Abstract
Learning and memory for extinction of conditioned fear is a basic mammalian mechanism for regulating negative emotion. Sleep promotes both the consolidation of memory and the regulation of emotion. Sleep can influence consolidation and modification of memories associated with both fear and its extinction. After brief overviews of the behavior and neural circuitry associated with fear conditioning, extinction learning and extinction memory in the rodent and human, interactions of sleep with these processes will be examined. Animal and human studies suggest that sleep can serve to consolidate both fear and extinction memory. In humans, sleep also promotes generalization of extinction memory. Time-of-day effects on extinction learning and generalization are also seen. REM may be a sleep stage of particular importance for the consolidation of both fear and extinction memory as evidenced by selective REM deprivation experiments. REM sleep is accompanied by selective activation of the same limbic structures implicated in the learning and memory of fear and extinction. Preliminary evidence also suggests extinction learning can take place during slow wave sleep. Study of low-level processes such as conditioning, extinction and habituation may allow sleep effects on emotional memory to be identified and inform study of sleep’s effects on more complex, emotionally salient declarative memories. Anxiety disorders are marked by impairments of both sleep and extinction memory. Improving sleep quality may ameliorate anxiety disorders by strengthening naturally acquired extinction. Strategically timed sleep may be used to enhance treatment of anxiety by strengthening therapeutic extinction learned via exposure therapy.
Keywords: sleep, fear conditioning, extinction, memory, habituation, anxiety
1. Introduction
Two research domains of intense and growing interest in biological psychology are the study of fear extinction (Graham & Milad, 2011; Milad & Quirk, 2012) and that of sleep dependent memory consolidation (Rasch & Born, 2013; Walker & Stickgold, 2006). Here we review their combination in studies that attempt to understand how the processes of memory consolidation and integration that take place during sleep may strengthen and generalize fear extinction, a separate form of memory that coexists with the conditioned fear that it opposes (Bouton, Westbrook, Corcoran, & Maren, 2006; Pavlov, 1927). Importantly, fear extinction is a form of memory for which neural circuits of encoding, consolidation and retrieval have been well described in animal models (Herry et al., 2010; Maren, 2011; Quirk & Mueller, 2008) and which is of increasing interest to clinical investigators as the neurobehavioral basis of exposure therapy (Craske et al., 2008; Hermans, Craske, Mineka, & Lovibond, 2006; McNally, 2007). After brief overviews of fear conditioning, extinction and sleep-dependent memory consolidation, we will examine the neural circuits of extinction studied in animals and increasingly in humans via functional neuroimaging. Next we will review experimental studies of sleep’s effects on fear and extinction memory in animals and, especially, in humans. Lastly, we will describe the clinical implications of this research that are of emerging interest to translational neuroscience.
2. Fear conditioning and extinction
Fear conditioning occurs when an emotionally neutral stimulus is associated with an inherently aversive experience (unconditioned stimulus or US). The neutral stimulus thereby becomes a conditioned stimulus (CS) with the capability, on its own, to evoke a fearful conditioned response (CR). Human studies of fear conditioning and extinction have generally used skin conductance response (SCR), a reliable measure of sympathetic activation (Milad & Quirk, 2012), or fear potentiation of the blink startle response (Grillon & Baas, 2003; Jovanovic & Ressler, 2010) as psychophysiological CR indices of fear. When the CS is subsequently presented repeatedly without the US, extinction (reduction) of the CR typically takes place. However, rather than erasing the CS−US association, extinction represents formation of a new memory that competitively inhibits the memory of the CS−US contingency when the CS is subsequently encountered (Bouton et al., 2006; Herry et al., 2010; Ji & Maren, 2007; Konorski, 1967; Maren, 2011; Milad & Quirk, 2012; Myers & Davis, 2002; Pavlov, 1927; Quirk & Mueller, 2008). Evidence that this is the case includes the following phenomena that were first discovered in animals and have since been demonstrated in humans: (1) Spontaneous recovery: An extinguished CR re-emerges in response to the CS with the simple passage of time (Pavlov, 1927; Quirk & Gehlert, 2003; Rescorla, 2004). (2) Renewal: The CR returns when the extinguished CS is presented in a context differing from the context within which extinction occurred (Bouton, 2002, 2004; Bouton et al., 2006; Milad, Orr, Pitman, & Rauch, 2005; Vervliet, Baeyens, Van den Bergh, & Hermans, 2013). (3) Reinstatement: An isolated US, presented in the absence of the CS, causes return of the CR when the CS is subsequently presented (Bouton & Bolles, 1979; Dirikx, Hermans, Vansteenwegen, Baeyens, & Eelen, 2007; Rescorla & Heth, 1975). (4) Savings: The CR to a previously conditioned and then extinguished CS is more readily re-acquired (Rescorla, 2002). Such findings demonstrate that the original CS−US association remains in memory, and its expression is sometimes able to overcome the later-learned extinction memory. It should also be noted, however, that recent studies suggest that, under certain circumstances, conditioned fear can indeed be erased, presumably by blockade of reconsolidation of a retrieved memory via pharmacological or behavioral interventions (Maren, 2011; Monfils, Cowansage, Klann, & LeDoux, 2009; Quirk et al., 2010; Schiller & Delgado, 2010; Schiller et al., 2010).
3. Sleep-dependent memory consolidation
As in other memory systems, extinction memory must be encoded, consolidated, and then retrieved in order to be expressed at a later time. Sleep has most often been associated with the consolidation stage of memory formation (Diekelmann & Born, 2010; Diekelmann, Wilhelm, & Born, 2009; Rasch & Born, 2013; Stickgold, 2005) including processes related to prioritization and integration of newly acquired memories with existing stores (Landmann et al., 2014; Pace-Schott, Nave, Morgan, & Spencer, 2012; Payne, Chambers, & Kensinger, 2012; Stickgold & Walker, 2013). More recent research shows evidence of sleep’s role in preparing the brain to encode new declarative memory (Yoo, Hu, Gujar, Jolesz, & Walker, 2007). Likewise, prior sleep may act at the retrieval stage to protect previously encoded memories from retroactive interference (Ellenbogen, Hulbert, Stickgold, Dinges, & Thompson-Schill, 2006) or to facilitate updating during reconsolidation (Deliens, Schmitz, et al., 2013).
4. Neural Circuits Associated with Fear Conditioning and Extinction
Translational studies directed toward greater understanding of extinction in humans have greatly benefitted from earlier and ongoing studies on the neural circuitry and neurochemistry of extinction in rodent models (Milad & Quirk, 2012). Recently, these findings have been augmented by a rapidly expanding literature on functional neuroimaging of extinction in healthy individuals and those with psychiatric disorders (reviewed in Milad & Quirk, 2012; Milad & Rauch, 2012; Shin & Liberzon, 2010). These animal and human findings are briefly discussed in the following sections (for more extensive reviews, see: Graham & Milad, 2011; Herry et al., 2010; Maren, 2011; Maren, Phan, & Liberzon, 2013; Maren & Quirk, 2004; Milad & Quirk, 2012; Milad & Rauch, 2012; Myers & Davis, 2007; Pape & Pare, 2010; Quirk & Mueller, 2008; Sotres-Bayon, Cain, & LeDoux, 2006).
Before proceeding, it bears repeating that any memory (of which both fear conditioning and fear extinction are examples in the emotional domain) are physically instantiated as plastic changes in neural circuitry. If such changes in circuitry are to be expressed behaviorally at times subsequent to their formation, the following steps must take place. Plasticity is first acquired as a result of experience (encoding or learning). It then must then be made to endure (strengthening or consolidation), a process during which additional plastic changes can occur (“off-line” integration, prioritization, etc.). Finally, then it must then be accessed (retrieved) in order to be behaviorally expressed. Sleep can influence all three of these processes (see sections 3. and 5.B.), however, the majority of studies, including those on fear conditioning and fear extinction, have focused on the consolidation process.
4.A. Fear conditioning and fear extinction circuitry in the rodent
In the rat, the plasticity associated with learning and storage of conditioned fear occurs within the basolateral amygdala (BLA) (Pape & Pare, 2010) and the prelimbic prefrontal cortex (PL) is necessary for the expression of this conditioned fear (Corcoran & Quirk, 2007a; Graham & Milad, 2011; Milad & Quirk, 2012). Although the plasticity associated with learning and storage of fear extinction also occurs within the BLA (Pape & Pare, 2010), the infralimbic prefrontal cortex (IL) is necessary for the consolidation and retrieval of extinction memory in the rat (Herry et al., 2010; Lebron, Milad, & Quirk, 2004; Milad & Quirk, 2002, 2012; Milad, Rauch, Pitman, & Quirk, 2006; Milad, Vidal-Gonzalez, & Quirk, 2004; Quirk, Garcia, & Gonzalez-Lima, 2006; Quirk & Mueller, 2008). When extinction is recalled, the IL activates gamma-aminobutyric acid (GABA)-ergic circuits within the amygdala that inhibit output from its central nucleus (CeA) to autonomic and brainstem circuits controlling fearful behavior (Maren & Quirk, 2004; Pape & Pare, 2010; Quirk & Mueller, 2008). N-methyl-D-aspartate (NMDA) receptor activations in the BLA are necessary for extinction learning and, in the IL, for consolidation of extinction memory (Pape & Pare, 2010; Santini, Muller, & Quirk, 2001). During extinction recall, hippocampal input provides contextual information that favors expression of extinction memory in contexts similar to those in which extinction was learned, but otherwise favors expression of conditioned fear (Bouton et al., 2006; Ji & Maren, 2007; Pape & Pare, 2010).
4.B. Fear conditioning and extinction circuitry in humans based on functional neuroimaging studies
In humans, functional neuroimaging studies of fear conditioning, extinction learning, and extinction recall have shown involvement of limbic and paralimbic areas homologous to those essential for these processes in the rat (Graham & Milad, 2011; Milad & Quirk, 2012). These structures include the ventromedial prefrontal cortex (vmPFC), believed to be homologous to the rat IL, and the dorsal anterior cingulate cortex (dACC), believed homologous to the rat IL (Vertes, 2006) as well as the amygdala and hippocampus. The human vmPFC is a functionally and anatomically diverse structure involved in multiple emotion-related processes such as consolidation of emotional memories (Nieuwenhuis & Takashima, 2011) including extinction (Corcoran & Quirk, 2007b), top-down regulation of amygdala activity (Myers-Schulz & Koenigs, 2012) and value-based decision making (O’Doherty, 2011). The dACC is a similarly diverse region for which the most generalized function has been suggested to be predicting the value of exerting effort and control (Shenhav, Botvinick, & Cohen, 2013) but, In the emotional domain, has been increasingly implicated in negative emotional responses (Etkin, Egner, & Kalisch, 2011; Etkin & Wager, 2007). As in other mammals, the human amygdala is involved in the affective labeling of stimuli particularly fear (Janak & Tye, 2015) and interacts continuously with the hippocampus in processing emotional aspects of episodic memories (Fanselow & Dong, 2010; McGaugh, 2004).
Milad and colleagues propose two distinct, mutually opposed networks for the expression of conditioned fear and extinction memory (Graham & Milad, 2011; Milad & Quirk, 2012; Milad & Rauch, 2012). The amygdala and dACC activate during expression of conditioned fear (Figure 1A) (Milad, Quirk, et al., 2007), whereas the vmPFC becomes activated during extinction learning and recall (Figure 1B) (Milad, Wright, et al., 2007). Therefore, activation of the fear network would promote the experience and expression of fear whereas activation of the extinction network would inhibit such fear, possibly by the top-down inhibition of amygdala output seen in the rodent. As in rodents, contextual factors determine whether or not extinction recall recruits the hippocampus (Kalisch et al., 2006; Milad, Wright, et al., 2007). Additional detail on findings from each stage of conditioning and extinction in humans follow.
Figure 1.
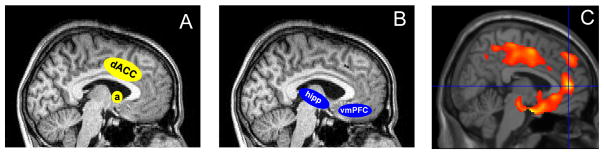
Neural circuits associated with expression of conditioned fear and fear extinction memory overlap with limbic areas that reactivate during REM sleep from quiescence state in NREM sleep. (A) fear expression network (B) fear extinction network. (C) anterior paralimbic REM activation area. Hipp, hippocampus; vmPFC, ventromedial prefrontal cortex; dACC, dorsal anterior cingulate cortex; a, amygdala.
4.B.1. Fear conditioning
Fear conditioning is associated with amygdala activation in both cued (Knight, Smith, Cheng, Stein, & Helmstetter, 2004; LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998; Linnman, Rougemont-Bucking, Beucke, Zeffiro, & Milad, 2011; Milad, Wright, et al., 2007; Phelps, Delgado, Nearing, & LeDoux, 2004) and contextual (Alvarez, Biggs, Chen, Pine, & Grillon, 2008) conditioning paradigms. For example, Milad et al., (2007) used the blood oxygen level dependent (BOLD) response of fMRI to show right dorsal amygdala activation during initial presentations of CS+/US pairs. Amygdala activity declined (habituated) over the course of conditioning but two areas of vmPFC remained deactivated throughout conditioning. A region homologous to the rat PL has been identified in the dACC in which both functional activity and cortical thickness are correlated with expression of conditioned fear (Linnman, Zeidan, Pitman, & Milad, 2012; Milad, Quirk, et al., 2007).
4.B.2. Extinction learning
Extinction learning has been associated with amygdala (Knight et al., 2004; Milad, Wright, et al., 2007; Phelps et al., 2004) and vmPFC activity (Milad, Wright, et al., 2007; Phelps et al., 2004). For example, (Milad, Wright, et al., 2007) showed that, by the end of extinction trials, SCR had equalized between the CS+ and CS− (i.e., psychophysiological extinction had occurred), but the BOLD response to the CS+ vs. CS− remained elevated in the vmPFC and left amygdala. Such enduring BOLD elevations, despite behavioral extinction by the end of the extinction-learning phase, may reflect persistence of the neural substrates of the original fear memory (i.e., that CS+ is more dangerous than CS−). At the behavioral level, the persistence of this memory trace has been repeatedly demonstrated by the phenomena of spontaneous recovery, contextual fear renewal, reinstatement and savings (see Section 2. above).
4.B.3. Extinction recall
fMRI studies have also shown that sites in the vmPFC become activated during extinction recall (Milad, Wright, et al., 2007; Rauch, Shin, & Phelps, 2006). Such vmPFC regions include the subgenual anterior cingulate cortex, an area homologous to the rat IL (Ongur, Ferry, & Price, 2003; Phelps & LeDoux, 2005; Vertes, 2004, 2006). For example, Phelps et al. (2004) showed that, whereas amygdala activity correlated with SCR during fear conditioning and initial extinction, delayed recall of extinction memory (tested 24 hours after fear conditioning and initial extinction) was correlated with activity in the subgenual anterior cingulate cortex. Moreover, Milad, Quinn, et al. (2005) showed that thickness of the vmPFC was positively related to the degree of psychophysiologically expressed extinction recall. Milad, Wright, et al. (2007) fear conditioned participants to two CS+ stimuli in one context and then extinguished fear of one of them in a second context. Extinction recall was tested 24 hours later in the extinction context and BOLD activity to the CS+E (conditioned and extinguished) was contrasted with that to the CS+U (conditioned only) thereby isolating activity unique to extinction recall while controlling for recall of fear conditioning. This contrast showed elevated activity in two areas of vmPFC close to those described during extinction recall by Phelps et al. (2004).
Contextual factors determine whether or not extinction recall recruits the hippocampus in addition to the vmPFC (Kalisch et al., 2006; Milad, Wright, et al., 2007). For example, during extinction recall in the context associated with extinction learning, activity in both the vmPFC and hippocampus correlated positively with the degree of psychophysiologically expressed extinction recall, and activities in the vmPFC and hippocampus were significantly correlated with each other (Milad, Wright, et al., 2007). Milad et al. (2007) suggested that contextual information, resulting from differentiation of the conditioning and extinction contexts, was represented by such hippocampal activity. Kalisch et al. (2006) also reported correlated activation of hippocampus and vmPFC during extinction recall when the CS+ was presented in the extinction context but not in the conditioning context. Kalisch et al. (2006) similarly concluded that contextual information from the hippocampus supported extinction recall in the vmPFC, but only in the presence of the extinction context. Therefore, activation of a hippocampal-vmPFC network during extinction recall has been replicated in two different experimental designs in which contextual information helped predict the outcome.
4.B.4. Extinction and higher-level, cognitive regulation of emotion
Activity in the dorsolateral prefrontal cortex (DLPFC) is correlated with that of the vmPFC during cognitive reappraisal of a fear-conditioned stimulus (Delgado, Nearing, Ledoux, & Phelps, 2008). Delgado et al. (2008) suggested that DLPFC areas subserving higher cognition recruit more phylogenetically primitive neuronal networks in the vmPFC that, in turn, regulate fear-associated activity in the amygdala during this uniquely human form of emotional regulation.
4.C. Sex differences in fear extinction memory
Recent publications by our research team have introduced comparisons between the sexes into a human model of extinction memory (Graham & Milad, 2013; Lebron-Milad, Abbs, et al., 2012; Lebron-Milad, Graham, & Milad, 2012; Lebron-Milad & Milad, 2012; Milad, Goldstein, et al., 2006; Milad & Quirk, 2012; Milad et al., 2010; Zeidan et al., 2011). Translational studies with both humans (Graham & Milad, 2013; Lebron-Milad, Abbs, et al., 2012; Milad, Goldstein, et al., 2006; Milad et al., 2010; Zeidan et al., 2011) and rodents (Graham & Milad, 2013; Milad, Igoe, Lebron-Milad, & Novales, 2009; Zeidan et al., 2011), have shown that extinction memory is sexually dimorphic (better in males) and that it varies across the menstrual cycle in females (Milad, Goldstein, et al., 2006; Milad et al., 2010). Moreover, conditions of high endogenous or exogenous estrogen enhance extinction memory in both women and female rats (Graham & Milad, 2013; Milad et al., 2010; Zeidan et al., 2011).
5. Interaction of Fear, Extinction and Sleep
Published studies on interactions of sleep with fear conditioning and its extinction have multiplied over the past decade. In the following sections, we briefly review activation patterns in the emotional brain during sleep noting evidence that REM sleep may be of particular importance to emotional memory processing. We then briefly review the neural bases of sleep-dependent memory consolidation. Then we review studies on sleep, fear conditioning and extinction in animals followed by those in humans. Lastly we attempt to reconcile, based upon findings to date, apparent discrepancies in these findings.
5.A. Activation of the emotional brain during sleep in humans and rodents
Mammalian sleep shows distinct changes in forebrain neuromodulation and regional activation that cycle predictably between REM and non-REM (NREM) sleep (Dang-Vu et al., 2010; Pace-Schott & Hobson, 2002). The transition from wake to NREM sleep is accompanied by global reductions in wake-promoting aminergic and cholinergic neuromodulation (reviewed in Pace-Schott & Hobson, 2002) and widespread forebrain de-activation (Braun et al., 1997; Maquet et al., 1997; Nofzinger et al., 2002) that decreases further with the deepening of NREM sleep (Dang-Vu et al., 2010; Kaufmann et al., 2006). However, with the transition from NREM to REM distinctive regions of the midline subcortex and cortex re-activate as was revealed by greater activation in REM versus NREM in early H215O positron emission tomography (PET) studies. Such reactivated regions included the pons and midbrain (Braun et al., 1997; Maquet et al., 1996), thalamus (Braun et al., 1997; Maquet et al., 1996), basal ganglia (Braun et al., 1997), amygdala (Maquet et al., 1996), hypothalamus, and ventral striatum (Braun et al., 1997), as well as limbic cortices including rostral and subcallosal anterior cingulate (Braun et al., 1997; Maquet et al., 1996), anterior insula, caudal orbitofrontal, paracingulate [Brodmann Area (BA) 32], medial prefrontal cortex (BA10) (Braun et al., 1997), parahippocampal gyrus and temporal pole (Braun et al., 1997). Very similar midline regions showing greater activation in REM compared to wakefulness were observed using 18Flurordeoxyglucose PET and have been characterized as an “anterior paralimbic REM activation area” (Nofzinger et al., 2004; Nofzinger, Mintun, Wiseman, Kupfer, & Moore, 1997) (Figure 1C). This area includes the septum, hypothalamus, ventral striatum and pallidum, hippocampus and uncus, as well as insular, anterior cingulate, orbitofrontal, and supplementary motor cortices (Nofzinger et al., 2004; Nofzinger et al., 1997) (Figure 1C). These paralimbic regions include many of the cortical and subcortical structures implicated in emotional learning, memory and expression including those of fear conditioning (e.g., amygdala and dACC) (Figure 1A) and extinction (e.g., vmPFC and hippocampus) (Figure 1B).
As in humans, there is an intimate association between REM sleep and activation of limbic regions in rodents. In the rat, the amygdala shows higher firing rates during wakefulness and REM sleep and lower firing rates during NREM (Jha, Ross, & Morrison, 2005). The amygdala is widely interconnected with limbic and brainstem regions that directly influence wakefulness and sleep as well as emotion (Jones, 2005). For example, inactivation of the amygdala decreases sleep latency, and increases slow wave activity (Tang, Yang, Liu, & Sanford, 2005), and its ablation increases sleep consolidation and total sleep time (Jha, Ross, et al., 2005).
5.B. Neurobiological bases of sleep-dependent consolidation of emotional memory
Sleep as a whole, its different stages as well as its phasic events can show distinct influences on the different memory systems (Ackermann & Rasch, 2014; Fogel & Smith, 2011; Rasch & Born, 2013). For example, the dual process hypothesis implicates REM sleep in consolidation of non-declarative memories resulting from procedural and emotional learning and NREM, particularly its slower EEG oscillations, in consolidation of newly acquired declarative memory (Ackermann & Rasch, 2014; Rasch & Born, 2013). Evidence also exists for a sequential processing model whereby both early-night slow wave sleep and REM during late sleep may be required for consolidation of particular types of learning (Stickgold & Walker, 2013; Stickgold, Whidbee, Schirmer, Patel, & Hobson, 2000). Processes occurring during REM sleep have consistently been invoked in the consolidation and regulation of emotional memory (reviewed in Deliens, Gilson, & Peigneux, 2014; Genzel, Spoormaker, Konrad, & Dresler, 2015; Goldstein & Walker, 2014; Walker & van der Helm, 2009), such as the dual functions for REM of episodic-content consolidation and emotional de-potentiation proposed by the “Sleep to Remember, Sleep to Forget” model (Walker & van der Helm, 2009). REM has also been suggested to be key to the integration of recently acquired information into existing networks in a flexible manner that allows novel associations to be expressed in the form of creativity (Cai, Mednick, Harrison, Kanady, & Mednick, 2009; Genzel et al., 2015; Stickgold, Hobson, Fosse, & Fosse, 2001). The unique neuromodulatory milieu of REM sleep, in which forebrain activation is modulated primarily by acetylcholine (ACh) rather than the complex aminergic, cholinergic and peptidergic activation of wakefulness (Pace-Schott & Hobson, 2002), is believed to impact the way in which memories are processed during this sleep stage (Stickgold et al., 2001; Stickgold & Walker, 2013).
Based upon the known neuroanatomy of different memory systems and rapidly emerging experimental findings, distinct sleep-dependent neurobiological mechanisms for consolidation in different memory systems have been proposed. For example, the widely accepted model of hippocampal-neocortical dialog (Buzsaki, 1996), proposing that memories initially encoded in the hippocampus are transferred to more permanent storage in the cortex during slow wave sleep (SWS), is a key tenet of explanatory theories on sleep dependent consolidation of declarative memory (Diekelmann & Born, 2010; Diekelmann et al., 2009; Hobson & Pace-Schott, 2002; Rasch & Born, 2013; Stickgold, 2005). Subcortical elements in addition to the hippocampus are posited in sleep-dependent mechanisms for consolidation in other memory systems such as the striatum in procedural memories (Albouy, King, Maquet, & Doyon, 2013) and the amygdala in emotional memory (Genzel et al., 2015). It is important to note that, although separable in theory, different memory systems interact in the consolidation of specific memories and share overlapping circuitry. For example, among emotional memory systems, the hippocampus plays a key role in contextual modulation of activity in the amygdala-vmPFC circuitry underlying extinction memory (Ji & Maren, 2007) and REM sleep differentially affects hippocampus–dependent versus hippocampus–independent tasks (Fu et al., 2007). Similarly, among procedural memory systems, the hippocampus provides contextual modulation of motor-sequence learning (Albouy et al., 2013). Moreover, a procedural task shown to consolidate across either wake or sleep when learned in isolation, requires sleep to consolidate when learned simultaneously with a declarative memory task (Robertson, 2009).
Despite such complexity, experimental findings on sleep-dependent memory consolidation in both animals and humans map well onto particular models, especially the hippocampal-neocortical processing of episodic learning. For example, in rats, firing patterns of hippocampal place-cells that accompany learning in waking are replayed during subsequent slow wave sleep (Skaggs & McNaughton, 1996; Wilson & McNaughton, 1994) in a compressed time frame (Nadasdy, Hirase, Czurko, Csicsvari, & Buzsaki, 1999). The coordinated occurrence during rat slow wave sleep of cortical EEG sleep spindles (phasic events, approximately 1 second in duration, consisting of 11–15 Hz oscillations with a pattern of waxing and waning amplitude) and EEG sharp-wave ripple complexes (a high amplitude slow wave co-occurring with a rapid approximately 200 Hz oscillation) that arise in the CA3 field of the hippocampus (Siapas, Lubenov, & Wilson, 2005; Siapas & Wilson, 1998) is widely believed to represent a mechanism whereby sleep promotes such hippocampal-neocortical memory transfer (Abel, Havekes, Saletin, & Walker, 2013; Rasch & Born, 2013). In humans, this model is supported by correlations seen between of memory for newly acquired declarative information learned before sleep (e.g., word associates, visual memory) and the quantity of Stage 2 NREM sleep spindles (Fogel & Smith, 2011) as well as delta frequency (0.5–4.0 Hz) and slow (<1 Hz) oscillations during the sleep following learning (Ackermann & Rasch, 2014; Rasch & Born, 2013). The slow (<1Hz) oscillation, believed to reflect synchronized alternation in cortical circuits of prolonged hyperpolarization (“down state”) and depolarization with rapid firing (“up state”), is assigned particular significance as a potential driver of both sleep spindles and sharp-wave ripple complexes (Abel et al., 2013; Rasch & Born, 2013). Such findings have, in the past decade, produced a voluminous literature, including reports on experimental enhancement of pre-sleep learning using electrical and auditory stimulation at slow-oscillation frequencies during human SWS (Marshall, Helgadottir, Molle, & Born, 2006; Ngo, Martinetz, Born, & Molle, 2013). While beyond the scope of this article, these findings are summarized in several outstanding recent reviews (Abel et al., 2013; Rasch & Born, 2013; Stickgold & Walker, 2013).
Recent speculation on the role of REM in fear conditioning and extinction memory suggests analogous offline processing of these emotion-based memories in the amygdala-vmPFC-hippocampal circuitry involved in the encoding and retrieval of extinction during wakefulness (Genzel et al., 2015). Notably, the rat p-wave, a REM-associated ascending phasic potential from the brainstem to the limbic subcortex, has been shown to be essential for extinction memory (Datta & O’Malley, 2013). In this study, among rats that underwent contextual fear conditioning followed by extinction training, only those who displayed an increase in p-waves during post-training sleep retained their extinction memory. The forebrain targets of the rat p-wave include the amygdala and hippocampus (Datta, Siwek, Patterson, & Cipolloni, 1998), p-waves increase during REM following learning trials on an active avoidance task (Datta, 2000) and this increase is accompanied by increased expression of plasticity related genes in these same limbic structures (Datta, Li, & Auerbach, 2008).
As might be expected given the shared circuitry among memory systems, recent animal experiments have shown evidence of neuronal replay during sleep following fear conditioning and extinction. Conditioned fear memories can be strengthened or weakened during sleep. Rolls et al. (2013) fear conditioned mice using an odor CS and footshock US. When the conditioned odor was presented during sleep, subsequent waking fear responses to this CS were strengthened. However these same responses could be weakened if a protein synthesis inhibitor was injected during sleep along with the CS. Such experiments demonstrate the possibility for reactivation of fear memory during sleep allowing either the reconsolidation or blockade of that reconsolidation, a phenomenon that has been demonstrated during wakefulness (Nader, Hardt, & Lanius, 2013). Further evidence of such replay in the rat was reported following fear conditioning during olfactomimetic stimulation of the olfactory bulb (Barnes & Wilson, 2014). After differential fear conditioning to olfactomimetic stimulation in a specific olfactory bulb locus (CS+), precise replay of CS+ olfactomimetic stimulation during SWS strengthened conditioned fear memory in subsequent waking. Additionally, olfactomimetic stimulation of a previously unstimulated locus during SWS promoted generalization of fear responses to stimulation at that same locus in subsequent waking. However precise replay of CS+ olfactomimetic stimulation during waking induced extinction of the CR, suggesting different connectivity between olfactory areas and fear and extinction circuitry during these two behavioral states.
In addition to studies on systems-level neuronal replay and oscillation, the genetic, molecular and synaptic mechanisms that underlie sleep’s influence on learning and memory are also areas of intense current investigation in animal models (for a comprehensive review, see Abel et al., 2013). Sleep- deprivation as well as natural-sleep paradigms have demonstrated that critical periods of post-learning sleep facilitate key synaptic, second messenger, gene transcriptional and protein synthetic processes required for memory consolidation (for reviews see Abel et al., 2013; Graves, Pack, & Abel, 2001). For example, sleep enhances and sleep deprivation diminishes expression of plasticity-related genes such as those involved in protein synthesis (da Costa Souza & Ribeiro, 2015) as well as the subsequent translation of their mRNA transcripts to proteins involved in learning and memory such as the cAMP-response element binding protein (CREB) (Abel et al., 2013). At the synapse, sleep may facilitate NMDA receptor-dependent memory processes as evidenced by impairment of hippocampal long-term potentiation (LTP) by sleep deprivation (McDermott et al., 2003) and sleep restriction (Kopp, Longordo, Nicholson, & Luthi, 2006) as well as by selective REM deprivation (Ishikawa et al., 2006). Alterations in glutamatergic neurotransmission, including both NMDA- and α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-receptor functioning, may underlie these effects (reviewed in Abel et al., 2013). Loss of extinction memory following REM deprivation (Fu et al., 2007) therefore suggests that REM sleep may facilitate NMDA-dependent consolidation of extinction memory, a process also facilitated by the NMDA partial agonist D-cycloserine (Ledgerwood, Richardson, & Cranney, 2005). At the synaptic level, evidence exists that NREM slow-wave sleep can lead to either the potentiation via LTP-like processes (Abel et al., 2013) or de-potentiation (Tononi, 2009; Tononi & Cirelli, 2003) of excitatory cortical synapses (the latter being a key tenet of the “synaptic homeostasis hypothesis”). Notably, both potentiation and de-potentiation can contribute to the synaptic plasticity that supports learning and memory (Abel et al., 2013).
5.C. Sleep effects of fear conditioning and fear extinction in rodents
5.C.1. Sleep and fear conditioning in the rat
High-severity stressors such as unavoidable electric shock, prolonged immobilization or fear conditioning reduce REM sleep in rodents (Pawlyk, Morrison, Ross, & Brennan, 2008). A major effect of fear conditioning in rats is to disrupt REM continuity by increasing the number of brief vs. continuous REM episodes (Madan et al., 2008). Contextual and discrete cues associated with past fear conditioning can also reduce and fragment REM in the rat (Jha, Brennan, Pawlyk, Ross, & Morrison, 2005; Pawlyk et al., 2008; Sanford, Tang, Ross, & Morrison, 2003) for as long as 2 weeks post-conditioning (Pawlyk et al., 2008). The BLA has been identified as a site at which both acute stress and fear-conditioned stimuli exert their sleep-disruptive effects (Wellman, Fitzpatrick, Machida, & Sanford, 2014) and these effects have been linked to central actions of corticotropin releasing factor (CRF) (Liu et al., 2011; Wellman, Yang, Ambrozewicz, Machida, & Sanford, 2013; Yang, Tang, Wellman, Liu, & Sanford, 2009). Notably, fear-conditioning-induced sleep disruption can be ameliorated by extinction training (Wellman, Yang, Tang, & Sanford, 2008). Importantly, however, the sleep-disruptive and, in particular, REM-disruptive effects of experimental stressors appear mainly with inescapable forms of stress and Pavlovian cued and contextual fear conditioning are canonical examples of such inescapable stress (Sanford, Suchecki, & Meerlo, 2014; Sanford, Yang, Wellman, Liu, & Tang, 2010). Conversely, escapable shock, such as occurs in active avoidance learning paradigms, can instead lead to enhanced total and REM sleep with robust rebound of any loss resulting from the stress manipulation (Sanford et al., 2014; Sanford et al., 2010; Suchecki, Tiba, & Machado, 2012). This intriguing difference is believed to reflect the fact that, during escapable shock, new instrumental learning is taking place whereas, for inescapable shock, only the unavoidable contingency is learned (Sanford et al., 2014; Sanford et al., 2010; Suchecki et al., 2012).
In the rat, total sleep deprivation (TSD) preceding (Ruskin, Liu, Dunn, Bazan, & LaHoste, 2004) or following (Graves, Heller, Pack, & Abel, 2003; Kumar & Jha, 2012) fear conditioning impairs consolidation of fear memory. For example, 6 hours of sleep deprivation immediately following simulated trauma in rats (predator scent exposure) attenuated a sensitization to this predator scent that was seen 7 days later in other rats that were allowed to sleep following initial exposure to that scent (Cohen et al., 2012).
Since TSD, of necessity, also produces REM sleep deprivation (REMD), reduced consolidation of fear conditioning following TSD might, in fact, be secondary to REMD. Evidence that this may be the case is the observation that, following fear conditioning, coherence of theta-frequency oscillations in REM among the amygdala, medial prefrontal cortex and hippocampus (a prominent feature of REM in the rat) is positively associated with the extent of later recall of conditioned fear (Popa, Duvarci, Popescu, Lena, & Pare, 2010). Surprisingly, however, there have been few animal studies on the effects of REMD on consolidation of fear conditioning per se and these few give conflicting results. One study (Tian et al., 2009) showed that REMD failed to block re-consolidation of fear conditioning (i.e., REMD followed fear memory reactivation). Other studies purporting to demonstrate prior REMD effects on acquisition of extinction (Silvestri, 2005; Silvestri & Root, 2008) might actually suggest that REMD strengthened fear memory. In these studies, REMD that followed cued fear conditioning but preceded subsequent extinction training by 48 hours, impaired acquisition of that later extinction (Silvestri, 2005; Silvestri & Root, 2008). These investigators argue that the possibility that REMD had strengthened consolidation of fear conditioning was ruled out because the initial extinction trials did not differ between REMD and control groups (Silvestri, 2005; Silvestri & Root, 2008). Nonetheless, an enduring (48-hour) blockade of the ability to encode extinction that is produced by post-conditioning REMD but does not involve strengthening of the fear memory is difficult to explain mechanistically. For example, it is possible that a stronger fear memory in the REMD group could have manifested as greater resistance to extinction despite not differing from control rats in the initial magnitude of freezing. Most notably, if the relationship of fear conditioning to REM is reciprocal, the abundant evidence that fear reduces duration and fragments REM (Pawlyk et al., 2008) suggests that, in fact, REM might oppose consolidation of conditioned fear.
5.C.2. Sleep and fear extinction in the rat
Following extinction training, Fu et al. (2007) have performed a comprehensive study showing that REMD blocked the consolidation and later recall of extinction of cued fear conditioning in the rat. These investigators tested effects of REMD on recall of extinction in both hippocampus-independent and hippocampus-dependent tasks. Hippocampus-independent tasks included cued fear conditioning, extinction learning, and extinction recall all in the same context (cued AAA) and cued fear conditioning in one context with both extinction and extinction recall in a second context (cued ABB). Hippocampus-dependent tasks included cued fear conditioning in one context, extinction in a second context and extinction recall in the conditioning context (cued ABA) and, contextual, un-cued conditioning, extinction, and extinction recall in a single context (contextual AAA). Rats were habituated to context then fear conditioning with foot-shock and, after 24 hours, extinction training was performed. Different groups then underwent either 6 hours of REMD immediately following extinction training, 6 hours of REMD following a 6-hour delay or they were immediately returned to their home cage without REMD. Finally, after 24 hours, extinction recall was tested. For hippocampus-dependent tasks there were no group differences in extinction recall. However, following training on hippocampus-independent tasks, those who underwent immediate REMD, compared with their controls, recovered significantly more fear after 24 hours (i.e., extinction memory was poorer) with these rats actually showing zero retention of extinction learning. Notably, however, extinction recall was intact when REMD was delayed for 6 hours following extinction training. It is likely, therefore, that immediate REMD overlapped a “REM window” (Smith, 2001) crucial to consolidating hippocampus-independent extinction memory.
5.C.3. Sleep influences both fear conditioning and extinction in the rat
In summary, in the rat, sleep appears important for memory of both fear conditioning and extinction. Although REM has been specifically shown to be important to the consolidation of extinction of cued conditioned fear (Fu et al., 2007), evidence for a role of REM in consolidation of the fear memory itself remains equivocal with indirect evidence suggesting that REM may, in some cases, oppose fear memory consolidation. Nonetheless, human studies discussed below suggest that REM may also help consolidate conditioned fear. After reviewing these human studies, we will attempt to reconcile these seemingly contradictory findings of a positive role for sleep, as well as possibly REM sleep, in consolidation of both fear and extinction.
5.D. Human studies of sleep and fear conditioning, extinction and extinction recall
Human studies of sleep’s interactions with fear conditioning and extinction have typically used SCR as the measure of conditioned fear (although see Marshall et al., 2014). Recent studies have combined SCR measures of fear and its extinction with polysomnography (PSG) and fMRI to investigate their sleep stage and functional anatomical correlates respectively. Studies dealing specifically with fear conditioning will first be discussed followed by those examining subsequent extinction learning and memory.
5.D.1. Sleep and fear conditioning
As in the rat (Graves et al., 2003; Kumar & Jha, 2012), sleep’s role in promoting the consolidation of fear is implied by studies showing that TSD can attenuate consolidation of fear conditioning in humans. In one such study, Menz et al. (2013) fear conditioned participants in the evening, while fMRI scanning, to two reinforced (using electric shock) stimuli (CS+), each with its own un-reinforced stimulus (CS−). One of these CS+ (CS+E), but not the other (CS+N), was then extinguished. Half of the subjects then underwent total sleep deprivation (TSD) for the subsequent night whereas the other half slept with PSG monitoring. On the following night all subjects slept (i.e., recovery sleep for the TSD group) and they were then tested for fear and extinction recall the following morning using shock expectancy ratings, SCR and fMRI. At this recall session, responses to the un-extinguished CS+N indexed fear recall whereas those to the extinguished CS+E indexed extinction memory. For the CS+N, the absolute values of neither shock expectancy nor the SCR differed between groups. However, in the group that slept on the night following fear conditioning, the difference in expectancy of receiving a shock between the CS+N and its respective CS− (CS−N) was significantly greater than this difference in the group who underwent TSD. Similarly, the difference between SCR to the CS+N and its CS−N was greater in the group that had slept and, in that group, this difference was correlated with the amount of time spent in REM sleep. Additionally, in the group that slept, both the difference in SCR between the CS+N and CS−N at the time of recall and post-learning REM quantity were associated with greater amygdala activation at fear recall testing. For the extinguished stimulus (CS+E), however, neither shock expectancy nor differential SCR differentiated those who slept from those who underwent TSD. Menz et al. (2013) point out, however, that the lesser difference between the SCR to the CS+N vs. CS−N in their TSD group resulted from a higher response to their un-reinforced CS (CS−N) rather than a lower response to their reinforced CS (CS+N). Therefore, sleep might also serve to prevent fear generalization (i.e., from the CS+N to its CS−N).
Another study suggested that sleep might play a role both in consolidating and in generalizing conditioned fear (Kuriyama, Soshi, & Kim (2010) associated particular pictorial contexts with neutral (uneventful driving) or aversive (gory motor vehicle accident) outcomes in brief video clips shown from the driver’s perspective. These investigators then showed that sleep deprivation on the first night subsequent to encoding, followed by recovery sleep, prevented generalization of subjective and physiologically expressed fear from pictures associated with the aversive event to those associated with the neutral context. They therefore suggested that posttraumatic insomnia might adaptively serve to diminish the generalization of conditioned fear that could follow trauma. However, in a subsequent study (Kuriyama, Honma, Yoshiike, & Kim, 2013), when these same investigators added an active memory suppression procedure (directed forgetting) following encoding on the same task, TSD produced a generalized increase rather than decrease in physiological fear expression, while TSD following directed remembering of the fearful stimuli replicated the previously observed generalized decease in fear expression upon recall testing. These authors attributed this differing effect to interference with hippocampal processing of context and prefrontal regulation of amygdala responses that was produced by the directed forgetting procedure at encoding. Nonetheless, these opposing findings emphasize the fragility and experimental-conditions dependency of sleep effects on emotional memory. [It is also noteworthy that the TSD-mediated reduction in fear termed “extinction” by Kuriyama et al. (2010) does not actually involve extinction learning but rather is reduced consolidation and generalization of fear conditioning—a process more akin to fear “erasure” (Maren, 2011).]
Another group has shown that REM duration was significantly associated with both higher vmPFC activation to the CS+ during fear conditioning and lower SCR to initial presentations of the CS+ at the beginning of extinction-training trials 24 hours later (Spoormaker, Gvozdanovic, Samann, & Czisch, 2014). Partial correlation analysis showed that REM duration accounted statistically for the relationship between vmPFC activation during conditioning and SCR 24 hours later. However, unlike Menz et al. (2013), this relationship was negative with regard to SCR. As these authors note, Menz et al. (2013) used differential SCR (i.e., CS+ minus its corresponding CS−) that decreases in response to either a CS− increase or a CS+ decrease whereas they simply measured SCR to the CS+. Therefore these findings again implicate REM in the processing of fear memory, but highlight the complexity and possible bidirectionality of this relationship. The Spoormaker group has also examined whether the rodent findings of REM disruption following fear conditioning (Pawlyk et al., 2008) and the subsequent amelioration of this disruption by extinction training (Wellman et al., 2008) could be duplicated in humans (Sturm, Czisch, & Spoormaker, 2013). To do so, they varied the intensity of a shock US (high vs. low) and extinction learning (present vs. absent following fear conditioning) and followed each condition with a PSG-monitored nap. Neither shock intensity nor subsequent extinction affected REM. However, higher shock intensity increased NREM Stage 1, the lightest stage of sleep, whereas extinction favored NREM Stage 4, the deepest.
5.D.2. Sleep and extinction learning and recall—behavioral studies
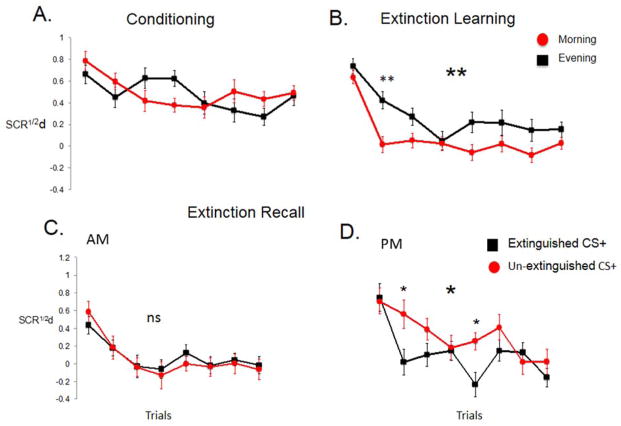
In the first human study on the effects of sleep on extinction memory (Pace-Schott et al., 2009), among healthy young adults of both sexes, a conditioned SCR was established to two color stimuli (CS+) using an electric shock while a third stimulus was unreinforced (CS−). One CS+ stimulus was then immediately extinguished (CS+E) whereas the other remained un-extinguished (CS+U). The Sleep group then underwent a 12-hour PM to AM delay with intervening sleep while the Wake group underwent a 12-hour AM to PM delay of continuous wakefulness. At extinction recall, in the Sleep group, not only did SCR remain reduced to the CS+E, but it was also reduced, to the same degree, to the CS+U. In the Wake group, however, SCR to the CS+U remained significantly greater than to the CS+E. Because SCR to the CS+E did not differ between groups, generalization of extinction memory to the CS+U following sleep was hypothesized. And, because SCR at fear conditioning and extinction did not differ between groups (Figure 2A, B), a key role for intervening sleep in the observed generalization was suggested (Figure 2C, D). Subsequent studies replicated the finding of generalized extinction memory over a night of sleep (Pace-Schott et al., 2013; Pace-Schott et al., 2014).
Figure 2.
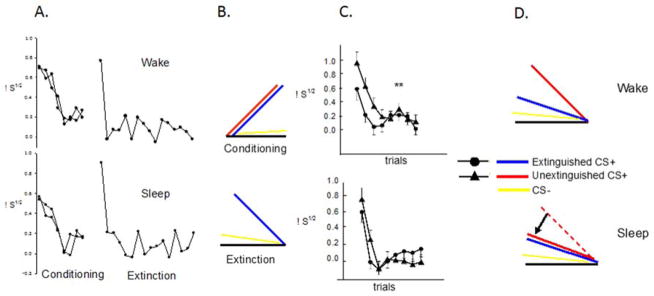
Intervening sleep promoted generalization of extinction of conditioned fear in comparison of two groups: Wake (trained in the morning and tested in the evening, upper panels) and Sleep (trained in the evening and tested in the morning, lower panels). A. Average trial by trial SCR during Fear Conditioning and Extinction Learning. There were no differences between the Sleep and Wake group. B. Schematic of the acquisition of conditioned fear (upper panel) and extinction learning (lower panel). Note that although acquisition of fear is schematically depicted as an ascending curve, actual SCR responses in humans tend to decrease across conditioning trials. C. Average trial by trial SCR to the extinguished (CS+E) and un-extinguished (CS+U) CS+ in the two groups during each trial of the Extinction Recall phase occurring 12 hours after Fear Conditioning and Extinction Learning phases. D. Schematic interpretation of results in C., i.e., because groups did not initially differ (A.), sleep promoted generalization of extinction learning. μS1/2, square-root transformed SCR in micro-Siemens,** p < 0.01, bars are SEM.
However, because Sleep and Wake groups had been tested at different times of day, extinction memory might also have been affected by circadian or sleep-homeostatic factors. Therefore, in a follow-up study (Pace-Schott et al., 2013), delays of 3 (within AM or PM), 12 (AM-PM, PM-AM) or 24 hours (AM-AM, PM-PM) were interposed between extinction learning and extinction recall in healthy young adult males using the same protocol and measuring, at each phase, differential SCR (CS+ minus temporally corresponding CS−). There were no effects of the duration of these delays on extinction recall nor were there time-of-day effects on fear conditioning itself (Figure 3A). However, both extinction learning (Figure 3B) and the generalization of extinction recall (Figure 3C, D) were significantly better in the morning. Therefore a complex interaction of sleep with circadian and sleep-homeostatic mechanisms, as well as sex differences (see above), may determine the degree to which extinction is learned, remembered and generalized. In males, circadian factors may promote AM extinction learning and/or retrieval via the co-occurring morning acrophase of cortisol and testosterone, the ratio of which was positively correlated with extinction learning (Pace-Schott et al., 2013). Evidence that this may be the case is the fact that, in this same group, individuals with later timing of sleep (later midpoint) and greater subjective “eveningness” (reviewed in Adan et al., 2012) showed an exaggerated time-of-day effect in the evening (i.e., yet poorer generalization of extinction memory), whereas those with earlier timing of sleep and greater subjective “morningness” derived the greatest morning benefit for extinction learning (Pace-Schott et al., under review).
Figure 3.
Fear conditioning did not differ between morning and evening (A) whereas extinction was significantly better learned in the morning (B). Extinction Recall in the morning showed generalization of extinction memory (C) whereas recall in the evening preserved the differentiation of the extinguished and unextinguished CS+ and favored expression of a greater overall degree of conditioned fear (D). Significance indicated for the Morning vs. Evening and CS+E vs. CS+U main effect (large asterisks) and trial-by-trial differences (small asterisks). SCR½d: differential SCR, * p < .05, ** p < .01. Error bars depict standard error of the mean.
This time-of-day effect could be due to either or both elements of the Two-Process model of sleep propensity (Borbely, 1982)—circadian rhythms (Process C: controlled by the endogenous 24-h clock) or sleep homeostasis (Process S: controlled by duration of prior waking). In the case of Process C, morning may constitute a particularly favorable physiological milieu for encoding or retrieval due to circadian fluctuations in hormonal and/or central influences (Pace-Schott et al., 2013). Further suggestion of circadian influences includes differential responses to time-of-day in high-morningness versus high-eveningness individuals (see above). In addition, Kuriyama, Mishima, Soshi, Honma, & Kim (2011) have shown that recall of aversive material is enhanced when encoded 3 hours after versus 3 hours before habitual sleep onset time in women but not in men. In the case of Process S, encoding or retrieval of extinction might benefit from the rested, morning post-sleep condition with lowered levels of putative somnogens such as adenosine (Porkka-Heiskanen & Kalinchuk, 2011).
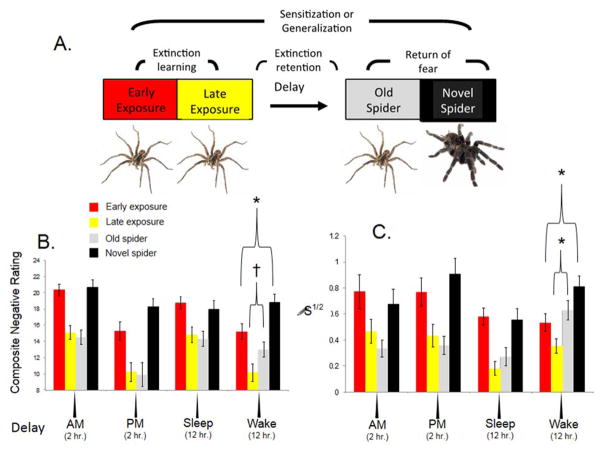
Nonetheless, evidence that lower habitual sleep quality also predicts poorer extinction recall (Pace-Schott et al., under review) and that time-of-day does not fully explain extinction learning (Pace-Schott et al., 2009) or recall (Pace-Schott, Verga, Bennett, & Spencer, 2012b) suggests that sleep itself may act upon the consolidation phase of extinction memory. For example, an extinction study using a clinically relevant paradigm (simulated exposure therapy) controlled for time-of-day (Pace-Schott, Verga, et al., 2012b). Spider-fearing, young adult women viewed multiple, identical 1-min videos of a behaving spider at a first session that was followed by 12-hr delays containing either a normal night’s sleep or continuous daytime wakefulness, or 2-hr delays containing continuous wakefulness in either the morning or evening. At the second session following the delay, all groups viewed the original video followed by videos of a novel spider. Only in the group that remained awake for 12 hours did subjective fear ratings and SCR for the original spider increase from the end of the first session to the beginning the second indicating loss of extinction memory. Moreover, only in this 12-hour awake group did ratings and SCR to the novel spider exceed those to the original spider at the start of the first session (when it too was novel), indicating sensitization of responses (Figure 4). In contrast, in only the 12-hour sleep group was heart rate acceleration less to the novel spider in the second session that it was to the original spider in the first session. Moreover, only in this 12-hour sleep did group corrugator supercili EMG not show sensitization from the original to the novel spider. None of these effects differentiated morning- and evening-awake groups arguing against circadian explanations for observed differences. Thus, sleep following exposure promoted retention and generalization of extinction learning and prevented sensitization to novel exemplars in a feared category of stimuli. Similar findings have been reported in a recent nap study (a procedure that also controls for time-of-day) using virtual-reality exposure therapy for spider phobia (Kleim et al., 2013). Therefore, for some forms of extinction learning, sleep effects have been identified independently of potential time-of-day confounders.
Figure 4.
Superior retention and generalization of extinction memory across sleep versus wakefulness during simulated exposure therapy for spider phobia. A. Experimental design showing putative learning and memory processes taking place between phases. B. Summed Disgust, Fear and Unpleasantness ratings. C. SCR in response to spider video. Note that extinction generalization in the Sleep group prevented sensitization to a novel spider video. Control groups tested following wakefulness entirely in the morning or evening (right panel of each graph) show no differences in extinction retention and generalization; a finding inconsistent with a circadian explanation of the sleep-wake differences. Bars are the standard error of the mean for groups computed by mixed ANOVA. † p < 0.10, * p < 0.05, μS1/2 square-root transformed SCR in micro-Siemens.
5.D.3. Sleep and extinction learning and recall—neuroimaging studies
In the first fMRI study of sleep and extinction memory, Spoormaker et al. (2010) showed that greater extinction recall (lower SCR to an extinguished stimulus), as well as vmPFC activation, to the extinguished stimulus were greater in individuals who achieved REM during a 90-min afternoon nap that followed fear conditioning and extinction learning. However, in this study, individuals who achieved REM also showed greater habituation to the unconditioned (shock) stimuli during fear conditioning in parallel with lower brainstem activity. This latter finding led these authors to suggest that trait-like differences in reactivity might have produced both the sleep and extinction-recall effects. Specifically, less aroused individuals may possess both better ability to enter REM in a nap and better extinction recall—i.e., correlational findings could reflect shared causation by a mutually associated third variable.
Therefore, in a subsequent fMRI study (Spoormaker et al., 2012), these investigators directly manipulated one of these variables (REM amount) following extinction learning by comparing REMD to NREM-deprived (NREMD) controls. Fear conditioning, extinction learning and extinction recall were performed on three successive evenings. At fear conditioning, two CS+ were reinforced and a third was not (CS−). At extinction learning, one CS+ (CS+U) was still reinforced but the other was extinguished (CS+E). All three stimuli were then presented at extinction recall on the third evening, These investigators observed elevated differential (CS+ minus CS−), range-corrected SCR to the CS+E (i.e., poorer extinction recall) in the REMD vs. NREMD group that became apparent during the second but not the first block of 3 extinction-recall trials. [Notably, however, assessment of extinction memory is often restricted to the initial 2–4 trials of the extinction recall phase in order to avoid confounding extinction memory with new extinction learning taking place during the extinction recall phase itself (e.g., Milad et al. 2007).] fMRI analyses comparing REMD vs. NREMD using the contrast between extinction learning and extinction recall for each type of stimulus showed no group differences. However when a type of contrast thought to reflect temporal prediction error (CS+E onset vs. offset) was used, greater activation for REMD than NREMD appeared in a number of temporal, occipital and orbitofrontal cortical areas. These investigators suggest that these effects reflect not only greater disruption of extinction-memory consolidation, but also disrupted temporal prediction-error signaling following REMD vs. NREMD. Preliminary evidence from another experiment also suggested that greater retention of extinction learning was associated with REM percent in intervening overnight sleep (Pace-Schott et al., 2014). Results to date on sleep and extinction memory from different laboratories are difficult to compare due to differing methodologies, such as whether or not extinction learning immediately follows conditioning or includes a reinforced CS+U.
NREM sleep, and especially SWS, may also play a role in fear and extinction learning and memory. Hauner, Howard, Zelano, & Gottfried (2013) performed human differential fear conditioning with two different CS+ partially reinforced with a shock US using an SCR conditioned response. During conditioning, each CS+ was accompanied by a different specific odor one of which was then presented, unreinforced, during a subsequent nap whenever subjects entered SWS. After the nap, the CS+ whose odor had been presented during SWS showed greater reduction in differential SCR relative to the CS+ whose odor had not been presented. Moreover the degree of this reduction was positively correlated with the duration of SWS during which the odor was presented. In the post-nap fMRI, the CS+ whose odor was presented during the nap, relative to the one whose odor was not presented, showed reduced activation of the hippocampus, ACC and insula as well as greater change of activity patterns in the left amygdala. Therefore memory traces encoded in fear and extinction networks during waking can be re-activated and modified by re-exposure during subsequent SWS, and such changes can endure to influence post-sleep retrieval. These findings were recently replicated by a study in which auditory stimuli that were fear-conditioned in waking using an electric shock US, when re-presented either during SWS or during waking were equally effective in producing later expression of extinction memory in waking (He et al., 2014). Therefore extinction can take place during SWS by presentation of stimuli in at least two sensory modalities.
5.D.4. Bidirectional effect of sleep and the consolidation of fear and extinction memory
One notable finding in the animal literature has been the bidirectional interaction between sleep architecture and these stressors (Pawlyk et al., 2008). Specifically, in rodents, fear conditioning disrupts REM sleep, extinction can restore continuous REM, and sleep may be necessary for the consolidation of both fear and extinction memory (detailed above). The reciprocal effect of fear conditioning and its extinction on REM sleep in the human has not yet been experimentally demonstrated (Sturm et al., 2013). It should be noted, however, that the severity of stress imposed on experimental animals cannot be achieved experimentally in humans. Therefore the prospective study of sleep in survivors of actual trauma may be necessary to observe comparable effects of stress on REM sleep. Nonetheless, if the subtle effects of experimentally imposed stress on human NREM sleep (e.g., (Sturm et al., 2013) were to persist over many nights following an actual trauma, they could, over time, magnify the effects of traumatic stress on sleep-dependent memory processes. Unlike experimental stressors, the study reviewed in the next section suggests that experimentally induced safety learning may augment subsequent REM in humans (Marshall et al., 2014).
5.D.5. Sleep and Safety Learning
Other forms of learning and their underlying plasticity, in addition to fear extinction, can produce enduring reduction of fear. These include safety learning (Gazendam, Kamphuis, & Kindt, 2013; Ostroff, Cain, Bedont, Monfils, & Ledoux, 2010) and between-session habituation (Bolivar, 2009). Recently, relationships between REM sleep and safety learning in a fear-potentiated startle paradigm have been reported in a large sample of young adults (Marshall et al., 2014). Participants spent 3 consecutive nights in the sleep laboratory and, after the second, baseline night, a differential fear conditioning procedure, using an electric shock US, established higher startle to a fear (CS+) versus a safety (CS−) signal. REM percent, latency and continuity, were related to fear conditioning and recall using canonical correlation. These investigators reported that the degree of safety-signal learning (decline in response to CS− over conditioning) predicted 28% of variance in REM consolidation on the night following conditioning. In turn, REM consolidation significantly predicted 23% of the variance in retention of differential conditioning (response to CS+ versus CS−) the following day. These investigators suggest, therefore, that REM sleep may play a role in consolidating memory for safety, and thereby facilitate, in subsequent wakefulness, better differentiation of safety and danger.
5.D.6. Sleep and between-session habituation
Between-session habituation is a non-associative memory that consolidates simultaneously with the consolidation of extinction (an associative memory) following sustained exposure to fearful stimuli (Craske et al., 2008; McSweeney & Swindell, 2002). Although theoretically dissociable, it is difficult to differentiate consolidation of this non-associative process from the associative processes taking place during extinction (McSweeney & Swindell, 2002). Therefore, the above-described effects of sleep on retention and generalization of extinction for spider fear (Kleim et al., 2013; Pace-Schott, Verga, Bennett, & Spencer, 2012) may reflect, in part, a concomitant consolidation of between-session habituation.
Nonetheless, within an experimental non-associative paradigm, sleep has been shown to promote between-session habituation. For example, following habituation to highly aversive International Affective Picture System (IAPS) stimuli, a 2-hour nap compared to 2 hours of quiet wakefulness at the same time-of-day resulted in greater retention of prior within-session habituation expressed as SCR and corrugator EMG (Pace-Schott et al., 2011). Greater between-session habituation of SCR was associated with the presence versus absence of SWS in the nap. Notably, the design of this experiment controlled for time-of-day effects. In another experiment, between-session habituation to a loud-tone stimulus, as measured by orbicularis oculi EMG and SCR, was greater across a night of sleep than across a day of continual wakefulness (Pace-Schott et al., 2014). Notably, in this study, time-of-day alone could not account for these sleep effects and percent SWS in the intervening nocturnal sleep was again associated with the degree of between-session habituation.
In a fear-conditioning paradigm with an electric shock US, one night of partial sleep deprivation relative to a full night’s sleep, has been shown to impair subsequent within-session habituation of both SCR and activation of the hypothalamus (fMRI) in response to the US (Peters et al., 2014). These central (hypothalamus) and peripheral (SCR) autonomic measures were, in addition, positively correlated with one another. Interestingly, however, partial sleep deprivation did not influence the associative learning represented by differentiation of the CS+ and CS−.
The process opposite to between-session habituation, namely sensitization, can also occur and may contribute to the development of anxiety symptoms following traumatic experiences in humans (Smid et al., 2012) and experimental stressors in animal models (Corley, Caruso, & Takahashi, 2012; Olson et al., 2011). In this regard, it is notable that sleep, following within-session habituation, appears to prevent between-session sensitization seen in the corresponding wake group in corrugator EMG (Pace-Schott et al., 2011; Pace-Schott, Verga, et al., 2012) or heart-rate decleration (Pace-Schott et al., 2014).
6. Does sleep promote fear or extinction?
The animal and human studies of sleep, fear conditioning and extinction reviewed above suggest that sleep can promote consolidation of both fear and extinction memory. Such bivalent findings appear less paradoxical when taking into account that these are both forms of associative emotional memory with neural circuitry and neuromodulation in common. For example, at encoding, both fear conditioning and extinction share a macroscopic anatomic locus in the BLA, although their specific supporting circuitry differs (Herry et al., 2010; Pape & Pare, 2010; Quirk & Mueller, 2008). Furthermore, similar bivalent memory enhancement has been reported for the stress hormone cortisol that, at high levels, can enhance both fear conditioning and the extinction of this conditioning (de Quervain, Aerni, Schelling, & Roozendaal, 2009; de Quervain et al., 2011). Similarly, enhanced noradrenergic signaling may augment both fear conditioning (LaLumiere, Buen, & McGaugh, 2003; Roozendaal, McEwen, & Chattarji, 2009) and its extinction (Pena, Engineer, & McIntyre, 2013; Smits et al., 2013). Moreover, the NMDA partial agonist d-cycloserine (DCS) can enhance both fear conditioning (Bolkan & Lattal, 2013; Kalisch et al., 2009; Silvestri & Root, 2008) and extinction of fear conditioning (Davis, 2011; Ledgerwood et al., 2005; Norberg, Krystal, & Tolin, 2008) depending on timing of its administration relative to encoding. Therefore it is not surprising that the complex neuroendocrine changes taking place across sleep, that include amygdala and vmPFC activation in REM as well as fluctuations in cortisol, norepinephrine and NMDA-receptor mediated LTP, should also promote consolidation of both fear and extinction memory. Similarly, sleep-dependent generalization of both fear conditioning (Kuriyama, Soshi, & Kim, 2010; Menz et al., 2013) and extinction learning (Pace-Schott et al., 2009; Pace-Schott et al., 2014; Pace-Schott, Verga, et al., 2012a) can also be explained by their shared substrates as emotional memory processes.
Nonetheless, it should be noted that sleep has not been shown to simultaneously enhance both fear and extinction. For example, Menz et al. (2013) showed that, although TSD versus sleep reduced fear to an un-extinguished CS+, it did not differentially affect responses to an extinguished CS+. Similarly, whereas sleep enhanced extinction generalization from an extinguished to an un-extinguished CS+, it did not enhance extinction memory for an extinguished CS+ (Pace-Schott et al., 2009). Therefore, interactions between fear and extinction circuitry likely take place during consolidation as well as, competitively, during retrieval. Given the fact that memory traces for both a fear and its extinction coexist, opportunity for such interaction is an enduring possibility.
7. Sleep and emotional declarative memory
7.A. Neural substrates of emotional declarative memory
Neuroimaging studies of sleep effects on emotional memory (e.g., comparing memory following TSD versus normal sleep) often employ visual stimuli (e.g., IAPS images) and a post-delay declarative memory task during which both recognition memory and changes in subjective emotional valence are probed (e.g., Sterpenich et al., 2007; Yoo, Gujar, Hu, Jolesz, & Walker, 2007). Contrasts used in such studies have revealed sleep effects on limbic structures similar to those implicated in fear conditioning and extinction. For example, successful recall of emotional stimuli was accompanied by greater activation of the amygdala, hippocampus and vmPFC in a group that slept versus one that was sleep deprived following learning, a difference still seen a full 6 months later (Sterpenich et al., 2007; Sterpenich et al., 2009). Similarly, following sleep deprivation, activation was increased in the amygdala in response to emotional stimuli (Yoo, Gujar, et al., 2007) whereas it was reduced in the vmPFC (Thomas et al., 2000; Yoo, Gujar, et al., 2007), a region known to inhibit expression of negative emotion (Diekhof, Geier, Falkai, & Gruber, 2011) as well as express extinction memory (Milad & Quirk, 2012). Moreover, sleep deprivation disrupted the functional connectivity between the vmPFC and the amygdala (Yoo, Gujar, et al., 2007). A recent fMRI study has further shown that the sleep debt accrued by prolonged sleep restriction can produce a similar hyper-responsivity of the amygdala to emotional faces as well as reduce its functional connectivity with the vmPFC (ventral anterior cingulate) (Motomura et al., 2013). Additionally, these same investigators showed that sleep-debt induced amygdala hyperactivity also extends to subliminally presented (backward masked) emotional-face stimuli (Motomura et al., 2014). In addition to increased reactivity of neural structures to negative stimuli, TSD has also been shown to increase the reactivity of the mesolimbic reward system to positive stimuli as well as decrease its functional connectivity with regulatory prefrontal regions suggesting that the emotional regulatory function of sleep may be important in a bivalent manner (Gujar, Yoo, Hu, & Walker, 2011). Another fMRI study suggested that retrieval of an emotional memory following a night of sleep engaged a more discrete and integrated set of limbic structures than retrieval following a day awake (Payne & Kensinger, 2011).
7.B. The role of REM sleep in regulation of emotions linked to declarative memory
Despite somewhat consistent findings from neuroimaging studies, findings on the roles of specific sleep stages, and particularly REM, in the consolidation and processing of emotional declarative memory have been mixed (For a review see Deliens et al., 2014). In the “Sleep to Remember Sleep to Forget” (SRSF) model, Walker and colleagues suggest that REM serves to simultaneously consolidate contents of an emotional memory and down-regulate its associated (self-reported) negative affect thereby serving an adaptive emotional de-potentiation function (Walker, 2009; Walker & van der Helm, 2009). Support for this model includes lessened reactivity to facial expressions of negative emotion (anger and fear) across a day with a REM-containing nap compared to a day without such a nap (Gujar, McDonald, Nishida, & Walker, 2010). These investigators also showed that, following exposure to negative stimuli, the spectral power of high frequency gamma (>30Hz) EEG oscillations during subsequent nocturnal REM (hypothesized to be a proxy for higher norepinephrine and, consequently, a poorer emotion regulatory milieu) was negatively associated with the overnight decrease in both subjective negative ratings and amygdala activation to previously seen stimuli (van der Helm et al., 2011). Further support for the SRSF model were findings that, compared to a baseline night, a night with selective REMD showed an increase in behavioral and neural reactivity to emotional images that was not observed following selective NREM deprivation (Rosales-Lagarde et al., 2012). Partial support was also provided by a study that showed that recall of word lists, tested 3 days after encoding during a mood induction procedure, lacked the mood-dependent memory (MDM) effect (viz., memories encoded in one emotional state are better retrieved in that same emotional state) if normal sleep was obtained each subsequent night, but MDM remained if TSD occurred on the first night (Deliens, Gilson, Schmitz, & Peigneux, 2013). However, partial sleep deprivation of either SWS-rich early-night or REM-rich late-night sleep did not alter the MDM effect (Deliens, Neu, & Peigneux, 2013) nor did a single full night of sleep (Deliens & Peigneux, 2014). These investigators, therefore conclude that the affective de-potentiation suggested by SRSF requires several nights. (For a review see Deliens et al., 2014).
However, another study examining the effect of REMD on emotional verbal memory found no differences compared with normal sleep (Morgenthaler et al., 2014). Moreover, other studies have led to findings for the effects of REM on emotional memory exactly opposite to those predicted by the SRSF model. For example, rather than de-potentiating the self-reported emotions associated with a declarative memory (e.g., recognition of an IAPS image), REM has instead been associated with their preservation (Baran, Pace-Schott, Ericson, & Spencer, 2012; Groch, Wilhelm, Diekelmann, & Born, 2013; Lara-Carrasco, Nielsen, Solomonova, Levrier, & Popova, 2009; Pace-Schott et al., 2011; Wagner, Fischer, & Born, 2002). Of clinical importance, such findings have led to the suggestion that depriving traumatized patients of sleep during the first night post-trauma may serve to attenuate consolidation of traumatic memories (Baran et al., 2012; Kuriyama et al., 2010; Menz et al., 2013; Wagner, Hallschmid, Rasch, & Born, 2006).
7.C. What more can be learned from low-level emotional versus declarative emotional memory processes?
Interactions between post-learning sleep and low-level emotional memory processes, such as fear conditioning and extinction, may help in disentangling seeming contradictions in findings on the specific role of REM sleep in the emotion regulation accompanying declarative memory. It is noteworthy that the above experiments rely upon recognition tasks that are highly dependent on the hippocampal formation. Hippocampus-dependent tasks have, in turn, been those most clearly linked with the NREM-sleep oscillatory activity such as percent SWS, sleep spindles, sharp-wave ripple events and the < 1Hz slow oscillation (Diekelmann & Born, 2010; Rasch & Born, 2013). As described above, the encoding, consolidation and retrieval of fear conditioning and extinction engage limbic circuits that utilize hippocampal input to disambiguate contextual factors, but that can also operate in a hippocampus-independent manner if the context is ambiguous or uninformative (Fu et al., 2007; Kalisch et al., 2006; LaBar & Phelps, 2005). Notably, in the one animal study that differentiated hippocampus-dependent versus independent tasks, post-extinction learning REMD was found to specifically impair hippocampus-independent versus hippocampus-dependent tasks (Fu et al., 2007). Similarly, in the human study showing most clearly a REM dependency for extinction memory, specific steps were taken to reduce context dependency in this cued paradigm, viz. the CS+U was shocked during extinction of the CS+E to avoid the experimental setting itself becoming an extinction context (Spoormaker et al., 2012). Notably also, the study showing SWS-dependent extinction occurring within sleep presented the context (odor) and not the cue (faces) during subjects’ naps (Hauner, Howard, Zelano, & Gottfried, 2013) [although a study replicating this effect used a simple auditory cue during both conditioning and sleep (He et al., 2014)].
Therefore, experimental paradigms that test effects of sleep on emotional memory by eliciting both recognition memory and subjective ratings of stimulus valence (e.g., Baran et al., 2012; Nishida, Pearsall, Buckner, & Walker, 2009; van der Helm et al., 2011) are, of necessity, testing a compound stimulus. Such stimuli consist, minimally, of components that could be classified, respectively, as declarative versus (potentially) implicit, hippocampus-dependent versus amygdala-dependent, or, as in the case of the SRSF model, the declarative memory itself versus its affective “charge” (Goldstein & Walker, 2014; Walker & van der Helm, 2009). Retrieval of such stimuli should, therefore, require those elements of sleep physiology (and their brain substrates) that support consolidation in all memory systems that underlie the constituent parts of such compound stimuli. Therefore, experiments that are able to dissociate these differing bases of an emotional memory may be better able to disambiguate the effects of REM and NREM sleep on the consolidation and modification of an emotional memory trace. Importantly, in the case of fear conditioning and extinction taking place in the absence of informative contextual cues, the memory trace itself may consist of plastic changes among BLA neurons that encode both the contingent stimuli and their elicited output via the central nucleus of the amygdala (Herry et al., 2010; Pape & Pare, 2010; Quirk & Mueller, 2008).
8. Related Clinical Findings
8.A. Sleep disturbance in anxiety disorders—a symptom and a risk factor
Sleep disruption is an important concomitant of certain anxiety disorders (Mellman, 2008a). In Generalized Anxiety Disorder, sleep disruption is among its DSM-5 (American Psychiatric Association, 2013) diagnostic criteria. Similarly, sleep problems are common in Panic Disorder (Lepola, Koponen, & Leinonen, 1994; Sheikh, Woodward, & Leskin, 2003). Among the anxiety-related Trauma and Stressor-Related Disorders, sleep disruption and nightmares of a repetitive nature are core symptoms of DSM-5 Posttraumatic Stress Disorder (PTSD) falling into the “alterations in arousal and reactivity” and “intrusion symptoms” criterion categories respectively. Similarly, among anxiety-related Obsessive Compulsive and Related Disorders, more subtle disruptions of sleep quality or timing are often present (Paterson, Reynolds, Ferguson, & Dawson, 2013). Among yet other anxiety disorders such as Social Anxiety Disorder, poorer sleep quality has been shown to predict disorder severity (Kushnir, Marom, Mazar, Sadeh, & Hermesh, 2014), and better sleep quality the success of therapeutic intervention (Zalta et al., 2013). Importantly, there is high comorbidity of insomnia with mood and anxiety disorders (Alvaro, Roberts, & Harris, 2013; Baglioni et al., 2011; Riemann, 2007; Stepanski & Rybarczyk, 2006) and evidence that, in some cases, insomnia itself can be preexisting risk factor for incident anxiety disorders (Breslau, Roth, Rosenthal, & Andreski, 1996; Ford & Kamerow, 1989; Johnson, Roth, & Breslau, 2006; Neckelmann, Mykletun, & Dahl, 2007) especially PTSD (Babson & Feldner, 2010; Koren, Arnon, Lavie, & Klein, 2002; Mellman, Bustamante, Fins, Pigeon, & Nolan, 2002; Mellman, Pigeon, Nowell, & Nolan, 2007).
8.B. Sleep, PTSD and extinction
Extinction learning and memory as well as sleep have been shown to be impaired in PTSD (Milad et al., 2008; Milad, Pitman, et al., 2009). Patients with PTSD, compared to trauma-exposed controls (TENC), extinguish fear conditioning more poorly (Blechert, Michael, Vriends, Margraf, & Wilhelm, 2007; Orr et al., 2000) and, after 24 hours, recall less extinction (Milad et al., 2008; Milad, Pitman, et al., 2009). PTSD patients also show structural and functional abnormalities in limbic regions associated with extinction recall including the perigenual anterior cingulate, amygdala and hippocampus (Milad et al., 2013; Milad et al., 2008; Milad, Pitman, et al., 2009; Milad, Rauch, et al., 2006; Rauch et al., 2006; Shin, Rauch, & Pitman, 2006). Compared to TENC, individuals with PTSD showed poorer retention of extinction as measured by SCR (Milad et al., 2008) and, during extinction learning, there was greater activation of the amygdala, while, during extinction recall, there was lesser activation of the vmPFC and hippocampus but greater activation of the dACC (Milad, Pitman, et al., 2009). Therefore, in PTSD, there is both hyper-activation of the fear expression and hypo-activation of the fear-extinction memory networks (Graham & Milad, 2011; Milad & Quirk, 2012).
Notably, both of the fear expression and extinction memory networks lie within regions selectively re-activated in the transition from NREM to REM sleep (see Section 5.A. and Figure 1C) suggesting that alterations of REM may contribute to extinction impairment in PTSD. Indeed, a recent meta-analysis found that increased average number of rapid eye movements per minute in REM sleep (REM density), along with increased Stage 1 NREM and decreased SWS, were the most consistent alterations of sleep in PTSD compared to control groups (Kobayashi, Boarts, & Delahanty, 2007). Moreover, as detailed above, both SWS and REM sleep have been linked, experimentally, with the consolidation of extinction memory. Interestingly, the repetitive, highly veridical nightmares of PTSD could play a significant role in the relative balance of fear and extinction memory in such patients, for example, by reinforcing conditioned fear via re-traumatization (see Levin & Nielsen, 2007).
An important limitation of sleep research in PTSD has been the focus on male subjects, a limitation that extends to the animal literature where studies of fear conditioning and extinction are primarily carried out on male animals (Lebron-Milad & Milad, 2012). However, gender differences in the relationship between PTSD and sleep have now begun to be reported (Kobayashi, Cowdin, & Mellman, 2012; Kobayashi & Mellman, 2012; Richards et al., 2013). For example, observed sex differences in the sleep symptoms of PTSD may vary with hormonal levels and phase of the menstrual cycle in women (Kobayashi et al., 2012). Additionally, REM alterations relative to controls are greater in females whereas SWS abnormalities are more pronounced in males (Richards et al., 2013). Such findings might, in the future, help to better understand sex differences in the prevalence of this disorder.
Sleep abnormalities that either precede or follow traumatic experiences, predict later development of PTSD (reviewed in Babson & Feldner, 2010; Mellman & Hipolito, 2006; Wright et al., 2011) leading to the view that sleep disturbances, rather than being secondary to the development of PTSD, may play a key role in the etiology of this disorder (Alvaro et al., 2013; Babson, Badour, Feldner, & Bunaciu, 2012; Babson, Blonigen, Boden, Drescher, & Bonn-Miller, 2012; Babson & Feldner, 2010; Germain, 2013; Germain, Buysse, & Nofzinger, 2008; Mellman, 2008b; Spoormaker & Montgomery, 2008; Wright et al., 2011). For example, motor vehicle accident survivors who later developed PTSD, unlike survivors who did not, had more severe immediately post-trauma sleep disturbances that failed to normalize over time (Koren et al., 2002). Similarly, Mellman and colleagues showed that subjective insomnia, nightmare severity and abnormalities of REM, especially its fragmentation, in the early aftermath of a traumatic injury predicted later development of PTSD (Mellman et al., 2002; Mellman & Hipolito, 2006; Mellman, Knorr, Pigeon, Leiter, & Akay, 2004; Mellman et al., 2007). Such sleep disruption might impede normal processing of emotional memories following trauma (Mellman & Hipolito, 2006) including the ability to consolidate memory for the extinction of fear associated with traumatic memories, and this may be one mechanism by which sleep disruption contributes to the development of this disorder. However, future longitudinal studies of sleep in traumatized individuals will be necessary to firmly establish any causal links between sleep disruption, extinction and PTSD.
8.C. Application of sleep interventions for anxiety disorders
The ability to remember fear extinction is key to the treatment of PTSD and other anxiety disorders treated using exposure therapy (Craske et al., 2008; Hermans et al., 2006; McNally, 2007). One mechanism by which sleep disturbance might precipitate or perpetuate such disorders is by preventing the consolidation and generalization of naturally occurring or therapeutically induced extinction memories during sleep (Germain et al., 2008). The degree to which extinction learning can generalize will also strongly impact the efficacy of such therapy (Craske et al., 2008; Pace-Schott, Verga, et al., 2012a; Rowe & Craske, 1998; Vansteenwegen et al., 2005; Vansteenwegen et al., 2007; Vervliet, Craske, & Hermans, 2013). For example, fearful responding may re-emerge when the patient encounters an exemplar of a feared category of objects (e.g., spiders) that differs from the specific exemplar (e.g., species of spider) for which fear was extinguished in therapy (Rowe & Craske, 1998; Vervliet, Vansteenwegen, Baeyens, Hermans, & Eelen, 2005). Extinction generalization may be particularly relevant to the treatment of PTSD, a disorder in which the opposing effect, generalization of fear responses, is ubiquitous (Sauerhofer et al., 2012). Moreover, in PTSD, the same traumatic event can produce conditioned fear to multiple stimuli in multiple perceptual modalities (Ehlers et al., 2002). Generalization and multiplication of fear responses in PTSD may occur via processes such as second-order fear conditioning to primary trauma-reminder cues (Wessa & Flor, 2007). Therefore improved consolidation and generalization of extinction learning might mitigate mechanisms by which fear generalization exacerbates the number and fear-relevance of trauma reminders.
Our initial studies suggest that sleep itself might be employed to help strengthen and generalize extinction. Our recent finding that sleep can enhance retention and generalization of extinction memory following simulated exposure therapy for Spider Phobia (Pace-Schott, Verga, et al., 2012a) is detailed above. These findings have been replicated in a recent study that used virtual-reality exposure therapy for DSM-IV diagnosed Spider Phobia (Kleim et al., 2013). Yet more recently, a large study of cognitive behavioral therapy in Social Anxiety Disorder has shown that better self-reported baseline sleep was associated with better post-treatment outcome on measures of anxiety (Zalta et al., 2013). Therefore, the memory enhancing function of sleep might be exploited to strengthen therapeutic extinction learned in exposure-based therapy by using strategically timed sleep bouts (Kleim et al., 2013; Pace-Schott, Verga, et al., 2012a).
9. Concluding remarks
The memory-integrative function of sleep is of particular theoretical importance to the retrieval of extinction memory because, as noted, extinction is an inhibitory memory that competes for expression with the fear memory that it opposes when a CS is encountered. Because the relative strengths of a particular fear and its extinction vary, in part, based upon past experience (e.g., strength of original fear encoding, extent of extinction learning, temporal proximity of extinction), their relative degree of consolidation, as well as any interaction between these opposing memory traces that takes place during consolidation, will affect their relative expression at retrieval. Human and animal evidence suggests that sleep can promote consolidation of both fear and its extinction and that REM sleep may be of particular importance to both processes. Sleep may serve to select memories for storage and integration with existing stores based upon salience that is determined, in turn, by the simultaneously encoded associated emotion (Stickgold & Walker, 2013). One possibility, therefore, is that REM may be a sleep stage during which the relative strengths of opposing fear and extinction memories are weighed and selectively adjusted. The neurobiology of REM appears particularly well suited to this function since it involves selective activation of the same limbic structures implicated in the learning and memory of fear and extinction. Nonetheless, extrinsic and intrinsic factors acting in proximity to retrieval also serve an important gating function for the expression of fear versus extinction. Extrinsic factors include the well-known phenomena of contextual renewal and reinstatement and, as described, intrinsic factors may include circadian and sleep homeostatic influences. In addition to conditioning and extinction per se, sleep effects on plasticity underlying between-session habituation may also influence ultimate fear expression. In ancestral animals, learning and memory that determine the extent of fear expressed would be expected to have evolved under intense selective pressure given the immense importance of fear responses to survival. Unlike extrinsic factors, sleep is a predictable, periodic behavior upon which such selective pressure could act. In more practical terms, sleep may represent a modifiable behavior through which extinction memory can be strengthened and generalized for the clinical purposes of preventing and treating anxiety disorders.
Acknowledgments
Research supported by MH090357, USAMRAA Log11293006, MH097965. The authors would like to thank Marie-France Marin, Ph.D., Lisa Maeng, Ph.D., and Kara Cover for helpful comments.
References
- Abel T, Havekes R, Saletin JM, Walker MP. Sleep, plasticity and memory from molecules to whole-brain networks. Current Biology. 2013;23(17):R774–788. doi: 10.1016/j.cub.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann S, Rasch B. Differential effects of non-REM and REM sleep on memory consolidation? Curr Neurol Neurosci Rep. 2014;14(2):430. doi: 10.1007/s11910-013-0430-8. [DOI] [PubMed] [Google Scholar]
- Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, Randler C. Circadian typology: a comprehensive review. Chronobiology International. 2012;29(9):1153–1175. doi: 10.3109/07420528.2012.719971. [DOI] [PubMed] [Google Scholar]
- Albouy G, King BR, Maquet P, Doyon J. Hippocampus and striatum: dynamics and interaction during acquisition and sleep-related motor sequence memory consolidation. Hippocampus. 2013;23(11):985–1004. doi: 10.1002/hipo.22183. [DOI] [PubMed] [Google Scholar]
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. Journal of Neuroscience. 2008;28(24):6211–6219. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvaro PK, Roberts RM, Harris JK. A Systematic Review Assessing Bidirectionality between Sleep Disturbances, Anxiety, and Depression. Sleep. 2013;36(7):1059–1068. doi: 10.5665/sleep.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American_Psychiatric_Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Babson KA, Badour CL, Feldner MT, Bunaciu L. The relationship of sleep quality and PTSD to anxious reactivity from idiographic traumatic event script-driven imagery. Journal of Traumatic Stress. 2012;25(5):503–510. doi: 10.1002/jts.21739. [DOI] [PubMed] [Google Scholar]
- Babson KA, Blonigen DM, Boden MT, Drescher KD, Bonn-Miller MO. Sleep quality among U.S. military veterans with PTSD: a factor analysis and structural model of symptoms. Journal of Traumatic Stress. 2012;25(6):665–674. doi: 10.1002/jts.21757. [DOI] [PubMed] [Google Scholar]
- Babson KA, Feldner MT. Temporal relations between sleep problems and both traumatic event exposure and PTSD: a critical review of the empirical literature. Journal of Anxiety Disorders. 2010;24(1):1–15. doi: 10.1016/j.janxdis.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Riemann D. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders. 2011;135(1–3):10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Baran B, Pace-Schott EF, Ericson C, Spencer RM. Processing of emotional reactivity and emotional memory over sleep. Journal of Neuroscience. 2012;32(3):1035–1042. doi: 10.1523/JNEUROSCI.2532-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DC, Wilson DA. Slow-wave sleep-imposed replay modulates both strength and precision of memory. Journal of Neuroscience. 2014;34(15):5134–5142. doi: 10.1523/JNEUROSCI.5274-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behaviour Research and Therapy. 2007;45(9):2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ. Intrasession and intersession habituation in mice: from inbred strain variability to linkage analysis. Neurobiology of Learning and Memory. 2009;92(2):206–214. doi: 10.1016/j.nlm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolkan SS, Lattal KM. Opposing effects of d-cycloserine on fear despite a common extinction duration: Interactions between brain regions and behavior. Neurobiology of Learning and Memory. 2013 doi: 10.1016/j.nlm.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Human Neurobiology. 1982;1(3):195–204. [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52(10):976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning and Memory. 2004;11(5):485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. Journal of Experimental Psychology: Animal Behavior Processes. 1979;5(4):368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biological Psychiatry. 2006;60(4):352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, Herscovitch P. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biological Psychiatry. 1996;39(6):411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. The hippocampo-neocortical dialogue. Cerebral Cortex. 1996;6(2):81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC. REM, not incubation, improves creativity by priming associative networks. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(25):10130–10134. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kozlovsky N, Matar MA, Kaplan Z, Zohar J, Cohen H. Post-exposure sleep deprivation facilitates correctly timed interactions between glucocorticoid and adrenergic systems, which attenuate traumatic stress responses. Neuropsychopharmacology. 2012;37(11):2388–2404. doi: 10.1038/npp.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. Journal of Neuroscience. 2007a;27(4):840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Recalling safety: cooperative functions of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS Spectr. 2007b;12(3):200–206. doi: 10.1017/s1092852900020915. [DOI] [PubMed] [Google Scholar]
- Corley MJ, Caruso MJ, Takahashi LK. Stress-induced enhancement of fear conditioning and sensitization facilitates extinction-resistant and habituation-resistant fear behaviors in a novel animal model of posttraumatic stress disorder. Physiology and Behavior. 2012;105(2):408–416. doi: 10.1016/j.physbeh.2011.08.037. [DOI] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy. 2008;46(1):5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- da Costa Souza A, Ribeiro S. Sleep Deprivation and Gene Expression. Curr Top Behav Neurosci. 2015 doi: 10.1007/7854_2014_360. [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Schabus M, Desseilles M, Sterpenich V, Bonjean M, Maquet P. Functional neuroimaging insights into the physiology of human sleep. Sleep. 2010;33(12):1589–1603. doi: 10.1093/sleep/33.12.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. Avoidance task training potentiates phasic pontine-wave density in the rat: A mechanism for sleep-dependent plasticity. Journal of Neuroscience. 2000;20(22):8607–8613. doi: 10.1523/JNEUROSCI.20-22-08607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Li G, Auerbach S. Activation of phasic pontine-wave generator in the rat: a mechanism for expression of plasticity-related genes and proteins in the dorsal hippocampus and amygdala. European Journal of Neuroscience. 2008;27(7):1876–1892. doi: 10.1111/j.1460-9568.2008.06166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, O’Malley MW. Fear extinction memory consolidation requires potentiation of pontine-wave activity during REM sleep. Journal of Neuroscience. 2013;33(10):4561–4569. doi: 10.1523/JNEUROSCI.5525-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Siwek DF, Patterson EH, Cipolloni PB. Localization of pontine PGO wave generation sites and their anatomical projections in the rat. Synapse. 1998;30(4):409–423. doi: 10.1002/(SICI)1098-2396(199812)30:4<409::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Davis M. NMDA receptors and fear extinction: implications for cognitive behavioral therapy. Dialogues Clin Neurosci. 2011;13(4):463–474. doi: 10.31887/DCNS.2011.13.4/mdavis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Frontiers in Neuroendocrinology. 2009;30(3):358–370. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Bentz D, Michael T, Bolt OC, Wiederhold BK, Margraf J, Wilhelm FH. Glucocorticoids enhance extinction-based psychotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(16):6621–6625. doi: 10.1073/pnas.1018214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59(5):829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliens G, Gilson M, Peigneux P. Sleep and the processing of emotions. Experimental Brain Research. 2014;232(5):1403–1414. doi: 10.1007/s00221-014-3832-1. [DOI] [PubMed] [Google Scholar]
- Deliens G, Gilson M, Schmitz R, Peigneux P. Sleep unbinds memories from their emotional context. Cortex. 2013;49(8):2221–2228. doi: 10.1016/j.cortex.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Deliens G, Neu D, Peigneux P. Rapid eye movement sleep does not seem to unbind memories from their emotional context. Journal of Sleep Research. 2013 doi: 10.1111/jsr.12065. [DOI] [PubMed] [Google Scholar]
- Deliens G, Peigneux P. One night of sleep is insufficient to achieve sleep-to-forget emotional decontextualisation processes. Cogn Emot. 2014;28(4):698–706. doi: 10.1080/02699931.2013.844105. [DOI] [PubMed] [Google Scholar]
- Deliens G, Schmitz R, Caudron I, Mary A, Leproult R, Peigneux P. Does recall after sleep-dependent memory consolidation reinstate sensitivity to retroactive interference? PLoS ONE. 2013;8(7):e68727. doi: 10.1371/journal.pone.0068727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev. 2009;13(5):309–321. doi: 10.1016/j.smrv.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58(1):275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Dirikx T, Hermans D, Vansteenwegen D, Baeyens F, Eelen P. Reinstatement of conditioned responses in human differential fear conditioning. Journal of Behavior Therapy and Experimental Psychiatry. 2007;38(3):237–251. doi: 10.1016/j.jbtep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Hackmann A, Steil R, Clohessy S, Wenninger K, Winter H. The nature of intrusive memories after trauma: the warning signal hypothesis. Behaviour Research and Therapy. 2002;40(9):995–1002. doi: 10.1016/s0005-7967(01)00077-8. [DOI] [PubMed] [Google Scholar]
- Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Current Biology. 2006;16(13):1290–1294. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neuroscience and Biobehavioral Reviews. 2011;35(5):1154–1165. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Fu J, Li P, Ouyang X, Gu C, Song Z, Gao J, Hu B. Rapid eye movement sleep deprivation selectively impairs recall of fear extinction in hippocampus-independent tasks in rats. Neuroscience. 2007;144(4):1186–1192. doi: 10.1016/j.neuroscience.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Gazendam FJ, Kamphuis JH, Kindt M. Deficient safety learning characterizes high trait anxious individuals. Biological Psychology. 2013;92(2):342–352. doi: 10.1016/j.biopsycho.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Genzel L, Spoormaker V, Konrad BN, Dresler M. The role of rapid eye movement sleep for amygdala-related memory processing. Neurobiology of Learning and Memory. 2015 doi: 10.1016/j.nlm.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Germain A. Sleep Disturbances as the Hallmark of PTSD: Where Are We Now? American Journal of Psychiatry. 2013;170(4):372–382. doi: 10.1176/appi.ajp.2012.12040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses. Sleep Med Rev. 2008;12(3):185–195. doi: 10.1016/j.smrv.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AN, Walker MP. The Role of Sleep in Emotional Brain Function. Annual Reviews of Clinical Psychology. 2014 doi: 10.1146/annurev-clinpsy-032813-153716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. The study of fear extinction: implications for anxiety disorders. American Journal of Psychiatry. 2011;168(12):1255–1265. doi: 10.1176/appi.ajp.2011.11040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. Blockade of Estrogen by Hormonal Contraceptives Impairs Fear Extinction in Female Rats and Women. Biological Psychiatry. 2013;73(4):371–378. doi: 10.1016/j.biopsych.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves L, Pack A, Abel T. Sleep and memory: a molecular perspective. Trends in Neurosciences. 2001;24(4):237–243. doi: 10.1016/s0166-2236(00)01744-6. [DOI] [PubMed] [Google Scholar]
- Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learning and Memory. 2003;10(3):168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clinical Neurophysiology. 2003;114(9):1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Groch S, Wilhelm I, Diekelmann S, Born J. The role of REM sleep in the processing of emotional memories: evidence from behavior and event-related potentials. Neurobiology of Learning and Memory. 2013;99:1–9. doi: 10.1016/j.nlm.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Gujar N, McDonald SA, Nishida M, Walker MP. A Role for REM Sleep in Recalibrating the Sensitivity of the Human Brain to Specific Emotions. Cerebral Cortex. 2010;21(1):115–123. doi: 10.1093/cercor/bhq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar N, Yoo SS, Hu P, Walker MP. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. Journal of Neuroscience. 2011;31(12):4466–4474. doi: 10.1523/JNEUROSCI.3220-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauner KK, Howard JD, Zelano C, Gottfried JA. Stimulus-specific enhancement of fear extinction during slow wave sleep. Nature Neuroscience. 2013 doi: 10.1038/nn.3527. (published online 22 September 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Sun HQ, Li SX, Zhang WH, Shi J, Ai SZ, Lu L. Effect of Conditioned Stimulus Exposure During Slow Wave Sleep on Fear Memory Extinction in Humans. Sleep. 2014 doi: 10.5665/sleep.4502. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D, Craske MG, Mineka S, Lovibond PF. Extinction in human fear conditioning. Biological Psychiatry. 2006;60(4):361–368. doi: 10.1016/j.biopsych.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. European Journal of Neuroscience. 2010;31(4):599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci. 2002;3(9):679–693. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Kanayama Y, Matsumura H, Tsuchimochi H, Ishida Y, Nakamura S. Selective rapid eye movement sleep deprivation impairs the maintenance of long-term potentiation in the rat hippocampus. European Journal of Neuroscience. 2006;24(1):243–248. doi: 10.1111/j.1460-9568.2006.04874.x. [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517(7534):284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha SK, Brennan FX, Pawlyk AC, Ross RJ, Morrison AR. REM sleep: a sensitive index of fear conditioning in rats. European Journal of Neuroscience. 2005;21(4):1077–1080. doi: 10.1111/j.1460-9568.2005.03920.x. [DOI] [PubMed] [Google Scholar]
- Jha SK, Ross RJ, Morrison AR. Sleep-related neurons in the central nucleus of the amygdala of rats and their modulation by the dorsal raphe nucleus. Physiology and Behavior. 2005;86(4):415–426. doi: 10.1016/j.physbeh.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17(9):749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. Journal of Psychiatric Research. 2006;40(8):700–708. doi: 10.1016/j.jpsychires.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Jones BE. Basic mechanisms of sleep-wake states. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4. Philadelphia: Elsevier; 2005. pp. 136–153. [Google Scholar]
- Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. American Journal of Psychiatry. 2010;167(6):648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Holt B, Petrovic P, De Martino B, Kloppel S, Buchel C, Dolan RJ. The NMDA agonist D-cycloserine facilitates fear memory consolidation in humans. Cerebral Cortex. 2009;19(1):187–196. doi: 10.1093/cercor/bhn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. Journal of Neuroscience. 2006;26(37):9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann C, Wehrle R, Wetter TC, Holsboer F, Auer DP, Pollmacher T, Czisch M. Brain activation and hypothalamic functional connectivity during human non-rapid eye movement sleep: an EEG/fMRI study. Brain. 2006;129(Pt 3):655–667. doi: 10.1093/brain/awh686. [DOI] [PubMed] [Google Scholar]
- Kleim B, Wilhelm FH, Temp L, Margraf J, Wiederhold BK, Rasch B. Sleep enhances exposure therapy. Psychological Medicine. 2013:1–9. doi: 10.1017/S0033291713001748. [DOI] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cogn Affect Behav Neurosci. 2004;4(3):317–325. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44(4):660–669. doi: 10.1111/j.1469-8986.2007.537.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Cowdin N, Mellman TA. One’s sex, sleep, and posttraumatic stress disorder. Biol Sex Differ. 2012;3(1):29. doi: 10.1186/2042-6410-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I, Mellman TA. Gender differences in sleep during the aftermath of trauma and the development of posttraumatic stress disorder. Behav Sleep Med. 2012;10(3):180–190. doi: 10.1080/15402002.2011.654296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konorski J. Integrative Activity of the Brain. Chicago: University of Chicago Press; 1967. [Google Scholar]
- Kopp C, Longordo F, Nicholson JR, Luthi A. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. Journal of Neuroscience. 2006;26(48):12456–12465. doi: 10.1523/JNEUROSCI.2702-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren D, Arnon I, Lavie P, Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. American Journal of Psychiatry. 2002;159(5):855–857. doi: 10.1176/appi.ajp.159.5.855. [DOI] [PubMed] [Google Scholar]
- Kumar T, Jha SK. Sleep deprivation impairs consolidation of cued fear memory in rats. PLoS ONE. 2012;7(10):e47042. doi: 10.1371/journal.pone.0047042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama K, Honma M, Yoshiike T, Kim Y. Memory suppression trades prolonged fear and sleep-dependent fear plasticity for the avoidance of current fear. Sci Rep. 2013;3:2227. doi: 10.1038/srep02227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama K, Soshi T, Kim Y. Sleep deprivation facilitates extinction of implicit fear generalization and physiological response to fear. Biological Psychiatry. 2010;68(11):991–998. doi: 10.1016/j.biopsych.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Kushnir J, Marom S, Mazar M, Sadeh A, Hermesh H. The link between social anxiety disorder, treatment outcome, and sleep difficulties among patients receiving cognitive behavioral group therapy. Sleep Med. 2014;15(5):515–521. doi: 10.1016/j.sleep.2014.01.012. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20(5):937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behavioral Neuroscience. 2005;119(3):677–686. doi: 10.1037/0735-7044.119.3.677. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Buen TV, McGaugh JL. Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. Journal of Neuroscience. 2003;23(17):6754–6758. doi: 10.1523/JNEUROSCI.23-17-06754.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann N, Kuhn M, Piosczyk H, Feige B, Baglioni C, Spiegelhalder K, Nissen C. The reorganisation of memory during sleep. Sleep Med Rev. 2014 doi: 10.1016/j.smrv.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Lara-Carrasco J, Nielsen TA, Solomonova E, Levrier K, Popova A. Overnight emotional adaptation to negative stimuli is altered by REM sleep deprivation and is correlated with intervening dream emotions. Journal of Sleep Research. 2009;18(2):178–187. doi: 10.1111/j.1365-2869.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- Lebron K, Milad MR, Quirk GJ. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learning and Memory. 2004;11(5):544–548. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- Lebron-Milad K, Abbs B, Milad MR, Linnman C, Rougemount-Bucking A, Zeidan MA, Goldstein JM. Sex differences in the neurobiology of fear conditioning and extinction: a preliminary fMRI study of shared sex differences with stress-arousal circuitry. Biol Mood Anxiety Disord. 2012;2(1):7. doi: 10.1186/2045-5380-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron-Milad K, Graham BM, Milad MR. Low estradiol levels: a vulnerability factor for the development of posttraumatic stress disorder. Biological Psychiatry. 2012;72(1):6–7. doi: 10.1016/j.biopsych.2012.04.029. [DOI] [PubMed] [Google Scholar]
- Lebron-Milad K, Milad MR. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biol Mood Anxiety Disord. 2012;2(1):3. doi: 10.1186/2045-5380-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biological Psychiatry. 2005;57(8):841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Lepola U, Koponen H, Leinonen E. Sleep in panic disorders. Journal of Psychosomatic Research. 1994;38(Suppl 1):105–111. doi: 10.1016/0022-3999(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Levin R, Nielsen TA. Disturbed dreaming, posttraumatic stress disorder, and affect distress: a review and neurocognitive model. Psychological Bulletin. 2007;133(3):482–528. doi: 10.1037/0033-2909.133.3.482. [DOI] [PubMed] [Google Scholar]
- Linnman C, Rougemont-Bucking A, Beucke JC, Zeffiro TA, Milad MR. Unconditioned responses and functional fear networks in human classical conditioning. Behavioural Brain Research. 2011;221(1):237–245. doi: 10.1016/j.bbr.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Zeidan MA, Pitman RK, Milad MR. Resting cerebral metabolism correlates with skin conductance and functional brain activation during fear conditioning. Biological Psychology. 2012;89(2):450–459. doi: 10.1016/j.biopsycho.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wellman LL, Yang L, Ambrozewicz MA, Tang X, Sanford LD. Antagonizing corticotropin-releasing factor in the central nucleus of the amygdala attenuates fear-induced reductions in sleep but not freezing. Sleep. 2011;34(11):1539–1549. doi: 10.5665/sleep.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan V, Brennan FX, Mann GL, Horbal AA, Dunn GA, Ross RJ, Morrison AR. Long-term effect of cued fear conditioning on REM sleep microarchitecture in rats. Sleep. 2008;31(4):497–503. doi: 10.1093/sleep/31.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquet P, Degueldre C, Delfiore G, Aerts J, Peters JM, Luxen A, Franck G. Functional neuroanatomy of human slow wave sleep. Journal of Neuroscience. 1997;17(8):2807–2812. doi: 10.1523/JNEUROSCI.17-08-02807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquet P, Peters J, Aerts J, Delfiore G, Degueldre C, Luxen A, Franck G. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383(6596):163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- Maren S. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron. 2011;70(5):830–845. doi: 10.1016/j.neuron.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14(6):417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature Reviews Neuroscience. 2004;5(11):844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Marshall AJ, Acheson DT, Risbrough VB, Straus LD, Drummond SP. Fear conditioning, safety learning, and sleep in humans. Journal of Neuroscience. 2014;34(35):11754–11760. doi: 10.1523/JNEUROSCI.0478-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. Journal of Neuroscience. 2003;23(29):9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clinical Psychology Review. 2007;27(6):750–759. doi: 10.1016/j.cpr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- McSweeney FK, Swindell S. Common processes may contribute to extinction and habituation. Journal of General Psychology. 2002;129(4):364–400. doi: 10.1080/00221300209602103. [DOI] [PubMed] [Google Scholar]
- Mellman TA. Sleep and Anxiety Disorders. Sleep Medicine Clinics. 2008a;3:261–268. doi: 10.1016/j.jsmc.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman TA. Sleep and post-traumatic stress disorder: a roadmap for clinicians and researchers. Sleep Med Rev. 2008b;12(3):165–167. doi: 10.1016/j.smrv.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. American Journal of Psychiatry. 2002;159(10):1696–1701. doi: 10.1176/appi.ajp.159.10.1696. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Hipolito MM. Sleep disturbances in the aftermath of trauma and posttraumatic stress disorder. CNS Spectr. 2006;11(8):611–615. doi: 10.1017/s1092852900013663. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Knorr BR, Pigeon WR, Leiter JC, Akay M. Heart rate variability during sleep and the early development of posttraumatic stress disorder. Biological Psychiatry. 2004;55(9):953–956. doi: 10.1016/j.biopsych.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Pigeon WR, Nowell PD, Nolan B. Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. Journal of Traumatic Stress. 2007;20(5):893–901. doi: 10.1002/jts.20246. [DOI] [PubMed] [Google Scholar]
- Menz MM, Rihm JS, Salari N, Born J, Kalisch R, Pape HC, Buchel C. The role of sleep and sleep deprivation in consolidating fear memories. Neuroimage. 2013;75:87–96. doi: 10.1016/j.neuroimage.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Milad MR, Furtak SC, Greenberg JL, Keshaviah A, Im JJ, Falkenstein MJ, Wilhelm S. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry. 2013;70(6):608–618. doi: 10.1001/jamapsychiatry.2013.914. quiz 554. [DOI] [PubMed] [Google Scholar]
- Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, Rauch SL. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behavioral Neuroscience. 2006;120(6):1196–1203. doi: 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164(3):887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. Journal of Psychiatric Research. 2008;42(7):515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42(4):456–464. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annual Review of Psychology. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biological Psychiatry. 2007;62(10):1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16(1):43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biological Psychology. 2006;73(1):61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behavioral Neuroscience. 2004;118(2):389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168(3):652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324(5929):951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthaler J, Wiesner CD, Hinze K, Abels LC, Prehn-Kristensen A, Goder R. Selective REM-sleep deprivation does not diminish emotional memory consolidation in young healthy subjects. PLoS ONE. 2014;9(2):e89849. doi: 10.1371/journal.pone.0089849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motomura Y, Kitamura S, Oba K, Terasawa Y, Enomoto M, Katayose Y, Mishima K. Sleep Debt Elicits Negative Emotional Reaction through Diminished Amygdala-Anterior Cingulate Functional Connectivity. PLoS ONE. 2013;8(2):e56578. doi: 10.1371/journal.pone.0056578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motomura Y, Kitamura S, Oba K, Terasawa Y, Enomoto M, Katayose Y, Mishima K. Sleepiness induced by sleep-debt enhanced amygdala activity for subliminal signals of fear. BMC Neurosci. 2014;15:97. doi: 10.1186/1471-2202-15-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36(4):567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Molecular Psychiatry. 2007;12(2):120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Molecular Psychiatry. 2012;17(2):132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. Replay and time compression of recurring spike sequences in the hippocampus. Journal of Neuroscience. 1999;19(21):9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Hardt O, Lanius R. Memory as a new therapeutic target. Dialogues Clin Neurosci. 2013;15(4):475–486. doi: 10.31887/DCNS.2013.15.4/knader. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckelmann D, Mykletun A, Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep. 2007;30(7):873–880. doi: 10.1093/sleep/30.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo HV, Martinetz T, Born J, Molle M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78(3):545–553. doi: 10.1016/j.neuron.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis IL, Takashima A. The role of the ventromedial prefrontal cortex in memory consolidation. Behavioural Brain Research. 2011;218(2):325–334. doi: 10.1016/j.bbr.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cerebral Cortex. 2009;19(5):1158–1166. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofzinger EA, Buysse DJ, Germain A, Carter C, Luna B, Price JC, Kupfer DJ. Increased activation of anterior paralimbic and executive cortex from waking to rapid eye movement sleep in depression. Archives of General Psychiatry. 2004;61(7):695–702. doi: 10.1001/archpsyc.61.7.695. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Buysse DJ, Miewald JM, Meltzer CC, Price JC, Sembrat RC, Moore RY. Human regional cerebral glucose metabolism during non-rapid eye movement sleep in relation to waking. Brain. 2002;125(Pt 5):1105–1115. doi: 10.1093/brain/awf103. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Mintun MA, Wiseman M, Kupfer DJ, Moore RY. Forebrain activation in REM sleep: an FDG PET study. Brain Research. 1997;770(1–2):192–201. doi: 10.1016/s0006-8993(97)00807-x. [DOI] [PubMed] [Google Scholar]
- Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biological Psychiatry. 2008;63(12):1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Contributions of the ventromedial prefrontal cortex to goal-directed action selection. Annals of the New York Academy of Sciences. 2011;1239:118–129. doi: 10.1111/j.1749-6632.2011.06290.x. [DOI] [PubMed] [Google Scholar]
- Olson VG, Rockett HR, Reh RK, Redila VA, Tran PM, Venkov HA, Raskind MA. The role of norepinephrine in differential response to stress in an animal model of posttraumatic stress disorder. Biological Psychiatry. 2011;70(5):441–448. doi: 10.1016/j.biopsych.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comparative Neurology. 2003;460(3):425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109(2):290–298. [PubMed] [Google Scholar]
- Ostroff LE, Cain CK, Bedont J, Monfils MH, Ledoux JE. Fear and safety learning differentially affect synapse size and dendritic translation in the lateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(20):9418–9423. doi: 10.1073/pnas.0913384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci. 2002;3(8):591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Milad MR, Orr SP, Rauch SL, Stickgold R, Pitman RK. Sleep promotes generalization of extinction of conditioned fear. Sleep. 2009;32(1):19–26. [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Nave G, Morgan A, Spencer RM. Sleep-dependent modulation of affectively guided decision-making. Journal of Sleep Research. 2012;21(1):30–39. doi: 10.1111/j.1365-2869.2011.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Shepherd E, Spencer RM, Marcello M, Tucker M, Propper RE, Stickgold R. Napping promotes inter-session habituation to emotional stimuli. Neurobiology of Learning and Memory. 2011;95(1):24–36. doi: 10.1016/j.nlm.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Spencer RM, Vijayakumar S, Ahmed NA, Verga PW, Orr SP, Milad MR. Extinction of conditioned fear is better learned and recalled in the morning than in the evening. Journal of Psychiatric Research. 2013;47(11):1776–1784. doi: 10.1016/j.jpsychires.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Tracy LE, Rubin Z, Mollica AG, Ellenbogen JM, Bianchi MT, Orr SP. Interactions of time of day and sleep with between-session habituation and extinction memory in young adult males. Experimental Brain Research. 2014 doi: 10.1007/s00221-014-3829-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Verga PW, Bennett TS, Spencer RM. Sleep promotes consolidation and generalization of extinction learning in simulated exposure therapy for spider fear. Journal of Psychiatric Research. 2012;46:1036–1044. doi: 10.1016/j.jpsychires.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiological Reviews. 2010;90(2):419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson JL, Reynolds AC, Ferguson SA, Dawson D. Sleep and obsessive-compulsive disorder (OCD) Sleep Med Rev. 2013 doi: 10.1016/j.smrv.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Pavlov I. Conditioned Reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Pawlyk AC, Morrison AR, Ross RJ, Brennan FX. Stress-induced changes in sleep in rodents: models and mechanisms. Neuroscience and Biobehavioral Reviews. 2008;32(1):99–117. doi: 10.1016/j.neubiorev.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Chambers AM, Kensinger EA. Sleep promotes lasting changes in selective memory for emotional scenes. Front Integr Neurosci. 2012;6:108. doi: 10.3389/fnint.2012.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Kensinger EA. Sleep leads to changes in the emotional memory trace: evidence from FMRI. Journal of Cognitive Neuroscience. 2011;23(6):1285–1297. doi: 10.1162/jocn.2010.21526. [DOI] [PubMed] [Google Scholar]
- Pena DF, Engineer ND, McIntyre CK. Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biological Psychiatry. 2013;73(11):1071–1077. doi: 10.1016/j.biopsych.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AC, Blechert J, Samann PG, Eidner I, Czisch M, Spoormaker VI. One night of partial sleep deprivation affects habituation of hypothalamus and skin conductance responses. Journal of Neurophysiology. 2014;112(6):1267–1276. doi: 10.1152/jn.00657.2013. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Popa D, Duvarci S, Popescu AT, Lena C, Pare D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(14):6516–6519. doi: 10.1073/pnas.0913016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Kalinchuk AV. Adenosine, energy metabolism and sleep homeostasis. Sleep Med Rev. 2011;15(2):123–135. doi: 10.1016/j.smrv.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biological Psychiatry. 2006;60(4):337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Gehlert DR. Inhibition of the amygdala: key to pathological states? Annals of the New York Academy of Sciences. 2003;985:263–272. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33(1):56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Pare D, Richardson R, Herry C, Monfils MH, Schiller D, Vicentic A. Erasing fear memories with extinction training. Journal of Neuroscience. 2010;30(45):14993–14997. doi: 10.1523/JNEUROSCI.4268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Born J. About sleep’s role in memory. Physiological Reviews. 2013;93(2):681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biological Psychiatry. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Savings tests: separating differences in rate of learning from differences in initial levels. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28(4):369–377. [PubMed] [Google Scholar]
- Rescorla RA. Spontaneous recovery. Learning and Memory. 2004;11(5):501–509. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. Journal of Experimental Psychology: Animal Behavior Processes. 1975;1(1):88–96. [PubMed] [Google Scholar]
- Richards A, Metzler TJ, Ruoff LM, Inslicht SS, Rao M, Talbot LS, Neylan TC. Sex differences in objective measures of sleep in post-traumatic stress disorder and healthy control subjects. Journal of Sleep Research. 2013;22(6):679–687. doi: 10.1111/jsr.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann D. Insomnia and comorbid psychiatric disorders. Sleep Med. 2007;8(Suppl 4):S15–20. doi: 10.1016/S1389-9457(08)70004-2. [DOI] [PubMed] [Google Scholar]
- Robertson EM. From creation to consolidation: a novel framework for memory processing. PLoS Biol. 2009;7(1):e19. doi: 10.1371/journal.pbio.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10(6):423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Rosales-Lagarde A, Armony JL, Del Rio-Portilla Y, Trejo-Martinez D, Conde R, Corsi-Cabrera M. Enhanced emotional reactivity after selective REM sleep deprivation in humans: an fMRI study. Front Behav Neurosci. 2012;6:25. doi: 10.3389/fnbeh.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe MK, Craske MG. Effects of varied-stimulus exposure training on fear reduction and return of fear. Behaviour Research and Therapy. 1998;36(7–8):719–734. doi: 10.1016/s0005-7967(97)10017-1. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Liu C, Dunn KE, Bazan NG, LaHoste GJ. Sleep deprivation impairs hippocampus-mediated contextual learning but not amygdala-mediated cued learning in rats. European Journal of Neuroscience. 2004;19(11):3121–3124. doi: 10.1111/j.0953-816X.2004.03426.x. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Suchecki D, Meerlo P. Stress, Arousal, and Sleep. Curr Top Behav Neurosci. 2014 doi: 10.1007/7854_2014_314. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Tang X, Ross RJ, Morrison AR. Influence of shock training and explicit fear-conditioned cues on sleep architecture in mice: strain comparison. Behavior Genetics. 2003;33(1):43–58. doi: 10.1023/a:1021051516829. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Yang L, Wellman LL, Liu X, Tang X. Differential effects of controllable and uncontrollable footshock stress on sleep in mice. Sleep. 2010;33(5):621–630. doi: 10.1093/sleep/33.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. Journal of Neuroscience. 2001;21(22):9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerhofer E, Pamplona FA, Bedenk B, Moll GH, Dawirs RR, von Horsten S, Golub Y. Generalization of contextual fear depends on associative rather than non-associative memory components. Behavioural Brain Research. 2012;233(2):483–493. doi: 10.1016/j.bbr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn Sci. 2010;14(6):268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463(7277):49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh JI, Woodward SH, Leskin GA. Sleep in post-traumatic stress disorder and panic: convergence and divergence. Depression and Anxiety. 2003;18(4):187–197. doi: 10.1002/da.10066. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79(2):217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46(1):141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21(5):1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Silvestri AJ. REM sleep deprivation affects extinction of cued but not contextual fear conditioning. Physiology and Behavior. 2005;84(3):343–349. doi: 10.1016/j.physbeh.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Silvestri AJ, Root DH. Effects of REM deprivation and an NMDA agonist on the extinction of conditioned fear. Physiology and Behavior. 2008;93(1–2):274–281. doi: 10.1016/j.physbeh.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271(5257):1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- Smid GE, van der Velden PG, Lensvelt-Mulders GJ, Knipscheer JW, Gersons BP, Kleber RJ. Stress sensitization following a disaster: a prospective study. Psychological Medicine. 2012;42(8):1675–1686. doi: 10.1017/S0033291711002765. [DOI] [PubMed] [Google Scholar]
- Smits JA, Rosenfield D, Davis ML, Julian K, Handelsman PR, Otto MW, Powers MB. Yohimbine Enhancement of Exposure Therapy for Social Anxiety Disorder: A Randomized Controlled Trial. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biological Psychiatry. 2006;60(4):329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, Gvozdanovic GA, Samann PG, Czisch M. Ventromedial prefrontal cortex activity and rapid eye movement sleep are associated with subsequent fear expression in human subjects. Experimental Brain Research. 2014 doi: 10.1007/s00221-014-3831-2. [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12(3):169–184. doi: 10.1016/j.smrv.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, Schroter MS, Andrade KC, Dresler M, Kiem SA, Goya-Maldonado R, Czisch M. Effects of rapid eye movement sleep deprivation on fear extinction recall and prediction error signaling. Human Brain Mapping. 2012;33(10):2362–2376. doi: 10.1002/hbm.21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanski EJ, Rybarczyk B. Emerging research on the treatment and etiology of secondary or comorbid insomnia. Sleep Med Rev. 2006;10(1):7–18. doi: 10.1016/j.smrv.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Sterpenich V, Albouy G, Boly M, Vandewalle G, Darsaud A, Balteau E, Maquet P. Sleep-related hippocampo-cortical interplay during emotional memory recollection. PLoS Biol. 2007;5(11):e282. doi: 10.1371/journal.pbio.0050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterpenich V, Albouy G, Darsaud A, Schmidt C, Vandewalle G, Dang Vu TT, Maquet P. Sleep promotes the neural reorganization of remote emotional memory. Journal of Neuroscience. 2009;29(16):5143–5152. doi: 10.1523/JNEUROSCI.0561-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437(7063):1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Hobson JA, Fosse R, Fosse M. Sleep, learning, and dreams: off-line memory reprocessing. Science. 2001;294(5544):1052–1057. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. Sleep-dependent memory triage: evolving generalization through selective processing. Nature Neuroscience. 2013;16(2):139–145. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: A multi-step process occurring during sleep. Journal of Cognitive Neuroscience. 2000;12(2):246–254. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- Sturm A, Czisch M, Spoormaker VI. Effects of unconditioned stimulus intensity and fear extinction on subsequent sleep architecture in an afternoon nap. Journal of Sleep Research. 2013 doi: 10.1111/jsr.12074. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Tiba PA, Machado RB. REM Sleep Rebound as an Adaptive Response to Stressful Situations. Front Neurol. 2012;3:41. doi: 10.3389/fneur.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Yang L, Liu X, Sanford LD. Influence of tetrodotoxin inactivation of the central nucleus of the amygdala on sleep and arousal. Sleep. 2005;28(8):923–930. doi: 10.1093/sleep/28.8.923. [DOI] [PubMed] [Google Scholar]
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Redmond D. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. Journal of Sleep Research. 2000;9(4):335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Tian S, Huang F, Li P, Ouyang X, Li Z, Deng H, Yang Y. Rapid eye movement sleep deprivation does not affect fear memory reconsolidation in rats. Neuroscience Letters. 2009;463(1):74–77. doi: 10.1016/j.neulet.2009.07.044. [DOI] [PubMed] [Google Scholar]
- Tononi G. Slow wave homeostasis and synaptic plasticity. J Clin Sleep Med. 2009;5(2 Suppl):S16–19. [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Research Bulletin. 2003;62(2):143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- van der Helm E, Yao J, Dutt S, Rao V, Saletin JM, Walker MP. REM sleep depotentiates amygdala activity to previous emotional experiences. Current Biology. 2011;21(23):2029–2032. doi: 10.1016/j.cub.2011.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansteenwegen D, Hermans D, Vervliet B, Francken G, Beckers T, Baeyens F, Eelen P. Return of fear in a human differential conditioning paradigm caused by a return to the original acquistion context. Behaviour Research and Therapy. 2005;43(3):323–336. doi: 10.1016/j.brat.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Vansteenwegen D, Vervliet B, Iberico C, Baeyens F, Van den Bergh O, Hermans D. The repeated confrontation with videotapes of spiders in multiple contexts attenuates renewal of fear in spider-anxious students. Behaviour Research and Therapy. 2007;45(6):1169–1179. doi: 10.1016/j.brat.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142(1):1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Baeyens F, Van den Bergh O, Hermans D. Extinction, generalization, and return of fear: a critical review of renewal research in humans. Biological Psychology. 2013;92(1):51–58. doi: 10.1016/j.biopsycho.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Craske MG, Hermans D. Fear extinction and relapse: state of the art. Annu Rev Clin Psychol. 2013;9:215–248. doi: 10.1146/annurev-clinpsy-050212-185542. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Vansteenwegen D, Baeyens F, Hermans D, Eelen P. Return of fear in a human differential conditioning paradigm caused by a stimulus change after extinction. Behaviour Research and Therapy. 2005;43(3):357–371. doi: 10.1016/j.brat.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Wagner U, Fischer S, Born J. Changes in emotional responses to aversive pictures across periods rich in slow-wave sleep versus rapid eye movement sleep. Psychosomatic Medicine. 2002;64(4):627–634. doi: 10.1097/01.psy.0000021940.35402.51. [DOI] [PubMed] [Google Scholar]
- Wagner U, Hallschmid M, Rasch B, Born J. Brief sleep after learning keeps emotional memories alive for years. Biological Psychiatry. 2006;60(7):788–790. doi: 10.1016/j.biopsych.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Walker MP. The role of sleep in cognition and emotion. Annals of the New York Academy of Sciences. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep, memory, and plasticity. Annual Review of Psychology. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychological Bulletin. 2009;135(5):731–748. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman LL, Fitzpatrick ME, Machida M, Sanford LD. The basolateral amygdala determines the effects of fear memory on sleep in an animal model of PTSD. Experimental Brain Research. 2014 doi: 10.1007/s00221-014-3850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman LL, Yang L, Ambrozewicz MA, Machida M, Sanford LD. Basolateral amygdala and the regulation of fear-conditioned changes in sleep: role of corticotropin-releasing factor. Sleep. 2013;36(4):471–480. doi: 10.5665/sleep.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman LL, Yang L, Tang X, Sanford LD. Contextual Fear Extinction Ameliorates Sleep Disturbances Found Following Fear Conditioning in Rats. Sleep. 2008;31(7):1035–1042. [PMC free article] [PubMed] [Google Scholar]
- Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. American Journal of Psychiatry. 2007;164(11):1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265(5172):676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Wright KM, Britt TW, Bliese PD, Adler AB, Picchioni D, Moore D. Insomnia as predictor versus outcome of PTSD and depression among Iraq combat veterans. Journal of Clinical Psychology. 2011;67(12):1240–1258. doi: 10.1002/jclp.20845. [DOI] [PubMed] [Google Scholar]
- Yang L, Tang X, Wellman LL, Liu X, Sanford LD. Corticotropin releasing factor (CRF) modulates fear-induced alterations in sleep in mice. Brain Research. 2009 doi: 10.1016/j.brainres.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep--a prefrontal amygdala disconnect. Current Biology. 2007;17(20):R877–878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nature Neuroscience. 2007;10(3):385–392. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- Zalta AK, Dowd S, Rosenfield D, Smits JA, Otto MW, Simon NM, Pollack MH. Sleep Quality Predicts Treatment Outcome in Cbt for Social Anxiety Disorder. Depression and Anxiety. 2013 doi: 10.1002/da.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Milad MR. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biological Psychiatry. 2011;70(10):920–927. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]