Abstract
Upregulation of neuronal nicotinic acetylcholine receptors (AChRs) is a venerable result of chronic exposure to nicotine; but it is one of several consequences of pharmacological chaperoning by nicotine and by some other nicotinic ligands, especially agonists. Nicotinic ligands permeate through cell membranes, bind to immature AChR oligomers, elicit incompletely understood conformational reorganizations, increase the interaction between adjacent AChR subunits, and enhance the maturation process toward stable AChR pentamers. These changes and stabilizations in turn lead to increases in both anterograde and retrograde traffic within the early secretory pathway. In addition to the eventual upregulation of AChRs at the plasma membrane, other effects of pharmacological chaperoning include modifications to endoplasmic reticulum stress and to the unfolded protein response. Because these processes depend on pharmacological chaperoning within intracellular organelles, we group them as “inside-out pharmacology”. This term contrasts with the better-known, acute, “outside-in” effects of activating and desensitizing plasma membrane AChRs. We review current knowledge concerning the mechanisms and consequences of inside-out pharmacology.
Keywords: Chaperoning, nicotine, nicotine addiction, nicotinic receptors, unfolded protein response, upregulation
1. Introduction
In 1983, it was discovered that chronic exposure to nicotine leads to an increased binding of nicotine at neuronal nicotinic acetylcholine receptors (AChRs) (Breese et al., 1997; Mamede et al., 2007; Marks et al., 1983; Nashmi et al., 2007; Schwartz and Kellar, 1983). This phenomenon was soon summarized by the appropriate but mechanistically vague term, “upregulation”. Denotations and connotations of AChR upregulation have changed as the process has continued to be studied with advances in molecular and cellular biology, allowing applications of mechanistic understanding. Radioligand binding assays do continue to show an increase in AChR number and a more selective increase in the number of high-affinity nicotine binding sites (Bencherif et al., 1995; Benwell et al., 1988; Darsow et al., 2005; Flores et al., 1992; Govind et al., 2012; Marks et al., 1983; Peng et al., 1994; Vallejo et al., 2005). We now understand that 3H-nicotine or 3H-epibatidine binding to AChRs, which reveals increased high-affinity binding, is only a partial description. Often, an increase in total binding, beyond increased cell surface binding, has been reported. Therefore, upregulation of AChRs involves an increase in AChR abundance in several organelles (endoplasmic reticulum [ER], Golgi, etc.) and is certainly not limited to the PM. More recently, optical measurements using AChRs tagged with fluorescent proteins have provided measurements of increased AChR number without relying on ligand binding (Henderson et al., 2014; Nashmi et al., 2007; Renda and Nashmi, 2012; Richards et al., 2011; Srinivasan et al., 2011). Thus, upregulation is becoming understood as a change in AChR number, stoichiometry, and trafficking (Darsow et al., 2005; Kuryatov et al., 2005; Lester et al., 2009; Miwa et al., 2011; Nelson et al., 2003; Sallette et al., 2005). As noted in Section 4.3 below, at the PM, prolonged exposure to nicotine may also favor higher-affinity AChR conformations on the PM, which also fits some definitions of “upregulated” (Govind et al., 2009).
Upregulation of AChRs occurs in clonal cell lines, cultured neurons, in mice, and in humans (Lester et al., 2012; Miwa et al., 2011; Mukhin et al., 2008; Nashmi et al., 2007; Srinivasan et al., 2011). Upregulation of AChRs in humans has been detected by comparing [3H]nicotine binding to postmortem brains of smokers and non-smokers and in vivo by fMRI or PET imaging (Benwell et al., 1988; Breese et al., 1997; Brody et al., 2013; Brody et al., 2008; Brody et al., 2011; Brody et al., 2006; Cosgrove et al., 2009; Jasinska et al., 2013; Mamede et al., 2007; Perry et al., 1999; Staley et al., 2006; Wullner et al., 2008). Heavy smokers (>14 cigarettes/day) have 25%-330% more AChRs when compared to non-smokers (Mukhin et al., 2008). In rodents, pharmacologically relevant concentrations of nicotine, over 10 days, produce 34%-110% more AChRs (Henderson et al., 2014; Nashmi et al., 2007). In both humans and rodents, upregulation following chronic nicotine is found in the brainstem, cerebellum, prefrontal cortex, and corpus callosum (Brody et al., 2013; Doura et al., 2008; Henderson et al., 2014; Jasinska et al., 2013; Marks et al., 1992; Mukhin et al., 2008; Nashmi et al., 2007; Nguyen et al., 2003; Pauly et al., 1991). Given that upregulation occurs in many systems, it is likely that the cause(s) for upregulation is a process common to many cell types.
Yet, upregulation is also selective, as shown in more detail below. For instance, no upregulation has been detected in thalamus. There are region-specific or cell-specific parameters involved in upregulation (discussed further in section 2.5). In several brain regions, maintained nicotine administration produces half of the maximal upregulation of high sensitivity AChRs after just one day. Continued administration produces additional increases over one to several weeks (Marks et al., 1991; Pietila et al., 1998).
There are many suggestions about the mechanistic details of upregulation including: activation-based, desensitization-based, conformation-based, and turnover-based mechanisms. Recently, many studies have converged on the concept that nicotine acts inside cells to enhance a critical step(s) in the maturation process of AChRs (Henderson et al., 2014; Kuryatov et al., 2005; Sallette et al., 2005; Srinivasan et al., 2011). This intracellular enhancement process has been characterized as pharmacological chaperoning (Kuryatov et al., 2005; Lester et al., 2009), and it occurs at the nanomolar concentrations thought to persist in the brain for hours after a person smokes (50 – 200 nM) (Benowitz, 1990; Henningfield et al., 1990). Approximately such a mechanism was indeed suggested earlier (Bencherif et al., 1995). It is likely that upregulation of AChRs includes multiple contributions from these various suggested mechanisms.
Here, we discuss our current understanding of the upregulation of AChRs as a consequence of pharmacological chaperoning and maturational enhancement. This review is not a synopsis at upregulation in general, but a summation of pharmacological chaperoning of AChRs by nicotinic ligands. The process of pharmacological chaperoning is not unique to nicotine and occurs with many nicotinic ligands that readily permeate cell membranes. The events we describe are conceptualized under the perspective of ‘inside-out’ and ‘outside-in’ drug interactions. Outside-in pharmacology presents the classical view of drug-receptor pharmacology where drugs bind to receptors on the PM to exert an effect. Inside-out pharmacology presents a view where drugs exert their effects through events inside of the cell and not on the surface of the cell (Kuryatov et al., 2005; Lester et al., 2009; Nichols et al., 2014; Sallette et al., 2005). Beginning in the late 90’s, evidence began to emerge as to the intracellular actions of nicotine and nicotinic ligands. In 1995, Bencherif et al., (Bencherif et al., 1995) hypothesized that there existed an additional, reserve pool of AChRs that were undetectable by [3H]-nicotine binding; but are converted to ‘upregulated’, high-affinity AChRs following chronic nicotine exposure. It was suggested that this reserve population was of predominant intracellular localization. In 1998, Whiteaker et al., (Whiteaker et al., 1998) documented that roughly 85% of the high-affinity binding occurred intracellularly. Moreover, that study showed that in cases of nicotine-induced upregulation of PM AChRs, there is a greater increase of intracellular AChRs. This work also provided the early observations of nicotine and nicotinic ligands permeating membranes (Whiteaker et al., 1998). In 2005, two reports were made of observations inside the cell involving nicotine’s ability to act as a maturational enhancer (Sallette et al., 2005) and/or pharmacological chaperone (Kuryatov et al., 2005). In 2011, it was observed that nicotine’s effect on upregulation manipulates events associated with ER export (Richards et al., 2011; Srinivasan et al., 2011). These findings over time have led to a proposed inside-out mechanism upregulation by nicotinic ligands. The inside-out actions of nicotinic ligands include, but are presumably not limited to: 1) pharmacological chaperoning, 2) pharmacological matchmaking, and 3) Golgi-ER cycling.
In this review, we discuss the role that inside-out pharmacology of nicotinic ligands play on AChRs and how these actions relate to nicotine addiction and potential neuroprotection against Parkinson’s disease. Indeed, inside-out effects may predominate more widely in neuropharmacology, for instance in the therapeutic effects of antidepressant and antipsychotic drugs (Lester et al., 2012).
Despite the generality of upregulation with regard to the cellular assay system, this review shows that upregulation is also selective at every level examined. This selectivity extends to the brain region examined, the neuronal cell type within region, the somatodendritic vs. axon terminal region of the neuron, the subunits contributing to the AChR, the detailed stoichiometry of α vs β subunits within the AChR pentamer, and possibly auxiliary proteins that contribute to proteostasis and trafficking on the AChR.1
2. Upregulation of AChRs depends on AChR subtype
Neuronal AChRs are pentameric receptors. Neuronal AChRs can be composed of α (α2-α7) and β (β2-β4) AChR subunits (Chavez-Noriega et al., 1997; Kuryatov and Lindstrom, 2011). They assemble as homomers (α7) or heteromers (containing α and β AChR subunits). Muscle AChRs contain α1, β1, δ, and either γ or ε subunits. In the CNS, the primary AChRs are α7 and β2* (α4β2*, α6β2*, α4α6β2*) AChRs (*, IUPHAR nomenclature meaning “other subunits may be present”) (Lukas et al., 1999). α3* AChRs exist in the CNS but are not so prevalent as the α7 or α4* AChR subtypes. Instead, they are in high abundance in the peripheral nervous system. These various AChR subtypes exhibit a diverse range of sensitivities to nicotine and other nicotinic ligands (Albuquerque et al., 2009; Brown et al., 2007; Chavez-Noriega et al., 1997; Jensen et al., 2005; Kuryatov and Lindstrom, 2011; Tapia et al., 2007). Likewise, the various AChR subtypes seem to be chaperoned differently by nicotine, leading to vastly different properties of upregulation (see section 5.7).
2.1 – Upregulation of α4β2 AChRs
Of all the subtypes, α4β2 AChRs are the most extensively characterized and have been shown to upregulate by low (≤100 nM) (Henderson et al., 2014; Peng et al., 1994; Srinivasan et al., 2011) and high concentrations (>1 μM) (Peng et al., 1994) of nicotine. α4β2 AChRs are assembled into two distinct stoichiometries: the high-sensitivity (HS) (α4)2(β2)3 and low-sensitivity (LS) (α4)3(β2)2 AChRs (Nelson et al., 2003; Tapia et al., 2007). Interestingly, nicotine selectively upregulates the HS AChR stoichiometry (Henderson et al., 2014; Kuryatov et al., 2005; Nelson et al., 2003; Srinivasan et al., 2011) (discussed further below). The EC50 for nicotine-induced upregulation of α4β2 AChRs depends on the assay system but is usually tens to hundreds of nM (Peng et al., 1994), a pharmacologically relevant concentration, as the steady state plasma concentration of nicotine during repeated smoking is ~150 nM (Benowitz, 1990; Henningfield et al., 1990). In some cell lines, α4β2 AChRs reach maximal upregulation in 24-48 hours (Henderson et al., 2014; Srinivasan et al., 2011; Walsh et al., 2008). Other reports using cell lines show that the time course of nicotine-induced upregulation occurred over several days with maximal upregulation occurring in 3-14 days (Peng et al., 1994). These latter assays resemble in vivo concentrations where α4* AChR upregulation typically reaches maximal levels at 10-14 days (Henderson et al., 2014; Marks et al., 1983; Marks et al., 2004; Matta et al., 2007; Nashmi et al., 2007). α4β2 AChR upregulation on the PM may be accompanied by a dramatic increase in the number of AChRs in the ER (Kuryatov et al., 2005; Srinivasan et al., 2011; Whiteaker et al., 1998). In some studies, the fractional increase in ER-resident α4β2 AChRs actually exceeds the fractional increases in α4β2 AChRs inserted on the PM (Srinivasan et al., 2011; Srinivasan et al., 2012). Others have found no marked increase in the number of ER resident AChRs following chronic nicotine (Sallette et al., 2005; Vallejo et al., 2005). In any case, there is never a decrease in ER-resident AChRs. The increase of α4β2 AChR density in the ER is accompanied by an increase in export from the ER via COPII and an increase of insertion on the PM (Richards et al., 2011; Srinivasan et al., 2011). In cell lines, the upregulation of α4β2 AChRs may also be associated with an increase in the stability on the PM (a reduced turnover rate) (see section 4.5 for additional details
2.2 – Upregulation of α6* AChRs
In comparison to α4* AChRs, α6* AChRs are found in more restricted cell types. The best-characterized α6* AChR populations occur in catecholaminergic neurons of the mesolimbic and nigrostriatal regions (Henderson et al., 2014; Mackey et al., 2012; Quik and McIntosh, 2006). Several major α6* AChR subtypes have been identified in midbrain and striatal regions, including: α6(non-α4)β2* and α4α6β2* AChRs (Champtiaux et al., 2003; Champtiaux et al., 2002; Marubio et al., 2003). Many studies in rodents suggest that α6β2* AChRs do not upregulate following chronic nicotine exposure (McCallum et al., 2006a; McCallum et al., 2006b; Moretti et al., 2010; Mugnaini et al., 2006; Perry et al., 2007). Despite this, there have been recent reports of upregulation with α6* AChRs following chronic nicotine treatment (Henderson et al., 2014; Perez et al., 2008; Tumkosit et al., 2006; Walsh et al., 2008). This occurs in all brain regions where α6* AChRs are found: the ventral tegmental area (VTA), substantia nigra pars compacta (SNc), superior colliculus, and medial habenula (Henderson et al., 2014). In partial resolution of this confusing picture, Perez et al., (2008) has shown that α6β2* AChRs that do not contain α4 AChR subunits (α6[non-α4]β2) are upregulated by nicotine, while α4α6β2* AChRs are not upregulated by nicotine.
Recent in vitro experiments show that the upregulation of α6β2β3 AChRs is accompanied by an increased rate of insertion of receptors into the PM (Henderson et al., 2014). The fold increase in insertion to the PM can roughly account for the fold increase in AChR density on the PM. Like α4β2 AChRs, α6β2* AChRs also exhibit an increase in export from the ER following chronic nicotine treatment (Henderson et al., 2014). Therefore it is possible that the upregulation of α6* AChRs is principally due to an increased insertion of new AChRs rather than a change in the stability or turnover of pre-existing AChRs at the PM. In our view, the mechanism of PM insertion is not influenced by nicotine. However, upregulation has increased the pool of intracellular pentameric AChRs awaiting contact with the insertion machinery. Furthermore, we (and others) have found that α6β2* AChRs are upregulated by nicotine and likely require co-assembly with β3 AChR subunits to be upregulated (Henderson et al., 2014; Tumkosit et al., 2006). α6β4* AChRs were found to upregulate following chronic nicotine treatment in the presence and absence of the β3 AChR subunit (Henderson et al., 2014).
2.3 – Upregulation of α3* AChRs
α3* AChRs are primarily assembled in the peripheral nervous system and play important roles in the autonomic nervous system. In the CNS, α3* AChRs are assembled in the thalamus, hypothalamus, locus coeruleus, and habenula (Fowler and Kenny, 2012; Jensen et al., 2005; Shih et al., 2014).
α3β4* AChRs generally have been reported to not upregulate at nicotine concentrations pharmacologically relevant to the smoking brain (50-200 nM). They do undergo upregulation at concentrations of nicotine ≥10 μM (Mazzo et al., 2013; Peng et al., 1997). However, such nicotine concentrations may occur transiently in the airways during smoking (Benowitz et al., 1988). In vitro, α3β4 AChRs are less sensitive (by ~10-fold) to nicotine-induced upregulation when compared to α3β2 AChRs (Walsh et al., 2008). Recently, it has been documented that nicotine produces a change in stoichiometry on α3β4 AChRs as it does on α4β2 AChRs: (α3)2(β4)3 AChR stoichiometry is preferred over (α3)3(β4)2 AChR stoichiometry following chronic treatment with nicotine (Mazzo et al., 2013).
Where upregulation of α3β4 AChRs has been observed, only a small proportion of upregulation occurs at the PM (~30% increase in surface AChRs) while most of the upregulated AChRs reside in intracellular organelles (~95% of the upregulated α3β4 AChRs are found in the ER) (Peng et al., 1997). This amount of surface AChR upregulation is small compared to α4* and α6* AChRs (~2-Fold increase in surface AChRs) (Henderson et al., 2014; Srinivasan et al., 2011).
2.4 – Upregulation of α7* AChRs
α7 AChRs are another AChR subtype that is widely distributed in the brain. α7 AChRs are found in the spinal cord, amygdala, olfactory region, cortex, hippocampus, cerebellum, and hypothalamus (Jensen et al., 2005).
α7 AChRs are noted for their potential role in schizophrenia (Freedman et al., 2000) and lung carcinoma (Brown et al., 2013b). Of interest, the postmortem analysis of schizophrenic brains has found that α7 AChRs are decreased in the hippocampus, cortex, and thalamus (~ 50%) when compared to non-schizophrenic brains (Freedman et al., 1995). Nicotine-induced upregulation of α7 AChRs had been detected in rodents using [125I]-α-bungarotoxin (Collins et al., 1990; Marks et al., 1983; Marks et al., 1986). In the case of humans, upregulation of α7 AChRs was detected postmortem; but only in very heavy smokers (Leonard et al., 2000). In cell lines, when upregulation of α7 AChRs is observed, it occurs at concentrations that are higher than the pharmacologically relevant range for moderate smokers (Peng et al., 1997). In many cases, when α7 AChR upregulation is observed, the increase is much less than observed for α6* and α4* AChRs (i.e., 33% increase (Peng et al., 1997)).
There is evidence that α7 AChRs upregulate by a different mechanism than β2* and β4* AChRs (Peng et al., 1997). β2/4* AChRs do not require activation of surface AChRs to upregulate (discussed in section 4.1). α7 AChR upregulation has been shown to be attenuated by competitive AChR antagonists (Peng et al., 1997), suggesting that activation of α7 AChRs may be required for α7 AChR upregulation (Peng et al., 1997). Brown et al., (Brown et al., 2013a) contributed to these findings by reporting upregulation of α7 AChRs (along with an increase in mRNA levels) through recruitment of Sp1-GATA4 or Sp1-GATA6 (Brown et al., 2013a). Although Brown et al., (Brown et al., 2013a) reports an increase in α7 mRNA, many report no increase in mRNA following chronic nicotine (Peng et al., 1997). Interestingly, this upregulation occurred at concentrations of nicotine that are pharmacologically relevant to human smokers (100 nM). The caveat to these findings by Brown et al. is that the upregulation in vitro and in vivo were observed in model systems used to study carcinogenesis (SCC-L cell line and chorioallantoic membrane models (Brown et al., 2013a)). Other studies, which cannot be reviewed here, suggest that some α7 AChR signaling occurs via their uniquely high Ca2+ permeability, and this Ca2+ flux may also underlie the occasional observations of α7 AChR upregulation.
2.5. Cell-specific upregulation
In addition to AChR subtype being a factor of upregulation, there also appears to be region- and cell-specific features that influence AChR upregulation. We have shown that α4* AChRs upregulate robustly in midbrain GABAergic neurons of the substantia nigra pars reticulata (SNr), SNc and VTA (Nashmi et al., 2007). Despite this, there was no significant upregulation of α4* AChRs in dopaminergic neurons of the VTA and SNc. The same trend was found when electrophysiological assays were used to document functional upregulation of α4β2* AChRs in midbrain regions (Nashmi et al., 2007; Xiao et al., 2009). What is the mechanism regulating that α4β2 AChRs in GABAergic neurons upregulate and α4β2 AChRs in dopaminergic neurons do not? It may be that different assemblies or stoichiometries of AChRs exist among GABAergic and dopaminergic neurons. In fact, GABAergic neurons in midbrain regions have been suggested to express α4α5β2 AChRs in addition to α4β2 AChRs (McClure-Begley et al., 2009). Dopaminergic neurons express many β2* AChRs (α4β2*, α4α6β2*, and α6(non-α4)β2*) (Champtiaux et al., 2003; Champtiaux et al., 2002; Gotti et al., 2005; Marubio et al., 2003). There is evidence that α4α5β2* and α4α6β2* AChRs do not upregulate (Moretti et al., 2010; Perez et al., 2008) and many reports show that α6(non-α4)β2* and α4β2* AChRs upregulate (Henderson et al., 2014; Nashmi et al., 2007; Perez et al., 2008; Renda and Nashmi, 2012). It is possible that the upregulation observed in GABAergic neurons is primarily α4(non-α5)β2. The absence of α4β2 AChR upregulation in dopaminergic neurons (Nashmi et al., 2007) may suggest that the majority of α4* AChRs in midbrain dopaminergic neurons are α4α6β2 AChRs which do not upregulate (Perez et al., 2008). The remainder of AChRs in midbrain dopaminergic neurons may be α6(non-α4)β2 AChRs as we have detected α6* upregulation in midbrain dopaminergic neurons of the VTA and SNc (Henderson et al., 2014). Together, these observations may at least partially explain the cell-specific upregulation of α4β2* AChRs.
2.6. Differential exposure with nicotine influences upregulation
In the preceding section, we discussed observations of upregulation of GABAergic neurons of the VTA and SNr with no change in dopaminergic neurons of the VTA and SNc. Recently, it has been demonstrated that intermittent exposure to nicotine (one dose on alternate days) does evoke a transient (hours) functional upregulation of AChRs in VTA dopaminergic neurons (Baker et al., 2013). This work also documented that intermittent nicotine exposure produced no functional upregulation on VTA GABAergic neurons. Furthermore, Baker et al., showed that intermittent activation and upregulation of AChRs by nicotine played a major role in behavioral sensitization to nicotine. This is extremely interesting, because many beginning smokers intermittently consume cigarettes. This also provides another example of cell-specific or region-specific upregulation as Baker et al., documented upregulation of AChRs in VTA dopaminergic neurons; but no upregulation of AChRs in VTA GABAergic neurons or neurons in the nucleus accumbens (Baker et al., 2013). Baker et al. suggested that the transient upregulation in their experiments may involve α6β2* AChRs, rather than the α4β2* AChRs studied previously (Nashmi et al., 2007).
3. Molecular, Cellular and circuit consequences of upregulation
3.1 – Transcriptional events accompanying upregulation
In this age of transcriptomics, readers often assume that upregulation of a protein results from gene activation. This is not the case for the effects of chronic nicotine on heteromeric AChRs.
There is agreement that mRNA levels of AChRs are not changed significantly following chronic nicotine exposure (Marks et al., 1992). Northern blot assays in cultured M10 cells revealed no change in the mRNA levels of AChRs following chronic nicotine treatment despite an increase in protein binding (Bencherif et al., 1995; Peng et al., 1994). Binding studies clearly show that despite the fact that there is no change in mRNA levels, protein levels of AChRs are increased following chronic nicotine treatment. Furthermore, even when experimenters inject fixed amounts of cRNA in Xenopus oocytes, nicotine-induced upregulation still occurs. More importantly, in brain the upregulation of AChRs does not appear to be accompanied by an increase in mRNA either (Marks et al., 1992; Pauly et al., 1996).This suggests that upregulation is independent of transcriptional events and is likely to occur through post-transcriptional mechanisms (Albuquerque et al., 2009; Lester et al., 2009; Miwa et al., 2011). Similar studies were conducted in mouse fibroblasts (Flores et al., 1992; Marks et al., 1992) and further suggest that nicotine-induced upregulation occurs through a post-transcriptional mechanism. Exceptions were found for α7 AChRs by Lam et al., (Lam et al., 2007) and Brown et al., (Brown et al., 2013a) as both showed that chronic nicotine treatment increased mRNA levels of α7 AChRs (mentioned above).
Although mRNA levels have been found to remain unchanged following chronic nicotine, are new AChR subunits synthesized so that upregulation may occur? When the protein synthesis inhibitor cycloheximide was added with nicotine, α4β2 AChRs still upregulated ~10-fold (Wang et al., 1998). This suggests that AChR upregulation does not require the synthesis of new protein as the existing pool of AChR subunits can be used for the enhanced stable assembly of pentamers.
3.2 – Functional and pharmacological products of upregulation
Nicotine-induced upregulation does not alter the “steady-state” affinity of nicotine on α4β2 AChRs, as measured by assays that incubate ligands and membranes for 20 min (Peng et al., 1994). While AChRs upregulate ~2.5 fold, the steady-state Kd of nicotine on untreated and nicotine treated cells was 3.8 ± 0.8 and 5.6 ± 1.0 nM, respectively (Peng et al., 1994). Although the affinity for nicotine on α4β2 AChRs is unchanged, upregulation is accompanied by a change in AChR sensitivity. That is, maintained nicotine exposure results in a selective increase in HS α4β2 AChRs (Kuryatov et al., 2005; Nelson et al., 2003; Tapia et al., 2007). The proportion of HS α4β2 AChRs on the PM increases at the expense of the proportion of LS α4β2 AChRs. As a result, sensitivity to agonist stimulation and desensitization is increased following chronic treatment with nicotine as additional α4β2 AChRs respond to lower concentrations of ACh and nicotinic agonists.
As mentioned (section 2.5), upregulation of α4* AChRs occurs in the GABAergic neurons of the VTA; but may not occur in the dopaminergic neurons which are inhibited by these same GABAergic neurons (Xiao et al., 2009). Selective upregulation of α4* in GABAergic neurons increases the baseline firing rate and the excitatory effect of nicotine in GABAergic neurons; but decreases the baseline firing rate and excitatory effect of nicotine in dopaminergic neurons (Nashmi and Lester, 2007; Xiao et al., 2009). This, in part, may be an explanation for tolerance to the chronic effects of nicotine (Nashmi et al., 2007).
Cognitive sensitization is apparent by many smokers’ accounts that they are able to think better when they smoke. Likewise, rodents exposed to nicotine exhibit improved spatial working memory (Levin et al., 1996; Levin et al., 1990) and improved contextual fear conditioning (Davis et al., 2005a; Davis et al., 2005b). These cognitive enhancements of nicotine exposure may be explained by α4* AChR upregulation in the hippocampus. Chronic nicotine increases α4* AChRs on glutamatergic axons of the medial perforant path (Nashmi et al., 2007). As a result, nicotine exposure lowers the threshold for induction of long-term potentiation in the medial perforant path. Upregulation of AChRs, assumed to be in glutamatergic neurons, was also observed in the anterior cingulate cortex, another region involved in cognition (Nashmi et al., 2007). Together, these may explain why cognitive enhancement has been observed with nicotine exposure. At more modest and intermittent nicotine doses, the transient upregulation in dopaminergic neurons may play a role in locomotor sensitization (Baker et al., 2013).
It is important to note that at pharmacologically relevant concentrations of nicotine, found in smokers, many AChRs are desensitized (Miwa et al., 2011). Desensitization, like “upregulation”, has vastly different uses across biological experiments, occurs on several time scales, and has experience a series of meanings since the first report (Katz and Thesleff, 1957). Neuroscientists now measure desensitization as a decrease in response to agonist (i.e., nicotine) after repetitive AChR activation; the extent of desensitization is both time and concentration dependent (Karlin, 2002; Katz and Thesleff, 1957; Wang and Sun, 2005). The biophysics of desensitization is discussed more fully in 5.5 below. It is not likely that activation or desensitization of PM AChRs play a major role in AChR upregulation (discussed in sections 4.1 and 4.2); but it is likely that both play a role altering nicotine-mediated behavior (Picciotto et al., 2008). A key concept is shown graphically in Miwa et al., 2011, Figure 3: upregulation of AChRs magnifies the effect of an acute nicotine dose, whether the dominant acute effect is activation or desensitization. Desensitization of AChRs may contribute to the salience of environmental cues related with smoking behavior (Mansvelder et al., 2002; Wooltorton et al., 2003). Both activation and desensitization of AChRs may play a role in primary and conditioned drug reward sensation (Brunzell et al., 2006; Tapper et al., 2004). For an extensive review of this topic, please refer to Picciotto et al., (2008).
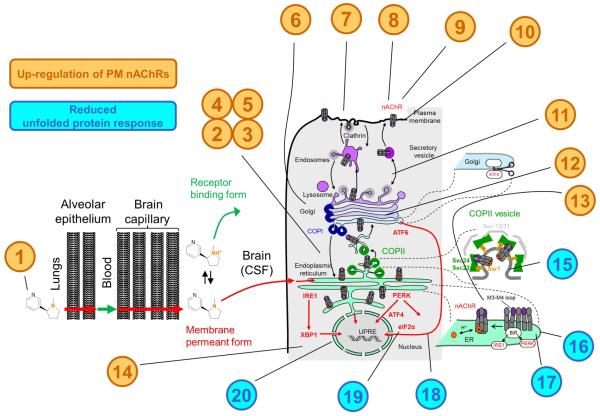
Figure 3. Evidence Supporting Inside-out pharmacology of nicotine and nicotinic ligands.
(1) Nicotine permeates lung epithelium, blood brain barrier and permeates cell membranes to enter intracellular organelles. (2) Nicotine enhances maturation of pentameric AChRs, increasing assembly in the ER (Kuryatov et al., 2005; Sallette et al., 2005). (3) ER retention is necessary for upregulation (Henderson et al., 2014; Srinivasan et al., 2011). (4) Cycling between the Golgi and ER is necessary for upregulation (Henderson et al., 2014). (5) Nicotinic ligands change the area of the peripheral ER (Henderson et al., 2014; Srinivasan et al., 2011). (6) The changes in AChR stoichiometry have occurred by the time AChRs have reached the Golgi (Henderson et al., 2014; Srinivasan et al., 2011). (7) Nicotine enhances the PM insertion rate of vesicles carrying α4β2 and α6β2β3 AChRs (Henderson et al., 2014; Richards et al., 2011). (8) Nicotinic ligands have differential effects on PM stoichiometry (Henderson et al., 2014; Nichols et al., 2014; Richards et al., 2012; Richards et al., 2011; Srinivasan et al., 2011; Srinivasan et al., 2012). (9) Nicotine and cytisine upregulate α4β2 and α6β2β3 AChRs at concentrations that activate ≤0.4% of PM AChRs (Henderson et al., 2014; Richards et al., 2012; Richards et al., 2011; Srinivasan et al., 2011). (10) Quaternary ammonium nicotinic ligands that permeate membranes poorly upregulate AChRs more slowly than nicotine and other tertiary ammonium ligands (Kuryatov et al., 2005). (11) Nicotine increases the number of trans-Golgi network bodies (Henderson et al., 2014; Srinivasan et al., 2011). (12) Nicotine enhances α4β2 AChR glycosylation. (13) β2 AChR subunit mutations that enhance ER exit change stoichiometry, similar to nicotine (Srinivasan et al., 2011). (14) Blocking proteasome activity upregulates AChRs. (15) Nicotine enhances the number of ER exit sites (Henderson et al., 2014; Srinivasan et al., 2011). (16) ER exit sites are increased by β2 AChR subunit M3-M4 loop mutations that introduce ER exit motifs (Srinivasan et al., 2011). (17) ER exit sites are increased by β2 AChR subunit M3-M4 loop mutations that eliminate ER retention motifs (Srinivasan et al., 2011). (18) Nicotinic ligands decrease ATF6 translocation to the nucleus (Srinivasan et al., 2012). (19) Nicotinic ligands decrease eIF2α phosphorylation (Srinivasan et al., 2012). (20) Nicotinic ligands reduce ER stress at concentrations that activate ≤0.4% of PM α4β2 AChRs. Brown font denotes events involved with upregulation of PM AChRs; Blue font denotes events involved with upregulation and the reduced unfolded protein response.
3.3 – Upregulation and the addiction to nicotine
Upregulation of AChRs in response to chronic nicotine plays a major role in nicotine dependence and, perhaps, in the inverse correlation between a person’s history of tobacco use and his or her susceptibility to Parkinson’s disease (PD) (Koob et al., 2004; Koob and Volkow, 2009; Ritz et al., 2007; Srinivasan et al., 2014). The fact that AChRs play a critical role in the addiction to nicotine is clear, as individual deletions of the α4, α6, or β2 AChR subunits are sufficient to block the self-administration of nicotine in mice (Pons et al., 2008). Moreover, The selective re-expression of these deleted subunits in the VTA is sufficient to re-instate self-administration of nicotine (Brunzell et al., 2010; Pons et al., 2008). Nicotine self-administration can be blocked by the selective antagonism of α6* (Jackson et al., 2009) or α4* (Yoshimura et al., 2007) AChRs. From this, it is clear that the AChRs mediating nicotine addiction include those that contain α4, α6, and β2 subunits (Picciotto et al., 1998; Pons et al., 2008; Tapper et al., 2004). The AChR subunits that have been found to upregulate with chronic nicotine in vivo include these three (Henderson et al., 2014; Nashmi et al., 2007; Staley et al., 2006). Therefore, it is likely that the upregulation of AChRs plays a prominent role the reward pathways driving the addiction to nicotine.
3.4 – Reduced ER stress and unfolded protein response
The symptoms of PD arise, in large part, from the selective degeneration of dopaminergic neurons. Dopaminergic neurons are subjected to Ca2+ influx and potentially toxic byproducts of dopamine metabolites that affect proteostasis (Surmeier et al., 2011). Under conditions of physiological stress, dopaminergic neurons display sustained unfolded protein responses (UPR). Maintained UPR activates the pro-apoptotic effecter, C/EBP homologous protein (CHOP) and this has been suggested to (at least partially) underlie dopaminergic neuron cell death in the progression of PD (Mercado et al., 2013). In dozens of retrospective epidemiological studies, there is an inverse correlation between a person’s history of tobacco use and the risk of developing PD (Hernan et al., 2002; Ritz et al., 2007; Tanner et al., 2002). We showed how this inverse correlation may be caused by the inside-out pharmacology of nicotinic ligands and their ability to reduce ER stress and the UPR (Srinivasan et al., 2012). Nicotinic ligands (nicotine, cytisine, and DHβE) reduced nuclear translocation of ATF6, a part of the UPR pathway and marker of ER stress. This occurred at concentrations that activated 0-0.4% of surface AChRs, showing that AChR activation does not play a major role in the reduced ER stress. In addition to its effect on ATF6 translocation, nicotine also suppressed phosphorylation of eukaryotic initiation factor 2α (eIF2α), another component of the UPR pathway. We suggest that as nicotine and nicotinic ligands accelerate ER export of AChRs, this suppresses ER stress and the UPR. Suppression of sustained UPR may provide an explanation to the apparent neuroprotective effect exhibited by nicotine.
We emphasize that reduction of the UPR occurs downstream from pharmacological chaperoning but not downstream from upregulation on the PM. Reduction of the UPR is a distinct consequence of pharmacological chaperoning by nicotine, but reduction of the UPR forms part of “inside-out” nicotinic pharmacology.
4. Potential Mechanisms of upregulation
4.1 – Outside-in pharmacology?
In cellular neuroscience, it was originally assumed that nicotine-induced upregulation resulted from chronic AChR activation, which triggers chronic Na+ and Ca2+ influx, leading in turn to a host of intracellular events that eventually “traffic” more AChRs to the PM. This assumed mechanism has lost favor since ~ 2005, for the majority of AChR subtypes (Kishi and Steinbach, 2006; Kuryatov et al., 2005; Lester et al., 2009; Sallette et al., 2005). The one exception may be α7 AChRs, which may upregulate following PM Ca2+ fluxes (Brown et al., 2013a) (discussed in section 2.4).
One argument against the AChR activation dependent mechanism is that ion flow through AChRs is not necessary for upregulation. In vivo, this has been demonstrated using chlorisondamine which causes persistent inhibition of AChR function in mice (el-Bizri and Clarke, 1994). Despite this persistent inhibition, AChR upregulation is not prevented following chronic treatment with nicotine. In vitro, the non-competitive antagonist (NCA) mecamylamine triggers upregulation despite the fact that it has no AChR agonist properties, functions as a NCA, and prevents ion flow through AChRs (Kishi and Steinbach, 2006; Peng et al., 1994). Mecamylamine had an additive effect with nicotine in its ability to trigger upregulation. Additionally, competitive antagonists d-tubocurarine and DHβE have been used as evidence that upregulation observed in AChRs does not require AChR activation (Kishi and Steinbach, 2006; Peng et al., 1997). Like mecamylamine, DhβE has no agonist properties on AChRs, acts as an antagonist; but upregulates AChRs (Kishi and Steinbach, 2006). Additionally, Kuryatov et al., (Kuryatov et al., 2005) showed that upregulation does not require activation of AChRs on the PM using mutations designed to allow nicotinic ligands to bind; but fail to gate ions. Here, a mutation found in autosomal nocturnal frontal lobe epilepsy patients in the α4 AChR subunit (S247F) was introduced to produce α4β2 AChRs that do not gate ions upon agonist binding. These mutant AChRs, despite being unable to gate ions, still upregulated following chronic nicotine treatment. Together, these data suggest that nicotinic ligands are not required to activate AChRs to trigger upregulation. However, it is likely that binding to the AChR is necessary (discussed more in section 4.5).
4.2 – Desensitization mediated upregulation
Despite a lack of support for the suggestion that AChR upregulation depends on AChR activation via an “outside-in” mechanism, a more subtle “outside-in” mechanism has arisen: that upregulation may be triggered by the as-yet unknown conformational changes that accompany AChR desensitization. Indeed, most AChRs desensitize following prolonged exposure (minutes and longer) to nicotine concentrations that only slightly activate PM AChRs when applied acutely (< 1 s). Thus, the desensitization hypothesis does allow for the common finding that nicotine-induced upregulation occurs at such concentrations (Henderson et al., 2014; Richards et al., 2012; Richards et al., 2011; Srinivasan et al., 2011; Srinivasan et al., 2012). For instance, in the case of α4β2 AChRs, we observed robust upregulation using 100 nM nicotine. This concentration of nicotine is sufficient to activate only <4% of surface HS α4β2 AChRs and 0% of LS α4β2 AChRs. In the case of α6β2β3 AChRs, we observed robust upregulation using 50 nM nicotine. This concentration of nicotine is sufficient to activate <5% of surface α6β2β3 AChRs (Henderson et al., 2014).
There is no evidence that desensitized AChRs on the PM have unique interactions with scaffolding proteins, adaptor proteins, kinases, transcription factors, chaperone proteins, phospholipids, or other molecules that might regulate their PM levels. “Inside-out” mechanisms described below do take account of “desensitized” AChRs, but as an example of stabilization by pharmacological chaperoning (see below, section 5.5).
4.3 – Upregulation as a result of conformational change
Vallejo et al., (2005) observed an alternative mechanism of upregulation, extending beyond the idea that upregulation is solely an increase in α4β2 AChR number. Two independent assays, biotinylation and antibody binding to surface AChRs, were used to document significant increase in 125I-epibatidine binding without a significant increase in the number of surface AChRs (Vallejo et al., 2005). Here, Vallejo et al., also observed no significant change in α4β2 AChR turnover following chronic treatment with nicotine. Interestingly, when blocking anterograde trafficking with brefeldin A, Vallejo et al., also found that there was no significant change in nicotine-induced increase in epibatidine binding. This suggests that trafficking through the secretory pathway is not necessary for creation of upregulated AChRs and that upregulation of AChRs may occur through a conformational change. Darsow et al., (2005) completed similar studies using brefeldin A, but found opposing results to Vallejo et al., (2005). Here, Darsow et al., did note an absence of nicotine-induced upregulation when forward trafficking from the ER is blocked with brefeldin A. Here, there was also a significant increase in PM α4β2 AChRs following chronic nicotine treatment. In considering these opposing results, we note that Vallejo et al., and Darsow et al., used different methods and systems: rat AChRs and ≥17.5 h brefeldin A treatment versus mouse AChRs and 10 h brefeldin A treatment, respectively (Darsow et al., 2005; Vallejo et al., 2005).
Govind et al., (2012) propose that upregulation of AChRs involves two components. The first component involves the original observations of Vallejo et al., (2005) but is expanded by Govind et al., (2012) and includes an event that is independent of an increase in AChR number. This component is likely transient, and proceeds faster than AChR degradation. An increase in surface AChR binding was detected, despite no increase in AChR number, therefore a change in confirmation is likely to be a transition from a resting low-affinity state and an ‘upregulated’ high-affinity state that was not associated with changes in AChR number (Govind et al., 2012; Vallejo et al., 2005). This event, in the context of this review, would be an “outside-in” mechanism.
During prolonged exposure to nicotine, AChR channels on the PM begin to close; and the earliest thermodynamic analysis suggested that these “desensitized” AChRs have higher affinity and altered conformation (Katz & Thesleff, 1957). In one interpretation, Vallejo et al., (2005) and Govind et al., (2012) may have found that additional exposure to agonists (e.g., nicotine) leads to further conformational changes, and to further increases in affinity, of these closed PM AChRs.
The second component proposed by Govind et al., (2012) actually occurs at an earlier step in AChR biosynthesis. The second component is caused by an increase in AChR number, distinct from the conformation-based mechanism. This second component involves the longer-lasting process of increased AChR number, resulting from reduced ER degradation, increased subunit assembly, and consequently, increased insertion of AChRs on the PM (Govind et al., 2012). Component two, in the context of this review, would be an inside-out mechanism, in agreement with many findings that have led to the proposal of inside-out nicotinic ligand chaperoning (Kishi and Steinbach, 2006; Kuryatov et al., 2005; Lester et al., 2009; Sallette et al., 2005; Srinivasan et al., 2011)(discussed further in following sections). In the context of blocking exocytic machinery with brefeldin A, Vallejo et al., presents an argument that in addition to changes in AChR stoichiometry (Lester et al., 2009; Srinivasan et al., 2011), changes in AChR number (Darsow et al., 2005; Henderson et al., 2014; Richards et al., 2011; Srinivasan et al., 2011), and changes in AChR sensitivity (Kuryatov et al., 2005), an additional change may occur, without trafficking, among AChRs.
Therefore in addition to the inside-out mechanisms that are described throughout this review, the Green lab finds evidence for an additional, perhaps complementary event: AChRs on the PM undergo a conformational change that results in higher-affinity AChRs.
4.4 – Does upregulation depend on basal PM density of AChRs?
An interesting finding in the study by Sallette et al., (Sallette et al., 2004) was that α3β4 AChRs maintained a high basal PM density (~3-fold higher than β2* AChRs) in addition to their observed resistance to upregulation. This occurs with α4β4 AChRs as well as α3β4 AChRs (Henderson et al., 2014; Richards et al., 2011; Srinivasan et al., 2011).
One key difference among β2* and β4* AChRs that likely clarifies this divergence are the differences in export and retention motifs found in each subunit (Srinivasan et al., 2011). The β4 AChR subunit contains an ER export motif that is not found in the β2 AChR subunit. Additionally the β4 AChR subunit lacks an ER retention motif that is found in the β2 AChR subunit. Together, these key differences lead to a more efficient export from the ER of β4* AChRs when compared to β2* AChRs. These data imply that upregulation may be influenced by the basal density of a particular AChR subtype on the PM. For example, β4* AChRs, due to their efficient ER export, may not upregulate since they maintain a high basal PM density (Henderson et al., 2014; Richards et al., 2011; Sallette et al., 2004; Srinivasan et al., 2011). As stated explicitly by Sallette et al., (Sallette et al., 2004) “α3β4 AChRs are constitutively upregulated, whereas α3β2 AChRs are weekly expressed, a feature surmounted by nicotine action.”
Additional evidence for this can be seen in the study presented by Srinivasan et al., (Srinivasan et al., 2011). Here, β2 AChR subunits were mutated to introduce the ER export motif found in the β4 AChR subunit (L349M) and the ER retention motif (found in the β2; but not in the β4 AChR subunit) was disrupted (365AAQA368). These mutated α4β2 (α4β2DM) AChRs exhibited a >2-fold increase in basal PM density compared to WT AChRs as a result in a greatly increase in the efficiency of anterograde trafficking. Interestingly, when these AChRs were exposed to nicotine only a small increase in PM density was observed (<20%).
In all cases where high basal PM density is observed (α4β4, α3β4, and α4β2DM AChRs), there has also been an observation of maximal anterograde trafficking of these AChRs (Richards et al., 2011; Srinivasan et al., 2011). Although the high basal PM density is the most readily observable feature, it may be more appropriate to suggest that the resistance of β4* AChRs is a result of their highly efficient export from the ER. Rather, β4* AChRs may be unaffected by pharmacological chaperoning because their already efficient export fulfills the roles played by maturation and pharmacological matchmaking.
4.5 – Upregulation as a result of increased AChR stability (decreased turnover)
It has been suggested that the increase of AChR number on the PM is a result of nicotine or nicotinic ligands stabilizing surface AChRs so that they are degraded or internalized more slowly. Evidence of this has been presented by Peng et al., (Peng et al., 1994). Here it was shown that AChRs treated with nicotine remain on the PM ≥4 days following inhibition of protein synthesis while in untreated conditions, AChRs on the surface are degraded to <50% of their original population in 24 hours. It is important to mention that this was observed using 5 μM nicotine. This is a concentration much higher than pharmacologically relevant: 10-100 times higher than observed in nicotine exposure through smoking (50-500 nM).
Kuryatov et al., (Kuryatov et al., 2005) used a more pharmacologically relevant concentration of nicotine (500 nM) in a surface biotinylation study. Here, it was demonstrated that α4β2 AChRs turnover with a half-life of 12.6 hours. Following nicotine treatment, the half-life was increased to 62.8 hours. This is strong evidence for the case of nicotine’s ability to increase AChR stability on the PM. Not all reports of AChR turnover are in agreement. It is quite possible that nicotine does not alter AChR stability on the PM. Vallejo et al., Darsow et al., and Sallette et al., (all 2005) showed that AChR turnover is not altered by chronic nicotine treatment using concentrations of 10 μM, 100 μM, and 1 mM, respectively. These three studies, while above pharmacologically relevant concentrations of nicotine, still provide evidence that AChR stability and turnover may not be altered by chronic nicotine exposure.
We have shown that chaperoning involves a process of increased anterograde traffic of stable AChR pentamers to the PM (Henderson et al., 2014; Richards et al., 2011). Using pharmacologically relevant concentrations of nicotine (50 or 100 nM) we have shown that chronic nicotine increases the rate of α4* and α6* AChR insertion onto the PM (Henderson et al., 2014; Richards et al., 2011; Srinivasan et al., 2011). As noted above, we believe that this increased insertion rate is a consequence of an increased pool of AChRs awaiting contact with the final steps of exocytosis machinery, not of a molecular modification in the exocytotic machinery. At this point, it is still not clear if increased stability (reduced turnover) is a contributor to AChR upregulation. If so, it is likely a component that is synergistic with increased insertion of AChRs onto the PM.
4.6 Binding of nicotinic ligands is a critical step in initiating upregulation
Even if activation and desensitization of AChRs do not contribute to upregulation, the binding of nicotinic ligands is necessary for the process of upregulation (Kishi and Steinbach, 2006). Mutating residues that are known to contribute to binding of nicotine and nicotinic ligands in the agonist binding site (W182F, W82F, and Y223F) resulted in a reduction or absence upregulation by nicotinic ligands (Kishi and Steinbach, 2006). At pharmacologically relevant concentrations of nicotine, mutations α4W182F, α4Y223F, α4Y126F, or β2W82F resulted in little or no upregulation (≤0.5 fold) of α4β2 AChRs when compared to WT α4β2 AChRs (~2.5 fold upregulation) (Kishi and Steinbach, 2006). Similar trends were observed for other nicotinic ligands: lobeline, carbamylcholine, and DhβE. Upregulation of these mutated AChRs occurred to some degree; but it was clear that the EC50 of upregulation and efficacy of upregulation were altered significantly upon mutating residues that contribute to the agonist binding site. This effect on AChR upregulation strongly suggests that binding to AChRs is necessary for upregulation.
It is a frequent result that nicotine concentrations required for upregulation, although far less than required to activate PM AChRs, are far greater than the steady-state Kd in equilibrium binding experiments at these AChRs (Gopalakrishnan et al., 1996; Kishi and Steinbach, 2006; Kuryatov et al., 2008). This also occurs with other nicotinic ligands: the concentration dependence for lobeline and DHβE does not match the steady-state affinities on resting or desensitized AChRs (Kishi and Steinbach, 2006). This suggests that the binding site of nicotinic ligands in triggering upregulation may not be to stable, mature pentamers. Kishi et al., (Kishi and Steinbach, 2006) suggest that these nicotinic ligands, are in fact binding to immature AChRs that reside in intracellular organelles.
4.7 – Inside-out pharmacology
The evidence gathered suggests an intracellular (“inside-out”) mechanism for nicotinic ligands in their ability to upregulate AChR number on the PM. Activation and desensitization based mechanisms have been proven unsatisfactory as AChR activation is not necessary. In parallel with evidence that activation of AChRs on the PM is not critical for upregulation to occur, other evidence suggests that nicotinic ligands manipulate upregulation through intracellular mechanisms. It is likely that AChR assembly in the ER and export through the secretory pathway is a rather inefficient process and the rate limiting step may occur at the level of the ER (Henderson et al., 2014; Srinivasan et al., 2011). Over the years we, along with several others, have begun to dissect the key intracellular events that manipulate upregulation. These include: 1) pharmacological chaperoning, 2) pharmacological matchmaking, and 3) Golgi-ER cycling. These will be covered explicitly in the following sections.
5. Inside-out pharmacology of nicotinic ligands
5.1 – Nicotinic ligands readily permeate membranes to act as pharmacological chaperones
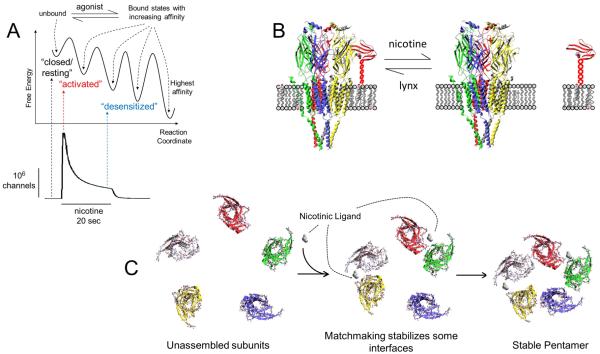
A pharmacological chaperone is a small molecule that stabilizes a protein by binding, as a substrate, agonist, antagonist, or allosteric modulator, at a pharmacologically relevant site on the target protein. This stabilization process is often associated with the pharmacological chaperone facilitating a protein in reaching its stable, low energy conformation (See Figure 2A). Binding primarily occurs within an organelle and typically occurs during biosynthesis and early trafficking of the target protein (Lester et al., 2012). Upon binding, a pharmacological chaperone facilitates the protein’s movement through the secretory pathway. Eventual insertion into the PM appears as usual. A pharmacological chaperone should not be confused with a chaperone protein; but the effect may be similar.
Figure 2. Three possible results of nicotinic ligand-AChR binding in the ER.
(A) Nicotinic ligand binding eventually favors stable, high-affinity states (a “chaperone”). (B) Nicotine may displace lynx, directing AChRs toward cholesterol-poor domains (an “escort” or “abductor”). (C) Nicotinic ligand binding at subunit interface acts as a maturational enhancer (a “matchmaker”) and results in the increased assembly of stable pentamers.
A critical component to the inside-out actions of nicotinic ligands is that nicotine readily passes through membranes to reach intracellular organelles. The process begins anew ~150 billion times a day, when a smoker puffs or vapes. Within 20 s, nicotine then permeates six membranes: in the lungs, endothelium of brain capillary and astrocytic end-feet (see Figure 3). Although there are hints of saturable, carrier-mediated transport in one or more of these membranes (Cisternino et al., 2013), most researchers agree that the simple membrane permeability of deprotonated nicotine accounts for most of this flux. This strongly suggests that nicotine may also enter intracellular organelles like the ER and Golgi (Kuryatov et al., 2005; Sallette et al., 2005; Xiu et al., 2009). It has been shown that many tertiary ligands, in addition to nicotine, permeate cell membranes and enter intracellular organelles (ER and Golgi) to upregulate AChRs (Kuryatov et al., 2005). These ligands cross the PM and intracellular membranes in their uncharged forms within minutes. This was exhibited when ligands such as nicotine and epibatidine, within a 20 minute incubation, proceeded to block all specific binding of [3H]nicotine on α4β2 AChRs (Kuryatov et al., 2005).
It is often asked whether a single “compartmentalization” experiment can provide a decisive distinction between outside-in and inside-out pharmacology. The answer is negative, as even molecules that are relatively impermeant on a time scale of seconds eventually enter cells. We believe that so-called “impermeant” drugs lose this adjective on times scales greater than ~2 h, so that upregulation and other aspects of pharmacological chaperoning, which occur over hours, days, and weeks, cannot be decisively tested with such drugs. For instance, quaternary amines (e.g., DMPP, ACh) may penetrate cells and trigger upregulation; but they require longer periods of time (~3 h) to do so when compared to nicotine and tertiary amines (Kuryatov et al., 2005).
It seems intuitive that nicotinic agonists such as cytisine, DMPP, and carbamylcholine are able to upregulate AChRs since they bind similarly to nicotine at the orthosteric site of AChRs (Kuryatov et al., 2005; Peng et al., 1994). Interestingly, even NCAs and allosteric modulators that do not bind to the orthosteric site, such as mecamylamine, have been shown to upregulate AChRs as well (Peng et al., 1994). The caveat is that this may require high concentrations (nearly mM) to do so. Some competitive antagonists have been shown to upregulate AChRs (i.e., DhβE) (Kuryatov et al., 2005). (−)-Lobeline, an AChR partial agonist (Farook et al., 2009), which also acts as an antagonist (Damaj et al., 1997), also upregulates AChRs (Kishi and Steinbach, 2006). Large, competitive antagonists, such as d-tubocurarine, are unable to upregulate AChRs and even prevent upregulation (Peng et al., 1994).This may be due to the fact that d-tubocurarine (and many competitive antagonists) is quaternary, large, and complex. As a result, d-tubocurarine may poorly penetrate cell membranes.
5.2 – Maturational enhancement
During the maturation process of AChRs, a sequence of glycosylation states typically appear (Sallette et al., 2005). Mature pentameric AChRs require complex glycosylations and trimming for successful export to the PM. Using metabolic labeling assays, Sallette et al., (Sallette et al., 2005) established that maturation of AChRs is a slow, inefficient process; but the speed and efficiency increase dramatically with nicotine treatment. Under basal conditions, 60% of α4 and β2 AChR subunits resident in the ER are glycosylated in the course of subunit processing while the remainder are degraded by cellular machinery. With nicotine treatment, > 90% of these AChR subunits become glycosylated. This process occurs shortly (as early as 30 minutes) after protein synthesis. Although maturation is a multi-step process, the model suggested by Sallette et al., (Sallette et al., 2005) clearly shows that nicotine promotes an early step in maturation of subunits that would otherwise be degraded, thereby increasing the number and stability of AChR subunits available for formation of stable, mature pentamers.
5.3 Pharmacological Matchmaking
It appears that nicotine binds to nicotine-sensitive precursors to promote a critical subunit-subunit interaction step that is limited in the processing of AChR subunits (Kuryatov et al., 2005; Sallette et al., 2004; Sallette et al., 2005) (depicted in Figure 2C). Our colleague Dennis Dougherty suggests that we summarize this increase in subunit-subunit interaction “pharmacological matchmaking” to distinguish it from “pharmacological chaperoning”.
There is an abundance of examples documenting this nicotine-induced increase in subunit-subunit interactions. Chronic nicotine treatment induces a dramatic increase in the co-immunoprecipitation between α4 and β2 AChR subunits in the ER, suggesting that nicotine increases subunit-subunit interaction (or assembly) of AChRs in the ER (Kuryatov et al., 2005; Sallette et al., 2005; Wang et al., 1998). Similarly, chronic nicotine treatment increases FRET among α4 and β2 AChR subunits, further suggesting that nicotine increase subunit-subunit interactions (Henderson et al., 2014; Son et al., 2009; Srinivasan et al., 2011). These FRET assays used whole-cell sections. Thereby, the observed FRET included AChRs resident both on the PM and within organelles. Given that transfected cells, as used in these assays, exhibit a large pool of AChRs in the ER (Kuryatov et al., 2005; Sallette et al., 2004; Sallette et al., 2005), it is likely that the majority of the observed FRET originates in intracellular organelles such as the ER (Moss et al., 2009). Using fluorescently tagged GalT, a marker for the trans-Golgi and trans-Golgi network (TG/TGN), selective examination of AChR assembly and stoichiometry in the TG/TGN is possible. Without nicotine treatment, a relatively equal proportion of high- and low-sensitivity stoichiometries of α4β2 AChRs are assembled in the TG/TGN (Srinivasan et al., 2011). Following nicotine treatment, a higher proportion of high-sensitivity α4β2 AChRs reside in the TG/TGN. This suggests that following chronic nicotine treatment, more HS α4β2 AChRs are assembled in the ER and subsequently chaperoned to the TG/TGN. Together, these FRET and immunoprecipitation assays support the hypothesis that nicotine acts as a matchmaker, increasing the subunit-subunit interactions of AChRs in ER, and chaperones AChRs through the secretory pathway to the TG/TGN, and finally to the PM.
5.4 Pharmacological Matchmaking and Maturational enhancement: importance of an extracellular microdomain
Systematic analysis of β2/β4 chimeras by Sallette et al., (Sallette et al., 2004) demonstrated that the extracellular domain may contribute a critical process for upregulation. Here it was shown that, residues 74-89 and 106-115 of the β2 AChR subunit’s extracellular domain greatly influence upregulation (Sallette et al., 2004). The β2 AChR residues of interest are near the subunit interface, face the orthosteric (agonist) binding site, and may play a key role in the pharmacological matchmaking process as a recognition site for nicotinic ligands. In this study, it was shown clearly that α3β2 AChRs are more responsive to upregulation when compared to α3β4 AChRs (as mentioned above), albeit at non-pharmacologically relevant concentrations of nicotine (≥1 mM). Sallette et al., (Sallette et al., 2004) created chimeric β4 AChR subunits containing the residues 74-89 and 106-115 of β2 AChR subunits; these α3β4Mutant AChRs upregulated to the same degree as α3β2 AChRs. Furthermore, Sallette et al., (Sallette et al., 2004) proposed that nicotine binds to immature AChR subunits, initiates conformational reorganization of this microdomain and this results in an enhanced interaction among AChR subunits which expedites the maturation process. This suggests that pharmacological matchmaking plays a role in the enhancement of the maturation process. We mentioned earlier that β2* and β4* AChRs may be differentially chaperoned by nicotinic ligands due to differences in retention and export motifs. The study by Sallette et al., (Sallette et al., 2004) focused exclusively on the extracellular domain in regions close to the subunit interfaces. This suggests that in additional to our knowledge about AChR retention and export motifs, a critical component that occurs at the interfaces of AChR subunits that allows matchmaking and maturational enhancement by nicotinic ligands to occur.
More recently, computational modeling has provided additional insights into interactions between nicotine and AChR subunits that may explain how the matchmaking and maturational enhancement process occurs. Gao et al., (Gao et al., 2005) showed that as ACh binds to the orthosteric site, conserved tryptophan residues from the principal and complementary subunits begin to form non-covalent interactions, most likely hydrogen bonds. These interactions may act as a ‘molecular glue’ and may be shared by other nicotinic drugs to stabilize the association of α and β AChR subunit intermediates through pharmacological matchmaking. Molecular dynamics have also provided insight into how ACh, nicotine, and other nicotinic ligands affect closure of the C-loop (Gao et al., 2005; Henderson et al., 2010; Pavlovicz et al., 2011b). Upon binding, agonists, partial agonists, antagonists, and allosteric modulators allow the C-loop to close with variable distances which allow additional non-covalent interactions with the complementary interface of an adjacent AChR subunit. This event is likely to provide another level of stabilization for pharmacological matchmaking and may play a significant role in the enhancement of the maturation process.
5.5 – Desensitization and conformational change in the context of pharmacological chaperoning
Inside-out mechanisms arise in part because agonists allow AChRs to reach additional states, beyond the resting states stabilized by antagonists (Figure 2A), both on the PM and inside the cell. Classical electrophysiological observations at the PM show that these additional states (the most detectable is the “channel-open state”) are metastable. We use the term in the same sense that after a depolarizing voltage jump, an open voltage-gated Na+ channel is metastable (Hille, 2001). Indeed the trace in Figure 2A resembles a conductance vs time trace for Na+ channels, a thousand times more slowly. When AChRs reside in open states for longer periods they can eventually, stochastically, surmount the energy barriers separating the open states from neighboring, additional states. Some of these neighboring states are more stable than open states (Jensen et al., 2005). Most of these more stable states have closed channels. There may be a selective advantage in these closings, for they avoid excitotoxic damage to neurons. There are doubtless several such closed states, accessible at increasingly prolonged times of agonist application as the agonist-receptor complex reaches states of lower free energy. When the AChR is on the PM, these increasingly stable closed states are loosely termed “desensitized”. Again there is a strong analogy with the several “inactivated” states of voltage-gated Na+ channels. We actually have no information about the number of “desensitized” states or their structure. However decades of research on desensitization states, and simple thermodynamics, assures us that that desensitized states bind agonist more tightly than either the resting or open-channel states (Albuquerque et al., 2009). This latter aspect of desensitization has no good parallel in Na+ channels. As expected from this explanation, the spectrum of stabilities of the stabilized states have led to suggestions that these states also have a spectrum of affinities for nicotine (Feltz and Trautmann, 1982).
Because of the strong evidence that ligands can also bind to AChRs inside cells (Whiteaker et al., 1998), agonists in particular can ease the transition to more stable states for AChRs inside cells. This is pharmacological chaperoning. We have very little information about these more stable states or their number; but some more stable states may have modified antigenicity (see section 4.3 above). Whether any of these more stable states are open is presently unknown and, in fact, unimportant for pharmacological chaperoning as presently conceived, because pharmacological chaperoning does not occur via ion fluxes (Kuryatov et al., 2005). The important aspect is that such stabilization helps to retard AChR degradation in the early secretory pathway (Mazzo et al., 2013; Sallette et al., 2005). Thus, in the present context the ready observability of desensitization at the PM exemplifies how agonists allow AChRs to find stable states within cells and on the PM.
5.6 – Intracellular cycling is necessary for upregulation
We, along with others, have demonstrated that chronic nicotine treatment enriches the ER with assembled AChRs (Henderson et al., 2014; Mazzo et al., 2013; Sallette et al., 2005; Srinivasan et al., 2011; Whiteaker et al., 1998). As mentioned above, this is the result of pharmacological matchmaking and maturational enhancement as a product of the inside-out pharmacology of nicotinic ligands.
One of the earlier observations concerning nicotine altering intracellular trafficking or exocytic machinery came from Darsow et al., (2005). Here, it was shown that brefeldin A, an inhibitor of transport from the ER to Golgi, prevents nicotine-induced upregulation. This indicates that nicotine exploits early exocytic machinery to upregulate AChRs. We have found that the increase in partially mature AChR pentamers leads to an increase in coat protein complex II (COPII) mediated anterograde traffic through the secretory pathway (Srinivasan et al., 2011). Sec24D, a component of the COPII machinery, can be used to identify ER exit sites, locations where ER resident proteins are packaged into COPII vesicles for export through the secretory pathway. Chronic nicotine dramatically increases the number of ER exit sites and the density of AChRs in ERES, indicating that export from the ER toward the PM is increased with nicotine (Henderson et al., 2014; Srinivasan et al., 2011; Srinivasan et al., 2012). Additionally, we have reported that chronic nicotine also increases the rate of insertion of AChRs on the PM (Henderson et al., 2014; Richards et al., 2011). As the density of AChRs increases in the ER, more stable pentameric AChRs are loaded into COPII export vesicles to move through the secretory pathway from the ER, to the ER Golgi intermediate compartment, to the Golgi, and then to the PM.
In addition to COPII mediated ER export, retrograde movement mediated by coat protein complex I (COPI) is an essential component to the upregulation of AChRs via nicotinic ligands (Henderson et al., 2014). COPI mediates retrograde traffic from the Golgi back to the ER and recognizes its cargo by binding to di-lysine motifs (KKxx or KxKxx) (Jackson et al., 2012; Ma and Goldberg, 2013). We used α6β2β3 AChRs as a model to investigate the importance of COPI in AChR upregulation, as the β3 mouse AChR subunit contains a KKK motif which satisfies both di-lysine motifs recognized by COPI (Henderson et al., 2014). Using mutations of the putative COPI retrieval motif in the β3 AChR subunit and an antagonist of COPI (CI-976) we showed that AChRs fail to upregulate when the interaction with COPI is prevented (Henderson et al., 2014). Upon inhibition of COPI retrograde traffic, there was a consequent increase of AChR density in the Golgi and a decrease of AChR density in the ER (Henderson et al., 2014). Despite this, there was no significant change in ERES. This indicates that there was no significant change in COPII mediated ER export. Interestingly, the basal levels of AChRs on the PM did not change when we compared the chronic nicotine treatment group to the no drug treatment group, following inhibition of COPI. When COPI function is normal, chronic nicotine treatment increases interactions between COPI and AChRs (as reported by FRET) and increases the density of AChRs in COPI vesicles (Henderson et al., 2014).
Perhaps many of the AChRs that reach the Golgi under upregulated conditions are still not fully ‘mature’. Instead of being targeted for traffic to the PM, they may fail a quality control check at the level of the Golgi and are then retrieved back to the ER, via COPI, for additional processing (additional post-translational modifications). Maturational enhancement by nicotinic ligands may assemble AChRs in a way that is premature. That is, their processing may occur at a rate that exceeds the capacity of post-translational modifications. Thereby, AChR pentamers that undergo maturational enhancement by nicotinic ligands are chaperoned out of the ER before they complete the required post-translational modifications that are required to exit the Golgi and be inserted into the PM. Therefore the cycling between the Golgi and ER may be a necessary and critical component to circumvent this deficiency and insure that only fully ‘mature’ AChRs reach the PM. When we add the fact that COPI interactions with AChRs and the AChR density in COPI vesicles increases following chronic nicotine treatment (Henderson et al., 2014), this argument is strengthened.
5.7 – Nicotinic ligands differentially chaperone AChRs
AChRs come in many different assemblies and some exhibit different stoichiometries. As mentioned, α4β2 AChRs are known to exist in either a high-sensitivity stoichiometry ((α4)2(β2)3) or a low-sensitivity stoichiometry ((α4)3(β2)2) (Nelson et al., 2003; Tapia et al., 2007). The term ‘high’ and ‘low’ sensitivity comes from the affinity and potency that nicotine exhibits on these two AChR stoichiometries. Nicotine selectively upregulates HS α4β2 AChRs (Kuryatov et al., 2005; Nelson et al., 2003; Son et al., 2009; Srinivasan et al., 2011; Tapia et al., 2007). When one considers the affinity of nicotine for HS α4β2 AChRs versus LS α4β2 AChRs (~100-fold higher (Kuryatov et al., 2005; Nelson et al., 2003)), this may explain how nicotine would act as a selective pharmacological chaperone for HS α4β2 AChRs and not for LS α4β2 AChRs. Further details will need to be revealed to fully understand the selective chaperoning of HS α4β2 AChRs. Despite being HS or LS, both stoichiometries of α4β2 AChRs contain (α4β2)2 and therefore two ‘high-affinity’ binding sites. The difference may be in how nicotine may interact with the interface of the auxiliary subunit whether it is α4 or β2. Recently, it was discovered that nicotinic ligands do bind to non-canonical binding sites with high affinity (Eaton et al., 2014). This has been characterized for the α4-α4 interface of LS α4β2 AChRs; but we do not know what may occur at the β2-β2 AChR interface of HS α4β2 AChRs.
Cytisine is a partial agonist that exhibits similar efficacy on both LS and HS α4β2 AChRs (Kuryatov et al., 2005). Cytisine exhibits robust upregulation of α4β2 AChRs (Kuryatov et al., 2005); but cytisine exerts different effects than nicotine. Chronic treatment with cytisine results in a PM population that favors LS α4β2 AChRs (Richards et al., 2012; Srinivasan et al., 2012). Essentially, cytisine primarily chaperones LS α4β2 AChRs. Recent data show that another nicotinic ligand likely chaperones low sensitivity α4β2 AChRs (Nichols et al., 2014). The endogenous allosteric modulator, lynx1, is well known for its role in ‘optimizing’ cholinergic tone via AChRs (Miwa et al., 1999; Miwa et al., 2012; Miwa et al., 2006). The apparent difference in AChR sensitivity to ACh in the absence and presence of lynx1, is likely due, in part, to lynx1 acting as a chaperone for LS α4β2 AChRs (Nichols et al., 2014). Lynx1 appears to stabilize α4-α4 AChR subunit dimers in the ER, as seen in co-immunoprecipitation, electrophysiological, and FRET assays (Nichols et al., 2014). This is similar to the way in which nicotine stabilized α4-β2 subunit dimers in co-immunoprecipitation assays (Sallette et al., 2005). Furthermore, this effect seems to be initiated in the ER (Nichols et al., 2014).
The fact that different nicotinic ligands chaperone a single type of AChR (α4β2), but with preference for different stoichiometries, suggest that there may be some specificity to the matchmaking and pharmacological chaperoning process depending on the nicotinic ligand. In the case of nicotine, we see agreement between the type of stoichiometry that is chaperoned and the sensitivity that nicotine has for that particular stoichiometry. Cytisine seems to activate, stabilize, and chaperone the other stoichiometry.
It is reasonable, on a thermodynamic basis, that nicotinic ligands chaperone the AChR subtype or stoichiometry in which they bind best. The case of lynx1 is however not a decisive test of this hypothesis. To clarify, lynx1 is the probable evolutionary antecedent to the snake venom toxin, α-bungarotoxin and lynx1 stabilizes α4-α4 AChR subunit interfaces (Miwa et al., 2012; Nichols et al., 2014). Recall that α-bungarotoxin (Miwa et al., 2012; Nichols et al., 2014) selectively binds to α7-α7 interfaces; but α-bungarotoxin also binds tightly to α-nonα interfaces of muscle AChRs. It is not known how lynx1’s affinity for α-α and α-β interfaces compares, as the GPI anchor complicates binding studies. Evidently lynx1 should be viewed as an incompletely understood blend of a pharmacological chaperone and a chaperone protein.
How is it that NCAs chaperone AChRs? We have thus far presented an intracellular mechanism where nicotinic ligands bind AChRs, foster maturational enhancement, stabilize nascent AChR pentamers, and chaperone AChRs through the secretory pathway to the PM. This has been suggested to depend upon binding at the agonist site (Kishi and Steinbach, 2006; Sallette et al., 2004). The upregulation of AChRs by competitive antagonists (e.g., DhβE) can be explained by the fact that they bind to the same site as nicotine and other nicotinic agonists (Hansen et al., 2006; Hansen et al., 2005; Pavlovicz et al., 2011a). On the other hand, NCAs, such as mecamylamine, do not bind the agonist site and chaperoning cannot be explained as clearly it is for nicotinic ligands that bind the orthosteric site. It is possible that binding at other sites, still at the interface of α and β AChR subunits may be sufficient. Mecamylamine likely binds within the luminal and non-luminal regions of the transmembrane domain at the interface of AChR subunits (Bondarenko et al., 2014; Charnet et al., 1990; Leonard et al., 1988). The binding of mecamylamine to the interface of these α4 and β2 AChR subunits may be sufficient, as a “matchmaking” event, to enhance maturation.
6. Summary: inside-out actions of nicotinic ligands
Many ligands have been shown to upregulate AChRs: agonists (ACh, epibatidine, nicotine, MCC, DMPP, cytisine), antagonists (mecamylamine, DhβE), and allosteric modulators (lynx1, genistein). We know that AChR upregulation is not triggered by activation or desensitization (see section 4.1 and 4.2); upregulation is triggered by binding of nicotinic ligands to AChRs in intracellular compartments (Kishi and Steinbach, 2006). Furthermore, this binding event does not occur on mature AChRs, but on immature AChRs which likely reside in the ER (see Figure 2 and Figure 3). Thus, AChR upregulation likely is initiated by a series of events that start in the ER and progress throughout the secretory pathway (See Figure 3).
First, nicotinic ligands enter organelles such as the ER and Golgi (Henderson et al., 2014; Kuryatov et al., 2005; Srinivasan et al., 2011). Here, nicotine or nicotinic ligands stabilize nascent AChR pentamers. By increasing assembly of dimers and trimers into pentamers, nicotinic ligands act as a ‘matchmaker’ or maturational enhancer (Sallette et al., 2005). Second, these additional AChR pentamers remain stabilized by nicotinic ligands that reside in the ER and are targeted for ER export via COPII. The increase of AChR pentamers in the ER results in an increase of ERES; thereby more AChR pentamers are packed in COPII vesicles that leave the ER and pass through the ER-Golgi intermediate compartment to reach the Golgi. Third, at the level of the Golgi there exists an incompletely understood mechanism, such as a quality control check, that prevents some ‘upregulated’ AChRs from exiting the Golgi to reach the PM. Regardless of the specific mechanism, many chaperoned AChRs are retrieved back to the ER via COPI (Henderson et al., 2014). Regarding Golgi-ER cycling of AChRs, nicotinic ligands may remain bound to AChRs while the AChRs reside in COPI and/or COPII vesicles. Here, processes related to pharmacological chaperoning and ’matchmaking’ may occur in these vesicles similarly to their binding in the ER and Golgi. Fourth, as nicotinic ligands increase the density of stable AChRs in the secretory pathway, the density of AChRs in secretory vesicles increase and the number of vesicles that are deployed to the PM increases paripassu (Henderson et al., 2014; Richards et al., 2011). Finally, once on the PM, AChRs might also remain stabilized by nicotinic ligands, reducing turnover and contributing to the increase in AChR number on the PM (Peng et al., 1994). As export from the ER increases with chronic treatment of nicotinic ligands, ER stress and the UPR is reduced. Nuclear ATF6 translocation and phosphorylation of eIF2α (markers of ER stress and the UPR) are attenuated by nicotinic ligands (Srinivasan et al., 2012). As a result, pro-apoptotic signaling is weakened and neuroprotection may occur.
7. Conclusion and Future Directions
As we continue to study the mechanistic basis of AChR upregulation, it becomes increasingly clear that many of the critical components occur in intracellular environments. It is likely that one locus is the ER; but we now know that the Golgi plays a significant role through the traffic of AChRs via COPI and COPII. In understanding the inside-out pharmacology of nicotinic ligands, we may be able to develop new therapies for addiction to nicotine (Lester et al., 2012; Lester et al., 2009) or for neuroprotection against PD (Srinivasan et al., 2014). Currently, most drug discovery efforts involve designing and optimizing ligands to be specific for AChR subtypes (likely α6* or α4* AChRs). However, it is quite possible that the therapeutic molecules found to be most effective in nicotine cessation or neuroprotection against PD will be the ones that are optimized for entry into the ER and for their ability to modulate maturational enhancement, matchmaking and chaperoning. These hypothetical ligands would be optimized based upon their affinity for specific subunit interfaces as opposed to their efficacy on PM AChRs. The state diagram of Figure 1A shows that it is unlikely that simple antagonists are likely targets to become pharmacological chaperones. However, inverse agonists, allosteric modulators (positive and negative), and noncompetitive channel blockers may stabilize AChRs quite effectively and likely in a manner similar to nicotine. In fact, allosteric modulators may be preferred as they do not interact with the orthosteric site and possess more potential for subtype-selective interactions (Henderson et al., 2012; Henderson et al., 2010).
Figure 1.
Structures of agonists (A), competitive antagonists (CA), non-competitive antagonists (NCA), and positive allosteric modulators (PAM) of AChRs. With the exception of d-tubocurarine, all molecules shown have been reported to upregulate AChRs.
In other biomedical fields, such as cancer and cystic fibrosis, it is taken for granted that a molecule with logP > 1 permeates cell membranes and binds to its targets intracellularly. Yet most neuroscientists, even today, remain skeptical when informed that nicotine may act intracellularly on the same targets that pass through the ER on their journey to the PM. We continue to assure our colleagues that the acute effects of nicotine follow “outside-in” mechanisms; but we continue to encounter resistance to the idea that some chronic effects follow “inside-out” pathways. Although several mechanistic uncertainties remain, this review may convince a few more skeptics.
Nicotinic ligands enter endoplasmic reticulum (ER) and bind to some nicotinic receptors (AChRs).
This “pharmacological chaperoning” alters traffic within early secretory pathways.
As a result, chronic exposure to nicotinic ligands upregulates some AChRs.
These results of intra-organellar binding are termed “inside-out pharmacology”.
“Inside-out” effects also modify ER stress and the unfolded protein response.
Acknowledgements
This work was supported by National Institutes of Health Grants AG033954, DA017279, DA019375, DA030396, NS034407, and DA033721, by the California Tobacco-Related Disease Research Program Grant 17RT0127, and by Louis and Janet Fletcher. We thank Sherry Leonard for contributing comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Non-standard abbreviations: ACh, acetylcholine; AChR, nicotinic acetylcholine receptor; DhβE, dihydro-β-erythroidine ; COPI, coat protein complex 1; COPII, coat protein complex II; DMPP, 1,1-dimethyl-4-phenylpiperazinium iodide; ER, endoplasmic reticulum; HS, high-sensitivity; LS, low-sensitivity; MCC, methylcarbamylcholine; NCA, non-competitive antagonist; PD, Parkinson’s disease, PM, plasma membrane; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata; TMA, tetramethylammonium; TG/TGN, trans-Golgi and trans-Golgi network; UPR, unfolded protein response; VTA, ventral tegmental area.
References
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89(1):73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LK, Mao D, Chi H, Govind AP, Vallejo YF, Iacoviello M, Herrera S, Cortright JJ, Green WN, McGehee DS, Vezina P. Intermittent nicotine exposure upregulates nAChRs in VTA dopamine neurons and sensitises locomotor responding to the drug. The European journal of neuroscience. 2013;37(6):1004–1011. doi: 10.1111/ejn.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencherif M, Fowler K, Lukas RJ, Lippiello PM. Mechanisms of up-regulation of neuronal nicotinic acetylcholine receptors in clonal cell lines and primary cultures of fetal rat brain. The Journal of pharmacology and experimental therapeutics. 1995;275(2):987–994. [PubMed] [Google Scholar]
- Benowitz NL. Pharmacokinetic considerations in understanding nicotine dependence. Ciba Foundation symposium. 1990;152:186–200. doi: 10.1002/9780470513965.ch11. discussion 200-189. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Sheiner L, Jacob P., 3rd Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clinical pharmacology and therapeutics. 1988;44(1):23–28. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (−)[3H]nicotine binding sites in human brain. Journal of neurochemistry. 1988;50(4):1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Bondarenko V, Targowska-Duda KM, Jozwiak K, Tang P, Arias HR. Molecular interactions between mecamylamine enantiomers and the transmembrane domain of the human alpha4beta2 nicotinic receptor. Biochemistry. 2014;53(5):908–918. doi: 10.1021/bi400969x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S. Effect of smoking history on [3H]nicotine binding in human postmortem brain. The Journal of pharmacology and experimental therapeutics. 1997;282(1):7–13. [PubMed] [Google Scholar]
- Brody A, Mukhin A, La Charite J, Ta K, Farahi J, Sugar C, Mamoun M, Vellios E, Archie M, Kozman M, Phuong J, Arlorio F, Mandelkern M. Up-regulation of nicotinic acetylcholine receptors in menthol cigarette smokers. Int J Neuropsychopharmacol. 2013;16:957–966. doi: 10.1017/S1461145712001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Costello MR, Abrams AL, Scheibal D, Farahi J, London ED, Olmstead RE, Rose JE, Mukhin AG. Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2008:1–12. doi: 10.1017/S146114570800922X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Khan A, Kozman D, Costello MR, Vellios EE, Archie MM, Bascom R, Mukhin AG. Effect of secondhand smoke on occupancy of nicotinic acetylcholine receptors in brain. Archives of general psychiatry. 2011;68(9):953–960. doi: 10.1001/archgenpsychiatry.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, Koren AO, Mukhin AG. Cigarette smoking saturates brain α4β2 nicotinic acetylcholine receptors. Archives of general psychiatry. 2006;63(8):907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KC, Perry HE, Lau JK, Jones DV, Pulliam JF, Thornhill BA, Crabtree CM, Luo H, Chen YC, Dasgupta P. Nicotine Induces the Up-regulation of the alpha7-Nicotinic Receptor (alpha7-nAChR) in Human Squamous Cell Lung Cancer Cells via the Sp1/GATA Protein Pathway. J Biol Chem. 2013a;288(46):33049–33059. doi: 10.1074/jbc.M113.501601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KC, Perry HE, Lau JK, Jones DV, Pulliam JF, Thornhill BA, Crabtree CM, Luo H, Chen YC, Dasgupta P. Nicotine induces the upregulation of the α7-nicotinic receptor (α7-nAChR) in human squamous cell lung cancer cells via the Sp1/GATA pathway. The Journal of biological chemistry. 2013b doi: 10.1074/jbc.M113.501601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RW, Collins AC, Lindstrom JM, Whiteaker P. Nicotinic α5 subunit deletion locally reduces high-affinity agonist activation without altering nicotinic receptor numbers. Journal of neurochemistry. 2007;103(1):204–215. doi: 10.1111/j.1471-4159.2007.04700.x. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, McIntosh JM. α-conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(3):665–673. doi: 10.1038/npp.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, Chang JR, Schneider B, Olausson P, Taylor JR, Picciotto MR. β2-Subunit-containing nicotinic acetylcholine receptors are involved in nicotine-induced increases in conditioned reinforcement but not progressive ratio responding for food in C57BL/6 mice. Psychopharmacology. 2006;184(3-4):328–338. doi: 10.1007/s00213-005-0099-z. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knockout mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(21):7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, McIntosh JM, Changeux JP. Distribution and pharmacology of α6-containing nicotinic acetylcholine receptors analyzed with mutant mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(4):1208–1217. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnet P, Labarca C, Leonard RJ, Vogelaar NJ, Czyzyk L, Gouin A, Davidson N, Lester HA. An open-channel blocker interacts with adjacent turns of α-helices in the nicotinic acetylcholine receptor. Neuron. 1990;2:87–95. doi: 10.1016/0896-6273(90)90445-l. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors hα2β2, hα2β4, hα3β2, hα3β4, hα4β2, hα4β4 and hα7 expressed in Xenopus oocytes. The Journal of pharmacology and experimental therapeutics. 1997;280(1) [PubMed] [Google Scholar]
- Cisternino S, Chapy H, Andre P, Smirnova M, Debray M, Scherrmann JM. Coexistence of passive and proton antiporter-mediated processes in nicotine transport at the mouse blood-brain barrier. The AAPS journal. 2013;15(2):299–307. doi: 10.1208/s12248-012-9434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AC, Bhat RV, Pauly JR, Marks MJ. Modulation of nicotine receptors by chronic exposure to nicotinic agonists and antagonists. Ciba Foundation symposium. 1990;152:68–82. doi: 10.1002/9780470513965.ch5. discussion 82-66. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Batis J, Bois F, Maciejewski PK, Esterlis I, Kloczynski T, Stiklus S, Krishnan-Sarin S, O’Malley S, Perry E, Tamagnan G, Seibyl JP, Staley JK. β2-Nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Archives of general psychiatry. 2009;66(6):666–676. doi: 10.1001/archgenpsychiatry.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Patrick GS, Creasy KR, Martin BR. Pharmacology of lobeline, a nicotinic receptor ligand. The Journal of pharmacology and experimental therapeutics. 1997;282(1):410–419. [PubMed] [Google Scholar]
- Darsow T, Booker TK, Pina-Crespo JC, Heinemann SF. Exocytic trafficking is required for nicotine-induced up-regulation of α4β2 nicotinic acetylcholine receptors. The Journal of biological chemistry. 2005;280(18):18311–18320. doi: 10.1074/jbc.M501157200. [DOI] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005a;25(38):8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Porter J, Gould TJ. Nicotine enhances both foreground and background contextual fear conditioning. Neurosci Lett. 2005b doi: 10.1016/j.neulet.2005.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doura MB, Gold AB, Keller AB, Perry DC. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain research. 2008;1215:40–52. doi: 10.1016/j.brainres.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton JB, Lucero LM, Stratton H, Chang Y, Cooper JF, Lindstrom JM, Lukas RJ, Whiteaker P. The unique alpha4+/-alpha4 agonist binding site in (alpha4)3(beta2)2 subtype nicotinic acetylcholine receptors permits differential agonist desensitization pharmacology versus the (alpha4)2(beta2)3 subtype. The Journal of pharmacology and experimental therapeutics. 2014;348(1):46–58. doi: 10.1124/jpet.113.208389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Bizri H, Clarke PB. Regulation of nicotinic receptors in rat brain following quasi-irreversible nicotinic blockade by chlorisondamine and chronic treatment with nicotine. Br J Pharmacol. 1994;113(3):917–925. doi: 10.1111/j.1476-5381.1994.tb17080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farook JM, Lewis B, Gaddis JG, Littleton JM, Barron S. Lobeline, a nicotinic partial agonist attenuates alcohol consumption and preference in male C57BL/6J mice. Physiol Behav. 2009;97(3-4):503–506. doi: 10.1016/j.physbeh.2009.02.031. [DOI] [PubMed] [Google Scholar]
- Feltz A, Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol (Lond) 1982;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat-brain is composed of α4 subunit and β2-subunit and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ. Habenular signaling in nicotine reinforcement. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(1):306–307. doi: 10.1038/npp.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Adams CE, Leonard S. The alpha7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. Journal of chemical neuroanatomy. 2000;20(3-4):299–306. doi: 10.1016/s0891-0618(00)00109-5. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38(1):22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Gao F, Bren N, Burghardt TP, Hansen S, Henchman RH, Taylor P, McCammon JA, Sine SM. Agonist-mediated conformational changes in acetylcholine-binding protein revealed by simulation and intrinsic tryptophan fluorescence. The Journal of biological chemistry. 2005;280(9):8443–8451. doi: 10.1074/jbc.M412389200. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan M, Monteggia LM, Anderson DJ, Molinari EJ, Piattoni-Kaplan M, Donnelly-Roberts D, Arneric SP, Sullivan JP. Stable expression, pharmacologic properties and regulation of the human neuronal nicotinic acetylcholine α4β2 receptor. The Journal of pharmacology and experimental therapeutics. 1996;276(1):289–297. [PubMed] [Google Scholar]
- Gotti C, Moretti M, Zanardi A, Gaimarri A, Champtiaux N, Changeux JP, Whiteaker P, Marks MJ, Clementi F, Zoli M. Heterogeneity and selective targeting of neuronal nicotinic acetylcholine receptor (nAChR) subtypes expressed on retinal afferents of the superior colliculus and lateral geniculate nucleus: identification of a new native nAChR subtype α3beta2(α5 or beta3) enriched in retinocollicular afferents. Molecular pharmacology. 2005;68(4):1162–1171. doi: 10.1124/mol.105.015925. [DOI] [PubMed] [Google Scholar]
- Govind AP, Vezina P, Green WN. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochemical pharmacology. 2009;78(7):756–765. doi: 10.1016/j.bcp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind AP, Walsh H, Green WN. Nicotine-induced upregulation of native neuronal nicotinic receptors is caused by multiple mechanisms. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(6):2227–2238. doi: 10.1523/JNEUROSCI.5438-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SB, Sulzenbacher G, Huxford T, Marchot P, Bourne Y, Taylor P. Structural characterization of agonist and antagonist-bound acetylcholine-binding protein from Aplysia californica. Journal of molecular neuroscience : MN. 2006;30(1-2):101–102. doi: 10.1385/JMN:30:1:101. [DOI] [PubMed] [Google Scholar]
- Hansen SB, Sulzenbacher G, Huxford T, Marchot P, Taylor P, Bourne Y. Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. The EMBO journal. 2005;24(20):3635–3646. doi: 10.1038/sj.emboj.7600828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BJ, Gonzalez-Cestari TF, Yi B, Pavlovicz RE, Boyd RT, Li C, Bergmeier SC, McKay DB. Defining the putative inhibitory site for a selective negative allosteric modulator of human α4β2 neuronal nicotinic receptors. ACS chemical neuroscience. 2012;3(9):682–692. doi: 10.1021/cn300035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BJ, Pavlovicz RE, Allen JD, Gonzalez-Cestari TF, Orac CM, Bonnell AB, Zhu MX, Boyd RT, Li C, Bergmeier SC, McKay DB. Negative allosteric modulators that target human α4β2 neuronal nicotinic receptors. The Journal of pharmacology and experimental therapeutics. 2010;334(3):761–774. doi: 10.1124/jpet.110.168211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BJ, Srinivasan R, Nichols WA, Dilworth CN, Gutierrez DF, Mackey ED, McKinney S, Drenan RM, Richards CI, Lester HA. Nicotine exploits a COPI-mediated process for chaperone-mediated up-regulation of its receptors. J Gen Physiol. 2014;143(1):51–66. doi: 10.1085/jgp.201311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, London ED, Benowitz NL. Arterial-venous differences in plasma concentrations of nicotine after cigarette smoking. JAMA. 1990;263(15):2049–2050. [PubMed] [Google Scholar]
- Hernan MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Annals of neurology. 2002;52(3):276–284. doi: 10.1002/ana.10277. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 3rd ed Sinauer; Sunderland, MA: 2001. [Google Scholar]
- Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI. The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. The Journal of pharmacology and experimental therapeutics. 2009;331(2):547–554. doi: 10.1124/jpet.109.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LP, Lewis M, Kent HM, Edeling MA, Evans PR, Duden R, Owen DJ. Molecular basis for recognition of dilysine trafficking motifs by COPI. Developmental cell. 2012;23(6):1255–1262. doi: 10.1016/j.devcel.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Zorick T, Brody AL, Stein EA. Dual role of nicotine in addiction and cognition: A review of neuroimaging studies in humans. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AA, Frolund B, Liljefors T, Krogsgaard-Larsen P. Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. Journal of medicinal chemistry. 2005;48(15):4705–4745. doi: 10.1021/jm040219e. [DOI] [PubMed] [Google Scholar]
- Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 2002;3(2):102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- Katz B, Thesleff S. A study of the ‘desensitization’ produced by acetylcholine at the motor end-plate. J Physiol. 1957;224:665–699. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi M, Steinbach JH. Role of the agonist binding site in up-regulation of neuronal nicotinic α4β2 receptors. Molecular pharmacology. 2006;70(6):2037–2044. doi: 10.1124/mol.106.029298. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neuroscience and biobehavioral reviews. 2004;27(8):739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharm. 2009;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Lindstrom J. Expression of functional human α6β2β3 acetylcholine receptors in Xenopus laevis oocytes achieved through subunit chimeras and concatamers. Molecular pharmacology. 2011;79(1):126–140. doi: 10.1124/mol.110.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Luo J, Cooper J, Lindstrom J. Nicotine acts as a pharmacological chaperone to up-regulate human α4β2 acetylcholine receptors. Molecular pharmacology. 2005;68(6):1839–1851. doi: 10.1124/mol.105.012419. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, Lindstrom J. Roles of accessory subunits in α4β2α5 nicotinic receptors. Molecular pharmacology. 2008;74(1):132–143. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- Lam DC, Girard L, Ramirez R, Chau WS, Suen WS, Sheridan S, Tin VP, Chung LP, Wong MP, Shay JW, Gazdar AF, Lam WK, Minna JD. Expression of nicotinic acetylcholine receptor subunit genes in non-small-cell lung cancer reveals differences between smokers and nonsmokers. Cancer research. 2007;67(10):4638–4647. doi: 10.1158/0008-5472.CAN-06-4628. [DOI] [PubMed] [Google Scholar]
- Leonard RJ, Labarca C, Charnet P, Davidson N, Lester HA. Evidence that the M2 membrane-spanning region lines the ion channel pore of the nicotinic receptor. Science. 1988;242:1578–1581. doi: 10.1126/science.2462281. [DOI] [PubMed] [Google Scholar]
- Leonard S, Breese C, Adams C, Benhammou K, Gault J, Stevens K, Lee M, Adler L, Olincy A, Ross R, Freedman R. Smoking and schizophrenia: abnormal nicotinic receptor expression. European journal of pharmacology. 2000;393(1-3):237–242. doi: 10.1016/s0014-2999(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Lester HA, Miwa JM, Srinivasan R. Psychiatric Drugs Bind to Classical Targets Within Early Exocytotic Pathways: Therapeutic Effects. Biol Psychiatry. 2012;72:905–915. doi: 10.1016/j.biopsych.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester HA, Xiao C, Srinivasan R, Son C, Miwa J, Pantoja R, Dougherty DA, Banghart MR, Goate AM, Wang JC. Nicotine is a Selective Pharmacological Chaperone of Acetylcholine Receptor Number and Stoichiometry. Implications for Drug Discovery. AAPS Journal. 2009;11:167–177. doi: 10.1208/s12248-009-9090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Kim P, Meray R. Chronic nicotine working and reference memory effects in the 16-arm radial maze: interactions with D1 agonist and antagonist drugs. Psychopharmacology. 1996;127(1):25–30. doi: 10.1007/BF02805971. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lee C, Rose JE, Reyes A, Ellison G, Jarvik M, Gritz E. Chronic nicotine and withdrawal effects on radial-arm maze performance in rats. Behav Neural Biol. 1990;53(2):269–276. doi: 10.1016/0163-1047(90)90509-5. [DOI] [PubMed] [Google Scholar]
- Lukas RJ, Changeux JP, Le Novere N, Albuquerque EX, Balfour DJ, Berg DK, Bertrand D, Chiappinelli VA, Clarke PB, Collins AC, Dani JA, Grady SR, Kellar KJ, Lindstrom JM, Marks MJ, Quik M, Taylor PW, Wonnacott S. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev. 1999;51(2):397–401. [PubMed] [Google Scholar]
- Ma W, Goldberg J. Rules for the recognition of dilysine retrieval motifs by coatomer. The EMBO journal. 2013;32(7):926–937. doi: 10.1038/emboj.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey ED, Engle SE, Kim MR, O’Neill HC, Wageman CR, Patzlaff NE, Wang Y, Grady SR, McIntosh JM, Marks MJ, Lester HA, Drenan RM. α6* Nicotinic Acetylcholine Receptor Expression and Function in a Visual Salience Circuit. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(30):10226–10237. doi: 10.1523/JNEUROSCI.0007-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamede M, Ishizu K, Ueda M, Mukai T, Iida Y, Kawashima H, Fukuyama H, Togashi K, Saji H. Temporal change in human nicotinic acetylcholine receptor after smoking cessation: 5IA SPECT study. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2007;48(11):1829–1835. doi: 10.2967/jnumed.107.043471. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33(6):905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. The Journal of pharmacology and experimental therapeutics. 1983;226(3):817–825. [PubMed] [Google Scholar]
- Marks MJ, Campbell SM, Romm E, Collins AC. Genotype influences the development of tolerance to nicotine in the mouse. The Journal of pharmacology and experimental therapeutics. 1991;259(1):392–402. [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12(7):2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Rowell PP, Cao JZ, Grady SR, McCallum SE, Collins AC. Subsets of acetylcholine-stimulated 86[Rb]+ efflux and 125[I]-epibatidine binding sites in C57BL/6 mouse brain are differentially affected by chronic nicotine treatment. Neuropharmacology. 2004;46(8):1141–1157. doi: 10.1016/j.neuropharm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Dose-response analysis of nicotine tolerance and receptor changes in two inbred mouse strains. The Journal of pharmacology and experimental therapeutics. 1986;239(2):358–364. [PubMed] [Google Scholar]
- Marubio LM, Gardier AM, Durier S, David D, Klink R, Arroyo-Jimenez MM, McIntosh JM, Rossi F, Champtiaux N, Zoli M, Changeux JP. Effects of nicotine in the dopaminergic system of mice lacking the α4 subunit of neuronal nicotinic acetylcholine receptors. The European journal of neuroscience. 2003;17(7):1329–1337. doi: 10.1046/j.1460-9568.2003.02564.x. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharm. 2007;190(3):269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Mazzo F, Pistillo F, Grazioso G, Clementi F, Borgese N, Gotti C, Colombo SF. Nicotine-Modulated Subunit Stoichiometry Affects Stability and Trafficking of α3β4 Nicotinic Receptor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(30):12316–12328. doi: 10.1523/JNEUROSCI.2393-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum SE, Parameswaran N, Bordia T, Fan H, McIntosh JM, Quik M. Differential regulation of mesolimbic α3*/α6*β2 and α4*β2 nicotinic acetylcholine receptor sites and function after long-term oral nicotine to monkeys. J Pharmacol Exp Ther. 2006a;318(1):381–388. doi: 10.1124/jpet.106.104414. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Parameswaran N, Bordia T, Fan H, Tyndale RF, Langston JW, McIntosh JM, Quik M. Increases in α4* but not α3*/α6* nicotinic receptor sites and function in the primate striatum following chronic oral nicotine treatment. J Neurochem. 2006b;96(4):1028–1041. doi: 10.1111/j.1471-4159.2005.03646.x. [DOI] [PubMed] [Google Scholar]
- McClure-Begley TD, King NM, Collins AC, Stitzel JA, Wehner JM, Butt CM. Acetylcholine-stimulated [3H]GABA release from mouse brain synaptosomes is modulated by α4β2 and α4α5β2 nicotinic receptor subtypes. Molecular pharmacology. 2009;75(4):918–926. doi: 10.1124/mol.108.052274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado G, Valdes P, Hetz C. An ERcentric view of Parkinson’s disease. Trends in molecular medicine. 2013;19(3):165–175. doi: 10.1016/j.molmed.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Miwa JM, Freedman R, Lester HA. Neural systems governed by nicotinic acetylcholine receptors: emerging hypotheses. Neuron. 2011;70(1):20–33. doi: 10.1016/j.neuron.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa JM, Ibanez-Tallon I, Crabtree GW, Sanchez R, Sali A, Role LW, Heintz N. lynx1, an endogenous toxin-like modulator of nicotinic acetylcholine receptors in the mammalian CNS. Neuron. 1999;23(1):105–114. doi: 10.1016/s0896-6273(00)80757-6. [DOI] [PubMed] [Google Scholar]
- Miwa JM, Lester HA, Walz A. Optimizing cholinergic tone through lynx modulators of nicotinic receptors: implications for plasticity and nicotine addiction. Physiology (Bethesda) 2012;27(4):187–199. doi: 10.1152/physiol.00002.2012. [DOI] [PubMed] [Google Scholar]
- Miwa JM, Stevens TR, King SL, Caldarone BJ, Ibanez-Tallon I, Xiao C, Fitzsimonds RM, Pavlides C, Lester HA, Picciotto MR, Heintz N. The Prototoxin lynx1 Acts on Nicotinic Acetylcholine Receptors to Balance Neuronal Activity and Survival In Vivo. Neuron. 2006;51(5):587–600. doi: 10.1016/j.neuron.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Moretti M, Mugnaini M, Tessari M, Zoli M, Gaimarri A, Manfredi I, Pistillo F, Clementi F, Gotti C. A comparative study of the effects of the intravenous self-administration or subcutaneous minipump infusion of nicotine on the expression of brain neuronal nicotinic receptor subtypes. Mol Pharmacol. 2010;78(2):287–296. doi: 10.1124/mol.110.064071. [DOI] [PubMed] [Google Scholar]
- Moss FJ, Imoukhuede PI, Scott K, Hu J, Jankowsky JL, Quick MW, Lester HA. GABA transporter function, oligomerization state, and anchoring: correlates with subcellularly resolved FRET. J Gen Physiol. 2009;134(6):489–521. doi: 10.1085/jgp.200910314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnaini M, Garzotti M, Sartori I, Pilla M, Repeto P, Heidbreder CA, Tessari M. Selective down-regulation of [(125)I]Y0-alpha-conotoxin MII binding in rat mesostriatal dopamine pathway following continuous infusion of nicotine. Neuroscience. 2006;137(2):565–572. doi: 10.1016/j.neuroscience.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Mukhin AG, Kimes AS, Chefer SI, Matochik JA, Contoreggi CS, Horti AG, Vaupel DB, Pavlova O, Stein EA. Greater nicotinic acetylcholine receptor density in smokers than in nonsmokers: a PET study with 2-18F-FA-85380. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2008;49(10):1628–1635. doi: 10.2967/jnumed.108.050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi R, Lester H. Cell autonomy, receptor autonomy, and thermodynamics in nicotine receptor up-regulation. Biochemical pharmacology. 2007;74(8):1145–1154. doi: 10.1016/j.bcp.2007.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, Huang Q, McClure-Begley T, Lindstrom JM, Labarca C, Collins AC, Marks MJ, Lester HA. Chronic nicotine cell specifically upregulates functional α4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(31):8202–8218. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y. Alternate stoichiometries of α4β2 nicotinic acetylcholine receptors. Molecular pharmacology. 2003;63(2):332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. The Journal of pharmacology and experimental therapeutics. 2003;307(3):1090–1097. doi: 10.1124/jpet.103.056408. [DOI] [PubMed] [Google Scholar]
- Nichols WA, Henderson BJ, Yu C, Parker RL, Richards CI, Lester HA, Miwa JM. Lynx1 shifts α4β2 nicotinic receptor subunit stoichiometry by affecting assembly in the endoplasmic reticulum. The Journal of biological chemistry. 2014 doi: 10.1074/jbc.M114.573667. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Gross SD, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. The Journal of pharmacology and experimental therapeutics. 1991;258(3):1127–1136. [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Robinson SF, van de Kamp JL, Collins AC. Chronic nicotine and mecamylamine treatment increase brain nicotinic receptor binding without changing α4 or β2 mRNA levels. The Journal of pharmacology and experimental therapeutics. 1996;278(1):361–369. [PubMed] [Google Scholar]
- Pavlovicz RE, Henderson BJ, Bonnell AB, Boyd RT, McKay DB, Li C. Identification of a negative allosteric site on human α4β2 and α3β4 neuronal nicotinic acetylcholine receptors. PloS one. 2011a;6(9):e24949. doi: 10.1371/journal.pone.0024949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovicz RE, Henderson BJ, Bonnell AB, Boyd RT, McKay DB, Li C. Identification of a negative allosteric site on human α4β2 and α3β4 neuronal nicotinic acetylcholine receptors. PloS one. 2011b;6(9):e24949. doi: 10.1371/journal.pone.0024949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Wang F, Lindstrom J. Chronic nicotine treatment up-regulates α3 and α7 acetylcholine receptor subtypes expressed by the human neuroblastoma cell line SH-SY5Y. Molecular pharmacology. 1997;51(5):776–784. doi: 10.1124/mol.51.5.776. [DOI] [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J. Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Molecular pharmacology. 1994;46(3):523–530. [PubMed] [Google Scholar]
- Perez XA, Bordia T, McIntosh JM, Grady SR, Quik M. Long-term nicotine treatment differentially regulates striatal α6α4β2* and α6(Nonα4)β2* nAChR expression and function. Molecular pharmacology. 2008;74(3):844–853. doi: 10.1124/mol.108.048843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. The Journal of pharmacology and experimental therapeutics. 1999;289(3):1545–1552. [PubMed] [Google Scholar]
- Perry DC, Mao D, Gold AB, McIntosh JM, Pezzullo JC, Kellar KJ. Chronic nicotine differentially regulates α6- and β3-containing nicotinic cholinergic receptors in rat brain. The Journal of pharmacology and experimental therapeutics. 2007;322(1):306–315. doi: 10.1124/jpet.107.121228. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: Activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Progress in neurobiology. 2008;84(4):329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391(6663):173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pietila K, Lahde T, Attila M, Ahtee L, Nordberg A. Regulation of nicotinic receptors in the brain of mice withdrawn from chronic oral nicotine treatment. Naunyn Schmiedebergs Arch Pharmacol. 1998;357(2):176–182. doi: 10.1007/pl00005152. [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W. Crucial role of α4 and α6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(47):12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, McIntosh JM. Striatal α6* nicotinic acetylcholine receptors: potential targets for Parkinson’s disease therapy. The Journal of pharmacology and experimental therapeutics. 2006;316(2):481–489. doi: 10.1124/jpet.105.094375. [DOI] [PubMed] [Google Scholar]
- Renda A, Nashmi R. Spectral confocal imaging of fluorescently tagged nicotinic receptors in knock-in mice with chronic nicotine administration. J Vis Exp. 2012;(60) doi: 10.3791/3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards CI, Luong K, Srinivasan R, Turner SW, Dougherty DA, Korlach J, Lester HA. Live-Cell Imaging of Single Receptor Composition Using Zero-Mode Waveguide Nanostructures. Nano Lett. 2012;12(7):11474–11480. doi: 10.1021/nl301480h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards CI, Srinivasan R, Xiao C, Mackey ED, Miwa JM, Lester HA. Trafficking of α4* nicotinic receptors revealed by superecliptic phluorin: effects of a β4 amyotrophic lateral sclerosis-associated mutation and chronic exposure to nicotine. The Journal of biological chemistry. 2011;286(36):31241–31249. doi: 10.1074/jbc.M111.256024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Ascherio A, Checkoway H, Marder KS, Nelson LM, Rocca WA, Ross GW, Strickland D, Van Den Eeden SK, Gorell J. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol. 2007;64(7):990–997. doi: 10.1001/archneur.64.7.990. [DOI] [PubMed] [Google Scholar]
- Sallette J, Bohler S, Benoit P, Soudant M, Pons S, Le Novere N, Changeux JP, Corringer PJ. An extracellular protein microdomain controls up-regulation of neuronal nicotinic acetylcholine receptors by nicotine. The Journal of biological chemistry. 2004;279(18):18767–18775. doi: 10.1074/jbc.M308260200. [DOI] [PubMed] [Google Scholar]
- Sallette J, Pons S, Devillers-Thiery A, Soudant M, Prado de Carvalho L, Changeux JP, Corringer PJ. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46(4):595–607. doi: 10.1016/j.neuron.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science. 1983;220(4593):214–216. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- Shih PY, Engle SE, Oh G, Deshpande P, Puskar NL, Lester HA, Drenan RM. Differential expression and function of nicotinic acetylcholine receptors in subdivisions of medial habenula. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(29):9789–9802. doi: 10.1523/JNEUROSCI.0476-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son CD, Moss FJ, Cohen BN, Lester HA. Nicotine normalizes intracellular subunit stoichiometry of nicotinic receptors carrying mutations linked to autosomal dominant nocturnal frontal lobe epilepsy. Molecular pharmacology. 2009;75(5):1137–1148. doi: 10.1124/mol.108.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Henderson BJ, Lester HA, Richards CI. Pharmacological chaperoning of nAChRs: A therapeutic target for Parkinson’s disease. Pharmacol Res. 2014 doi: 10.1016/j.phrs.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Pantoja R, Moss FJ, Mackey EDW, Son C, Miwa J, Lester HA. Nicotine upregulates α4β2 nicotinic receptors and ER exit sites via stoichiometry-dependent chaperoning. J Gen Physiol. 2011;137:59–79. doi: 10.1085/jgp.201010532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Richards CI, Xiao C, Rhee D, Pantoja R, Dougherty DA, Miwa JM, Lester HA. Pharmacological chaperoning of nicotinic acetylcholine receptors reduces the endoplasmic reticulum stress response. Mol Pharmacol. 2012;81(6):759–769. doi: 10.1124/mol.112.077792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, Dubin JA, Estok K, Brenner E, Baldwin RM, Tamagnan GD, Seibyl JP, Jatlow P, Picciotto MR, London ED, O’Malley S, van Dyck CH. Human tobacco smokers in early abstinence have higher levels of β2* nicotinic acetylcholine receptors than nonsmokers. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(34):8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Guzman JN, Sanchez-Padilla J, Schumacker PT. The role of calcium and mitochondrial oxidant stress in the loss of substantia nigra pars compacta dopaminergic neurons in Parkinson’s disease. Neuroscience. 2011;198:221–231. doi: 10.1016/j.neuroscience.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner CM, Goldman SM, Aston DA, Ottman R, Ellenberg J, Mayeux R, Langston JW. Smoking and Parkinson’s disease in twins. Neurology. 2002;58(4):581–588. doi: 10.1212/wnl.58.4.581. [DOI] [PubMed] [Google Scholar]
- Tapia L, Kuryatov A, Lindstrom J. Ca2+ permeability of the (α4)3(β2)2 stoichiometry greatly exceeds that of (α4)2(β2)3 human acetylcholine receptors. Molecular pharmacology. 2007;71(3):769–776. doi: 10.1124/mol.106.030445. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of α4* receptors: sufficient for reward, tolerance and sensitization. Science. 2004;306(5698):1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Tumkosit P, Kuryatov A, Luo J, Lindstrom J. β3 subunits promote expression and nicotine-induced up-regulation of human nicotinic α6* nicotinic acetylcholine receptors expressed in transfected cell lines. Molecular pharmacology. 2006;70(4):1358–1368. doi: 10.1124/mol.106.027326. [DOI] [PubMed] [Google Scholar]
- Vallejo YF, Buisson B, Bertrand D, Green WN. Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(23):5563–5572. doi: 10.1523/JNEUROSCI.5240-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh H, Govind AP, Mastro R, Hoda JC, Bertrand D, Vallejo Y, Green WN. Upregulation of nicotinic receptors by nicotine varies with receptor subtype. The Journal of biological chemistry. 2008;283:6022–6032. doi: 10.1074/jbc.M703432200. [DOI] [PubMed] [Google Scholar]
- Wang F, Nelson ME, Kuryatov A, Olale F, Cooper J, Keyser K, Lindstrom J. Chronic nicotine treatment up-regulates human α3β2 but not α3β4 acetylcholine receptors stably transfected in human embryonic kidney cells. The Journal of biological chemistry. 1998;273(44):28721–28732. doi: 10.1074/jbc.273.44.28721. [DOI] [PubMed] [Google Scholar]
- Wang H, Sun X. Desensitized nicotinic receptors in brain. Brain Res Brain Res Rev. 2005;48(3):420–437. doi: 10.1016/j.brainresrev.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Sharples CG, Wonnacott S. Agonist-induced up-regulation of α4β2 nicotinic acetylcholine receptors in M10 cells: pharmacological and spatial definition. Molecular pharmacology. 1998;53(5):950–962. [PubMed] [Google Scholar]
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(8):3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullner U, Gundisch D, Herzog H, Minnerop M, Joe A, Warnecke M, Jessen F, Schutz C, Reinhardt M, Eschner W, Klockgether T, Schmaljohann J. Smoking upregulates α4β2* nicotinic acetylcholine receptors in the human brain. Neurosci Lett. 2008;430(1):34–37. doi: 10.1016/j.neulet.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Xiao C, Nashmi R, McKinney S, Cai H, McIntosh JM, Lester HA. Chronic nicotine selectively enhances α4β2* nicotinic acetylcholine receptors in the nigrostriatal dopamine pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:12428–12439. doi: 10.1523/JNEUROSCI.2939-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu X, Puskar NL, Shanata JA, Lester HA, Dougherty DA. Nicotine binding to brain receptors requires a strong cation-π interaction. Nature. 2009;458(7237):534–537. doi: 10.1038/nature07768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura RF, Hogenkamp DJ, Li WY, Tran MB, Belluzzi JD, Whittemore ER, Leslie FM, Gee KW. Negative allosteric modulation of nicotinic acetylcholine receptors blocks nicotine self-administration in rats. The Journal of pharmacology and experimental therapeutics. 2007;323(3):907–915. doi: 10.1124/jpet.107.128751. [DOI] [PubMed] [Google Scholar]