overall pathogen detection methods labour intensive, time consuming and difficult to automate.
In order to address some of these concerns, a modification enabling simultaneous and rapid detection of some foodborne pathogens in a high throughput format was attempted. In this method, the assay utilizes a fluorogenic probe which has flanking GC-rich arm sequences complementary to one another (Chen et al. 2000; McKllip and Drake 2000). Also known as molecular beacon (MB) a fluorescent moiety is conjugated to one end of the sequence, and a quencher moiety is attached to the other end of the sequence. In the nonexistence of target DNA sequences, the MB assumes a hairpin conformation, with the two arms hybridizing to each other, thus bringing the quencher into close proximity to the fluorophore (which results in no or low background fluorescence). In the presence of target DNA, the sequence in the loop region hybridizes, the hairpin of the MB opens, and the fluorophore and the quencher separate. In the open conformation, the fluorophore of the MB emits a detectable signal that can directly be correlated with the quantity of the target template present in the assay (Higuchi et al. 1993; Tyagi and Kramer 1996).
In this communication, we report the application of MB probe being used for real-time, simultaneous detection of Bacillus cereus and Staphylococcus aureus. Also real- time assays based on isothermal amplification reaction coupled with MB have been exploited for practical applications with simple, fast, inexpensive, sensitive, method with a high-throughput format that enables the testing of many samples simultaneously, and, ideally, allows the detection of a series of different pathogenic microbes in the same assay tube.
Materials and methods
Bacterial test cultures
Bacillus cereus (MTCC 1272), Staphylococcus aureus (MTCC 96), Salmonella parathyphi (MTCC 735), Listeria monocytogenes (MTCC 1143), Streptococcus pneumonia (MTCC 655), Yersinia enterocolitica (MTCC 859), Escherichia coli (MTCC 729) used in the studies were procured from Microbial Type Culture Collection (MTCC), Institute of Microbial Technology (IMTECH), Chandigarh, India.
Design of molecular beacons
All oligonucleotide primers were synthesised and procured from Sigma-Aldrich Chemical Company, Bangalore, India.
MB primer was designed to be perfectly complementary to the hblA, bceT and entFM gene of Bacillus cereus and seB gene of Staphylococcus aureus. The fluorescent dye was tagged at the 5′ end and the quencher at the 3′ end. The stem sequence was selected so that they would not complement the sequences within the loop region. The length of the beacon was selected so that the annealing temperature is slightly higher than the annealing temperature of the PCR primers. The secondary structure and thermodynamic stability were studied using http://mfold.rna.albany.edu/?q=mfold/dna-folding-form. Only the primers having minimum secondary structure and stability were used for the assay. The Tm of the primers was higher than 30 °C to allow for better annealing. The beacons were resuspended in TE buffer, stored at −20 °C and protected from light. Aliquots (100 pM/μL) were prepared and used for subsequent studies. To enable simultaneous detection of B. cereus and S. aureus toxin producing genes each of the beacons primers were labelled with a different fluorophores: (6- Carboxyfluorescein [6-FAM], Triarylmethane [TAM], 5-Tetrachloro-Fluroscein Phosphoramadite [TET] and Texas Red [TxRd]) at the 5′ end and a quencher (BHQ) at the 3′ end. (Table 1). Initially, each of the primer pairs and molecular beacons was individually assessed. Following this, each individual assay was incorporated stepwise to form a single, optimized multiplex assay capable of the simultaneous real-time isothermal detection of all four target genes in a single strip well format.
Table 1.
Molecular beacon primer list
| Primer Name | 5′-3′ |
|---|---|
| C-HblF | TGCTATTTTGGGTCTACCAAT |
| C-HblR | GACATATAAGTAAGAGCGTTAA |
| HblMB | [TAM]-CTGACAGTAATATTAGTCAG-[BHQ2] |
| C-BcetF | GAAGTAATAAGCGTACCATCTG |
| C-BcetR | GAAGTAATAAGCGTACCATCTG |
| BcetMB | [6FAM]-CAGCGATATTTACAAGACCGCTG-[BHQ1] |
| C-EntFMF | CAAAACCAGCAGGTGTT |
| C-EntFMR | GGTTATGTAAGTGCAGACTTC |
| EntFMMB | [TET]-CGCTGAGGTGAAGCTGGTCAGCG-[BHQ1] |
| C-SEBF | GTTCGGGTATTTGAAGATGG |
| C-SEBR | TTGGTCAAATTTATCTCCTGG |
| SEBMB | [TxRd]-CGCATCAATAAGAAAAAGGGATGCG-[BHQ2] |
Isolation of DNA
DNA from bacterial tests cultures was isolated using the protocol given by (Sambrook et al. 1989). The copy number of the DNA isolated was determined using http://cels.uri.edu/gsc/cndna.html. The DNA obtained was serially diluted to yield a concentration of 102–108 copies.
Isothermal amplification Reaction
The isothermal amplification reaction was performed for a total volume of 50 μL. The reaction mixture consisted of 50 mM Tris-HCI, pH 7.5, 10 mM MgCI2, 1 mM dithiothreitol, 1 mM bovine serum albumin, Forward and reverse primers 80 μM, 300 pM of molecular beacons, 5 units of phi(Φ)29 DNA polymerase (Thermo Fisher Scientific, Bangalore, India), template DNA (102–108 copies), dNTP mix 2 mM (Sigma Aldrich, Bangalore, india) . The assay was performed in a 384 well black polystyrene NUNC plates (Thermo scientific, India). The reaction was carried out at 30 °C and the real-time readings were recorded at specific absorption-emission wavelengths of the specific dyes used using a Varioskan-flash microplate reader (Thermo Fischer). All the experiments were performed in triplicates. A separate platform was maintained for performing the isothermal reaction to avoid contamination.
Real-Time isothermal Analysis
The ability of MB to detect toxin genes of B. cereus and S. aureus in real-time isothermal assays was investigated. Different initial concentrations of template DNA isolated were used. DNA extracts were diluted with water, in 10-fold serial dilutions. 5 μL of template solution was added to each 25 μL of isothermal reaction. A no-template-control, in which sterile buffer was substituted for template DNA, was used in each experiment. This control was used to subtract any fluorescence that is not directly related to amplification.
Statistical Analysis
Limit of Blank (LOB): A set of fifteen blank samples (without template DNA), in triplicates were subjected to the assay conditions mentioned above. The LOB was calculated using the formula LoB = mean blank + 1.645(SDblank).
Results
Detection of B.cereus and S.aureus strains by conventional isothermal amplification assay and molecular beacon real-time isothermal amplification (MBRTIA).
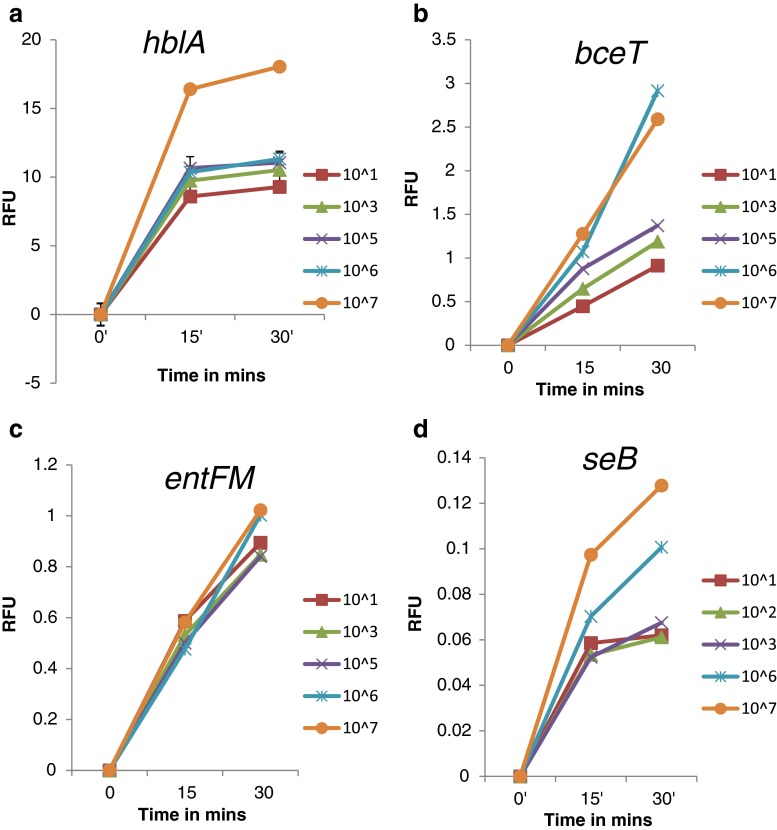
We examined the sensitivity of MB probe, real-time isothermal amplification methodology with reference to conventional isothermal amplification. Varying DNA concentrations ranging from 101 to107 copy number was used. A no-template control, containing sterile buffer was substituted for template DNA, in each experiment. This control was used to subtract any fluorescence that was not directly related to amplification. Fluorescence from the MB probe increased as the target DNA accumulated at the end of each successive round of amplification. The trend was same with all the toxin genes studied (Fig. 1). All data collected during the isothermal amplification cycle were used in the analysis and for quantifying the amplification of target DNA. Using the MB probe with real-time detection, it was possible to detect up to 101 copy number of becT, hblA, entFM genes of B.cereus and seB gene of S.aureus.
Fig. 1.
Molecular beacon based real-time isothermal amplification (MBRTIA) analysis for the detection of B. cereus and S. aureus toxin genes. MBRTIA analysis of a hblA gene, b bceT gene, c entFM gene, d seB gene (■) 101 copies, (▲) 103 copies, (×) 105 copies, (*) 106 copies, (•) 107 Copies
As the reaction was intended to be an isothermal reaction, phi(Φ)-29 DNA polymerase was used at 30 °C in MB reaction.
However, the efficiency of detection measured as threshold cycle value (Ct) varied slightly among different toxin genes. The critical threshold cycle (Ct) is defined as the cycle at which a significant increase in fluorescence is first recorded. The Ct value increased as the initial number of the available template molecules decreases. Thus, Ct values can potentially be used to quantify input target molecules.
The critical threshold cycle (Ct), defined as the cycle at which a significant increase in fluorescence is first recorded, increased as the initial number of template molecules DNA decreases (Heid et al. 1996). This was expected because samples containing low concentrations of template DNA would require more isothermal amplification reaction to replicate enough copies to produce a significant fluorescent signal. (Fig. 1). At higher initial target concentrations, the endpoint plateau at a lower fluorescent value than would be expected. This phenomenon has been attributed to late cycle inhibition (Heid et al. 1996).
The variability between different sample preparations was investigated. Six different sets of real-time LAMP assay were performed with initial template ranging from 10 1 to 107 DNA copy number. Each amplification was performed in duplicates. Comparison of Ct values for each duplicate sample showed minimal variation, indicating MBRTIA to be highly reproducible. Comparison of Ct values of the six different sets of assay also revealed little variability (Table 2). More significantly, the rate of fluorescent change at each template concentration was similar among the five different assays.
Table 2.
Precision of the MBRTIA assay
| Toxin gene | Copy number | Inter assay | Intra assay |
|---|---|---|---|
| SD | SD | ||
| bceT | 101 | 0.001 | 0.021 |
| 102 | 0.012 | 0.014 | |
| 103 | 0.011 | 0.022 | |
| 104 | 0.015 | 0.035 | |
| 105 | 0.024 | 0.034 | |
| 106 | 0.007 | 0.017 | |
| 107 | 0.034 | 0.036 | |
| hblA | 101 | 0.005 | 0.021 |
| 102 | 0.016 | 0.012 | |
| 103 | 0.019 | 0.017 | |
| 104 | 0.035 | 0.022 | |
| 105 | 0.044 | 0.034 | |
| 106 | 0.027 | 0.028 | |
| 107 | 0.038 | 0.024 | |
| EntFM | 101 | 0.018 | 0.008 |
| 102 | 0.021 | 0.017 | |
| 103 | 0.016 | 0.041 | |
| 104 | 0.015 | 0.022 | |
| 105 | 0.014 | 0.009 | |
| 106 | 0.027 | 0.027 | |
| 107 | 0.014 | 0.034 | |
| seB | 101 | 0.032 | 0.006 |
| 102 | 0.022 | 0.017 | |
| 103 | 0.011 | 0.021 | |
| 104 | 0.014 | 0.033 | |
| 105 | 0.044 | 0.032 | |
| 106 | 0.026 | 0.011 | |
| 107 | 0.037 | 0.032 |
Specificity of the reaction
To determine the specificity of primers used in the study, the assay was performed with DNA extracted from 6 different bacterial cultures. The reaction conditions used were same as mentioned above. MBRTIA assays showed that the MB, specific for becT, hblA, entFM genes of B.cereus and seB of S.aureus failed to detect the other bacterial templates (data not shown).
Discussion
The objective of this study was to optimize the performance of the MBs designed by us for simultaneous sdetection of B.cereus and S.aureus. The isothermal amplification -based MB assay provides the possibility of quantitative real-time detection of specific target DNA directly in the isothermal amplification tube or in well format. The reported assay could detect as low as 101 copy of both B.cereus and S.aureus and also cover a wide dynamic range of detection, all in a real-time manner. Detection of these pathogens using MB has been amazingly specific. Analysis of fluorescence data recorded at each amplification time provided a clear profile of the amplification process. The number of amplification cycles required before a significant increase in fluorescence from target–beacon hybrids was detected (critical threshold cycle Ct) and can be used to quantify the initial number of template molecules in the reaction. The critical cycle is inversely proportional to the logarithm of the initial number of target molecules. These data can be used to formulate a standard quantification curve for the detection of these pathogens. One additional benefit of using the Ct values for quantitation is that a much larger assay range is permitted than directly using total fluorescent emission, which has a dynamic range of approximately 1,000.
MBs labelled with different colour fluorophores can be used simultaneously for multiplex isothermal amplification for identification of different genes and different pathogens. In our studies this property was taken advantage of in detecting two bacterial pathogens simultaneously. This unique property has been exploited for a variety of applications, including the detection of point mutations in the methylenetetrahydrofolate reductase gene (Giessendorf et al. 1998), analysis of an 81-bp region of the Mycobacterium tuberculosis rpoB gene for mutations that confer resistance to the antibiotic rifampicin (Piatek et al. 1998), ecological studies with ruminal bacteria (Schofield et al. 1997), and real-time assay for HIV viruses (Vet et al. 1999). MB assays are simple and fast. Reagents are mixed in one step and reactions can be carried out in closed tubes or multi wells. Data can be recorded during each given time and results are automatically analyzed immediately after the reaction is completed, usually 1–2 h. However, in our studies the MB reaction reached optimum in 15 min and then reached a plateau. This means, the reaction is so fast that the detection could be completed in 15 min. Due to their high specificity and high sensitivity, MB can be effectively incorporated into real-time detection assays and provide a quick and accurate method for detection of specific nucleic acid sequences in homogeneous solutions. Molecular beacons (MB), due to their stable stem-and-loop structure, have been demonstrated to be significantly more specific than dyes such as SYBR green I and other types of probes. (Goto et al. 2009)
In conclusion we envisage that the speed and sensitivity of bacterial pathogen detection based on isothermal amplification assay method can be greatly enhanced with the application of MB. The application of the assay to environmental food samples suggests that the assay could be used for the sensitive and cost-effective monitoring of environmental and food samples. Importantly, this multiplex assay can be used to apply molecular beacons for the detection of multiple bacterial species and this tetraplex molecular-beacon coupled with real-time isothermal amplification assay can be used for the detection of a multiple bacterial species.
Acknowledgments
The authors thank the Director CSIR- CFTRI, for providing facilities to carry out the research work. The first author is grateful to the Indian Council of Medical Research (ICMR) for providing senior research fellowship to carry out the present work.
References
- CDC Vital signs: incidence and trends of infection with pathogens transmitted commonly through food-Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 1996–2010. MMWR Morb Mortal Wkly Rep. 2011;60:749–755. [PubMed] [Google Scholar]
- Chen J, Griffiths MW. Detection of Salmonella and simultaneous detection of Salmonella and Shiga-like toxin producing Escherichia coli using the magnetic capture hybridization polymerase chain reaction. Lett Appl Microbiol. 2001;32:7–11. doi: 10.1046/j.1472-765x.2001.00846.x. [DOI] [PubMed] [Google Scholar]
- Chen W, Martinez G, Mulchandani A. Molecular beacons: a real-time polymerase chain reaction assay for detecting Salmonella. Anal Biochem. 2000;280:166–172. doi: 10.1006/abio.2000.4518. [DOI] [PubMed] [Google Scholar]
- Giessendorf BA, Vet JAM, Tyagi S, Memsink EJMG, Trijbels FJM, Blom HJ. Molecular beacons: a new approach for semi automated mutation analysis. Clin Chem. 1998;44:482–486. [PubMed] [Google Scholar]
- Goto M, Honda E, Ogura A, Nomoto A, Hanaki K-I. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques. 2009;46:167–172. doi: 10.2144/000113072. [DOI] [PubMed] [Google Scholar]
- Harry EJ, Pogliano K, Losick R. Use of immunofluorescence to visualize cell-specific gene expression during sporulation in Bacillus subtilis. J Bacteriol. 1995;177:3386–3393. doi: 10.1128/jb.177.12.3386-3393.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Methods. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- Higuchi R, Fockler C, Dollinger G, Watson R. Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Biotechnology. 1993;11(9):1026–1030. doi: 10.1038/nbt0993-1026. [DOI] [PubMed] [Google Scholar]
- Hill WE, Datta AR, Feng P, Lampel KA, Payne WL. Identification of foodborne bacterial pathogens by gene probes. In: FDA, editor. Bacteriological Analytical Manual. Gaithersburg: AOAC International; 1998. [Google Scholar]
- McKllip JL, Drake MA. Molecular beacon polymerase chain reaction detection of Escherichia coli O157:H7 in milk. J Food Prot. 2000;63:855–859. doi: 10.4315/0362-028x-63.7.855. [DOI] [PubMed] [Google Scholar]
- NACMCF (National Advisory Committee on Microbiological Criteria for Foods) Microbiological safety evaluations and recommendations on fresh produce. Food Control. 1999;10:117–143. doi: 10.1016/S0956-7135(99)00026-2. [DOI] [Google Scholar]
- Nyachuba DG. Foodborne illness: is it on the rise? Nutr Rev. 2010;68:257–269. doi: 10.1111/j.1753-4887.2010.00286.x. [DOI] [PubMed] [Google Scholar]
- Piatek AS, Tyagi S, Pol AC, Telenti A, Miller LP, Kramer FR, Alland D. Molecular beacon sequence analysis for detecting drug resistance in Mycobacterium tuberculosis. Nat Biotechnol. 1998;16:359–363. doi: 10.1038/nbt0498-359. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring: Harbor laboratory press; 1989. [Google Scholar]
- Schofield P, Pell AN, Krause DO. Molecular beacons: trial of a fluorescence-based solution hybridization technique for ecological studies with ruminal bacteria. Appl Environ Microbiol. 1997;63:1143–1147. doi: 10.1128/aem.63.3.1143-1147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi S, Kramer FR. Molecular beacon: probes that fluoresce upon hybridization. Nat Biotechnol. 1996;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- Vet JAM, Majithia AR, Marras SAE, Tayai S, Dube S, Poiesz BJ, Kramer FR. Multiplex detection of four pathogenic retroviruses using molecular beacons. Proc Natl Acad Sci U S A. 1999;96:6394–6399. doi: 10.1073/pnas.96.11.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]