Abstract
Brain lesions that damage the insular cortex interrupt addictive behaviors, suggesting that drug addiction sensitizes the insula. However, neuroimaging studies seem to lead to an opposite picture: structural neuroimaging studies show reduced grey matter volume of the insular cortex (IC) of drug users, and functional neuroimaging studies show reduced IC activity when drug users perform decision-making tasks. These results have been interpreted as indicating that addictive behaviors are associated with reduced interoceptive signaling within the IC. We use this apparent contradiction to examine possible roles of the insula in addiction, identify open questions, and explore ways to address them.
Keywords: insular cortex, drug dependence, smoking, decision-making
The insular cortex (IC) has been known for a while as a receiver of interoceptive signals, and a necessary substrate for experiencing emotion and self-awareness [1–3]. More recently its role in attention and decision-making has been gathering increased attention [4–6]. A growing body of research indicates that the decision process is a dynamic interplay between an implicit or automatic appetitive system, which promotes cue-induced habitual behaviors, and the executive control/inhibitory prefrontal cortex system [7]. The insular cortex, activated by homeostatic imbalance (such as deprivation from drugs), or by reward cues, plays a key role in this balancing process [8]. Since addiction to substances is invariably associated with physiological states that give rise to strong interoceptive signals, and since it involves flaws in the decision process (e.g., choosing immediate rewards at the expense of long-term negative consequences), it is not surprising that the insular cortex was found to be strongly involved in addictive behaviors. However, the specific nature and mechanisms of insular involvement in addiction are unclear and what we do know seems somewhat paradoxical.
Brain damage that destroys the IC seems to correct at least some of these addictive behaviors. For instance, individuals addicted to nicotine have been known to stop smoking instantly and effortlessly following IC damage [9]. Studies also indicate that such lesions may disrupt some of the cognitive distortions that draw gamblers to gambling behaviors[10]. These results suggest that the insula/interoceptive system is sensitized in these cases of addiction, such that its damage seems to correct the pathological state of addiction. Yet, many functional neuroimaging studies have shown that individuals with substance dependence show reduced IC activity when engaged in decision-making tasks [11–13] and this decrease in IC activation is predictive of relapse after a period of abstinence[14,15]. Also many voxel-based morphometry studies have revealed that substance dependent individuals (e.g., cocaine dependent individuals) have reduced grey matter volumes in certain brain regions, including the insular cortex[16]. These studies suggest that addicts have a desensitized interoceptive insula system.
At first glance, the two lines of studies (lesion studies on the one hand, and functional neuroimaging and voxel-based morphometry on the other) seem contradictory. In this article, we review the animal literature, human lesion studies, and both structural and functional neuroimaging studies in order to identify open questions and potential avenues to reconcile these apparent contradictions. Our ultimate goal is to motivate and drive forward a more subtle conception of insular influence on addiction that will foster new research and eventually yield specific, mechanistic insights. We start with a brief review of the insular cortex's heterogeneous anatomy and sub-regions in order to map out more precisely its role in addiction.
Heterogeneity of the Insular Cortex and its role in addiction
Based on its internal structure, three major subdivisions of the IC have been identified: (1) the granular insula, which is located in the posterior dorsal portion of the IC, (2) the agranular insula located in the anterior ventral portion of the IC, and (3) the dysgranular insula – a large band occupying the middle portion of the IC [17]. Although these anatomical subdivisions have been identified both in humans and animals, in the addiction literature their use has been limited to animal studies. Functional subdivisions that only partially overlap with the anatomical ones (Figure 1) are used instead in the human neuroimaging studies. Additional work is necessary to explain and reconcile these differences. We will use the anatomical subdivisions when describing the animal literature and the functional subdivisions when discussing the human neuroimaging studies. Unfortunately, human lesion studies do not provide specific locations within the IC, predominantly due to a restricted study population.
Figure 1. Subregions of the Insular Cortex.
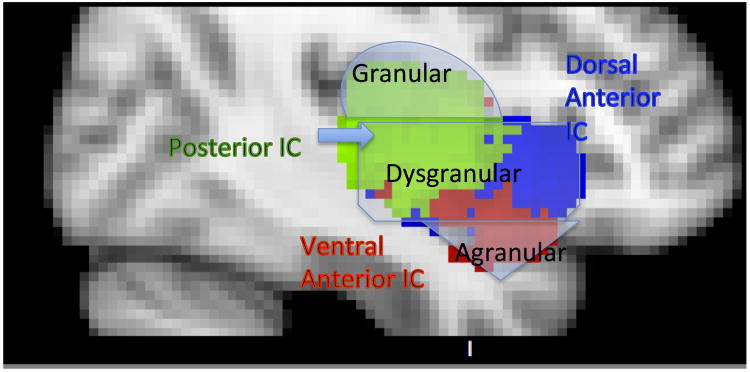
Sub-region masks [18] downloaded from NeuroVault.org: posterior (green), dorsal anterior (blue) and ventral anterior (red). Schematic anatomical subdivisions (granular, dysgranular and agranular) [17] are indicated in light grey, to provide association between functional and structural components.
Numerous functional parcellations of the IC have been performed utilizing different methodologies including functional connectivity analysis [18] and meta-analysis of neuroimaging data [18–22]. Most accounts converge on three sub-regions: (1) posterior insular cortex (PIC) responsible for sensorimotor, pain and language processing [18,23,24], (2) dorsal anterior insular cortex (dAIC) involved in higher cognition and executive control [18,25,26], and (3) ventral anterior insular cortex (vAIC) associated with social-emotional processing, chemosensation and autonomic function [18,27]. The most recent parcellation work [18] utilized a comprehensive multi-method approach by combining functional connectivity methods with a new meta-analysis framework to map out the networks of which insula sub-regions are a part, as well as their functionality. Their results identified three insular networks (Table 1).
Table 1.
Neural networks identified by functional connectivity analysis and by NeuroSynth meta-analysis consistent with insula subdivision into PIC, dAIC and vAIC [18].
| Network components identified by both analysis | Functionality | |
|---|---|---|
| Ventral-Anterior (vAIC) | Amygdala (AMY), ventral tegmental area (VTA), posterolateral orbitofrontal cortex | social-emotional processing, chemosensation and autonomic function |
| Dorsal-Anterior (dAIC) | anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (DLPFC) | higher cognition and executive control |
| Posterior (PIC) | supplementary motor area (SMA), somatosensory cortex | sensorimotor, pain and language processing |
The use of the full NeuroSynth database [28] (4393 studies at that time) in this study makes the findings generalizable. We used the masks resulting from that work (Figure 1) to remap prior published findings (for all studies that provided coordinates), because most authors identified the area of interest as simply insula, or anterior insula (AI), or posterior insula (PI). The portions of the current review that simply refer to “anterior/posterior insula” or “insula” discuss the findings for which coordinates were not available, and thus no greater anatomical specificity was possible.
One of the currently accepted notions on the role of the insula in addiction is that interoceptive signals generated from physiological states in the body (or somatic states), which are associated with the hedonic experience of drug use, initiate activation of the posterior insula. Signals are then transmitted to the anterior insula where somatic-marker representations reach awareness and are committed to memory [9], thus constituting affective learning of drug-effects and their associations with contexts. When a person is confronted with drug related stimuli after the learning is completed, the pathway involved during original drug exposure reverses and results in activation of the drug-seeking goal. Specifically, the previously stored somatic pattern associated with the experience of the drug is evoked by dAIC, represented through vAIC, which, in turn, initiates the physical sensation of craving that is processed by PIC. In addition, the lack of the desired substance may generate an urge that magnifies the value of the somatic marker representation (i.e., the conscious feelings of craving or urges may turn the volume up) hence amplifying the importance of the drug-seeking goal. These processes are facilitated by the insula's connections to what has been referred to as the impulsive system (which includes the amygdala, nucleus accumbens), as well as the reflective system (which includes hippocampus, anterior cingulate cortex (ACC) and ventromedial prefrontal cortex (VMPFC)) [7,9]. However, apparent contradictions seem to emerge when one considers lesion versus neuroimaging studies (both structural and functional). How are we to reconcile these findings and what can the apparent inconsistency tell us about IC involvement in addiction? Additional clues can be found in the large body of work studying the effects of IC lesions, both in animals and humans.
Evidence from lesions
Animal studies
The essential role of the IC in drug addiction is evident in the animal literature. Many of these studies used the conditioned place preference paradigm (CPP), in which rodents experience a drug administration in an environment that they do not normally prefer, and quickly develop a preference for this environment. A similar method is the conditioned place aversion (CPA) paradigm where an environment is paired with aversive stimuli.
In a CPP experiment with mice, the preference for the nicotine paired environment was extinguished after the experimenters lesioned the IC [29]. In another series of experiments using the CPP with rats, the learned preference for the amphetamine paired environment was extinguished after blocking IC function via injections of anesthetics (e.g., lidocaine [30], or anisomycin [31]).
In another group of studies, rats demonstrated reduced self-administration of nicotine after their granular IC function was blocked via an anesthetic injection [32], or a disabling electrical stimulation, i.e., the induction of a depolarization block [33]. One study [31] attempted to identify which subregion of the IC associates context and drug effects, however they obtained similar results (loss of CPP) irrespective of whether they injected anesthetic into posterior granular [30,31] or anterior agranular [31] regions of the IC. All these findings are consistent with the somatic marker theory outlined above: damage of the IC disrupts initiation of the drug seeking goal, damage of the AIC disrupts recall of the somatic pattern, and damage of the PIC disrupts initiation of craving sensations.
Interestingly, two of the above studies [32,33] that examined whether blocking IC function disrupts food seeking behavior, found no effect, thus suggesting that food motivation (which is fundamental to survival) may have alternate, non-insula mediated pathways.
Finally, to explore the insula's role (and its subdivisions) in affective learning that establishes the association between context and interoceptive drug effects, one study employed reversible inactivation of each of the two major subdivisions of the insula in rats, and they tested the effects of this inactivation on the acquisition of morphine-induced CPP, and CPA induced by naloxone-precipitated acute morphine withdrawal [34]. They found that inactivation of the posterior granular IC, but not the anterior agranular IC, disrupted acquisition of CPP. However the acquisition of CPA was disrupted by inactivation of either area. This suggests a potentially different mechanism for learning reward and punishment related cues. Additional research is needed to investigate whether this difference is specific to addiction, or it is a generic characteristic of the learning mechanism.
Altogether, both the granular and agranular insular cortices seem to play a major role in evoking associations between drug-cues and approach behavior, as well as in learning the aversive reactions associated with drug withdrawal; however, only the granular insular cortex is imperative in learning the association of positive drug-related sensations with context cues. Most importantly, all these studies suggest that lesions of the IC (or its experimentally induced inactivation) lead to the interruption of an addiction to a substance.
Human studies
Studies of brain lesions in humans are also consistent with animal studies. The first study that identified IC connection to smoking cessation found that patients, who were avid smokers prior to their suffering of stroke, were more likely to drop the habit if their brain damage included the insula [35], with the odds ratio over 136. Another study [36] found that strokes that involved the IC increased odds of quitting smoking within a year of the stroke fivefold compared to strokes that spared the IC. We note that one study that examined the effects of brain lesions on smoking behavior reported that increased likelihood of smoking cessation after insula lesion was not significant[37]. However, given that this study was conducted in a non-American culture, it was suggested [9] that a likely explanation for this discrepancy could relate to cultural differences in the perception of the long-term harms of smoking. Detailed analyses of the lesions revealed that the disruption of smoking addiction did not depend on whether it involved the anterior or posterior regions of the IC [35]. These findings replicate the outcomes of the animal studies discussed above and support the somatic marker account of the IC role in addiction: the AIC damage interferes with the recall of the somatic pattern and PIC damage interferes with induction of craving sensation; both steps seem to be necessary for initiating a drug seeking goal.
Interestingly, subsequent studies revealed that this disruption of smoking addiction is not exclusive to IC damage, i.e., damage to adjacent basal ganglia structures (e.g., the putamen region of the striatum) can also lead to smoking cessation [38]. However, when basal ganglia damage is combined with IC damage, the disruption of smoking addiction is intensified as reflected by a higher number of patients who quit smoking after their stroke (when compared to patients with only striatum lesions), and by a more sustained quitting over time [38], thus consistent with the notion that the IC plays a key role in addiction. These results are consistent with almost three decades of research showing that the basal ganglia (i.e., mesolimbic dopamine system and its projection to the ventral striatum) is necessary for drug motivation, and that when the addictive behavior becomes automatic and habitual, the dorsal striatum (which includes the putamen) becomes a key neural substrate [39]. But they are also consistent with the more recent findings that the insular cortex also plays a significant role in addiction. Hence the combined damage of the insula and the putamen leads to a stronger disruption of a substance addiction.
Together these studies suggest that disrupting IC functions actually abolishes the behavioral addiction to smoking, a finding that appears at odds with many neuroimaging studies (reviewed in the following section) that suggest that reduced volume or function of the insula reflects a compromised interoceptive system that underlies the decision to seek drugs, and the relapse to drug use after a period of abstinence.
Evidence from neuroimaging studies
Structural imaging studies examining gray matter volume and density differences between addicted individual and healthy controls show lower volume in the IC, in cocaine users [40–42], heroin users [40], cannabis users [43], methamphetamine users [42,44] and smokers [45,46]. The degree of this brain volume reduction seems to increase as a function of the number of years of drug use [41,47]. Among these studies, nearly all of those that allowed for inspection of the subdivisions of IC found differences in PIC.
A seemingly similar picture emerges from functional neuroimaging studies. Several studies have shown that methamphetamine dependent individuals show reduced activity in dAIC and PIC when performing decision-making tasks [12,13]. Other work has shown that substance users have reduced IC activation during positive and negative stimuli evaluation compared with non-using controls. Specifically, light adolescent smokers showed reduced dAIC activity when viewing “pleasurable” food stimuli [48]. Similarly, methamphetamine dependent individuals showed reduced insula activity when viewing IAPS (set of normed positive and negative emotional stimuli) images [49,50]. This reduction was evident to positive images in PIC [49] and negative images in dAIC [50]. These findings are not limited to visual stimuli, and they also extend to other interoceptive stimuli. Both current and recently abstinent methamphetamine dependent subjects showed a more attenuated PIC response, in response to interoceptive stimuli (e.g., pleasant touch) [51] and when exposed to negative interoceptive stimuli (e.g., breathing load manipulation) in PIC [13]. Finally, patients with alcohol dependence showed lower activity (compared to healthy controls) in the dAIC, and in the putamen, during tasks involving risky decisions [52]. All these findings have been interpreted as consistent with the notion of a desensitized interoceptive insula system in individuals with substance dependence.
Nonetheless, it is important to note that not all studies have yielded reduced IC activity in individuals addicted to various substances. For instance, one study of inhibition processing in smokers, using a monetary incentive Go-NoGo task, showed that successful inhibition of a NoGo response was linked to increased activation in the right dAIC (together with other prefrontal and parietal regions) in smokers compared to controls [53]. Another study reported that higher activation of the right dAIC in methamphetamine users was linked to greater likelihood of choosing a risky option [54]; however, in prior work with non-drug users the relationship between dAIC activation and risk preference was reversed [55]: an increase in insula activity led to a greater likelihood of choosing a safe option. Altogether, these two studies suggest that some substance users show increased, as opposed to decreased, IC activity during decision-making. It is not clear why these differences exist, but differences such as the severity of the addiction of the participants, abstinence status, or the behavioral performance of the participants on the experimental tasks (e.g., increased versus decreased performance scores) may play a role. It is also possible that the inconsistencies may have been caused by small sample size [56,57], or head motion [58].
Finally, several studies in individuals with substance use disorders reported increased IC responses across all IC subdivision to drug related cues and craving associated with those cues [8,59,60]. Importantly, these studies reporting increased IC activity in response to drug related cues did not involve decision-making or risky choice tasks.
In essence, with only a few exceptions, studies report reduced (as opposed to increased) IC activity for addicts when performing decision-making and evaluative tasks [11–13,48–52] (Fig. 2B). Because of some of these inconsistencies observed in IC activity in substance dependent individuals, some investigators have proposed that IC activation is possibly an “interoceptive” or “somatic marker” tuning mechanism that helps adjust the reward value of a stimulus to optimize the individual's choice in a way that best satisfies his/her internal and external environmental needs and goals [9,61,62]. Although these suggestions are intriguing and helpful in explaining potential differences in the dynamics of potentially different specialized functions mediated through different subdivisions of the insula itself, there is a fundamental question that remains unanswered, which is how does the IC's function change with addiction and, and what is the mechanism of this change. Obviously, answering this question is currently difficult. However, we will attempt to pose several directions for future research that could help address this key question.
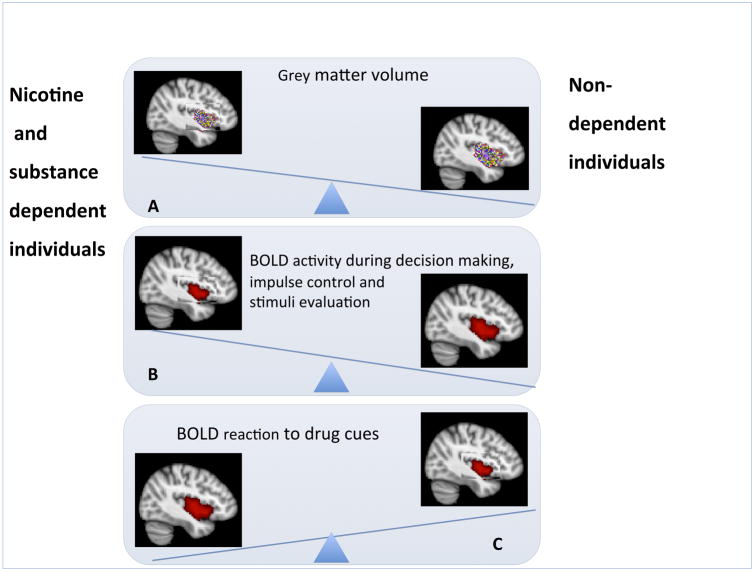
Figure 2. Summary of neuroimaging findings comparing nicotine and/or substance dependent and non-addicted individuals.
A. smaller grey matter volume in substance dependent individuals [40–46]. B. Most studies reveal decreased IC activity during decision-making [11–13] and stimuli evaluation [13,48–51] in substance dependent individuals, but also note exceptions [53,54]. C. Greater IC activity in response to drug cues in substance dependent individuals [8,59,60].
An insight from the functional connectivity literature
The network based approach considers the brain as a set of cohesive interconnected networks, as opposed to a collection of components. From examining the insula-centered networks, it has been proposed that the IC, together with the ACC, form a Salience Network (SN) that is responsible for coordinating resources between Default Mode (DMN: VMPFC & posterior cingulate cortex) and Central Executive (CEN: dorsolateral prefrontal cortex & posterior parietal cortex) networks [63]. Moreover, it has been shown that the IC plays a key role in this process by initiating network switching [4].
Some evidence from resting state functional connectivity (rsFC) studies suggests that the role of the IC in initiating network switching, as well as its synchronization with the amygdala and putamen, are deregulated in addicted individuals. This work suggests that functional connectivity of the IC is different in addicted individuals and may vary depending on their abstinence status. For example, weaker rsFC with ACC has been shown in opioid [64] and in cocaine users [65], suggesting that an overall weaker salience network is associated with drug use. This might be consistent with numerous earlier studies using functional neuroimaging or neuropsychological studies, which showed that drug users have a dysfunctional “reflective” system [7].
Recent examination of smokers after 24 hours of abstinence found that nicotine withdrawal (or deprivation) elevates the strength of amygdala-insula and insula–DMN connectivity, and the administration of nicotine down-regulates it [66]. Similarly, abstinent heroin users showed increased amygdala - IC connectivity [67]. Additional evidence that abstinence increases IC - amygdala rsFC was provided by another recent paper [65], that compared cocaine addicts who recently used (as evidenced by a positive urine test) with ones who did not (negative test). Abstinent users demonstrated stronger connectivity than active recent users. These findings, together with prior evidence that the insula plays a key role in monitoring and regulating homeostasis of bodily states [2,61,68], supports the hypothesis that the insula's influence on goal-directed drug seeking is achieved by increased synchronization of its activity with the amygdala, and by disrupting the salience network functionality [69]. When the goal is successfully achieved (drug is consumed), this imbalance is re-adjusted back to normal. Insula - amygdala synchronization may even be decreased in drug users compared to control subjects, as evidenced from a study with currently using (not abstinent) opiate users [64]. These findings are also consistent with earlier neural models of addiction that include the insula. For instance, the strengthening of an insula-amygdala system (which we consider as part of an “impulsive” system), while increasing an insula-DMN connectivity (which could be interpreted as a decreased engagement of an executive control or “reflective” system), is consistent with the proposal that withdrawal or deprivation exacerbates the influence of the “impulsive” system, while it “hijacks” the capacity of the “reflective” system to exert self-control [70]
Open questions
There are several potential avenues for future research that could help address this apparent inconsistency between lesion and neuroimaging studies. Although many studies have shown decreased gray matter in the insula of substance dependent individuals as reviewed earlier [40–46], unfortunately there are no studies that have attempted to look at an increase or decrease in the white matter associated with the IC itself. Also studies that address structural connectivity of the IC are currently lacking. Such studies should be conducted in the future to determine whether drug dependent individuals develop increased IC white matter, which could reflect an enhancement in function and automaticity, despite an observed decrease in gray matter. Similarly, efficiency of the insula-centric network should be examined based on diffusion tensor imaging data.
Another open question in the structural domain is whether the decreased PIC volume is the consequence or prerequisite of substance abuse. Studies that show a negative correlation between PIC volume and years of use [41,47] can be considered indirect evidence of the latter. However, this doesn't rule out the possibility of a bi-directional relationship. If smaller PIC volume is predictive of substance abuse vulnerability, it could be used together with family history and genetic predisposition to identify people at higher risk of drug abuse. A longitudinal study that would examine a large group starting in pre-adolescence, with follow-ups through adulthood, would be able to answer this question.
Concluding remarks
It is tempting to consider parsimonious theories such as ‘desensitized interoceptive insula system’ or ‘more efficient insula system’; both consistent with reduced activation, but the latter can also be used to explain why insula lesions often lead to loss of addiction. However, we must consider the fact that the IC is a part of a neural network, and its damage or lesion leads to changes in the network configuration that could alter its function. It is also likely that the changes in the insula-centered networks that are due to substance use or pre-existing differences in these networks predisposing to drug use can be responsible for the apparent contradictory roles of the IC in addiction. Several functional connectivity studies discussed earlier [64–69] provide initial evidence for the hypothesis that addiction is characterized by salience network dysregulation. More extensive research is necessary to confirm these preliminary findings and to determine the direction of these differences and the causal relationships.
Further understanding of the IC activity, insula-centered circuits, their functionality, and changes due to addiction can be promoted by not limiting ourselves to fMRI methodology. Specifically, use of interleaved transcranial magnetic stimulation (TMS) in combination with BOLD imaging may allow for a more experimental approach to these questions. A recent study demonstrates that optimized use of a TMS/BOLD sequence may allow for selective interrogation of cortical and subcortical [71]. Another promising approach is animal studies utilizing electrophysiological recordings and in vivo imaging [72,73]. This method may allow for understanding of neural computations performed by insular neurons, and visibility of potential structural changes with the IC. An advantage of this approach was demonstrated in a recent study that used in vivo imaging with mice and found that cocaine administration stimulated rapid increase in growth of new dendritic spines [73].
Finally, a potential explanation for this apparent contradiction between lesion and neuroimaging studies may lie in the heterogeneity of the IC that is only recently starting to be acknowledged in the functional neuroimaging literature, and which is yet to be examined in human lesion studies. When the mapping between anatomical and functional insula subdivisions is clarified, and we have a more precise understanding of each component's role in addiction, this apparent contradiction may be resolved.
Highlights.
Insula lesions lead to disruption of certain addictions (i.e., smoking)
Addicted individuals show reduced insula gray matter volume and activity.
These studies seem contradictory in explaining the role of the insula in addiction
We discuss open issues and future research directions to reconcile the contradiction
Acknowledgments
Authors Note: This manuscript was supported in part by NIDA Grant Number R01DA031626 to S. J. Read and NCI Grant Number R01CA152062 to A. Bechara.
Glossary of Terms
- Central executive network (CEN)
A network of brain regions that include the dorsolateral prefrontal and posterior parietal cortices, and which are active during executive control functions related to decision making and information maintenance in the context of goal-directed behavior [74]
- Conditioned Place Aversion (CPA)
Similar to CPP (below) except location is paired with aversive stimuli to condition avoidance
- Conditioned Place Preference (CPP)
Form of Pavlovian conditioning where location is paired with rewarding stimuli to condition preference
- Default Mode Network (DMN)
A network of brain regions that include the ventromedial prefrontal and posterior cingulate/ medial parietal cortices, and which are active when the individual is not focused on the outside world or during a passive state (i.e., when the individual is asked to do nothing) [74]
- Go-NoGo Task
Experimental task to evaluate attention and inhibition in which participant views a set of stimuli from one of two groups, and is instructed to press the button when the stimulus belongs to one group but not the other
- International Affective Picture System (IAPS)
A set of normed positive and negative emotional stimuli
- Resting-state Functional Connectivity (rsFC)
Approach that involves analyses of temporal synchronization of brain regions' activation patterns by measuring co-activation of resting state fMRI time series [75]
- Salience network (SN)
A network comprised of dorsal anterior insula and anterior cingulate cortex, responsible for identifying salient stimuli and events, and coordinating attention and working memory resources between central executive and default mode networks [63]
- Somatic-marker representations
Body-related responses that represent emotion [76]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Craig AD. Pain, temperature, and the sense of the body. In: Franzén PO, et al., editors. Somesthesis and the Neurobiology of the Somatosensory Cortex. Birkhäuser; Basel: 1996. pp. 27–39. [Google Scholar]
- 2.Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 3.Craig AD. The sentient self. Brain Struct Funct. 2010;214:563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- 4.Sridharan D, et al. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, et al. The Iowa Gambling Task in fMRI Images. Hum Brain Mapp. 2010;31:410–423. doi: 10.1002/hbm.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawrence NS, et al. Distinct Roles of Prefrontal Cortical Subregions in the Iowa Gambling Task. Cereb Cortex. 2009;19:1134–1143. doi: 10.1093/cercor/bhn154. [DOI] [PubMed] [Google Scholar]
- 7.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 8.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naqvi NH, et al. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci. 2014;1316:53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark L, et al. Damage to insula abolishes cognitive distortions during simulated gambling. Proc Natl Acad Sci. 2014;111:6098–6103. doi: 10.1073/pnas.1322295111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nestor L, et al. Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. NeuroImage. 2010;49:1133–1143. doi: 10.1016/j.neuroimage.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart JL, et al. Striatum and insula dysfunction during reinforcement learning differentiates abstinent and relapsed methamphetamine-dependent individuals: Striatum dysfunction associated with relapse. Addiction. 2014;109:460–471. doi: 10.1111/add.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart JL, et al. You Are the Danger: Attenuated Insula Response in Methamphetamine Users During Aversive Interoceptive Decision-Making. Drug Alcohol Depend. 2014 doi: 10.1016/j.drugalcdep.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo X, et al. Error processing and gender-shared and -specific neural predictors of relapse in cocaine dependence. Brain. 2013;136:1231–1244. doi: 10.1093/brain/awt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinha R. New Findings on Biological Factors Predicting Addiction Relapse Vulnerability. Curr Psychiatry Rep. 2011;13:398–405. doi: 10.1007/s11920-011-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackey S, Paulus M. Are there volumetric brain differences associated with the use of cocaine and amphetamine-type stimulants? Neurosci Biobehav Rev. 2013;37:300–316. doi: 10.1016/j.neubiorev.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morel A, et al. The human insula: Architectonic organization and postmortem MRI registration. Neuroscience. 2013;236:117–135. doi: 10.1016/j.neuroscience.2012.12.076. [DOI] [PubMed] [Google Scholar]
- 18.Chang LJ, et al. Decoding the Role of the Insula in Human Cognition: Functional Parcellation and Large-Scale Reverse Inference. Cereb Cortex. 2012;23:739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wager TD, Feldman Barrett L. From affect to control: Functional specialization of the insula in motivation and regulation. Publ Online PsycExtra. 2004 at < http://affective-science.org/pubs/2004/Wager_Edfest_submitted_copy.pdf>.
- 20.Mutschler I, et al. Functional organization of the human anterior insular cortex. Neurosci Lett. 2009;457:66–70. doi: 10.1016/j.neulet.2009.03.101. [DOI] [PubMed] [Google Scholar]
- 21.Kurth F, et al. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cauda F, et al. Meta-analytic clustering of the insular cortex. NeuroImage. 2012;62:343–355. doi: 10.1016/j.neuroimage.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 24.Wager TD. Placebo-Induced Changes in fMRI in the Anticipation and Experience of Pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 25.Eckert MA, et al. At the Heart of the Ventral Attention System: the Right Anterior Insula. Hum Brain Mapp. 2009;30:2530–2541. doi: 10.1002/hbm.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dosenbach NUF, et al. A Core System for the Implementation of Task Sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanfey AG, et al. The Neural Basis of Economic Decision-Making in the Ultimatum Game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- 28.Yarkoni T, et al. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott D, Hiroi N. Deconstructing craving: dissociable cortical control of cue reactivity in nicotine addiction. Biol Psychiatry. 2011;69:1052–1059. doi: 10.1016/j.biopsych.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Contreras M, et al. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- 31.Contreras M, et al. A Role for the Insular Cortex in Long-Term Memory for Context-Evoked Drug Craving in Rats. Neuropsychopharmacology. 2012;37:2101–2108. doi: 10.1038/npp.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forget B, et al. Granular Insular Cortex Inactivation as a Novel Therapeutic Strategy for Nicotine Addiction. Biol Psychiatry. 2010;68:265–271. doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 33.Pushparaj A, et al. Electrical Stimulation of the Insular Region Attenuates Nicotine-Taking and Nicotine-Seeking Behaviors. Neuropsychopharmacology. 2013;38:690–698. doi: 10.1038/npp.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li CL, et al. Effects of inactivating the agranular or granular insular cortex on the acquisition of the morphine-induced conditioned place preference and naloxone-precipitated conditioned place aversion in rats. J Psychopharmacol (Oxf) 2013;27:837–844. doi: 10.1177/0269881113492028. [DOI] [PubMed] [Google Scholar]
- 35.Naqvi NH, et al. Damage to the Insula Disrupts Addiction to Cigarette Smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suñer-Soler R, et al. Smoking Cessation 1 Year Poststroke and Damage to the Insular Cortex. Stroke. 2012;43:131–136. doi: 10.1161/STROKEAHA.111.630004. [DOI] [PubMed] [Google Scholar]
- 37.Bienkowski P, et al. Insular lesions and smoking cessation after first-ever ischemic stroke: A 3-month follow-up. Neurosci Lett. 2010;478:161–164. doi: 10.1016/j.neulet.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Gaznick N, et al. Basal Ganglia Plus Insula Damage Yields Stronger Disruption of Smoking Addiction Than Basal Ganglia Damage Alone. Nicotine Tob Res. 2014;16:445–453. doi: 10.1093/ntr/ntt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 40.Gardini S, Venneri A. Reduced grey matter in the posterior insula as a structural vulnerability or diathesis to addiction. Brain Res Bull. 2012;87:205–211. doi: 10.1016/j.brainresbull.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 41.Ersche KD, et al. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz DL, et al. Global and local morphometric differences in recently abstinent methampetamine dependrnt individuals. NeuroImage. 2010;50:1392–1401. doi: 10.1016/j.neuroimage.2010.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez-Larson MP, et al. Atered prefrontal and insular cortical thickness in adolescent marijuana users. Behav Brain Res. 2011;220:164–172. doi: 10.1016/j.bbr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakama H, et al. Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction. 2011;106:1474–1483. doi: 10.1111/j.1360-0443.2011.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durazzo TC, et al. Interactive effects of chronic cigarette smoking and age on brain volumes in controls and alcohol-dependent individuals in early abstinence: Interactive effects. Addict Biol. 2014;19:132–143. doi: 10.1111/j.1369-1600.2012.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morales AM, et al. Cigarette Exposure, Dependence, and Craving Are Related to Insula Thickness in Young Adult Smokers. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrós-Loscertales A, et al. Reduced striatal volume in cocaine-dependent patients. NeuroImage. 2011;56:1021–1026. doi: 10.1016/j.neuroimage.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 48.Rubinstein ML, et al. Adolescent Smokers Show Decreased Brain Responses to Pleasurable Food Images Compared With Nonsmokers. Nicotine Tob Res. 2011;13:751–755. doi: 10.1093/ntr/ntr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilman JM, Hommer DW. IMAGING STUDY: Modulation of brain response to emotional images by alcohol cues in alcohol-dependent patients. Addict Biol. 2008;13:423–434. doi: 10.1111/j.1369-1600.2008.00111.x. [DOI] [PubMed] [Google Scholar]
- 50.Kim YT, et al. The differences in neural network activity between methamphetamine abusers and healthy subjects performing an emotion-matching task: functional MRI study: NEURAL NETWORK OF METHAMPHETAMINE ABUSERS ON AN EMOTION TASK. NMR Biomed. 2011;24:1392–1400. doi: 10.1002/nbm.1702. [DOI] [PubMed] [Google Scholar]
- 51.May AC, et al. Current and former methamphetamine-dependent adults show attenuated brain response to pleasant interoceptive stimuli. Drug Alcohol Depend. 2014;140:e138. doi: 10.1016/j.drugalcdep.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li CR, et al. Altered Impulse Control in Alcohol Dependence: Neural Measures of Stop Signal Performance. Alcohol Clin Exp Res. 2009;33:740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luijten M, et al. Effects of reward and punishment on brain activations associated with inhibitory control in cigarette smokers: Reward, punishment and inhibition in smokers. Addiction. 2013 doi: 10.1111/add.12276. [DOI] [PubMed] [Google Scholar]
- 54.Gowin JL, et al. Altered cingulate and insular cortex activation during risk-taking in methamphetamine dependence: losses lose impact: Altered ACC and insula during risk among MD. Addiction. 2014;109:237–247. doi: 10.1111/add.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Button KS, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 57.David SP, et al. Potential Reporting Bias in fMRI Studies of the Brain. PLoS ONE. 2013;8:e70104. doi: 10.1371/journal.pone.0070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Dijk KRA, et al. The Influence of Head Motion on Intrinsic Functional Connectivity MRI. NeuroImage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Engelmann JM, et al. Neural substrates of smoking cue reactivity: A meta-analysis of fMRI studies. Neuroimage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schacht JP, et al. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review: Alcohol cue imaging. Addict Biol. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Upadhyay J, et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gu H, et al. Mesocorticolimbic Circuits are Impaired in Chronic Cocaine Users as Demonstrated by Resting State Functional Connectivity. NeuroImage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sutherland MT, et al. Down-Regulation of Amygdala and Insula Functional Circuits by Varenicline and Nicotine in Abstinent Cigarette Smokers. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.01.035. at < http://www.sciencedirect.com/science/article/pii/S0006322313001376>. [DOI] [PMC free article] [PubMed]
- 67.Xie C, et al. Identification of hyperactive intrinsic amygdala network connectivity associated with impulsivity in abstinent heroin addicts. Behav Brain Res. 2011;216:639–646. doi: 10.1016/j.bbr.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sutherland MT, et al. Insula's functional connectivity with ventromedial prefrontal cortex mediates the impact of trait alexithymia on state tobacco craving. Psychopharmacology (Berl) 2013;228:143–155. doi: 10.1007/s00213-013-3018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sutherland MT, et al. Resting state functional connectivity in addiction: Lessons learned and a road ahead. NeuroImage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noël X, et al. A neurocognitive approach to understanding the neurobiology of addiction. Curr Opin Neurobiol. 2013;23:632–638. doi: 10.1016/j.conb.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hanlon CA, et al. Probing the Frontostriatal Loops Involved in Executive and Limbic Processing via Interleaved TMS and Functional MRI at Two Prefrontal Locations: A Pilot Study. PLoS ONE. 2013;8:e67917. doi: 10.1371/journal.pone.0067917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lau B, Glimcher PW. Value Representations in the Primate Striatum during Matching Behavior. Neuron. 2008;58:451–463. doi: 10.1016/j.neuron.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Munoz-Cuevas FJ, et al. Cocaine-induced structural plasticity in frontal cortex correlates with conditioned place preference. Nat Neurosci. 2013;16:1367–1369. doi: 10.1038/nn.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic Reconfiguration of Structural and Functional Connectivity Across Core Neurocognitive Brain Networks with Development. The Journal of Neuroscience. 2011;31(50):18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. Http://doi.org/10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. European Neuropsychopharmacology. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 76.Bechara A, Damasio AR. The somatic marker hypothesis: A neural theory of economic decision. Games Econ Behav. 2005;52:336–372. [Google Scholar]