Abstract
A low plasma level of high-density lipoprotein (HDL) cholesterol (HDL-C) is a major risk factor for the development of atherosclerotic cardiovascular disease (ASCVD). However, several observations have highlighted the shortcomings of using cholesterol content as the sole reflection of HDL metabolism. In particular, several large randomized controlled trials of extended release niacin and cholesteryl-ester transfer protein (CETP) inhibitors on background statin therapy have failed to show improvement in ASCVD outcomes despite significant increases in HDL-C. Reverse cholesterol transport (RCT) is the principal HDL function that impacts macrophage foam cell formation and other functions such as endothelial activation of endothelial nitric oxide synthase, monocyte adhesion, and platelet aggregation. Cholesterol efflux from macrophages to plasma/serum reflects the first critical step of RCT and is considered a key anti-atherosclerotic function of HDL. Whether this function is operative in humans remains to be seen, but recent studies assessing cholesterol efflux in humans suggest that the cholesterol efflux capacity (CEC) of human plasma or serum is a potent marker of ASCVD risk. This review describes the methodology of measuring CEC ex vivo from human samples and the findings to date linking CEC to human disease. Studies to date confirm that CEC can be reliably measured using stored human blood samples as cholesterol acceptors and suggest that CEC may be a promising new biomarker for atherosclerotic and metabolic diseases. Further studies are needed to standardize measurements and clarify the role CEC may play in predicting risk of developing disease and response to therapies.
Keywords: HDL, Lipoprotein, Function, Cholesterol Efflux, coronary disease, heart disease, atherosclerosis
Introduction
A low plasma level of high-density lipoprotein (HDL) cholesterol (HDL-C) is a major risk factor for the development of atherosclerotic cardiovascular disease (ASCVD).1 However, several observations have highlighted the shortcomings of using cholesterol content as the sole reflection of HDL metabolism. HDL-C is lower in the insulin resistance state, which also confers increased ASCVD risk. This is evidenced by the fact that the association between low HDL-C and ASCVD is attenuated by adjustment for total low-density lipoprotein (LDL) particle concentration.2 In addition, genetic studies of low or high high-density lipoprotein (HDL) cholesterol (HDL-C ) have not shown association with increased or decreased risk, respectively.3,4 Lastly, several large randomized controlled trials of extended release niacin and cholesteryl-ester transfer protein (CETP) inhibitors on background statin therapy have failed to show improvement in ASCVD outcomes despite significant increases in HDL-C.5–8 These observations on the shortcomings of using cholesterol content of HDL as a marker of risk have focused attention on other parameters of HDL metabolism to improve prediction of ASCVD risk and response to therapy.
HDL exerts several key anti-atherosclerotic functions related to cholesterol transport, endothelial and vascular function, and inflammation. Reverse cholesterol transport (RCT), the ability of HDL to accept cholesterol from the periphery and deliver it to the liver for excretion, is the principal method for HDL biogenesis from nascent lipid-poor particles to mature cholesteryl-ester laden spherical particles;9 RCT is also considered the principal HDL function that impacts macrophage foam cell formation and other functions such as endothelial activation of endothelial nitric oxide synthase ( eNOS), monocyte adhesion, and platelet aggregation.10 Therefore, RCT is the overriding action of HDL on multiple cell types.
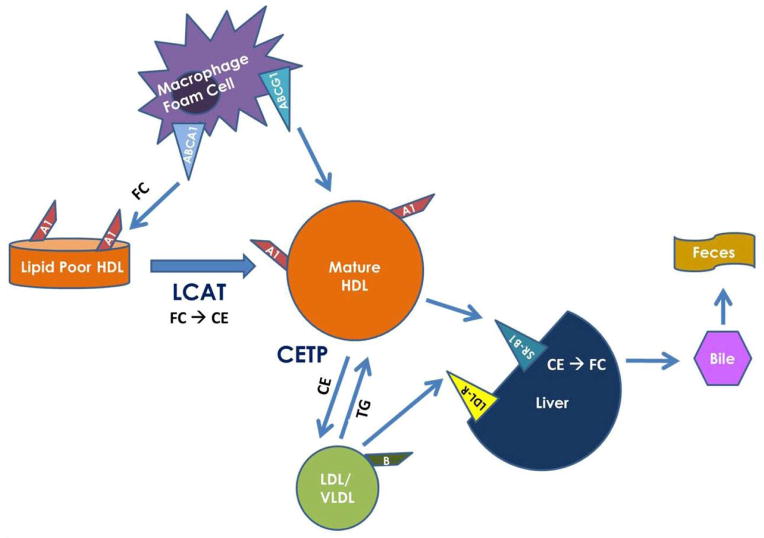
RCT is a complex process that has been well worked out in animal models.11 As summarized in Figure 1, lipid-poor apolipoprotein (apo) A-I (apoA-I) interacts with the ABCA1 receptor on hepatocytes and macrophages in the periphery to accept cellular cholesterol. This cholesterol is esterified by lecithin:cholesterol acyltransferase ( LCAT), leading to the formation of spherical HDL particles with a hydrophobic cholesteryl ester core. These enlarging HDL particles can continue to accept cholesterol from the periphery via other pathways and deliver it back to the liver for excretion into bile and feces. In addition, cholesterol within HDL particles can be exchanged with LDL and very low-density lipoprotein (VLDL) particles for triglycerides (TGs), leading to catabolism of HDL particles by lipases and either excretion via the kidney or re-participation in accepting cholesterol from the periphery.
Figure 1.
Reverse Cholesterol Transport. LCAT: Lecithin:Cholesterol Acyltransferase; CETP: Cholesteryl Ester Transfer Protein; FC: Free Cholesterol; CE: Cholesteryl Ester; TG: Triglycerides; LDL-R: Low-density lipoprotein receptor.
Macrophage-specific cholesterol efflux is the key initial step in RCT and has been shown in genetic and pharmacologic animal studies to be more closely associated with atherosclerosis than circulating levels of HDL-C.9 Whether this function is operative in humans remains to be seen, but recent studies assessing cholesterol efflux in humans suggest that the cholesterol efflux capacity (CEC) of human plasma or serum is a potent marker of ASCVD risk. This review serves to describe the methodology of measuring CEC ex vivo from human samples and the findings to date linking CEC to human disease.
Measuring Cholesterol Efflux Capacity (CEC) in Humans
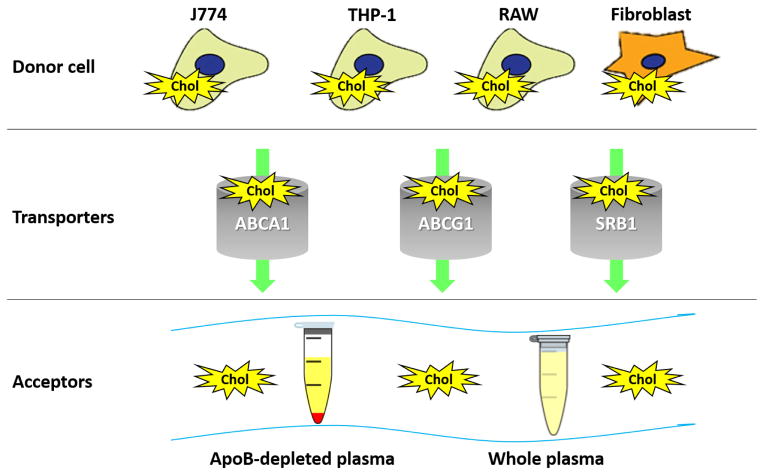
There is no standardized method for measuring CEC in humans and protocols vary considerably; however, they all measure the movement of labeled cholesterol from cells to an extracellular acceptor (Figure 2).12 In general, most studies in humans have only tested the cholesterol acceptor aspect of efflux, specifically, the differential capacity of human serum/plasma to accept cholesterol from cells in a unidirectional manner. This approach does not take into account the ability of a patient’s own macrophages to efflux cholesterol and does not assess cholesterol influx, or net efflux.
Figure 2.
Cholesterol Efflux Assay. The movement of labeled cholesterol from within cells to extracellular acceptors is quantified as cholesterol efflux. Choice of donor cells, cholesterol transporters interrogated, type of labeled cholesterol and cholesterol acceptor can affect efflux measurements. Chol = labeled cholesterol. ApoB = Apolipoprotein B. J774, THP-1, RAW = types of macrophage cell lines.
Macrophages are the most relevant cell type for studies of atherosclerosis given the central role of macrophage “foam” cells in disorders of lipid accumulation. Macrophages efflux cholesterol via several transporters, including adenosine tri-phosphate (ATP)-binding cassette transporters ABCA1 and ABCG1, scavenger receptor SRB1, as well as via aqueous diffusion. CEC assays can reflect all of these pathways in aggregate or can be modified to interrogate a specific transporter. Choice of cholesterol acceptor can have significant impact on assessment of CEC and is the largest source of variation across studies. Cholesterol acceptor mediums can range in specificity for HDL from isolated pure HDL to apo B-depleted plasma/serum to whole plasma/serum. The use of ApoB-depleted plasma eliminates the role of LDL and VLDL in assessing cholesterol efflux, making it more specific for HDL-mediated CEC. When whole or apoB-depleted plasma/serum is used, other cholesterol acceptors and shuttles such as albumin can also play a role in CEC; however, studies have shown that apoA-I, the main protein constituent of HDL particles, is responsible for ~75–80% of the CEC from macrophage cell lines with amplified ABCA1 transporter pathways.13,14 In one small study, CEC to apoB-depleted plasma moderately correlated with CEC to isolated HDL (r=0.46, p<0.02) but was not correlated at all with CEC to whole plasma (p>0.2).15 Ascertaining the specific methodology used to assess CEC is critical when evaluating the reported findings in human studies. Correlations between CEC and other lipid markers can vary widely whether using whole vs. apoB-depleted plasma/serum as the cholesterol acceptor.15
CEC and ASCVD
Studies assessing the association between CEC and ASCVD are summarized in Table 1. Perhaps the first reported study of CEC and coronary artery disease ( CAD) in humans, a small case-control study in the mid 1990’s showed that CEC was lower in patients with prevalent CAD and was the lowest in those with both CAD and diabetes mellitus (DM).16 Though the vast majority of studies have assessed the cholesterol acceptor capacity aspect of the efflux pathway, one of the earliest studies in humans tested the cholesterol donor capacity of patient-derived peripheral blood mononuclear cells to standardized recombinant HDL2 particles.17 Macrophages from patients with angiographic CAD had lower CEC than those derived from controls without angiographic CAD and inversely correlated with HDL and LDL particle size. The first large study of CEC in humans utilized a sample of 793 individuals without acute coronary syndrome presenting for coronary angiogram in a case-control design.18 In this study sample, CEC was positively correlated with HDL-C (r=0.51, p<0.0001). Increasing CEC was associated with decreased prevalence of angiographic CAD, even when adjusted for traditional risk factors and HDL-C. CEC was also inversely associated with severity of angiographic CAD and, in a separate cohort, with carotid intima media thickness. This study established the relevance of measuring CEC in humans with regard to ASCVD, but its cross-sectional design limited the ability to determine whether impaired efflux occurred prior to the onset of CAD. In a letter to the editor in response to this study, another group reported their observation using a slightly different assay in a nested case-control design that CEC was not associated with incident ASCVD events, suggesting that choice of assay methodology may affect findings.19
Table 1.
Studies Correlating Cholesterol Efflux Capacity with Human Disease
| Study | Efflux Specifics | Population | Endpoint | Efflux Result |
|---|---|---|---|---|
| ASCVD | ||||
| Linsel-Nitschke et al., 200917 |
|
|
|
Lower in CAD:
|
| Khera et al., 201118 |
|
|
|
Lower in CAD:
|
| De Vries et al., 201119 |
|
|
|
No difference:
|
| Li et al., 201313 |
|
|
|
Lower in outpatient CAD:
No difference in angiographic CAD:
Higher in incident CVD:
|
| Rohatgi et al., 201421 |
|
|
|
Inverse with CVD:
|
| CKD | ||||
| Shroff et al., 201425 |
|
|
|
|
| Kaseda et al., 201524 |
|
|
|
No difference |
| Yamamoto et al., 201223 |
|
|
|
Lower in ESRD:
|
| Auto-immune | ||||
| Charles-Schoeman et al., 201235 |
|
|
|
No difference but inverse with RA disease severity:
|
| Holzer et al., 201234 |
|
|
|
Lower in psoriasis:
Inverse with psoriasis disease severity:
|
| Mehta et al., 201233 |
|
|
|
Lower in psoriasis:
|
| Ronda et al., 201436 |
|
|
|
ABCA1 efflux:
ABCG1 efflux:
SRB1 efflux:
ACBG1 efflux:
|
| Roe et al., 201444 |
|
|
|
Lower in PCOS:
|
| Diabetes | ||||
| Cavallero et al., 199545 |
|
|
|
Lower in DM:
Higher in post-prandial state:
|
| Syvanne et al., 199616 |
|
|
|
Lower in DM and in CAD:
Highest in those without DM or CAD and lowest in those with DM and CAD:
|
| Brites et al., 199946 |
|
|
|
Lower in DM:
|
| Zhou et al., 200831 |
|
|
|
ABCA1-efflux:
SRB1-efflux:
|
| Dullaart et al., 200826 |
|
|
Higher in metsyn:
|
|
| de Vries et al., 200828 |
|
|
|
Higher in high vs. normal TG:
No different in DM with normal TG vs. controls |
| Zhou et al., 200947 |
|
|
|
Lower in DM:
|
| Nestel et al., 201230 |
|
|
|
Higher in IR vs. IS:
Inverse with ApoA-I:
|
| Low et al., 201215 |
|
|
|
Higher in DM: Increased by
|
| Dullart et al., 201227 |
|
|
|
No difference by glycemic status |
| Yassine et al., 201429 |
|
|
|
ABCA1 efflux:
|
| Kubota et al., 201432 |
|
|
|
Lower in glucose intolerance:
Inverse with OGTT measures:
|
The second large study assessed both the cross-sectional association of CEC and CAD as well as the longitudinal association between CEC and incident ASCVD events.13 Two convenience samples were used: one included 577 patients enrolled from outpatient clinics with CAD defined as a history of CAD, MI, or coronary revascularization, the second included 1150 patients presenting for coronary angiogram with CAD defined as at least 50% coronary stenosis. CEC was inversely associated with prevalent CAD in the outpatient cohort but not in the angiographic cohort. The angiographic cohort was followed prospectively for 3 years and CEC measured at the time of angiogram was surprisingly positively associated with incident cardiovascular (CV) events (MI, stroke, or death). One explanation of this observation could be the use of a convenience sample with a mix of patients both with and without angiographic CAD.20 It is also interesting to note the lack of a dose-response relationship across increasing tertiles of CEC (HR for tertile 2: 0.9; tertile 3: 1.9), suggesting a threshold effect. Regardless, it remains possible that the association between CEC and CV disease (CVD) may vary depending on the population being studied.
Our assessment of CEC in the Dallas Heart Study was the third large study of CEC and CAD and the first longitudinal study among an unselected healthy population.21 Of note, this study employed fluorescent-labeled cholesterol instead of radiolabeled cholesterol used in most other studies. Among 2924 healthy individuals free from cardiovascular disease, CEC was inversely associated with incident CV events (MI, stroke, coronary revascularization, or CV death). The inverse association was graded across increasing quartiles of CEC, was not attenuated by adjustment for CAD risk factors, HDL-C, or HDL-P, and was similar when CEC was analyzed as a continuous variable (HR 1SD: 0.68 [95% CI 0.55–0.84]). Furthermore, as the first multiethnic study of HDL function, the Dallas Heart Study showed no interaction by race/ethnicity, an important observation as therapies targeting HDL and HDL function are developed.
Another study of CEC and incident CAD was conducted in the EPIC-Norfolk cohort and is also a longitudinal study among an unselected population free from CVD.22 Using a nested case-control design with almost 1900 events, the top tertile of CEC, as compared to the bottom tertile, was inversely associated with incident CV events in fully adjusted analyses (OR 0.75, 95%CI 0.53–0.97). This study is consistent with the prior cross-sectional inverse associations with prevalent CAD and the longitudinal inverse association with incident CV events among a healthy population. Overall, these studies have established that CEC can be measured in high-throughput fashion in thousands of human subjects and exhibits a validated inverse association with incident CAD among healthy individuals.
CEC and Chronic Kidney Disease(CKD)
Patients with CKD and end-stage renal disease (ESRD) on dialysis are at marked increased risk for CVD events and CV death. Several studies have shown alterations in HDL function in this population including CEC (Table 1). A small case-control study of adults with ESRD on hemodialysis showed that isolated HDL from patients with ESRD on dialysis elicited significantly less cholesterol efflux than matched controls but did not correlate with inflammation.23 Using a similar methodology, a more recent case-control study in children showed that there was a trend toward lower CEC in children with CKD or ESRD on dialysis vs. controls (did not meet statistical significance).24 Both of these studies used gas chromatography instead of labeled cholesterol to quantify efflux. In the largest case-control study to date, CEC was worse among children with CKD compared to controls and was inversely associated with worsening CKD stage.25 Interestingly, in a subset of children followed prospectively after kidney transplantation, CEC did not improve. These reports suggest that CEC can be impaired in children before the onset of traditional ASCVD risk factors in adulthood. Although not the subject of this review, all of these studies also assessed HDL action on endothelial function and vascular inflammation, showing consistent worsening of these HDL functions among adults and children with CKD and on dialysis.
CEC and DM/Metabolic Syndrome(MetS)
DM and MetS are characterized by insulin resistance and dyslipidemia, in particular, high TG and low HDL-C levels, and confer increased risk for ASCVD; CEC has been tested in several studies in these populations (Table 1). The same first case-control study of CEC and CAD in humans was also one of the first to show that CEC was lower in patients with DM, and lowest in those with both DM and CAD.16 Additional case-control studies, however, have revealed that CEC is, in contrast, often higher in patients with DM compared to controls in states of hypertriglyceridemia or prevalent MetS but is not different with respect to DM status absent these conditions.15,26–30 This suggests that plasma/serum from individuals with insulin resistance may confer increased efflux ex vivo, perhaps due to increased CETP activity (whole plasma)29 or increased circulating levels of lipid-poor pre-beta HDL particles (whole and apoB-depleted plasma).28 In contrast to these studies, pathway-specific analyses revealed that ABCA1-mediated CEC was shown to be lower in those with DM but only those with urinary protein, and SRB1-mediated CEC was lower in all patients with DM regardless of proteinuria status.31 In the only true cohort study, which consisted of 439 Japanese-Americans, oral glucose tolerance testing revealed 8.6% with a new diagnosis of DM and 16% with a new diagnosis of impaired glucose tolerance. Efflux was lower in this newly diagnosed, treatment-naïve glucose intolerance group compared to those with normal glucose tolerance.32 These studies suggest that factors such as duration of DM and degree of proteinuria and insulin resistance may impact associations with CEC.
CEC and Auto-immune Disorders
Many auto-immune disorders are associated with increased atherosclerosis and ASCVD events. In a moderately-sized case-control study, CEC was lower in patients with psoriasis compared to controls and correlated with larger HDL particle size among those with psoriasis.33 Another case-control study reported that CEC was inversely correlated with psoriasis disease severity but PON-1 activity and HDL anti-oxidative capacity were no different by disease status.34 Among patients with rheumatoid arthritis, one study suggested an inverse correlation between CEC and disease severity35 and another reported lower ABCG1-mediated CEC but not ABCA1- or SRB1-mediated CEC as compared to controls. In systemic lupus patients, ABCA1- and ABCG1- but not SRB1-mediated CEC was lower as compared to controls.36 Taken together, these initial studies suggest that CEC is inversely related to auto-immune disease severity, but the associations may be cholesterol-transporter specific and may not be consistent with other HDL function measurements in this population.
CEC and Lifestyle Interventions
With regard to the effect of lifestyle changes on CEC, very few studies with active comparators or placebo controls have been published (Table 2). Several have suggested increased CEC with diets enriched in polyphenols37 and alcohol intake38 and no effect with unsaturated fat.39,40 Only one small study testing exercise and CEC had a control arm,41 suggesting no improvement with exercise training among patients with DM. Other uncontrolled published studies on exercise and CEC have been summarized in a recent review.42 The largest of these included 100 overweight women and found that reduction in caloric intake by 500 kcals and increase in physical activity by 5000 steps/day led to a 6% decrease in HDL-C and a 10% decrease in ABCA1-CEC (p=0.006) which was attenuated when adjusted for apoA-I levels.43
Table 2.
Effect of Lifestyle Interventions on Cholesterol Efflux Capacity in Humans
| Study | Efflux Assay | Study design/Population | Intervention | Effect on Efflux |
|---|---|---|---|---|
| Diet | ||||
| Beulens et al., 200438 |
|
|
|
|
| Buonacorso et al., 200739 |
|
|
|
|
| Kralova Lesna et al., 200840 |
|
|
|
|
| Kralova Lesna et al., 201048 |
|
|
|
|
| Aicher et al., 201243 |
|
|
|
|
| Hernaez et al., 201437 |
|
|
|
|
| Exercise | ||||
| Ribiero et al., 200841 |
|
|
|
|
| Blazek et al., 201342 |
|
|||
Conclusion
In summary, cholesterol efflux from macrophages to plasma/serum reflects the first critical step of RCT and is considered a key anti-atherosclerotic function of HDL. Studies to date confirm that CEC can be reliably measured using stored human blood samples as cholesterol acceptors and suggest that CEC may be a promising new biomarker for atherosclerotic and metabolic diseases. Further studies are needed to standardize measurements and clarify the role CEC may play in predicting risk of developing disease and response to various therapies.
Abbreviations
- Apo
Apolipoprotein
- ASCVD
Atherosclerotic cardiovascular disease
- ATP
Adenosine triphosphate
- CAD
Coronary artery disease
- CEC
Cholesterol efflux capacity
- CETP
Cholesterol Ester Transport Protein
- CKD
Chronic kidney disease
- CV
Cardiovascular
- CVD
Cardiovascular disease
- DM
Diabetes mellitus
- eNOS
Endothelial nitric oxide synthase
- ESRD
End-stage renal disease
- HDL
High-density lipoprotein
- HDL-C
High-density lipoprotein cholesterol
- LCAT, Lecithin
cholesterol acyltransferase
- LDL
Low-density lipoprotein
- LDL-C
Low-density lipoprotein cholesterol
- MetS
Metabolic syndrome
- MI
Myocardial infarction
- RCT
Reverse cholesterol transport
- TGs
Triglycerides
- VLDL
Very low-density lipoprotein
Footnotes
Disclosures: Anand Rohatgi is supported by the National Heart, Lung, and Blood Institute of the NIH under Award Number K08HL118131.
Research grant, Merck, Significant.
Advisory Board, Astra Zeneca, modest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 [Google Scholar]
- 2.Kathiresan S, Otvos JD, Sullivan LM, et al. Increased small low-density lipoprotein particle number: a prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation. 2006;113:20–9. doi: 10.1161/CIRCULATIONAHA.105.567107. [DOI] [PubMed] [Google Scholar]
- 3.Waterworth DM, Ricketts SL, Song K, et al. Genetic Variants Influencing Circulating Lipid Levels and Risk of Coronary Artery Disease. Arterioscler Thromb Vasc Biol. 2010;30:2264–76. doi: 10.1161/ATVBAHA.109.201020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edmondson AC, Brown RJ, Kathiresan S, et al. Loss-of-function variants in endothelial lipase are a cause of elevated HDL cholesterol in humans. J Clin Invest. 2009;119:1042–50. doi: 10.1172/JCI37176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Effects of Extended-Release Niacin with Laropiprant in High-Risk Patients. N Engl J Med. 2014;371:203–12. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 6.Barter P, Gotto AM, LaRosa JC, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–10. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 7.Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–99. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 9.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50(Suppl):S189–94. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mineo C, Shaul PW. Regulation of signal transduction by HDL. J Lipid Res. 2013;54:2315–24. doi: 10.1194/jlr.R039479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rader DJ, Hovingh GK. HDL and cardiovascular disease. The Lancet. 2014;384:618–25. doi: 10.1016/S0140-6736(14)61217-4. [DOI] [PubMed] [Google Scholar]
- 12.Rothblat GH, de la Llera-Moya M, Favari E, Yancey PG, Kellner-Weibel G. Cellular cholesterol flux studies: methodological considerations. Atherosclerosis. 2002;163:1–8. doi: 10.1016/s0021-9150(01)00713-4. [DOI] [PubMed] [Google Scholar]
- 13.Li XM, Tang WH, Mosior MK, et al. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol. 2013;33:1696–705. doi: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fielding CJ, Fielding PE. Evidence for a lipoprotein carrier in human plasma catalyzing sterol efflux from cultured fibroblasts and its relationship to lecithin:cholesterol acyltransferase. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:3911–4. doi: 10.1073/pnas.78.6.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Low H, Hoang A, Forbes J, et al. Advanced glycation end-products (AGEs) and functionality of reverse cholesterol transport in patients with type 2 diabetes and in mouse models. Diabetologia. 2012;55:2513–21. doi: 10.1007/s00125-012-2570-9. [DOI] [PubMed] [Google Scholar]
- 16.Syvänne M, Castro G, Dengremont C, et al. Cholesterol efflux from Fu5AH hepatoma cells induced by plasma of subjects with or without coronary artery disease and non-insulin-dependent diabetes: importance of LpA-I:A-II particles and phospholipid transfer protein. Atherosclerosis. 1996;127:245–53. doi: 10.1016/s0021-9150(96)05962-x. [DOI] [PubMed] [Google Scholar]
- 17.Linsel-Nitschke P, Jansen H, Aherrarhou Z, et al. Macrophage cholesterol efflux correlates with lipoprotein subclass distribution and risk of obstructive coronary artery disease in patients undergoing coronary angiography. Lipids in Health and Disease. 2009;8 doi: 10.1186/1476-511X-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–35. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cholesterol Efflux Capacity and Atherosclerosis. N Engl J Med. 2011;364:1472–5. doi: 10.1056/NEJMc1101853. [DOI] [PubMed] [Google Scholar]
- 20.Khera AV, Rader DJ. Cholesterol efflux capacity: full steam ahead or a bump in the road? Arterioscler Thromb Vasc Biol. 2013;33:1449–51. doi: 10.1161/ATVBAHA.113.301519. [DOI] [PubMed] [Google Scholar]
- 21.Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–93. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saleheen D, Scott R, Javad S, et al. Abstract 19753: Hdl Cholesterol Efflux Capacity is Inversely Associated With Incident Chd Events Independent of Hdl-c and Apoa-i Concentrations. Circulation. 2014;130:A19753. [Google Scholar]
- 23.Yamamoto S, Yancey PG, Ikizler TA, et al. Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol. 2012;60:2372–9. doi: 10.1016/j.jacc.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaseda R, Jabs K, Hunley TE, et al. Dysfunctional high-density lipoproteins in children with chronic kidney disease. Metabolism. 2015;64:263–73. doi: 10.1016/j.metabol.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shroff R, Speer T, Colin S, et al. HDL in Children with CKD Promotes Endothelial Dysfunction and an Abnormal Vascular Phenotype. J Am Soc Nephrol. 2014;25:2658–68. doi: 10.1681/ASN.2013111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dullaart RPF, Groen AK, Dallinga-Thie GM, Vries Rd, Sluiter WJ, Tol Av. Fibroblast cholesterol efflux to plasma from metabolic syndrome subjects is not defective despite low high-density lipoprotein cholesterol. Eur. 2008;158:53–60. doi: 10.1530/EJE-07-0451. [DOI] [PubMed] [Google Scholar]
- 27.Dullaart RPF, Annema W, de Boer JF, Tietge UJF. Pancreatic β-cell function relates positively to HDL functionality in well-controlled Type 2 diabetes mellitus. Atherosclerosis. 2012;222:567–73. doi: 10.1016/j.atherosclerosis.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 28.de Vries R, Groen AK, Perton FG, et al. Increased cholesterol efflux from cultured fibroblasts to plasma from hypertriglyceridemic type 2 diabetic patients: roles of pre beta-HDL, phospholipid transfer protein and cholesterol esterification. Atherosclerosis. 2008;196:733–41. doi: 10.1016/j.atherosclerosis.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 29.Yassine HN, Belopolskaya A, Schall C, Stump CS, Lau SS, Reaven PD. Enhanced cholesterol efflux to HDL through the ABCA1 transporter in hypertriglyceridemia of type 2 diabetes. Metab Clin Exp. 2014;63:727–34. doi: 10.1016/j.metabol.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nestel P, Hoang A, Sviridov D, Straznicky N. Cholesterol efflux from macrophages is influenced differentially by plasmas from overweight insulin-sensitive and -resistant subjects. International journal of obesity (2005) 2012;36:407–13. doi: 10.1038/ijo.2011.170. [DOI] [PubMed] [Google Scholar]
- 31.Zhou H, Tan KCB, Shiu SWM, Wong Y. Cellular cholesterol efflux to serum is impaired in diabetic nephropathy. Diabetes Metab Res Rev. 2008;24:617–23. doi: 10.1002/dmrr.895. [DOI] [PubMed] [Google Scholar]
- 32.Kubota M, Nakanishi S, Hirano M, et al. Relationship between Serum Cholesterol Efflux Capacity and Glucose Intolerance in Japanese-Americans. J Atheroscler Thromb. 2014;21:1087–97. doi: 10.5551/jat.24315. [DOI] [PubMed] [Google Scholar]
- 33.Mehta NN, Li R, Krishnamoorthy P, et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis. 2012;224:218–21. doi: 10.1016/j.atherosclerosis.2012.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holzer M, Wolf P, Curcic S, et al. Psoriasis alters HDL composition and cholesterol efflux capacity. J Lipid Res. 2012;53:1618–24. doi: 10.1194/jlr.M027367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Annals of the Rheumatic Diseases. 2012;71:1157–62. doi: 10.1136/annrheumdis-2011-200493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronda N, Favari E, Borghi MO, et al. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Annals of the Rheumatic Diseases. 2014;73:609–15. doi: 10.1136/annrheumdis-2012-202914. [DOI] [PubMed] [Google Scholar]
- 37.Hernáez Á, Fernández-Castillejo S, Farràs M, et al. Olive Oil Polyphenols Enhance High-Density Lipoprotein Function in Humans A Randomized Controlled Trial. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014 doi: 10.1161/ATVBAHA.114.303374. [DOI] [PubMed] [Google Scholar]
- 38.Beulens JWJ, Sierksma A, Van Tol A, et al. Moderate alcohol consumption increases cholesterol efflux mediated by ABCA1. J Lipid Res. 2004;45:1716–23. doi: 10.1194/jlr.M400109-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Buonacorso V, Nakandakare ER, Nunes VS, Passarelli M, Quintão ECR, Lottenberg AMP. Macrophage cholesterol efflux elicited by human total plasma and by HDL subfractions is not affected by different types of dietary fatty acids. The American Journal of Clinical Nutrition. 2007;86:1270–7. doi: 10.1093/ajcn/86.5.1270. [DOI] [PubMed] [Google Scholar]
- 40.Kralova Lesna I, Suchanek P, Kovar J, Stavek P, Poledne R. Replacement of dietary saturated FAs by PUFAs in diet and reverse cholesterol transport. J Lipid Res. 2008;49:2414–8. doi: 10.1194/jlr.M800271-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Ribeiro ICD, Iborra RT, Neves MQTS, et al. HDL Atheroprotection by Aerobic Exercise Training in Type 2 Diabetes Mellitus. Medicine & Science in Sports & Exercise. 2008;40:779–86. doi: 10.1249/MSS.0b013e3181632d2d. [DOI] [PubMed] [Google Scholar]
- 42.Blazek A, Rutsky J, Osei K, Maiseyeu A, Rajagopalan S. Exercise-mediated changes in high-density lipoprotein: Impact on form and function. Am Heart J. 2013;166:392–400. doi: 10.1016/j.ahj.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 43.Aicher BO, Haser EK, Freeman LA, et al. Diet-induced weight loss in overweight or obese women and changes in high-density lipoprotein levels and function. Obesity (Silver Spring, Md) 2012;20:2057–62. doi: 10.1038/oby.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roe A, Hillman J, Butts S, et al. Decreased cholesterol efflux capacity and atherogenic lipid profile in young women with PCOS. J Clin Endocrinol Metab. 2014;99:E841–7. doi: 10.1210/jc.2013-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cavallero E, Brites F, Delfly B, et al. Abnormal reverse cholesterol transport in controlled type II diabetic patients. Studies on fasting and postprandial LpA-I particles. Arteriosclerosis, Thrombosis, and Vascular Biology. 1995;15:2130–5. doi: 10.1161/01.atv.15.12.2130. [DOI] [PubMed] [Google Scholar]
- 46.Brites FD, Cavallero E, de Geitere C, et al. Abnormal capacity to induce cholesterol efflux and a new LpA-I pre-beta particle in type 2 diabetic patients. Clin Chim Acta. 1999;279:1–14. doi: 10.1016/s0009-8981(98)00155-7. [DOI] [PubMed] [Google Scholar]
- 47.Zhou H, Shiu SWM, Wong Y, Tan KCB. Impaired serum capacity to induce cholesterol efflux is associated with endothelial dysfunction in type 2 diabetes mellitus. Diabetes & vascular disease research: official journal of the International Society of Diabetes and Vascular Disease. 2009;6:238–43. doi: 10.1177/1479164109344934. [DOI] [PubMed] [Google Scholar]
- 48.Králová Lesná I, Suchánek P, Stávek P, Poledne R. May Alcohol-Induced Increase of HDL Be Considered as Atheroprotective? Physiological Research. 2010;59:407–13. doi: 10.33549/physiolres.931769. [DOI] [PubMed] [Google Scholar]