Abstract
Docosahexaenoic acid (22:6n3, DHA) is an n-3 polyunsaturated fatty acid (PUFA) known to affect numerous biological functions. While DHA possesses many properties that impact cell survival such as suppressing cell growth and inducing apoptosis, the exact molecular and cellular mechanism(s) remain unknown. Peroxisome proliferator-activated receptors (PPARs) are a family of nuclear receptors that regulate many cell pathways including cell death. As DHA acts as a ligand to PPARs the aim of this study was to examine the involvement of PPARδ in DHA-mediated cytotoxicity toward H9c2 cells. Treatment with DHA (100 µM) resulted in a significant decline in cell viability, cellular metabolic activity and total antioxidant capacity coinciding with increased total proteasome activities and activity of released lactate dehydrogenase (LDH). No changes in reactive oxygen species (ROS) production or accumulation of lipid peroxidation products were observed but DHA promoted apoptotic cell death as detected by flow cytometry, increased caspase-3 activity and decreased phosphorylation of Akt. Importantly, DHA enhanced PPARδ DNA binding activity in H9c2 cells strongly signifying that the cytotoxic effect of DHA might be mediated via PPARδ signaling. Co-treatment with the selective PPARδ antagonist GSK 3787 (1 µM) abolished the cytotoxic effects of DHA in H9c2 cells. Cytotoxic effects of DHA were attenuated by co-treatment with myriocin, a selective inhibitor of serine palmitoyl transferase (SPT), preventing de novo ceramide biosynthesis. LC/MS analysis revealed that treatment with DHA resulted in the accumulation of ceramide, which was blocked by GSK 3787. Interestingly, inhibition of cytochrome P450 (CYP) oxidase with MS-PPOH (50 µM) abolished DHA-mediated cytotoxicity suggesting downstream metabolites as the active mediators. We further demonstrate that CYP oxidase metabolites of DHA, methyl epoxy docosapentaenoate (EDP methyl esters,1 µM) (mix 1:1:1:1:1:1; 4,5-, 7,8-, 10,11-, 13,14-,16,17- and 19,20-EDP methyl esters) and 19,20-EDP cause cytotoxicity via activation of PPARδ signaling leading to increased levels of intracellular ceramide. These results illustrate novel pathways for DHA-induced cytotoxicity that suggest an important role for CYP-derived metabolites, EDPs.
Keywords: Docosahexaenoic acid, Epoxydocosapentaenoic acids, H9c2 ?cells, PPARδ, Apoptosis, Ceramide
1. Introduction
Polyunsaturated fatty acids (PUFAs) such as docosahexaenoic acid (DHA, C22:6n-3) and eicosapentaenoic acid (EPA, C20:5n-3) are metabolites of α-linolenic acid essential to cell survival. PUFAs participate in regulating many functions both at the cellular and systemic level (Schmitz and Ecker, 2008). For example, they are important components of cell membranes affecting their fluidity, composition and structure (Das 2006; Siddiqui et al., 2011). Numerous studies report a pronounced positive effect of DHA towards the cardiovascular system (Stanley et al., 2012), brain development (Ruxton et al., 2005), prevention of dementia and major depressive episodes (Barberger-Gateau et al., 2002). Evidence demonstrates that DHA can produce various effects in cancer and immortal cell lines (Jakobsen et al., 2008; Kang et al., 2010), such as increased apoptosis (Xiong et al., 2012). Previously, we demonstrated that DHA triggered a concentration-dependent death of the immortalized H9c2 cardiac cells but not in primary neonatal cardiomyocytes (Qadhi et al., 2013). The fundamental difference between immortalized and primary cardiac cells dictates the importance of obtaining a better understanding of DHA-mediated biological events, which is important for the development of DHA-based therapeutic strategies to treat various pathogenic conditions.
DHA has been shown to initiate a cytotoxic response in several cancer cell lines through peroxisome proliferator-activated receptors (PPARs) (Tuller et al., 2009). PPARs are ligand-activated transcription factors and members of the nuclear hormone receptor superfamily (Issemann and Green, 1990; Kliewer et al., 1999). PPARs function as nuclear receptors to various molecules, including lipid mediators, regulating cellular functions associated with fatty acid metabolism (Desvergne and Wahli,1999; Peters and Gonzalez, 2009) and lipid transport (Kersten et al., 2000). Additionally, PPARs have a role in inflammatory responses (Peters and Gonzalez, 2009; Varga et al., 2011), cell differentiation (Barak et al., 1999) and tissue development (Rosen et al., 1999). There are three PPAR isotypes described (α, γ, and β/δ) regulating physiologically distinct processes (Yue et al., 2008). PPARα is pro-carcinogenic in hepatocytes, while PPARγ activity leads to terminal differentiation in prostate and breast cancer cells, as well as a reduction in infarct size in hearts (Kersten et al., 2000; Niemoller and Bazan, 2010).
PPARs are involved in regulating numerous pathways impacting both physiological and pathophysiological conditions such as cardiovascular disease and cancer. Activation of PPARs can result in intracellular accumulation of ceramide, which mediates down-stream effects such as apoptosis (Jiang et al., 2009; Wang et al., 2006). DHA has previously been shown to increase the formation of ceramide in vitro (Lu et al., 2010). In addition, DHA acts as a ligand for PPARs (Gani and Sylte, 2008b). The role of PPARδ in carcinogenesis appears to be the least studied and the most controversial (Peters et al., 2012; Yang et al., 2013). PPARδ is ubiquitously expressed in all adult tissues including the heart (Planavila et al., 2005; Yue et al., 2008). Given the physiological proximity of immortalized cell lines with cancer cells and the fact that H9c2 cells are known to abundantly express PPARδ isoform (Gilde et al., 2003), our objective in this study was to elucidate the role of PPARδ signaling associated with ceramide accumulation in DHA-induced cytotoxicity.
2. Materials and methods
2.1. Chemicals and reagents
DHA (cat # 90310), N-methylsulphonyl-6-(2-proparglyloxyphenyl) hexanamide (MS-PPOH) (cat # 75770) and 19, 20 – EDP (cat # 10175) were purchased from Cayman Chemicals (Ann Arbor, MI, USA). Tissue culture materials were obtained from Invitrogen (Burlingt on, ON, Canada); Bradford protein assay solution from Bio-Rad Laboratories (Mississauga, ON, Canada); Primary and secondary antibodies from Cell Signaling (Pickering, ON, Canada); Trypan blue, MTT, 2′, 7′-Dichlorofluorescin diacetate, myriocin from Sigma–Aldrich (Oakville, ON, Canada). Annexin V-FITC and propidium iodide from BDsciences; Caspase 3 assay kit from Sigma (Saint Louis, MO, USA cat# CASPC3C-1KT); Total 20S proteasome assay kit from Chemicon (EMD Millipore, Etobicoke, ON, Canada cat # APT280); Lipid peroxidation (MDA) cat# ab118970; PPARδ transcription factor assay kit Cayman Chemicals (Ann Arbor, MI, USA cat # 90,310). GSK 3787 cat# 3961; Ceramide cat# 0744 from Tocris bioscience (Bristol, UK). Cell Counting Kit-8 (CCK-8) from Dojindo Molecular Technologies Inc. (Burlington, ON, Canada) cat# CK04–05. EDP methyl esters regioisomer mixtures (1:1:1:1:1:1; 4,5-, 7,8-, 10,11-, 13,14-, 16,17- and 19,20-EDP) were prepared as described previously (Morisseau et al., 2010).
2.2. Cell culture
H9c2 cells (American Type Culture Collection, Manassas, VA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% penicillin, and streptomycin at 37 °C in an atmosphere of (5% CO2/95% N2). H9c2 cells were plated at 25–30% confluency and cultured until they reached 75–80% confluence, upon which were treated with 0 or 100 µM DHA, then were harvested and subjected to various assay s. During some experiments cells were treated in the presence or absence of the selective PPARδ antagonist 4-chloro-N-[2-[[5-(trifluorom ethyl)-2-pyridinyl]sulfonyl]ethyl]benzamide (GSK 3787, Tocris Bioscience, Bristol, UK). GSK 3787 was used at a concentration of 1 µM based on previously established data (Palkar et al., 2010). DHA was used at a concentration of 100 µM dissolved in ethanol, which was based on our previous study demonstrating DHA has a concentration-dependent response triggering cytotoxicity in H9c2 cells (Qadhi et al., 2013). Control groups were administered vehicle solution (<5% ethanol, ddH20).
2.3. Western blotting
Samples were subjected to Western blot analysis as previously described (Qadhi et al., 2013). Briefly, H9c2 cell samples (20 µg protein) were resolved in 12% SDS-polyacrylamide gel and then transferred electrophoretically to nitrocellulose membranes, that were blocked in TBS-T buffer (0.15 M NaCl, 3 mM KCl, 25 mM trishydroxymethyl methylamine and 0.1% tween-25, pH 7.4) with 5% skim milk for 2 h at room temperature. Membranes were washed four times with TBS-T buffer, and then incubated with a phosphor-Akt antibody for Ser473 (1:500, Cell Signaling, cat #9271) overnight at 4 °C. Membranes were washed as described above and then incubated with horseradish-peroxidase linked anti-rabbit IgG secondary antibody (1:10,000, Cell Signaling, cat #7074) for 2 h at room temperature. A similar procedure was used to probe membranes for total Akt (1:1000, Cell Signaling, cat #9272S), and we utilized GAPDH (1:1000, Cell Signaling, cat #2118S) and ULK-1 (1:1000, Cell Signaling, cat #8054) as loading controls. Chemiluminscence solution was used to detect signals. Relative band intensity was expressed in arbitrary units, and was assessed by densitometry using Image J software (NIH, Bethesda, MD, USA).
2.4. Cell viability and metabolic activity
Cell viability was estimated based on a modified Trypan blue exclusion method as previously described (Qadhi et al., 2013). Briefly, 0.4% of Trypan blue solution was added to cells for 15 min at room temperature. Cells were then centrifuged at 1000 × g for 5 min at room temperature. The supernatant was gently aspirated and cells were washed three times with ice-cold PBS. 1% sodium dodecyl sulfate solution was added to lysed cells. Optical density was read spectrophotometrically at 595 nm. In a separate set of experiments, CCK-8 was utilized as a measure of cellular viability (Stone et al., 2008; Tang et al., 2010). This assay utilizes the water-soluble tetrazolium salt [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-8). Colorimetric changes representing cellular viability are assessed by the reduction of WST-8 via cellular dehydrogenases produced an orange colored formazan dye. CCK-8 was added to H9c2 cells in 96-well plates, and incubated in the dark for 2 h. Optical density was read spectrophotometrically at 450 nm. Cellular activity was assessed by the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolim bromide (MTT) to formazan crystals as described previously (Qadhi et al., 2013). The MTT assay is a widely used colorimetric assay for estimation of cellular activity (Freimoser et al., 1999). Briefly, MTT solution was added to incubated cells for 6 h at 37 °C prior to assessment. Optical density was measured spectrophotometrically at 595 nm and the cellular activity ratio was represented relative to control. Activity of released of LDH was measured colorimetrically using a modified MTT protocol as described by (Abe and Matsuki, 2000).
20S total proteasome activity was measured using an assay kit (EMD Millipore, Billerica, MA) based on the detection of 7-amino-4-methylcoumarin (AMC) fluorescence in cell lysates after cleavage of the peptide LLVY-AMC. Fluorescence was measured at 380 nm excitation and 460 nm emission. A standard curve was established with AMC. Caspase-3 was assessed as described previously (Qadhi et al., 2013), using the selective substrate Ac-DEVD-AMC. Briefly, caspase-3 activity was determined in cell lysates by detection of AMC fluorescence after cleavage of the peptide. The fluorescence was monitored at 460 nm and excited at 380 nm. The activity was calculated by using a linear standard curve found with AMC.
2.5. PPARδ DNA binding activity
PPARδ DNA binding activity was measured in cell lysates by ELISA assay Cayman Chemicals (Ann Arbor, MI, USA cat # 10006914), based on a double stranded DNA sequence that containing the peroxisome proliferator response element (PPRE). Activated PPARδ present in the cell lysates binds to the PPRE and is detected via primary and secondary antibodies.
2.6. Markers of oxidative stress
Lipid peroxidation was assessed in cell lysates (Abcam Inc. Toronto, ON), based on measuring of the levels of malondialdehyde and 4-hydroxynonenal fluorometrically at 553 nm (emission) and excited at 532 nm. Reactive oxygen species (ROS) generation was measured using the cell permeable probe 2′, 7′-Dichlorofluorescin diacetate (H2DCF-DA) that is converted to the fluorescent product 2′, 7′- dichlorofluorescein upon removal of its diacetate groups via intracellular esterases, which allows for the overall quantification of intracellular redox status (LeBel et al., 1992). H9c2 cells were seeded in 24-well plates, and the production of ROS was determined after 6, 12, and 24 h of DHA treatment (0, 1, 10, 100 µM) by incubating cells with 30 µM of H2DCF-DA in pre-warmed phenol-free Hank's buffer for 1 h (in the dark). Following this, the media was removed and replaced with pre-warmed phenol-free Hank's buffer on its own, and positive controls were treated with 100 µM of H202 for 30 min. Changes in fluorescence were recorded using a fluorescence plate reader (excitation 480 nm, emission 520 nm). Total antioxidant capacity (TAC) was measured using an Antioxidant Assay Kit (Sigma–Aldrich, Co, Oakville, ON). The specific assay is based on the formation of a ferryl myoglobin radical from myoglobin and hydrogen peroxide, which oxidizes the ABTS (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) to produce a radical cation ABTS+, a soluble green color chromogen that was determined spectrophotometrically at 405 nm. In the presence of antioxidants the radical cation is suppressed to an extent dependent on the activity of the antioxidant and the color intensity is decreased proportionally. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was used as a positive control antioxidant (Samokhvalov et al., 2013).
2.7. Flow cytometric detection of apoptosis
Flow cytometry was employed to detect apoptosis as previously described (Jurasz et al., 2011). In brief, trypsinized cells were washed with binding buffer and centrifuged at a low speed (220 × g). Following this, binding buffer was used to resuspend the cells with Annexin V- fluorescein isothiocyanate (FITC) and propidium iodide (PI), followed by incubation in the dark for 15 min, then diluted to a total volume of 500 µl. Fluorescence was induced with a 488 nm argon laser and detected on FL1 (525 nm BP filter), and FL3 (620 nm SP filter) on a Beckman Coulter Cytomics FC500 flow cytometer (10,000 events per sample). Spectral compensation was performed using Cell Lab Quanta analysis software. Number of apoptotic cells was quantified by combining events falling in the lower and upper right quadrants (early and late apoptotic cells, res pectively).
2.8. LC/MS measurement of ceramide
The extraction of ceramides from H9c2 cells was performed in accordance to a previously published protocol (Bose and Kolesnick, 2000). Cells were centrifuged at 500 × g for 5 min then rinsed with cold PBS and centrifuged again at 500 × g for 5 min. 300 µL of a 0.4% NaCL solution and 1 mL of a chloroform–methanol–1N HCL (100:100:1, v/v/v) mixture were added to the samples. Following this the samples were wrapped in parafilm and vortexed at 1000 rpm (at room temperature) for 20 min. Samples were then centrifuged at 15,000 rpm for 2 min, and the resulting organic phase was separated and transferred into new vials. The samples were then evaporated using a Savant DNA 120 SpeedVac Concentration system (Thermo Fisher). The resulting pellets were then dispersed in the chloroform–methanol–HCL solution, and combined with 1 µg/mL of freshly prepared yohimbine hydrochloride, which was used as an internal standard.
The samples were resolved using liquid chromatography–mass spectrometry (LC/MS) analysis, comprised of a Waters ZQ 4000 Mass Spectrometer coupled to a Waters 2795 Separations Module (LC + autosampler). Ceramides were detected using a single ion recording in electrostatic ionization mode on a Waters Xterra C18 column (2.1 × 50 mm, 3 µm). The parents ions were observed at m/z = 343.2 and 355.2 for ceramide and yohimbine, respectively. An A:B:C gradient system (0.225 mL/min) was used composed of acetonitrile (containing 0.005% acetic acid), water containing 0.2% NH4OH and 0.05% NH4OAc, and methanol. During the acquisition of the data, the mass spectrometer was maintained at a source and desolation temperature of 140 °C and 250 °C, and a cone voltage of 20 V and 24 V, respectively. The resulting values were then calculated using a ceramide standard curve and expressed as mg weight per mg of cellular mass, and then further normalized to account for the fold change between wet and dry cellular mass for quantification purposes.
2.9. Statistical analysis
Values are expressed as mean ± SEM. Statistical significance was determined by the use of ANOVA. To determine whether significant differences exist between the groups, a Bonferonni post hoc test was performed. Values are considered significant if p < 0.05.
3. Results
3. 1. Treatment with DHA results in a dramatic decline in cell viability and cellular metabolic activity via PPARδ-signaling
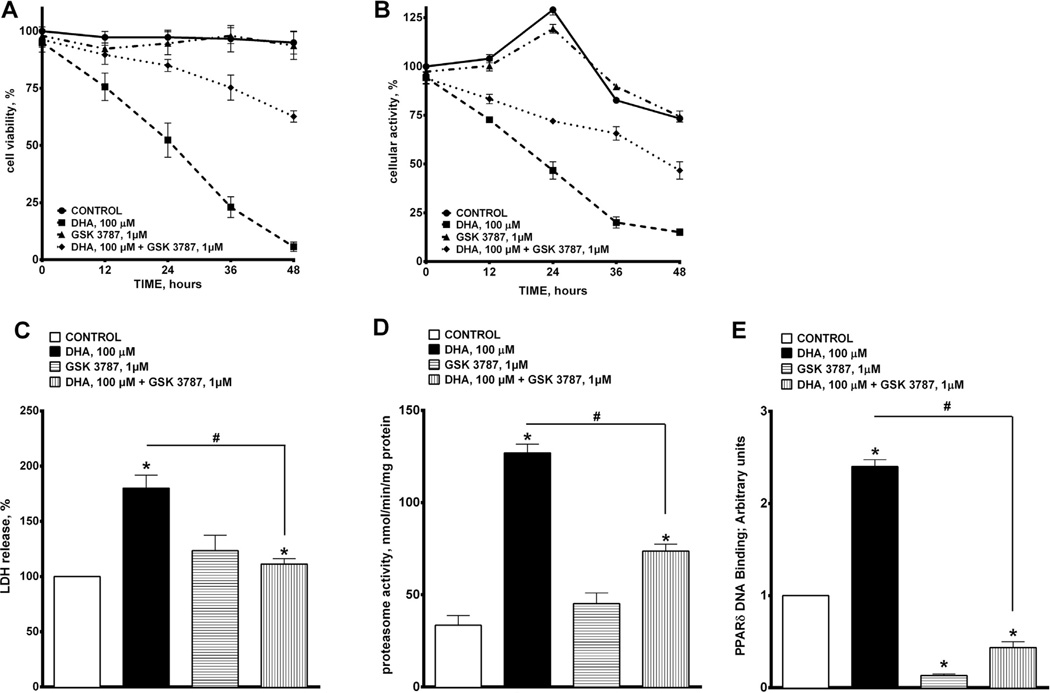
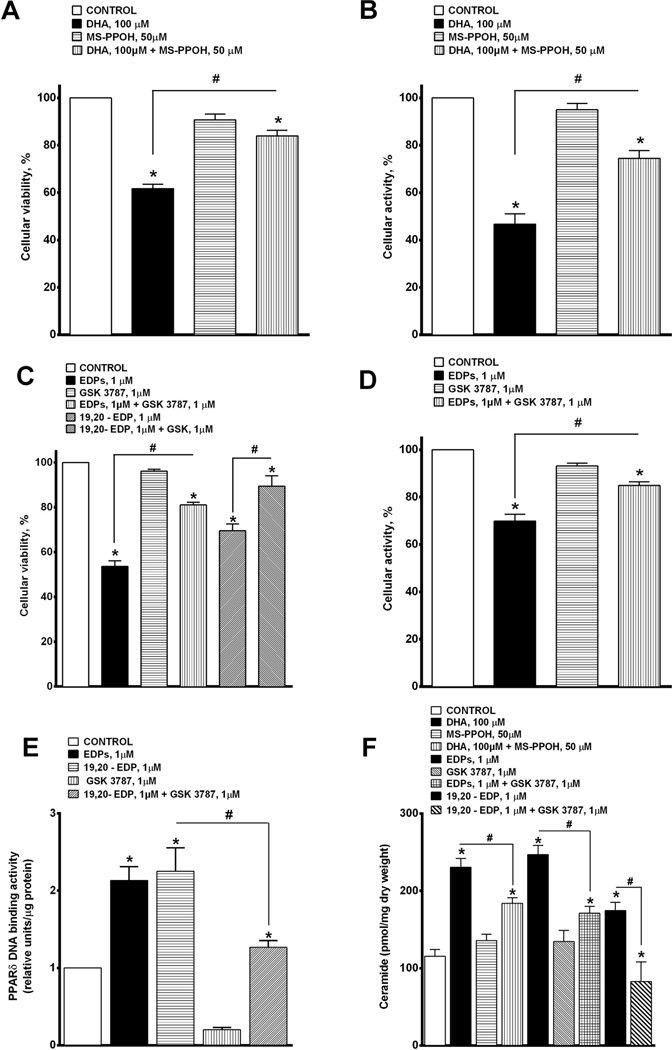
We demonstrated that treatment with DHA (100 µM) induced a pronounced decrease in cell viability as assessed by Trypan Blue exclusion that persisted for up to 48 h (Fig 1A), which is consistent with our previously published study (Qadhi et al., 2013). The deleterious effects of DHA were attenuated by co-treatment with the selective PPARδ antagonist, GSK 3787 (1 µM, Fig. 1A). Further evidence of DHA-induced cytotoxicity was observed by a decrease in cellular metabolic activity, based on detection of the reduced form of MTT in mitochondria. Fig. 1B shows that DHA progressively lowered cellular metabolic activity in H9c2 cells over the 48 h time course of exposure. Similarly, addition of GSK 3787 reduced the cytotoxic effect of DHA toward cellular metabolic activity in H9c2 cells. Assessment of the release of LDH from H9c2 cells exposed to DHA provided more evidence of a cytotoxic effect. DHA triggered a large release of LDH from cells within 24 h, which was attenuated by co-treatment with GSK 3787 (Fig. 1C). Total proteasome activity reveals intracellular accumulation of ubiquinated proteins, thus representing a marker of unspecific cellular degenerative processes. Fig. 1D demonstrates that treatment with DHA robustly increased total proteasome activity in H9c2 cells, which was suppressed by co-treatment with GSK 3787.
Fig. 1.
Effect of DHA exposure on cellular viability and metabolic activity. (A) Cell viability was estimated based on the Trypan Blue exclusion method. H9c2 cells were treated with 0 or 100 µM of DHA, and assessed at 24 h. (B) Cellular metabolic activity was estimated by the oxidation of MTT to formazan. (C) The percentage of LDH release was quantified colorimetrically. (D) Proteasomal activity was assessed by the detection of 7-amino-4-methylcourmarin (AMC) in cell lysates post cleavage from the peptide LLVY-AMC. (E) PPARδ DNA binding was quantified using an ELISA kit. Values are expressed as mean ± SEM; N = 3; *p < 0.05 DHA 100 µM vs. control, #p < 0.05 DHA 100 µM vs. DHA 100 µM and GSK 3787 1 µM.
Over all, DHA-induced cytotoxicity was greatly attenuated by a selective inhibitor of PPARδ suggesting there was an involvement of PPARδ-signaling. In order to further validate the role of PPARδ signaling in mediating DHA cytotoxicity, we measured PPARδ DNA binding activity in H9c2 cells exposed to DHA with or without GSK 3787. This assay revealed that treatment with DHA for 24 h resulted in a marked increase in PPARδ DNA binding activity. As expected, co-treatment with GSK 3787 prevented DHA-induced activation of PPARδ DNA binding activity (Fig. 1E). These results suggest that DHA-induced cytotoxicity involves activation of PPARδ signaling in H9c2 cells.
3.2. Treatment with DHA has no effect on oxidative stress in H9c2 cells
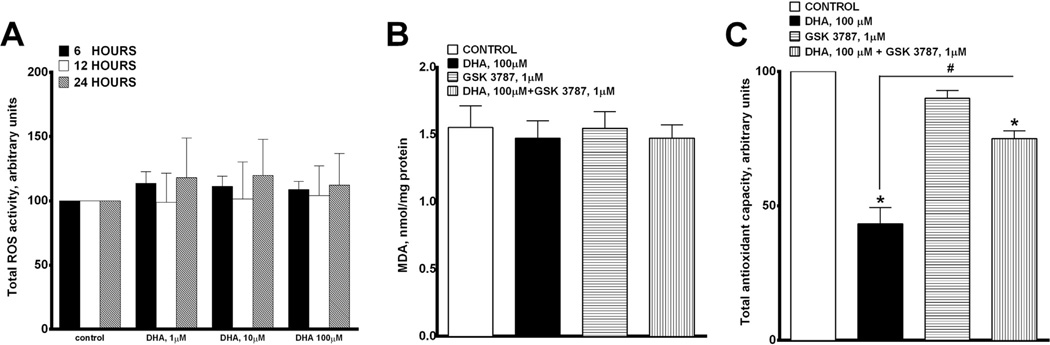
To examine whether the DHA-induced cytotoxicity occurred via activation of a general stress response such as oxidative stress rather than through an involvement of selective molecular mechanisms we assessed ROS production and accumulation of TBA-active products (MDA and 4-NEN) in H9c2 cells. We found that H9c2 cells treated with H2O2 (100 µM) induced a 9-fold increase in ROS production (data not shown) while DHA treatment triggered a mild and non-significant increase in ROS (~1.3-fold) (Fig. 2A and B). As endogenous antioxidant capacity of cells is an important component in understanding cellular responses to stress, we next assessed the effect of DHA on TAC in H9c2 cells. Interestingly, DHA treatment significantly depleted the antioxidant capacity in H9c2 cells after 24 h (Fig. 2C). This effect was attenuated by co-treatment with GSK 3787 (Fig 2C). These data suggest that DHA triggered ROS production was neutralized by the endogenous antioxidant system in H9c2 cells. Moreover, the DHA effect specifically involved PPARδ signaling and the contribution of unspecific stress reactions such as oxidative stress in DHA-induced cytotoxicity was not significant.
Fig. 2.
Effect of DHA on oxidative stress. (A) Histogram representing the generation of reactive oxygen species in H9c2 cells treated with 0, 1, 10 or 100 µM of DHA at 6, 12 and 24 h. (B) Lipid peroxidation was determined by the accumulation of TBA-active products after 24 h of treatment. H9c2 cells treated with 0 or 100 µM of DHA with and without GSK 3787 (1 µM). (C) Total antioxidant capacity was measured in H9c2 cells following 24 h treatment with 0 or 100 µM of DHA with and without GSK 3787 (1 µM). Values are expressed as mean ± SEM; N = 3; *p < 0.05 DHA 100 µM vs. control, #p < 0.05 DHA 100 µM vs. DHA 100 µM and GSK 3787 1 µM.
3.3. DHA induces apoptosis in H9c2 cells mediated via PPARδ-signaling
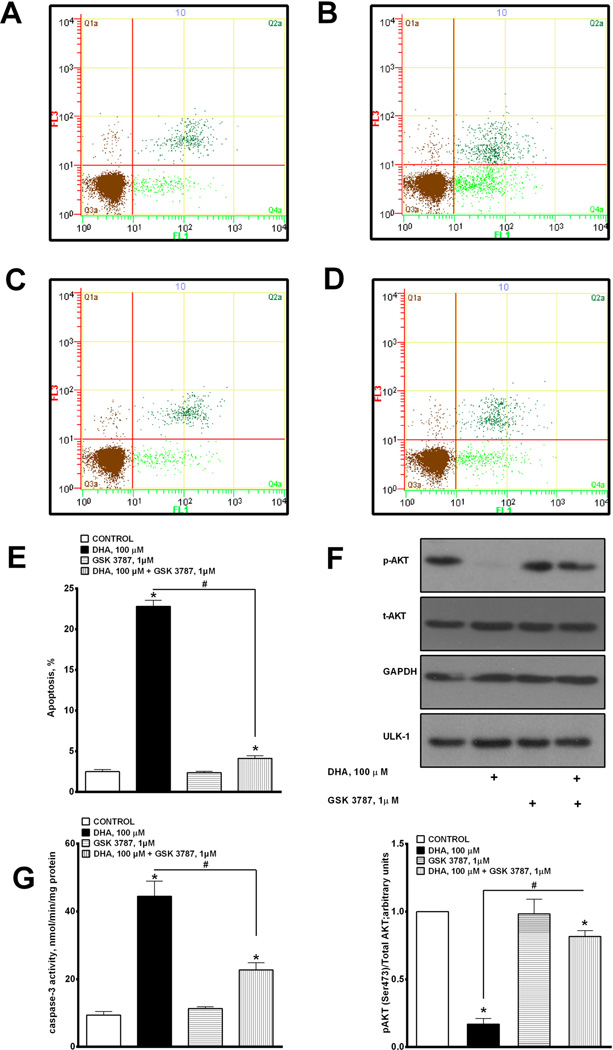
DHA-mediated cytotoxicity is believed to involve a complex interplay of numerous cell death/survival pathways. In this part of our study we estimated the overall apoptotic response based on the measurement of Annexin-V FITC binding, phosphorylation of Akt and caspase-3 activity in H9c2 cells exposed to DHA. We implemented flow cytometric analysis utilizing Annexin-V FITC and propidium iodide (PI) to further examine the mechanisms of DHA-mediated apoptosis. DHA treatment led to a large increase in apoptosis (Fig. 3A–C). Interestingly, co-incubation of DHA with GSK 3787 led to a significant attenuation of the apoptotic response induced by DHA treatment (Fig 3D). The specific values for all four treatment conditions are summarized in Fig 3E.
Fig. 3.
DHA-induced apoptosis mediated via PPARδ-signaling. (A–D) Representative bivariate dot plots of Annexin-V FITC and propidium iodide binding. Apoptotic cells were assessed by combining values falling in the upper and lower right quadrants. (E) Histogram summarizing flow cytometry data demonstrating induction of DHA-induced apoptosis. (F) Cells were harvested and subjected to western blotting to assay the level of p-AKT (upper insert), and quantified (lower insert). (G) Caspase-3 activity was 19,20-EDP with or without GSK 3787 1 µM and assessed at 24 h. (C) Cell viability and (D) cellular metabolic activity were measured. (E) PPARδ DNA binding was quantified using an ELISA kit. Values are expressed as mean ± SEM; N = 3; *p < 0.05 EDPs or 19,20-EDP 1 µM vs. control, #p < 0.05 19,20-EDP 1 µM vs. 19,20-EDP 1 µM and GSK 3787 1 µM. (F) LC/MS analysis was employed to measure the accumulation of ceramide following treatment with 0 or 100 µM of DHA with or without MS-PPOH 50 µM, EDPs mix 1 µM (1:1:1:1:1; 7,8-, 10,11-, 13,14-, 16,17- and 19,20-EDP) or 1 µM 19,20-EDP with or without GSK 3787 1 µM and assessed at 24 h. Values are expressed as mean ± SEM; N = 3; *p < 0.05 EDP 1 µM vs. control, #p < 0.05 EDPs 1 µM vs. EDPs 1 µM and GSK 3787 1 µM, #p < 0.05 19,20-EDP 1 µM vs. 19,20-EDP 1 µM and GSK 3787 1 µM.
In order to further validate the apoptotic response we examined if treatment with DHA affected phosphorylation of protein kinase B (Akt), a key component of intracellular anti-apoptotic mechanisms (Datta et al., 1997). We demonstrated that 24 h of exposure to DHA resulted in reduction of Akt phosphorylation in H9c2 cells (Fig. 3F). This corresponded with a dramatic increase in caspase-3 activity (Fig. 3G), strongly suggesting activation of apoptosis. Co-treatment with GSK 3787 prevented the loss of phosphorylated Akt and reduced caspase-3 activity.
3.4. Pharmacological inhibition of de novo ceramide synthesis attenuates DHA-induced cytotoxicity
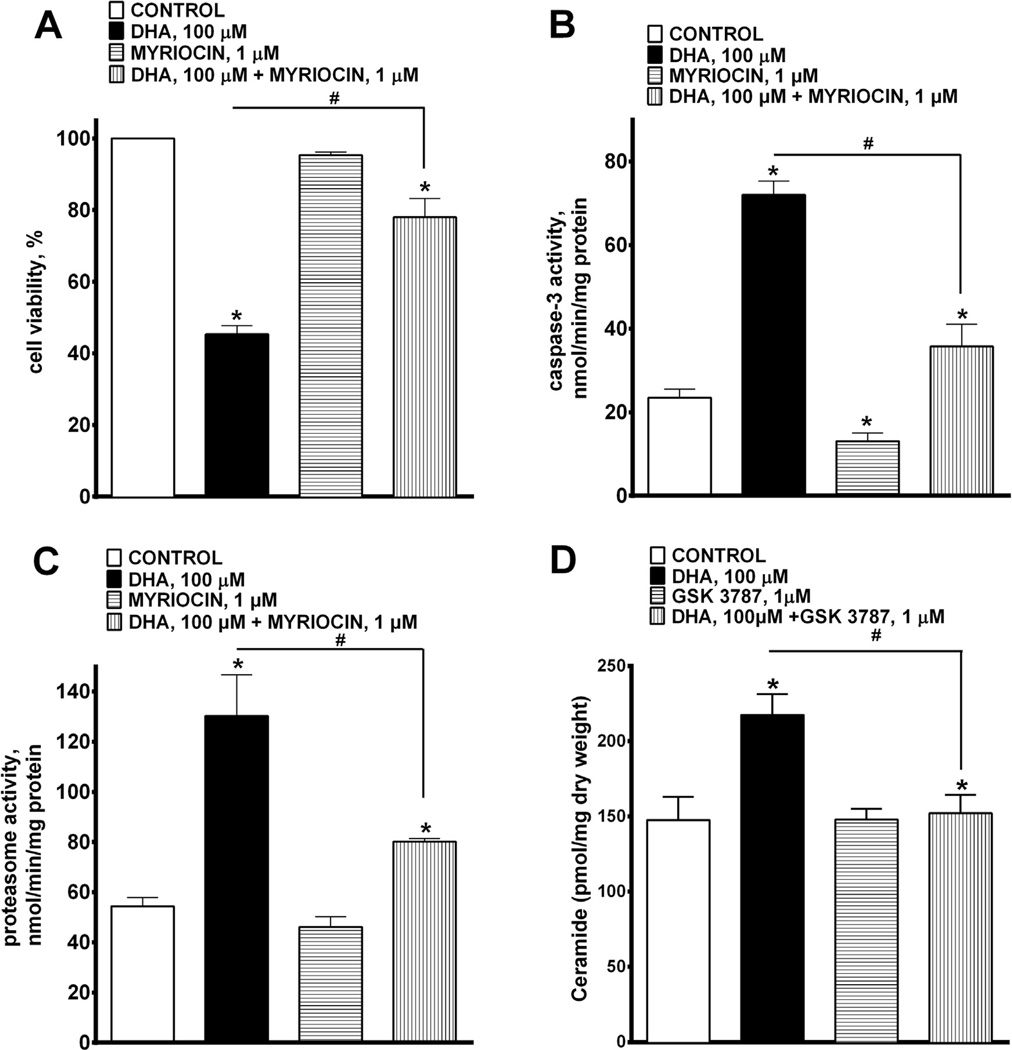
Accumulation of ceramide has been linked to major perturbations in cell metabolism resulting in apoptotic cell death (Wang et al., 2006), and evidence suggests that DHA may increase the level of intracellular ceramide (Lu et al., 2010). Furthermore, activation of PPAR-mediated signaling, particularly via PPARδ, can also enhance accumulation of ceramide in cells (Jiang et al., 2009). To determine the role of this pathway we treated cells with myriocin (1 µM), which is a potent inhibitor of serine palmitoyltransferase (SPT) in order to block the de novo synthesis of ceramide. We demonstrate that co-treatment with myriocin attenuated DHA-mediated cell death (Fig. 4A), prevented DHA-triggered increase in caspase-3 (Fig. 4B) and total proteasome activities (Fig 4C). Evidence of de novo ceramide synthesis was supported by the marked increase in ceramide levels following 24 h treatment with DHA (Fig. 4D). Inhibition of PPARδ activation with GSK 3787 attenuated the DHA-mediated accumulation of ceramide (Fig 4D). These results support a suggestion that PPARδ play s a crucial role in DHA-mediated cytotoxicity associated with ceramide accumulation.
Fig. 4.
DHA-induced cytotoxicity is attenuated by pharmacological inhibition on de novo ceramide synthesis. H9c2 cells were treated with 0 or 100 µM of DHA with or without myriocin 1 µM, an inhibitor of SPT, and assessed at 24 h. (A) Cell viability, (B) caspase-3 and (C) total proteasomal activities were measured. Co-treatment of DHA with myriocin significantly abolished DHA-induced cytotoxity. Values are expressed as mean ± SEM; N = 3; *p < 0.05 DHA 100 µM vs. control, #p < 0.05 DHA 100 µM vs. DHA 100 µM and myriocin 1 µM. (D) LC/MS analysis was employed to measure the accumulation of ceramide following treatment with 0 or 100 µM of DHA with or without GSK 3787 1 µM, a PPARδ antagonist, and assessed at 24 h. Values are expressed as mean ± SEM; N = 3; *p < 0.05 DHA 100 µM vs. control, #p < 0.05 DHA 100 µM vs. DHA 100 µM and GSK 3787 1 µM.
3.5. CYP oxidase metabolites of DHA, epoxydocosapentaenoic acids (EDPs), demonstrate cytotoxicity toward H9c2 cells
Recent literature has suggested that cytochrome P450 oxidase metabolites of DHA, epoxydocosapentaenoic acids (EDPs), act as autocrine and paracrine mediators to regulate cellular processes (Morisseau et al., 2010; Ye et al., 2002). In order to explore this notion, we performed experiments to investigate the role of EDPs in the DHA-mediated response. First, we treated H9c2 cells with DHA and/or MS-PPOH, an inhibitor of CYP oxidases, to determine if epoxide metabolites of DHA were involved in the cytotoxic response. Inhibition of CYP oxidase activity with MS-PPOH (50 µM) blocked DHA-induced cell loss and metabolic activity (Fig. 5A and B). Thus, we next tested the actions of EDPs using a mixture of six different EDP methyl ester regioisomers (1 µM) (1:1:1:1:1:1; 4,5-, 7,8-, 10,11-, 13,14-, 16,17- and 19,20-EDP methyl ester) or the individual isomer 19,20-EDP (1 µM). Interestingly, both the mixture of EDPs and 19,20-EDP alone caused a significant loss in cell viability and metabolic activity at a 100-fold lower concentration than DHA. The adverse effects of EDPs were attenuated by co-treatment with GSK 3787 (1 µM) suggesting EDP-mediated events involved the activation of PPARδ (Fig. 5C and D). In order to confirm that EDP-mediated effects involved PPARδ, we performed a PPARδ DNA binding assay. We found that both the EDPs mixture and pure form of 19, 20-EDP increased PPARδ DNA binding activity, which was significantly diminished by co-treatment with GSK 3787 (Fig 5E). To further explore the signaling mechanisms, we assessed changes in ceramide accumulation. (Fig. 5F). The inhibition of CYP oxidases with MS-PPOH blocked the DHA-induced ceramide accumulation, while the EDPs mixture and 19,20-EDP alone triggered the accumulation of ceramide at a 100-fold lower concentration (Fig. 5F). The role of PPARδ in the EDP response was confirmed when GSK 3787 blocked ceramide production. These results support our hypothesis that the DHA metabolites, EDPs, specifically 19,20-EDP, induce cytotoxicity via a PP ARδ-dependent pathway triggering ceramide production.
Fig. 5.
EDPs demonstrate cytotoxicity toward H9c2 cells. H9c2 cells were treated with 0 or 100 µM of DHA with or without MS-PPOH 50 µM and assessed at 24 h. (A) Cell viability was assessed using CCK-8, and (B) cellular metabolic activity was assessed via MTT. Values are expressed as mean ± SEM; N = 3; *p < 0.05 DHA 100 µM vs. control, # p < 0.05 DHA 100 µM vs. DHA 100 µM and MS-PPOH 50 µM. H9c2 cells were treated with 0, 1 µM of EDP mix (1:1:1:1:1; 7,8-, 10,11-, 13,14-, 16,17- and 19,20-EDP) or 1 µM
4. Discussion
Our results demonstrate a novel mechanism through which DHA and its epoxy metabolites, EDPs, trigger a cytotoxic response in immortalized H9c2 cells. We found that DHA- and EDP-induced cytotoxicity is mediated via PPARδ signaling resulting in de novo ceramide biosynthesis.
In our previous study we found that DHA produced a concentration-dependent cytotoxic effect in H9c2 cells (Qadhi et al., 2013). Consistent with that previous study, we found that DHA induced a rapid decline in viability of H9c2 cells, which persisted up to 48 h. Furthermore, the significant release of LDH into the cell culture medium indicated loss of membrane integrity due to DHA cytotoxicity (Abe and Matsuki, 2000; Parhamifar et al., 2013). To further explore the type of injury we demonstrated that DHA treatment led to the activation of total proteasome activity, the ubiquitin-proteasome pathway involved in removal of irreversibly denatured proteins. Activation of total proteasome activity therefore appears to be a marker of the rate of overall protein degradation as well as turnover, providing an estimate of cell injury (Jakobsen et al., 2008). DHA has previously been shown to lead to an increase in reactive oxygen species (ROS) levels leading to oxidative damage of membranes and lipids (Al-Gubory, 2012; Kang et al., 2010). Considering PUFAs are susceptible to oxidation, this can trigger significant lipid peroxidation cascades leading to cell death (Al-Gubory, 2012; Obajimi et al., 2005; Premanathan et al., 2011; Xiong et al., 2012). While evidence suggests that DHA-induced cytotoxicity can involve initiation of oxidative stress, our results demonstrated that neither ROS production nor lipid peroxidation were markedly increased in our experimental model. However, the decreased total antioxidant capacity suggests the endogenous systems in H9c2 cells suppressed any significant increase in ROS production. Importantly, the data indicate DHA cytotoxicity occurred through a more specific cell death pathway in H9c2 cells, which involved PPAR signaling.
PPARs are involved in regulating a range of cellular processes including metabolism, cell growth and differentiation (Barak and Lee, 2010). Interestingly, the involvement of PPARs in regulating carcinogenesis has a dichotomous role both in the prevention and initiation of tumor transformation of normal cells (Michalik et al., 2004). The contribution of PPARs in regulating cellular homeostasis is multifaceted, whereby modulation of PPAR functional activity leads to profound alterations in many pivotal cell functions (Lee et al., 2013; Scatena et al., 2004). Evidence from the literature demonstrates that DHA activates PPARs, including PPARδ (Diep et al., 2000; Gani and Sylte, 2008a). Importantly, H9c2 cells are known to abundantly express the PPARδ isoform whereas the other isoforms are almost undetectable (Gilde et al., 2003). The fact that we were able to attenuate DHA-mediated events by inhibiting PPARδ, and DHA enhanced PPARδ DNA binding activity in H9c2 cells supports the notion that PPARδ signaling is involved in DHA-mediated cytotoxicity.
DHA has demonstrated unique characteristics, whereby depending upon the cell type it can promote either cell survival or cell death (Fasano et al., 2012; Kim et al., 2006b). Importantly, it remains unresolved how DHA triggers a selective cell survival or cell death pathway. In the current study using the immortalized H9c2 cells, DHA clearly induced cytotoxicity via activation of an apoptotic response. Flow cytometry analyses demonstrated that DHA increased Annexin-V binding reflecting activation of apoptosis (Brune et al., 2013; Xiong et al., 2012). We further demonstrated that DHA caused marked effects causing decreased Akt phosphorylation and increased caspase-3 activity in H9c2 cells. Phosphorylation of Akt is known to promote cell survival and suppress apoptosis via downstream pathways such as phosphorylation of BAD (Datta et al., 1999, 1997). Our results provide evidence that PPARδ signaling is important for DHA-triggered apoptosis. However, research on the role of PPARδ signaling in apoptotic responses is unclear (Peters and Gonzalez, 2009). In contrast to our data demonstrating that PPARδ has a role in triggering apoptosis, previous reports show that PPARδ activation prevented apoptosis in endothelial progenitor cells (Han et al., 2008). While data in keratinocytes demonstrates that PPARδ induces apoptotic signaling may occur via induction of terminal differentiation (Kim et al., 2006a). These results may be partially explained by differences in the expression profiles of PPAR isoforms between various cell types (Ritzenthaler et al., 2009).
Ceramides are an important family of bioactive lipids composed of sphingosine and a fatty acid. Emerging evidence suggests they are involved in a variety of cellular processes ranging from cell pro liferation to cell death and they are well-known activators of apoptosis (Liu et al., 2013; Parra et al., 2013). Ceramides can be generated by de novo synthesis, sphingomyelin hydrolysis or through the salvage pathway, all of which can be activated in response to various stimuli. In cancer cells endogenous levels of ceramide are typically low, but after treatment with chemotherapeutic drugs these levels are increased triggering cell death (Liu et al., 2013). Whereas in the heart, excessive lipid and ceramide accumulation is involved in cardiac contractile dysfunction, increased oxidative stress, and cardiomyocyte apoptosis (Delpy et al., 1999; Young et al., 2002; Zhou et al., 2000). DHA can increase synthesis of ceramide via activation of serinepalmitoyl transferase (SPT) a key enzyme in de novo sphingolipid biosynthesis from palmitate (Lu et al., 2010). Interestingly, PPAR receptors are involved in regulating lipid homeostasis, where agonist activation of PPARs in rat hearts was shown to increase SPT activity and ceramide levels (Baranowski et al., 2007). In the current study, we inhibited DHA-mediated events by blocking PPARδ-induced ceramide production, as well as SPT activity, suggesting a novel pathway for DHA-induced apoptosis in H9c2 cells. Furthermore, our observation of increased accumulation of ceramide in DHA-treated cells correlates with the loss of Akt phosphorylation, as ceramide has been reported to impede Akt phosphorylation (Bourbon et al., 2002).
Metabolism of n-3 PUFAs such as DHA, can occur by cyclooxygenase, lipoxygenases and cytochrome P450 (CYP) oxidases to numerous metabolites with various characteristics including anti-inflammatory and anti-angiogenic properties (Morisseau et al., 2010; Zhang et al., 2013). CYP oxidases can convert DHA to epoxydocosapentaenoic acids (EDPs) (Arnold et al., 2010), which have demonstrated novel properties impacting angiogenesis, thrombosis and cancer (Jung et al., 2012; Zhang et al., 2013). EDPs have been suggested as the active mediator leading to protection against arrhythmias within the heart previously attributed to the actions of DHA (Ulu et al., 2013, 2014). Although man y DHA metabolites have been shown to be biologically active, 19,20-EDP is of particular interest due to its various pronounced biological effects, particularly toward cardiovascular system. (Morin et al., 2011; Ulu et al., 2014). Inhibition of CYP oxidase activity with MS-PPOH has been shown to reduce the production of DHA-derived epoxy metabolites (Morin et al., 2011). Experimental evidence indicates that the addition of MS-PPOH can inhibit the hyperactivity protective effects of the n-3 PUFA EPA, in a human bronchi model (Morin et al., 2009). In our model, the simultaneous addition of MS-PPOH prevented the DHA-mediated cytotoxicity suggesting the involvement of CYP oxidase metabolites. The cytotoxic effect of EDPs, specifically 19,20-EDP, further supported the idea that the epoxy metabolites of DHA are active lipid mediators. Indeed, the fact that the EDP-mediated increase in ceramide levels were blocked by the PPARδ antagonist demonstrates a novel mechanism of action. Collectively, these data highlight epoxy metabolites of DHA as important biologically active lipids regulating cellular function. Evidence has demonstrated that diols of some fatty acids such as linoleic acid oxide (leukotoxin) can be significantly more toxic as compared to their pro genitor epoxides (Moghaddam et al., 1997). Therefore, it remains unknown whether the EDPs are responsible for the cellular response we observed or it is attributed to soluble epoxide hydrolase derived diol metabolites. Nevertheless, EDPs have potential therapeutic value as they possess strong biological effects and are relatively stable chemically (Morisseau et al., 2010; Zhang et al., 2013). In summary, we show that PPARδ signaling leading to the de novo synthesis of ceramide appears to be a crucial step in a DHA-induced cytotoxic cascade in H9c2 cells. Furthermore, our findings demonstrating inhibition of CYP oxidase activity and accumulation of ceramide significantly adds to our understanding of the biological function of epoxy metabolites of DHA. This study provides new insights to understanding the unknown properties of n-3 lipids.
Supplementary Material
HIGHLIGHTS.
Investigated the mechanisms of DHA mediated cytotoxicity in H9c2 cardiac cells.
DHA treatment decreased cell viability and triggered apoptosis in H9c2 cells.
DHA induced cell death involved PPARδ signaling.
DHA treatment induced de novo ceramide production but EDPs were 100× more potent.
CYP-derived metabolites of DHA, epoxydocosapentaenoic acids, had an important role.
Acknowledgments
The authors would like to thank Dr. Vishwa Somayaji for his expertise with the LC/MS analysis. JMS is the recipient of a Health Scholar Award from Alberta Innovates Health Solutions. This work was supported by a grant from the Q2 National Science and Engineering Research Council (NSERC) of Canada (JMS), and partially supported by NIEHS grant R01 ES002710, NIH CounterAct U54NS079202 and NIEHS Superfund Research Program grant P42 ES004699 (BDH).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxlet.2014.09.029.
Footnotes
Conflict of interest statement
No conflict of interests declared.
References
- Abe K, Matsuki N. Measurement of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction activity and lactate dehydrogenase release using MTT. Neurosci. Res. 2000;38:325–329. doi: 10.1016/s0168-0102(00)00188-7. [DOI] [PubMed] [Google Scholar]
- Al-Gubory KH. Mitochondria: omega-3 in the route of mitochondrial reactive oxygen species. Int. J. Biochem. Cell Biol. 2012;44:1569–1573. doi: 10.1016/j.biocel.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Arnold C, Markovic M, Blossey K, Wallukat G, Fischer R, Dechend R, Konkel A, von Schacky C, Luft FC, Muller DN, Rothe M, Schunck WH. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. J. Biol. Chem. 2010;285:32720–32733. doi: 10.1074/jbc.M110.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Y, Lee CH. The molecular basis of PPAR function. PPAR Res. 2010;2010:510530. doi: 10.1155/2010/510530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- Baranowski M, Blachnio A, Zabielski P, Gorski J. PPAR alpha agonist induces the accumulation of ceramide in the heart of rats fed high-fat diet. J. Physiol. Pharmacol. 2007;58:57–72. [PubMed] [Google Scholar]
- Barberger-Gateau P, Letenneur L, Deschamps V, Peres K, Dartigues JF, Renaud S. Fish, meat, and risk of dementia: cohort study. BMJ. 2002;325:932–933. doi: 10.1136/bmj.325.7370.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose R, Kolesnick R. Measurement of ceramide levels by the diacylglycerol kinase reaction and by high-performance liquid chromatography-fluorescence spectrometry. Methods Enzymol. 2000;322:373–378. doi: 10.1016/s0076-6879(00)22034-x. [DOI] [PubMed] [Google Scholar]
- Bourbon NA, Sandirasegarane L, Kester M. Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J. Biol. Chem. 2002;277:3286–3292. doi: 10.1074/jbc.M110541200. [DOI] [PubMed] [Google Scholar]
- Brune M, Muller M, Melino G, Bierhaus A, Schilling T, Nawroth PP. Depletion of the receptor for advanced glycation end products (RAGE) sensitizes towards apoptosis via p53 and p73 posttranslational regulation. Oncogene. 2013;32:1460–1468. doi: 10.1038/onc.2012.150. [DOI] [PubMed] [Google Scholar]
- Das UN. Essential fatty acids – a review. Curr. Pharm. Biotechnol. 2006;7:467–482. doi: 10.2174/138920106779116856. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Delpy E, Hatem SN, Andrieu N, de Vaumas C, Henaff M, Rucker-Martin C, Jaffrezou JP, Laurent G, Levade T, Mercadier JJ. Doxorubicin induces slow ceramide accumulation and late apoptosis in cultured adult rat ventricular myocytes. Cardiovasc. Res. 1999;43:398–407. doi: 10.1016/s0008-6363(99)00142-x. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Diep QN, Touyz RM, Schiffrin EL. Docosahexaenoic acid, a peroxisome proliferator-activated receptor-alpha ligand, induces apoptosis in vascular smooth muscle cells by stimulation of p38 mitogen-activated protein kinase. Hypertension. 2000;36:851–855. doi: 10.1161/01.hyp.36.5.851. [DOI] [PubMed] [Google Scholar]
- Fasano E, Serini S, Piccioni E, Toesca A, Monego G, Cittadini AR, Ranelletti FO, Calviello G. DHA induces apoptosis by altering the expression and cellular location of GRP78 in colon cancer cell lines. Biochim. Biophys. Acta. 2012;1822:1762–1772. doi: 10.1016/j.bbadis.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Freimoser FM, Jakob CA, Aebi M, Tuor U. The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay is a fast and reliable method for colorimetric determination of fungal cell densities. Appl. Environ. Microbiol. 1999;65:3727–3729. doi: 10.1128/aem.65.8.3727-3729.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gani OA, Sylte I. Ligand-induced stabilization and activation of peroxisome proliferator-activated receptor gamma. Chem. Biol. Drug Des. 2008a;72:50–57. doi: 10.1111/j.1747-0285.2008.00677.x. [DOI] [PubMed] [Google Scholar]
- Gani OA, Sylte I. Molecular recognition of docosahexaenoic acid by peroxisome proliferator-activated receptors and retinoid-X receptor alpha. J. Mol. Graph Model. 2008b;27:217–224. doi: 10.1016/j.jmgm.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Gilde AJ, van der Lee KA, Willemsen PH, Chinetti G, van der Leij FR, van der Vusse GJ, Staels B, van Bilsen M. Peroxisome proliferator-activated receptor (PPAR) alpha and PPARbeta/delta, but not PPARgamma, modulate the expression of genes involved in cardiac lipid metabolism. Circ. Res. 2003;92:518–524. doi: 10.1161/01.RES.0000060700.55247.7C. [DOI] [PubMed] [Google Scholar]
- Han JK, Lee HS, Yang HM, Hur J, Jun SI, Kim JY, Cho CH, Koh GY, Peters JM, Park KW, Cho HJ, Lee HY, Kang HJ, Oh BH, Park YB, Kim HS. Peroxisome proliferator-activated receptor-delta agonist enhances vasculogenesis by regulating endothelial progenitor cells through genomic and nongenomic activations of the phosphatidylinositol 3-kinase/Akt pathway. Circulation. 2008;118:1021–1033. doi: 10.1161/CIRCULATIONAHA.108.777169. [DOI] [PubMed] [Google Scholar]
- Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Jakobsen CH, Storvold GL, Bremseth H, Follestad T, Sand K, Mack M, Olsen KS, Lundemo AG, Iversen JG, Krokan HE, Schonberg SA. DHA induces ER stress and growth arrest in human colon cancer cells: associations with cholestero l and calcium homeostasis. J. Lipid Res. 2008;49:2089–2100. doi: 10.1194/jlr.M700389-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YJ, Uchida Y, Lu B, Kim P, Mao C, Akiyama M, Elias PM, Holleran WM, Grunfeld C, Feingold KR. Ceramide stimulates ABCA12 expression via peroxisome proliferator-activated receptor {delta} in human keratinocytes. J. Biol. Chem. 2009;284:18942–18952. doi: 10.1074/jbc.M109.006973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurasz P, Yurkova N, Kirshenbaum L, Stewart DJ. VEGF masks BNIP3-mediated apoptosis of hypoxic endothelial cells. Angiogenesis. 2011;14:199–207. doi: 10.1007/s10456-011-9204-6. [DOI] [PubMed] [Google Scholar]
- Kang KS, Wang P, Yamabe N, Fukui M, Jay T, Zhu BT. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PloS One. 2010;5:e10296. doi: 10.1371/journal.pone.0010296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Bility MT, Billin AN, Willson TM, Gonzalez FJ, Peters JM. PPARbeta/delta selectively induces differentiation and inhibits cell proliferation. Cell Death Differ. 2006a;13:53–60. doi: 10.1038/sj.cdd.4401713. [DOI] [PubMed] [Google Scholar]
- Kim HK, Della-Fera M, Lin J, Baile CA. Docosahexaenoic acid inhibits adipocyte differentiation and induces apoptosis in 3T3-L1 preadipocytes. J. Nutr. 2006b;136:2965–2969. doi: 10.1093/jn/136.12.2965. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Lehmann JM, Willson TM. Orphan nuclear receptors: shifting endocrinology into reverse. Science. 1999;284:757–760. doi: 10.1126/science.284.5415.757. [DOI] [PubMed] [Google Scholar]
- LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- Lee TI, Kao YH, Chen YC, Huang JH, Hsiao FC, Chen YJ. Peroxisome proliferator-activated receptors modulate cardiac dysfunction in diabetic cardiomyopathy. Diabetes Res. Clin. Pract. 2013;100:330–339. doi: 10.1016/j.diabres.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Liu J, Beckman BS, Foroozesh M. A review of ceramide analogs as potential anticancer agents. Future Med. Chem. 2013;5:1405–1421. doi: 10.4155/fmc.13.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CY, Li CC, Liu KL, Tsai CW, Lii CK, Chen HW. Docosahexaenoic acid down-regulates phenobarbital-induced cytochrome P450 2B1 gene expression in rat primary hepatocytes via the sphingomyelinase/ceramide pathway. J. Nutr. Biochem. 2010;21:338–344. doi: 10.1016/j.jnutbio.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat. Rev. Cancer. 2004;4:61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat. Med. 1997;3:562–566. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin C, Fortin S, Rousseau E. 19,20-EpDPE, a bioactive CYP450 metabolite of DHA monoacyglyceride, decreases Ca2+ sensitivity in human pulmonary arteries. Am. J. Physiol. Heart Circulatory Physiol. 2011;301:H1311–H1318. doi: 10.1152/ajpheart.00380.2011. [DOI] [PubMed] [Google Scholar]
- Morin C, Sirois M, Echave V, Rizcallah E, Rousseau E. Relaxing effects of 1 7(18)-EpETE on arterial and airway smooth muscles in human lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296:L130–L139. doi: 10.1152/ajplung.90436.2008. [DOI] [PubMed] [Google Scholar]
- Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, Hammock BD. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J. Lipid Res. 2010;51:3481–3490. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemoller TD, Bazan NG. Docosahexaenoic acid neurolipidomics. Prostaglandins Other Lipid Mediators. 2010;91:85–89. doi: 10.1016/j.prostaglandins.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obajimi O, Black KD, MacDonald DJ, Boyle RM, Glen I, Ross BM. Differential effects of eicosapentaenoic and docosahexaenoic acids upon oxidant-stimulated release and uptake of arachidonic acid in human lymphoma U93 7 cells. Pharmacol. Res.: Off. J. Ital. Pharmacol. Soc. 2005;52:183–191. doi: 10.1016/j.phrs.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Palkar PS, Borland MG, Naruhn S, Ferry CH, Lee C, Sk UH, Sharma AK, Amin S, Murray IA, Anderson CR, Perdew GH, Gonzalez FJ, Muller R, Peters JM. Cellular and pharmacological selectivity of the peroxisome proliferator-activated receptor-beta/delta antagonist GSK3787. Mol. Pharmacol. 2010;78:419–430. doi: 10.1124/mol.110.065508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parhamifar L, Andersen H, Moghimi SM. Lactate dehydrogenase assay for assessment of polycation cytotoxicity. Methods Mol. Biol. 2013;948:13–22. doi: 10.1007/978-1-62703-140-0_2. [DOI] [PubMed] [Google Scholar]
- Parra V, Moraga F, Kuzmicic J, Lopez-Crisosto C, Troncoso R, Torrealba N, Criollo A, Diaz-Elizondo J, Rothermel BA, Quest AF, Lavandero S. Calcium and mitochondrial metabolism in ceramide-induced cardiomyocyte death. Biochim. Biophys. Acta. 2013;1832:1334–1344. doi: 10.1016/j.bbadis.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Gonzalez FJ. Sorting out the functional role(s) of peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) in cell proliferation and cancer. Biochim. Biophys. Acta. 2009;1796:230–241. doi: 10.1016/j.bbcan.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Shah YM, Gonzalez FJ. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat. Rev. Cancer. 2012;12:181–195. doi: 10.1038/nrc3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planavila A, Rodriguez-Calvo R, Jove M, Michalik L, Wahli W, Laguna JC, Vazquez-Carrera M. Peroxisome proliferator-activated receptor beta/delta activation inhibits hypertrophy in neonatal rat cardiomyocytes. Cardiovasc. Res. 2005;65:832–841. doi: 10.1016/j.cardiores.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Premanathan M, Karthikeyan K, Jeyasubramanian K, Manivannan G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed.: Nanotechnol. Biol. Med. 2011;7:184–192. doi: 10.1016/j.nano.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Qadhi R, Alsaleh N, Samokhvalov V, El-Sikhry H, Bellenger J, Seubert JM. Differential responses to docosahexaenoic acid in primary and immortalized cardiac cells. Toxicol. Lett. 2013;219:288–297. doi: 10.1016/j.toxlet.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Ritzenthaler JD, Roman J, Han S. PPARbeta/delta agonist increases the expression of PGE2 receptor subtype EP4 in human lung carcinoma cells. Methods Mol. Biol. 2009;512:309–323. doi: 10.1007/978-1-60327-530-9_17. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Ruxton CH, Calder PC, Reed SC, Simpson MJ. The impact of long-chain n-3 polyunsaturated fatty acids on human health. Nutr. Res. Rev. 2005;18:113–129. doi: 10.1079/NRR200497. [DOI] [PubMed] [Google Scholar]
- Samokhvalov V, Alsaleh N, El-Sikhry HE, Jamieson KL, Chen CB, Lopaschuk DG, Carter C, Light PE, Manne R, Falck JR, Seubert JM. Epoxyeicosatrienoic acids protect cardiac cells during starvation by modulating an autophagic response. Cell Death Dis. 2013;4:e885. doi: 10.1038/cddis.2013.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scatena R, Martorana GE, Bottoni P, Giardina B. Mitochondrial dysfunction by synthetic ligands of peroxisome proliferator activated receptors (PPARs) IUBMB Life. 2004;56:477–482. doi: 10.1080/15216540400008416. [DOI] [PubMed] [Google Scholar]
- Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Siddiqui RA, Harvey KA, Xu Z, Bammerlin EM, Walker C, Altenburg JD. Docosahexaenoic acid: a natural powerful adjuvant that improves efficacy for anticancer treatment with no adverse effects. Biofactors. 2011;37:399–412. doi: 10.1002/biof.181. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Khairallah RJ, Dabkowski ER. Update on lipids and mitochondrial function: impact of dietary n-3 polyunsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care. 2012;15:122–126. doi: 10.1097/MCO.0b013e32834fdaf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S tone MK, Kolling GL, Lindner MH, Obrig TG. p38 mitogen-activated protein kinase mediates lipopolysaccharide and tumor necrosis factor alpha induction of shiga toxin 2 sensitivity in human umbilical vein endothelial cells. Infect. Immun. 2008;76:1115–1121. doi: 10.1128/IAI.01300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, Zeh HJ, Lotze MT. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010;29:5299–5310. doi: 10.1038/onc.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuller ER, Brock AL, Yu H, Lou JR, Benbrook DM, Ding WQ. PPARα signaling mediates the synergistic cytotoxicity of clioquinol and docosahexaenoic acid in human cancer cells. Biochem. Pharmacol. 2009;77:1480–1486. doi: 10.1016/j.bcp.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Ulu A, Harris TR, Morisseau C, Miyabe C, Inoue H, Schuster G, Dong H, Iosif AM, Liu JY, Weiss RH, Chiamvimonvat N, Imig JD, Hammock BD. Anti-inflammatory effects of omega-3 polyunsaturated fatty acids and soluble epoxide hydrolase inhibitors in angiotensin-II-dependent hypertension. J. Cardio vasc. Pharmacol. 2013;62:285–297. doi: 10.1097/FJC.0b013e318298e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulu A, Stephen Lee KS, Miyabe C, Yang J, Hammock BG, Dong H, Hammock BD. An omega-3 epoxide of docosahexaenoic acid lowers blood pressure in angiotensin-II dependent hypertension. J. Cardiovasc. Pharmacol. 2014 doi: 10.1097/FJC.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta. 2011;1812:1007–1022. doi: 10.1016/j.bbadis.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lv X, Shi J, Hu X. Ceramide induces apoptosis via a peroxisome proliferator-activated receptor gamma-dependent pathway. Apoptosis. 2006;11:2043–2052. doi: 10.1007/s10495-006-0191-9. [DOI] [PubMed] [Google Scholar]
- Xiong A, Yu W, Tiwary R, Sanders BG, Kline K. Distinct roles of different forms of vitamin E in DHA-induced apoptosis in triple-negative breast cancer cells. Mol. Nutr. Food Res. 2012;56:923–934. doi: 10.1002/mnfr.201200027. [DOI] [PubMed] [Google Scholar]
- Yang L, Zhou J, Ma Q, Wang C, Chen K, Meng W, Yu Y, Zhou Z, Sun X. Knockdown of PPAR delta gene promotes the growth of colon cancer and reduces the sensitivity to bevacizumab in nude mice model. PLoS One. 2013;8:e60715. doi: 10.1371/journal.pone.0060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D, Zhang D, Oltman C, Dellsperger K, Lee HC, VanRollins M. Cytochrome p-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J. Pharmacol. Exp. Ther. 2002;303:768–776. doi: 10.1124/jpet.303.2.768. [DOI] [PubMed] [Google Scholar]
- Young ME, McNulty P, Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: Part II: potential mechanisms. Circulation. 2002;105:1861–1870. doi: 10.1161/01.cir.0000012467.61045.87. [DOI] [PubMed] [Google Scholar]
- Yue TL, Nerurkar SS, Bao W, Jucker BM, Sarov-Blat L, Steplewski K, Ohlstein EH, Willette RN. In vivo activation of peroxisome proliferator-activated receptor-delta protects the heart from ischemia/reperfusion injury in Zucker fatty rats. J. Pharmacol. Exp. Ther. 2008;325:466–474. doi: 10.1124/jpet.107.135327. [DOI] [PubMed] [Google Scholar]
- Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, Wettersten HI, Ulu A, Hu X, Tam S, Hwang SH, Ingham ES, Kieran MW, Weiss RH, Ferrara KW, Hammock BD. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc. Natl. Acad. Sci. U. S. A. 2013;110:6530–6535. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.