Summary
Contact precautions are widely recommended to prevent multidrug-resistant organism (MDRO) transmission. However, conflicting data exist regarding their effectiveness. Prior systematic reviews examined contact precautions as part of a larger bundled approach, limiting ability to understand their effectiveness. The aim of this review was to characterize the effectiveness of contact precautions alone against transmission of any MDRO among adult acute care patients. Directed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement, comprehensive searches of four electronic scientific literature databases were conducted for studies published in English from January 2004 to June 2014. Studies were included if interventional, original research, evaluating contact isolation precautions against MDRO transmission among inpatients. Searches returned 284 studies, six of which were included in the review. These studies measured four different MDROs with one study showing a reduction in transmission. Whereas studies were of high quality regarding outcome operationalization and statistical analyses, overall quality was moderate to low due to poor intervention description, population characterization and potential biases. Where compliance was measured (N = 4), it presented a threat to validity because it included select parts of the intervention, ranged from 21% to 87%, and was significantly different across study phases (N = 2). The poor quality of evidence on this topic continues to limit interpretation of these data. Hence, this conflicting body of literature does not constitute evidence for or against contact precautions. We recommend that researchers consider power calculation, compliance monitoring, non-equivalent concurrent controls when designing future studies on this topic.
Keywords: Contact precautions, Infection control, Infection prevention, Multidrug-resistant organism, Nosocomial
Introduction
In 2014, the World Health Organization (WHO) declared antimicrobial resistance a worldwide problem that requires urgent action.1 The WHO's report states that most global regions have high rates of resistance to antimicrobial drugs among bacteria such as Staphylococcus aureus, Klebsiella pneumonia, and Escherichia coli, and that these bacteria are moreover frequently resistant to multiple antimicrobials.1 Multidrug-resistant organisms (MDROs) are considered to be serious threats to global security as infections with these organisms have higher mortality than those of non-drug-resistant strains, and are more difficult and costly to treat.1, 2, 3, 4 Therefore, identifying and employing effective techniques to control the spread of MDROs is of high importance to manage health outcomes and reduce healthcare costs.3
Isolation precautions are the preferred technique to control transmission of pathogens with high morbidity, mortality, or epidemiological significance, but controversy remains regarding the effectiveness of isolation precautions.3, 5, 6, 7, 8, 9, 10 This debate intensified following transmission of Ebola virus to healthcare workers despite use of isolation precautions.11 Like Ebola virus, MDROs are spread through direct or indirect contact.3 Therefore, contact precautions, which include isolation in a private room, if possible, and use of gowns and gloves, are recommended to reduce transmission of MDROs.6
However, evidence regarding the effectiveness of contact precautions against MDRO transmission is limited in methodology and content. Prior studies have predominantly reported outbreak scenarios and therefore lack equivalent control group(s) and are subject to performance bias.12, 13 Additionally, most have focused on meticillin-resistant S. aureus (MRSA) or vancomycin-resistant enterococci (VRE).13 The effectiveness of contact precautions against emerging MDROs such as carbapenem-resistant Acinetobacter baumannii and β-lactamase-producing Enterobacteriaceae has not been established.14, 15 More evidence may have become available regarding emerging MDROs since publication of previous reviews. Previous systematic reviews of this topic are similarly limited in the types of MDROs studied and outcomes measured, and have had mixed results.13, 16, 17, 18 More importantly, contact precautions in all of these reviews were grouped with other infection control practices such as active surveillance.13, 16, 17, 18, 19 Thus, gaps in the literature exist regarding effects of contact precautions alone and against emerging MDROs.14, 15
The objective of this systematic review is to characterize the effectiveness of contact isolation precautions alone against transmission of any MDRO among adult patients from interventional studies in which contact precautions are not included bundled with other interventions. In order to increase consistency between included studies and to better isolate the effect of contact precautions, this review focuses on acute care, as other settings such as skilled nursing facilities have different potential for infection transmission.20
Methods
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.21 Inclusion criteria for studies in this systematic review were: (1) original research, (2) published in peer-reviewed, scientific journals, (3) in English, (4) involved human inpatients, (5) conducted in acute care settings, (6) outcomes were infection or colonization with one or more bacterial organisms identified as multidrug resistant by the US Centers for Disease Control and Prevention (CDC), (7) experimental or quasi-experimental design (i.e. interventional), and (8) with intervention of contact isolation precautions (as either the control or experimental exposure).3 The components of contact precautions required for inclusion were: placement of the infected or colonized patient in a single room or in a cohort facility, application of standard precautions and disposable gown and glove use for close patient contact.6 Searches were limited to the past 10 years (January 1st, 2004 to June 2014) to target the most recent literature (i.e. with emergent pathogen outcomes), including that published subsequent to national clinical guidelines. Editorials, correspondences, commentaries, letters, or proceeding papers were excluded. Studies in which the effectiveness of isolation precautions was indistinguishable from that of a larger intervention bundle were also excluded.
Search strategy
With the help of a university librarian, searches of PubMed, Ovid Medline, EBSCO Cumulative Index of Nursing and Allied Health Literature (CINAHL) and Cochrane Central Register of Clinical Trials were conducted using the following terms: (1) isolation precautions, (2) multidrug resistance, (3) bacterial infections, and (4) healthcare-associated infection. The names of specific MDROs identified by the CDC were included as both keyword and medical subject headings (MeSH) terms, where applicable, to maximize search results.3 Searches also included synonyms, related phrases, and pluralized terms (see Appendix A, Supplementary material online). Hand searches of reference lists were also conducted.
Study selection
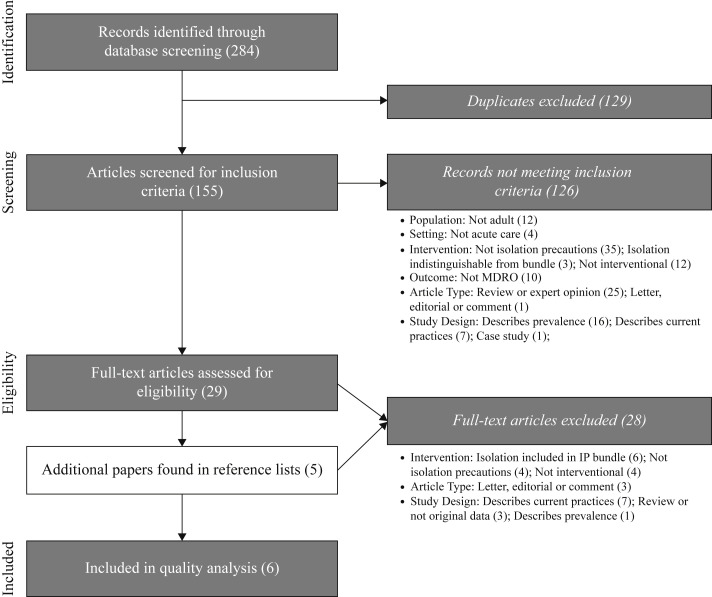
Two reviewers (C.C.C. and B.C.) screened search results to determine whether titles and abstracts met the inclusion and exclusion criteria. Full texts of articles were obtained and screened for eligibility when the title and abstract appeared to meet the criteria. All reasons for exclusion were recorded (Figure 1 ).
Figure 1.
Flow diagram of search results and eligibility analysis. Boxes on the left represent stages of evaluation of the publication returned through electronic database searches. The boxes on the right outline the number of articles excluded by the primary reason for exclusion. MDRO, multidrug-resistant organism; IP, infection prevention.
Data abstraction
A data abstraction tool of relevant criteria from The Cochrane Collaboration data collection form for intervention review of randomized controlled trials (RCTs) and non-RCTs was tailored for use in this review.22 C.C.C. pilot-tested the modified tool (see Appendix B, Supplementary material online) with two randomly selected eligible papers to confirm appropriateness of the tool and then used it to systematically collect data. These data included rationale for inclusion, methods, participants, intervention groups, outcomes, data and analysis, as well as funding sources, key conclusions, and reported conflicts of interest. C.C.C. contacted the publication's corresponding author if study details were unclear.
Quality appraisal
Each study was appraised using the quality assessment tool that was developed, piloted, and employed by Aboelela and colleagues to review publications regarding isolation precaution effectiveness (see Appendix C, Supplementary material online).13 This tool has items regarding sample representativeness, bias and confounding, description of the intervention, outcomes and follow-up, and statistical analysis, which are each ranked 1–4, where 4 is the highest quality. Each paper was assessed as to whether it addressed the aforementioned categories in a manner that was ‘completely adequate’, ‘partially adequate’, ‘inadequate, not stated or impossible to tell’ or ‘not applicable’. The authors performed component quality analysis independently and discussed results to consensus, as necessary.23
Results
The search strategy described above returned 284 publications (Ovid: 165; PubMed: 112; CINAHL: 6; Cochrane: 1). Having excluding 129 duplicates, C.C.C. and B.C. reviewed the titles and abstracts of 155 remaining papers. Of these, 126 did not meet the inclusion criteria. The remaining 29 publications underwent full-text review. Hand search yielded five additional papers for eligibility assessment (Figure 1).
The most usual reasons for study exclusion were testing an intervention other than isolation precautions (N = 39) or a bundled intervention (N = 9); reviewing or presenting data that were not original (N = 28); describing the prevalence of MDRO (N = 17) or infection prevention practices (N = 14); or examining isolation through observation alone (N = 16). Of these, three attempted to estimate isolation precaution efficacy using mathematical models.24, 25, 26
Characteristics of included studies
Six studies met the inclusion criteria and were included for the final review (Table I ). Four studies were non-randomized quasi-experimental studies comparing pre- and post-intervention MDRO rates, whereas two studies had a repeated treatment design.27, 28, 29, 30, 31, 32 One study took place during an MDRO outbreak.27 Studies included in this review had four different MDROs as primary outcomes, many comparators, and varying methods of identifying MDRO colonization and infection. These fundamental differences prevented meaningful use of meta-analysis to evaluate the effectiveness of contact precautions against MDRO transmission. The small number of studies included in the review and difficulty in identifying and locating unpublished studies also precluded us from an assessment of publication bias.33
Table I.
Summary of key characteristics of publications included in the systematic review
| Article | Study design | Setting and population | Intervention and comparison | Primary outcome | Time horizon (dates) | Key conclusions |
|---|---|---|---|---|---|---|
| Bearman et al.30 | One group pretest–post-test (two intervention phases) | Medical ICU at academic hospital (USA) |
|
Prevalence and incidence of MRSA or VRE colonization or infection |
|
No differences in the proportion of patients acquiring VRE (14% vs 18%, P = 0.19) or MRSA (5.7% vs 5% P = 0.92) in the two study phases |
| Bearman et al.29 | One group pretest–post-test (two intervention phases) | Surgical ICU at academic hospital (USA) |
|
Prevalence of MRSA or VRE |
|
Compared with contact precautions, universal gloving with emollient-impregnated gloves, no statistically significant change in the rates of device-associated infection, CDI, or patient MDRO acquisition was observed |
| Cepeda et al.31 | Repeated treatment | All inpatients with stay >12 h in three medical–surgical ICUs of two academic hospitals (Great Britain) |
|
Incidence of MRSA colonization or infection |
|
Risks of acquiring MRSA were similar in the move and non-move phases; combined hazard ratio 0.73 (95% CI: 0.49–1.10), P = 0.94 one-sided and for hospital A and B individually [0.72 (0.44–1.17), P = 0.91 and 0.76 (0.37–1.58), P = 0.77] |
| Cheng et al.28 | One group pretest–post-test with non-equivalent, concurrent control (three phases) | Patients of an ICU in one university-affiliated teaching hospital (Hong Kong) |
|
‘Changes in the trend or level of incidence density of ICU onset infection due to MRSA’ (p. 3) |
|
No difference in level or trend change of the incidence density of ICU onset infections due to MRSA and ESBL-producing organisms across different phases during the study period |
| Cohen et al.27 | One group pretest–post-test (four intervention phases) | All inpatients of a tertiary care medical centre (Israel) |
|
CRKP colonization or infection ‘episodes’ |
|
Contact precautions alone are not sufficient for controlling an outbreak of CRKP colonization and infection; significant changes in incidence rate corresponding with phases 2 and 3 |
| Gbaguidi-Haore et al.32 | Repeated treatment | Academic hospital (France) |
|
Acinetobacter baumannii colonization or infection |
|
Implementation of isolation precautions was negatively associated with A. baumannii colonization incidence [RR: 0.50 (95% CI: 0.40–0.64); P < 0.001] |
ICU, intensive care unit; MRSA, meticillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci; CDI, Clostridium difficile infection; MDRO, multidrug-resistant organism; CI, confidence interval; EBSL, extended spectrum β-lactamase; CRKP, carbapenem-resistant Klebsiella pneumoniae; ED, emergency department; RR, risk ratio.
Population and setting
Included studies were conducted in France, Great Britain, Israel, Hong Kong, and the USA.27, 28, 29, 30, 31, 32 Most studies were conducted in a single acute care centre (N = 5); one study included two hospitals with analyses done by subgroup. Four settings were noted as academic centres, another as a tertiary care centre, and in one study the setting was not described.30 Four studies took place in an intensive care unit (ICU); the other two applied their intervention throughout the whole hospital. Whereas most studies did not state inclusion or exclusion criteria for the individual patients, one indicated that all hospital admissions were included, and another included those admitted to the ICU for >48 h.
Interventions and comparisons
Almost all papers offered a description of the intervention. Two papers described the intervention by citing CDC guidelines.29, 30 Variations to these practices and/or additional descriptions regarding the intervention were single-room isolation alone, staff cohorting, regular environmental cleaning and/or environmental cleaning at discharge and reserving healthcare devices (e.g. stethoscopes) for each infected patient.27, 28, 31
Most studies (N = 5) compared the effectiveness of contact precautions with the effectiveness of another infection control intervention.27, 28, 29, 30, 31 These included universal gloving, gowning without moving infected individuals to private rooms, and cohorting patients and staff.27, 28, 29, 30, 31 Cohen et al. also included two additional phases of cohorting, which were increased surveillance in the ICU (phase 3) and active surveillance in the emergency department (phase 4).27 Bearman et al. also performed active surveillance, but it was not clear who was subject to screening.29 One study compared contact isolation precautions against no intervention to prevent transmission of MDRO colonization or infection.32
In most of the studies, the authors initiated isolation precautions at the time of a positive MDRO culture (i.e. isolation was not pre-emptive) and the precautions were initiated for either colonization or infection.29, 30, 31, 32 Some protocols included cohorting nurse staff members to care for the MDRO-positive patients.27, 28, 31 One study mentioned that it was possible for patients to be removed from isolation if the patient was MDRO-negative for six months.27 None of the publications included how long patients were observed to detect occurrence of the outcome. One publication noted that patients who were present during a study phase change were subsequently treated with the intervention of the new phase.31
Five studies used pretests and post-tests to compare interventions in the different phases, though most aggregated results by phase or by year.27, 28, 30, 31, 32 Authors of one study compared MDRO infection rates between the pretest (phase 1) and the removed-treatment phase (phase 2), and phase 2 to the following phase where contact precautions were reintroduced (phase 3).32 Another study included a concurrent group, though this was a non-equivalent control as MRSA incidence managed with contact precautions was compared to extended spectrum beta-lactamase (ESBL)-producing organism incidence managed with standard precautions (i.e. a different outcome was measured in each group).28
Outcomes
MDROs of interest in the included papers were MRSA, VRE, carbapenem-resistant Klebsiella pneumonia (CRKP), and drug-resistant A. baumannii. 27, 28, 29, 30, 31, 32 Three studies included measures of more than one MDRO: VRE and MRSA as primary outcomes and EBSL-producing organisms.28, 29, 30 All of the papers' primary outcomes included colonization with the pathogen of interest in addition to active infection. However, screening procedures to identify cases differed substantially. One study tested the roommates, providers, and immediate environment of active cases to track pathogen spread (active, snowball sampling); another swabbed all patients for MDROs within 24 h of admission, weekly, at discharge, and as clinically indicated.27, 31 Two studies tested participants on admission and then every four days or as clinically indicated; the other two tested for MDRO when deemed clinically necessary.28, 29, 30, 32
Analyses
Two of the papers used Student's t-test and χ2 or Fisher's exact test to compare continuous and categorical variables, respectively.29, 30 Others used a Cox proportional hazards model, Poisson multiple regression analysis, or segmented linear regression (including change-point analysis).27, 28, 31, 32 Two studies reported power calculations to ensure sufficient sample size to detect the anticipated change in infection rate, though in one of these studies the data analysis plan was amended and the power calculation was not changed to reflect the new strategy.29, 31
Study conclusions
Five out of the six studies concluded that contact precautions did not represent a statistically significant improvement in MDRO infection control beyond that of the comparator(s).27, 29, 30, 31 However, one showed a decreased colonization rate of drug-resistant A. baumannii during periods of contact precautions use compared to a period with no patient isolation (relative risk: 0.5; 95% confidence interval: 0.40–0.64; P < 0.001).32
Quality appraisal
Quality of the included papers ranged widely. Whereas overall quality could be considered moderate for each paper, poor performance on key quality items such as bias and cofounding limited usefulness of this body of literature. Table II displays a visual representation of each paper's quality along the concepts identified by Aboelela et al. All had at least one quality concept that showed clear opportunity for improvement. Gabaguidi-Hoare et al. did not have a portion of the quality appraisal tool that was deemed ‘inadequate’, but had more ‘not applicable’ items on the quality assessment tool. The following sections outline the rationale for the quality assessment of each paper.
Table II.
Quality assessment results for each included papera
| Quality criterion | Bearman et al.30 | Bearman et al.29 | Cepeda et al.31 | Cheng et al.28 | Cohen et al.27 | Gbaguidi-Haore et al.32 |
|---|---|---|---|---|---|---|
| Representativeness | ||||||
| Study population description | 3 | 2 | 4 | 2 | 2 | 4 |
| Inclusion/exclusion criteria | 1 | 1 | 4 | 1 | 1 | 1 |
| Location/setting descriptionb | 4 | 4 | 4 | 4 | 4 | 4 |
| Bias and confounding | ||||||
| Study population corresponded to larger population in all key factors | 1 | 1 | 1 | 1 | 1 | 1 |
| Masking | 1 | 1 | 1 | 1 | 1 | 1 |
| How similar was the assessment of outcomes between groups | 1 | 1 | 1 | 1 | 1 | 1 |
| Involvement from author | 1 | 1 | 1 | 1 | 1 | 1 |
| Accounted for confounding interventions | 3 | 2 | 4 | 4 | 2 | 1 |
| Compliance rate | 4 | 3 | 2 | 2 | 2 | 1 |
| Description of intervention | ||||||
| Replication possible given descriptions of intervention | 2 | 3 | 4 | 4 | 3 | 4 |
| Outcomes and follow-up | ||||||
| Outcome assessment procedure clearly defined | 4 | 4 | 4 | 4 | 3 | 3 |
| Groups equivalent in attrition/LOS/death/patient days | 4 | 4 | 4 | 2 | 2 | 4 |
| Statistical analysis | ||||||
| Description and appropriateness of methods | 4 | 4 | 4 | 4 | 4 | 4 |
| Tested differences between groups and variability | 2 | 4 | 4 | 2 | 2 | 4 |
LOS, length of stay.
Key: 1, not applicable; 2, inadequate, not stated; 3, partially adequate; 4, completely adequate. Columns represent each concept outlined on the quality assessment tool and each row represents an included paper.
Added to quality assessment tool described by Aboelela et al. (2006).
Representativeness
Excepting Cepeda et al. which provided extensive details of the study setting, inclusion and exclusion criteria, and patient population characteristics, representativeness of the included studies was difficult to determine given the poor quality of population and inclusion criteria descriptions. The reviewers determined that most descriptions of the sample population were inadequate or partially adequate because these descriptions, if included at all, frequently lacked immunocompromised status or device use among the included sample, which are known risk factors for infection.3, 27, 28, 29, 30 Further, two studies explicitly stated the inclusion and exclusion criteria for enrolment of participants within these setting(s).27, 31 Given that outcomes appear to include the whole unit or hospital, where no criteria were stated, the reviewers assumed that all patients were included in the study and determined this criterion to be ‘not applicable’ on the quality score. Nevertheless, failing to state this fact represents poor transparency of reporting. All studies provided adequate information regarding setting characteristics, including size and type of facility, type of unit (if applicable) and the hospital location. It was not stated in any study how settings and units within these facilities were chosen for participation, potentially subjecting the included studies to selection bias.34
Bias and confounding
The six papers received their lowest evaluations on the quality measures related to bias and confounding. Regarding the potential for sampling and selection bias, the reviewers assumed that the entire facility or unit was included unless otherwise stated. Therefore, the quality criterion for comparing the sample population characteristics to that of a larger population was deemed ‘not applicable’.
The studies had wide quality variation in accounting for confounding interventions. The reviewers interpreted adequacy on this item as noting broad or systemic changes potentially affecting healthcare delivery and attempting to mitigate the effects of the confounder(s), where possible. Two studies were deemed completely adequate in this respect.28, 31 Cheng et al. identified the severe acute respiratory syndrome (SARS) epidemic and corresponding systemic change as a potential confounder in their study and revised the statistical analysis to account for resulting bias. Cepeda et al. mentioned that environmental services protocols remained unchanged throughout the study and monitored hand hygiene to ensure consistent adherence rates. One study was deemed partially adequate as the facility ICU underwent renovations that doubled the number of beds in preparation for phase 2 of the study, though the authors confirmed that nurse:staff ratios were identical across the phases.30 This suggests that new nursing staff may have been hired. Though the presence of new personnel may lead to performance bias, this was not addressed in study design.
Some studies were classified as inadequate due to inherent confounding in the study design itself, such as the addition of multiple ‘bundled’ interventions simultaneously.27 Others were considered inadequate because potential confounders, such as changes in unit occupancy and hand hygiene adherence, were tracked, but differences across study phases or groups were not accounted for in the statistical analysis.29 Further, Cohen et al. mentioned that national regulations for infection control changed during the course of the study, making it possible that a novelty effect may have presented a threat to construct validity.35 Gbaguidi-Haore et al. mentioned no potentially confounding interventions and that study was therefore scored as ‘not applicable’ on this item.
The level of intervention compliance and quality of compliance monitoring in these studies was mixed and often inadequate. One did not track or report compliance.32 Another reported compliance inconsistently across different phases of the study, and others recorded compliance for particular components of the intervention (e.g. gowning compliance among nurses, but not among other healthcare workers).27, 28, 31 In contrast, both articles by Bearman et al. measured compliance for all components of the intervention and reported rates during each phase.29, 30 Given the compliance rates reported by these studies, compliance was determined to be completely and partially adequate, respectively.
Description of intervention
Half the studies' intervention descriptions were completely adequate.28, 31, 32 Those not deemed to be completely adequate lacked descriptions of the compliance monitoring process, whether gloves were donned upon contact with the patient's immediate environment during isolation, and how compliance was enforced when enforcement was mentioned.27, 29, 30 Bearman et al. was inadequate on this item as description of the survey component lacked critical information that would be needed to repeat these methods, including survey format and distribution.30
Outcomes and follow-up
The majority of the included papers had high-quality operational definitions and assessment description such that methods were reasonably repeatable.28, 29, 30, 31 Four of six papers completely addressed whether pre-intervention and intervention phase groups were equivalent in follow-up/attrition by showing that length of stay and/or death among participants was equivalent between phases.29, 30, 31, 32
Statistical analysis
Statistical validity was generally acceptable in the included studies. Three studies' analyses were deemed ‘completely adequate’, as each included appropriate statistical methods, clear description of methods, and comparisons between groups.29, 31, 32 The remaining papers were ‘partially adequate’ as they did not test for differences between groups or variability within them.
Discussion
We reviewed six studies regarding the effectiveness of contact precautions against MDRO infections. Five of the six studies did not find significant association between contact precautions and reductions in MDRO transmission. One study investigating contact precautions for A. baumannii colonization or infection compared against no intervention demonstrated a reduction in the number of cases in phases where isolation precautions were implemented.32
Limitations of this review are that it does not include papers published in languages other than English or grey literature. As with all literature reviews, it is also subject to publication bias. However, our findings are consistent with previous literature. De Angelis and colleagues combined results of three studies (including Bearman et al. 29) in a fixed effects model, despite differences in study interventions, and concluded that contact precautions were not effective against VRE acquisition.16 Another limitation of our review, shared by previous literature, may be a failure to address droplet or airborne transmission of these bacteria, which may explain inconsistent effectiveness of contact precautions.36 However, study quality regarding low compliance rates, bias and confounding, and failure to adjust for confounders and/or confirm equivalency between pre- and post-test groups, preclude ability to draw strong conclusions from this evidence base regardless of these studies' findings.12
Implications for clinicians
The quality of this body of literature does not justify changes in practice. Conflicting data from studies with poor design and/or low compliance does not constitute evidence against contact precautions; rather, these data are inconclusive. Whereas the study that performed best on our quality score found no significance between contact precautions and not isolating patients, this study did not consistently assess intervention compliance in the various study phases.31 The included study that showed a difference in MDRO transmission with use of contact precautions did not report compliance rates and could not be assessed for quality on any of the other bias and confounding items of the quality assessment tool.32 Inconsistencies and absences in compliance monitoring and reporting make it impossible to tell whether protocols were completed as intended, threatening the internal validity of these studies.34 The CDC recently faced similar difficulty interpreting health outcomes after two healthcare workers were infected with Ebola virus in Texas, as it was unclear whether transmission had been due to inadequate isolation precaution protocols or to a protocol breach.37 In practice, healthcare facilities should be regularly monitoring compliance, and investigating potential lapses when cross-transmission is documented, to potentially resolve systems-based inefficiencies. It is also important for researchers to monitor and report compliance to understand what effectiveness level can be reasonably expected in practice where compliance may be lower than in clinical studies. Given the quality of evidence presented here, it may be advisable that healthcare workers and administrators continue to devote focus and resources to improve components of contact precautions and other infection prevention techniques with strong effectiveness evidence, such as hand hygiene technique and compliance.3
Implications for researchers
Although the included studies have limitations that have been well described in the literature on this topic, such as lack of intervention allocation concealment, some demonstrate realistic opportunities for improvement in future studies. First, two of the included papers contained a power calculation.29, 31 As MDRO infections are rare events and most studies on this topic include small patient samples due to feasibility and cost concerns, future studies should also conduct a calculation to determine whether the study is adequately powered to detect differences in infection rates between intervention phases.12 Second, four of the included studies attempted to control for time trends in healthcare-associated infections and other confounders through statistical analysis.27, 28, 31, 32 A concurrent, non-equivalent control, such as in Cheng et al., may address this issue, but concurrent controls are not always feasible. In the future, longitudinal studies with multiple pre-intervention collection points could add even stronger evidence by directly measuring and accounting for infection trends that are not related to the intervention, as in Bearman et al. 38 Third, these studies differ from most previous publications by attempting to monitor intervention compliance.12, 39 Previous studies that monitored compliance demonstrated improved adherence (i.e. with hand hygiene) when an isolation precautions intervention was implemented.40, 41 However, this was not consistent with levels of compliance reported in the studies reviewed here.28, 29, 30, 31 Low compliance with contact precautions could be the reason that this intervention appears to be equally or less effective than other interventions.31 This body of evidence demonstrates the implementation of a number of improvements in study design which, when combined in future studies, may yield substantially stronger evidence.
Another consideration for future studies is inclusion of patient-centred outcomes. Whereas benefits of isolation precautions are uncertain, adverse consequences of isolation precautions to the isolated individual, such as increased depression, anxiety, and anger, are well documented.42, 43 A number of papers returned in our search discussed negative consequences associated with isolation, but none of the included papers incorporated patient-centred measures such as anxiety and depression.10, 12, 39, 43, 44, 45 Considering that patient isolation is relatively resource intensive compared to other infection prevention activities, cost-utility analyses in future studies may be a good option to incorporate health outcomes, patient preferences, and costs to evaluate the effectiveness of contact isolation precautions against MDRO infection.46
Acknowledgements
We thank A. Meret for her expert guidance regarding the electronic literature database searches.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jhin.2015.05.003.
Conflict of interest statement
None declared.
Funding sources
The National Institutes of Nursing Research generously provided funding for this paper (NINR T32NR013454 and F31 NR015176-01). Study sponsors had no role in study design, collection, analysis, interpretation of data or manuscript generation.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.World Health Organization . WHO; Geneva: 2014. Antimicrobial resistance. Global report on surveillance.http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1 Available at: [last accessed May 2015] [Google Scholar]
- 2.Cosgrove S.E., Sakoulas G., Perencevich E.N., Schwaber M.J., Karchmer A.W., Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 3.Siegel J.D., Rhinehart E., Jackson M., Chiarello L. The Healthcare Infection Control Practices Advisory Management of multidrug-resistant organisms in health care settings, 2006. Am J Infect Control. 2006;35:S165–S193. doi: 10.1016/j.ajic.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Capitano B., Nicolau D.P. Evolving epidemiology and cost of resistance to antimicrobial agents in long-term care facilities. J Am Med Directors Assoc. 2003;4:S90–S99. doi: 10.1097/01.JAM.0000066029.00660.5A. [DOI] [PubMed] [Google Scholar]
- 5.Liu C., Bayer A., Cosgrove S.E. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 6.Siegel J.D., Rhinehart E., Jackson M., Chiarello L., Healthcare Infection Control Practices Advisory Committee . 2007. Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings.http://www.cdc.gov/ncidod/dhqp/pdf/isolation2007.pdf Available at: [last accessed May 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strausbaugh L.J., Siegel J.D., Weinstein R.A. Preventing transmission of multidrug-resistant bacteria in health care settings: a tale of 2 guidelines. Clin Infect Dis. 2006;42:828–835. doi: 10.1086/500408. [DOI] [PubMed] [Google Scholar]
- 8.Smith P.W., Bennett G., Bradley S. SHEA/APIC guideline: infection prevention and control in the long-term care facility, July 2008. Infect Control Hosp Epidemiol. 2008;29:785–814. doi: 10.1086/592416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regional Office for Western Pacific, Regional Office for South-East Asia. Practical guidelines for infection control in health care facilities. Available at: http://www.who.int/water_sanitation_health/emergencies/infcontrol/en/ [last accessed May 2015].
- 10.Zastrow R.L. Emerging infections: the contact precautions controversy. Am J Nurs. 2011;111:47–53. doi: 10.1097/10.1097/01.NAJ.0000395242.14347.37. [DOI] [PubMed] [Google Scholar]
- 11.Tavernise S. C.D.C. Director becomes face of nation's worry and flawed response. The New York Times. October 15th, 2014 http://www.nytimes.com/2014/10/16/us/cdc-director-becomes-face-of-nations-worry-and-flawed-response.html Available from: [last accessed May 2015] [Google Scholar]
- 12.Landelle C., Pagani L., Harbarth S. Is patient isolation the single most important measure to prevent the spread of multidrug-resistant pathogens? Virulence. 2013;4:163–171. doi: 10.4161/viru.22641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aboelela S.W., Saiman L., Stone P., Lowy F.D., Quiros D., Larson E. Effectiveness of barrier precautions and surveillance cultures to control transmission of multidrug-resistant organisms: a systematic review of the literature. Am J Infect Control. 2006;34:484–494. doi: 10.1016/j.ajic.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Apisarnthanarak A., Khawcharoenporn T., Mundy L.M. Practices to prevent multidrug-resistant Acinetobacter baumannii and methicillin-resistant Staphylococcus aureus in Thailand: a national survey. Am J Infect Control. 2013;41:416–421. doi: 10.1016/j.ajic.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Tschudin-Sutter S., Frei R., Dangel M., Stranden A., Widmer A.F. Rate of transmission of extended-spectrum beta-lactamase-producing enterobacteriaceae without contact isolation. Clin Infect Dis. 2012;55:1505–1511. doi: 10.1093/cid/cis770. [DOI] [PubMed] [Google Scholar]
- 16.De Angelis G., Cataldo M.A., De Waure C. Infection control and prevention measures to reduce the spread of vancomycin-resistant enterococci in hospitalized patients: a systematic review and meta-analysis. J Antimicrob Chemother. 2014;69:1185–1192. doi: 10.1093/jac/dkt525. [DOI] [PubMed] [Google Scholar]
- 17.Cooper B.S., Stone S.P., Kibbler C.C. Isolation measures in the hospital management of methicillin resistant Staphylococcus aureus (MRSA): systematic review of the literature. BMJ. 2004;329:533. doi: 10.1136/bmj.329.7465.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyce J.M., Havill N.L., Kohan C., Dumigan D.G., Ligi C.E. Do infection control measures work for methicillin-resistant Staphylococcus aureus? Infect Control Hosp Epidemiol. 2004;25:395–401. doi: 10.1086/502412. [DOI] [PubMed] [Google Scholar]
- 19.Munoz-Price L.S., Quinn J.P. Deconstructing the infection control bundles for the containment of carbapenem-resistant Enterobacteriaceae. Curr Opin Infect Dis. 2013;26:378–387. doi: 10.1097/01.qco.0000431853.71500.77. [DOI] [PubMed] [Google Scholar]
- 20.Mody L., Bradley S.F., Galecki A. Conceptual model for reducing infections and antimicrobial resistance in skilled nursing facilities: focusing on residents with indwelling devices. Clin Infect Dis. 2011;52:654–661. doi: 10.1093/cid/ciq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D., Liberati A., Tetzlaff J., Altman D.G. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Cochrane Collaboration . April 18th, 2014. Data collection form.http://www.cochrane.org/sites/default/files/uploads/forums/u389/ERC%20data%20collection%20form%20for%20intervention%20reviews%20for%20RCTs%20and%20non-RCTs.doc Available at: [last accessed May 2015] [Google Scholar]
- 23.Lundh A., Gotzsche P.C. Recommendations by Cochrane Review Groups for assessment of the risk of bias in studies. BMC Med Res Methodol. 2008;8:22–31. doi: 10.1186/1471-2288-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chow K., Wang X., Curtiss R., 3rd, Castillo-Chavez C. Evaluating the efficacy of antimicrobial cycling programmes and patient isolation on dual resistance in hospitals. J Biol Dynam. 2011;5:27–43. doi: 10.1080/17513758.2010.488300. [DOI] [PubMed] [Google Scholar]
- 25.D’Agata E.M., Horn M.A., Ruan S., Webb G.F., Wares J.R. Efficacy of infection control interventions in reducing the spread of multidrug-resistant organisms in the hospital setting. PLoS One. 2012;7:e30170. doi: 10.1371/journal.pone.0030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kypraios T., O’Neill P.D., Huang S.S., Rifas-Shiman S.L., Cooper B.S. Assessing the role of undetected colonization and isolation precautions in reducing methicillin-resistant Staphylococcus aureus transmission in intensive care units. BMC Infect Dis. 2010;10:29. doi: 10.1186/1471-2334-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen M.J., Block C., Levin P.D. Institutional control measures to curtail the epidemic spread of carbapenem-resistant Klebsiella pneumoniae: a 4-year perspective. Infect Control Hosp Epidemiol. 2011;32:673–678. doi: 10.1086/660358. [DOI] [PubMed] [Google Scholar]
- 28.Cheng V.C.C., Tai J.W.M., Chan W.M. Sequential introduction of single room isolation and hand hygiene campaign in the control of methicillin-resistant Staphylococcus aureus in intensive care unit. BMC Infect Dis. 2010;10:263. doi: 10.1186/1471-2334-10-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bearman G., Rosato A.E., Duane T.M. Trial of universal gloving with emollient-impregnated gloves to promote skin health and prevent the transmission of multidrug-resistant organisms in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2010;31:491–497. doi: 10.1086/651671. [DOI] [PubMed] [Google Scholar]
- 30.Bearman G.M., Marra A.R., Sessler C.N. A controlled trial of universal gloving versus contact precautions for preventing the transmission of multidrug-resistant organisms. Am J Infect Control. 2007;35:650–655. doi: 10.1016/j.ajic.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Cepeda J.A., Whitehouse T., Cooper B. Isolation of patients in single rooms or cohorts to reduce spread of MRSA in intensive-care units: prospective two-centre study. Lancet. 2005;365:295–304. doi: 10.1016/S0140-6736(05)17783-6. [DOI] [PubMed] [Google Scholar]
- 32.Gbaguidi-Haore H., Legast S., Thouverez M., Bertrand X., Talon D. Ecological study of the effectiveness of isolation precautions in the management of hospitalized patients colonized or infected with Acinetobacter baumannii. Infect Control Hosp Epidemiol. 2008;29:1118–1123. doi: 10.1086/592697. [DOI] [PubMed] [Google Scholar]
- 33.Soeken K.L., Sripusanapan A. Assessing publication bias in meta-analysis. Nurs Res. 2003;52:57–60. doi: 10.1097/00006199-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 34.LoBiondo-Wood G., Haber J. 6th ed. Mosby/Elsevier; St Louis, MO: 2006. Nursing research: methods and critical appraisal for evidence-based practice. [Google Scholar]
- 35.Shadish W.R., Cook T.D., Campbell D.T. Houghton Mifflin; Boston: 2002. Experimental and quasi-experimental designs for generalized causal inference. [Google Scholar]
- 36.Diab-Elschahawi M., Lusignani L.S., Starzengruber P. The strength of coughing may forecast the likelihood of spread of multi-drug resistant microorganisms from the respiratory tract of colonized patients. Antimicrob Resist Infect Control. 2014;3:38. doi: 10.1186/s13756-014-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park A. Nurses ‘infuriated’ by suggestion of Dallas Ebola protocol breach. Time. October 14th, 2014 http://time.com/3506907/nurses-protocol-breach-ebola/ Available at: [last accessed May 2015] [Google Scholar]
- 38.Shardell M., Harris A.D., El-Kamary S.S., Furuno J.P., Miller R.R., Perencevich E.N. Statistical analysis and application of quasi experiments to antimicrobial resistance intervention studies. Clin Infect Dis. 2007;45:901–907. doi: 10.1086/521255. [DOI] [PubMed] [Google Scholar]
- 39.Kirkland K.B. Taking off the gloves: toward a less dogmatic approach to the use of contact isolation. Clin Infect Dis. 2009;48:766–771. doi: 10.1086/597090. [DOI] [PubMed] [Google Scholar]
- 40.Weber D.J., Sickbert-Bennett E.E., Brown V.M. Compliance with isolation precautions at a university hospital. Infect Control Hosp Epidemiol. 2007;28:358–361. doi: 10.1086/510871. [DOI] [PubMed] [Google Scholar]
- 41.Swoboda S.M., Earsing K., Strauss K., Lane S., Lipsett P.A. Isolation status and voice prompts improve hand hygiene. Am J Infect Control. 2007;35:470–476. doi: 10.1016/j.ajic.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Abad C., Fearday A., Safdar N. Adverse effects of isolation in hospitalised patients: a systematic review. J Hosp Infect. 2010;76:97–102. doi: 10.1016/j.jhin.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan D.J., Diekema D.J., Sepkowitz K., Perencevich E.N. Adverse outcomes associated with contact precautions: a review of the literature. Am J Infect Control. 2009;37:85–93. doi: 10.1016/j.ajic.2008.04.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santos R.P., Mayo T.W., Siegel J.D. Healthcare epidemiology: active surveillance cultures and contact precautions for control of multidrug-resistant organisms: ethical considerations. Clin Infect Dis. 2008;47:110–116. doi: 10.1086/588789. [DOI] [PubMed] [Google Scholar]
- 45.Masse V., Valiquette L., Boukhoudmi S. Impact of methicillin resistant Staphylococcus aureus contact isolation units on medical care. PLoS One. 2013;8:e57057. doi: 10.1371/journal.pone.0057057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drinka P., Faulks J.T., Gauerke C., Goodman B., Stemper M., Reed K. Adverse events associated with methicillin-resistant Staphylococcus aureus in a nursing home. Archs Intern Med. 2001;161:2371–2377. doi: 10.1001/archinte.161.19.2371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.