Abstract
Growing evidence demonstrates that prolonged exposure to general anesthetics during brain development induces widespread neuronal cell death followed by long-term memory and learning disabilities in animal models. These studies have raised serious concerns about the safety of anesthetic use in pregnant women and young children. However, the underlying mechanisms of anesthetic-induced neurotoxicity are complex and are not well understood. MicroRNAs are endogenous, small, non-coding RNAs that have been implicated to play important roles in many different disease processes by negatively regulating target gene expression. A possible role for microRNAs in anesthetic-induced developmental neurotoxicity has recently been identified, suggesting that microRNA-based signaling might be a novel target for preventing the neurotoxicity. Here we provide an overview of anesthetic-induced developmental neurotoxicity and focus on the role of microRNAs in the neurotoxicity observed in both human stem cell-derived neuron and animal models. Aberrant expression of some microRNAs has been shown to be involved in anesthetic-induced developmental neurotoxicity, revealing the potential of microRNAs as therapeutic or preventive targets against the toxicity.
Keywords: microRNAs, Anesthetics, Developmental Neurotoxicity, Stem Cells
Introduction
In 1979, Steen and Michenfelder compiled several clinical case studies into a review article and highlighted the possible issue of anesthetic-induced neurotoxicity [1]. The case reports in this article detailed children having adverse effects to anesthetic administration. However, it was around this time that the terms “apoptosis” and “excitotoxicity” were just being coined and as such, much of the research in the field relied solely on observations in the clinic rather than on experimental findings. It wasn’t until 1999 that Olney and colleagues first reported, using an experimental animal model, that anesthetics could induce toxicity in the developing brain and that this toxicity was specific to the period of rapid synaptogenesis in the brain [2, 3]. They observed that blockade of the N-methyl-D-aspartate receptor (NMDA) receptor could induce significant cell death in the brains of 7-day old rat pups and suggested that their findings “may have relevance to human neurodevelopmental disorders involving prenatal (drug-abusing mothers) or postnatal (pediatric anesthesia) exposure to drugs that block NMDA receptors”. Since the majority of anesthetic agents act either as type-A γ-aminobutyric acid receptor (GABAA receptor) agonists or NMDA receptor antagonists, this seminal study raised important questions about the toxic effects of anesthetics on the developing brain. Understanding whether all anesthetics or only a subset of anesthetics could induce this toxicity and the mechanisms by which this toxicity occurs became the focus of additional studies.
Evidence of developmental neurotoxicity from different models
Animal Studies
It has been reported in several studies that the developing brain is most vulnerable to anesthetics during the period of rapid synaptogenesis [4-6]. This is a time in the developing brain in which many synapses are being formed between neurons. This period ranges differently among species. For example, in rodents it lasts about the first 2 weeks of life, while in humans it ranges from about the 3rd trimester of pregnancy through the 2nd or 3rd year of life [7, 8]. Several studies done in developing rodent models found that nearly all anesthetics including isoflurane, sevoflurane, ketamine, propofol and anesthetic combinations could induce cell death in the brains of these animals and lead to learning and memory impairments later in life [9-13]. For example, Shen and colleagues found that postnatal day (PD) 3 Sprague-Dawley rats displayed significant impairments in spatial learning and memory, as assessed by the Morris Water Maze test following a single exposure to 1% sevoflurane [11]. They found that these effects were dose and exposure number dependent. They also found that 7-week-old rats were insensitive to sevoflurane exposure and displayed equivalent performance in the Water Maze test with or without sevoflurane exposure, confirming that vulnerability to anesthetics is confined to an early period in brain development. In addition, Pesic and colleagues have shown that propofol administration induced neuroapoptosis in the cortex and thalamus of PD7 rat pups through an extrinsic pathway and activation of caspase-3 [14]. Liu and colleagues also observed increases in neuroapoptosis in the frontal cortex of PD7 rat pups exposed multiple times to ketamine. They also found that rat pups exposed to ketamine had altered expression of apoptotic related genes as assessed by microarray [15].
Although these initial rodent studies were extremely important, questions were raised about the translatability of these findings to humans. Brambrink, Creeley and others moved to using Rhesus Macaques to study the effects of anesthetics on the developing brain. This allowed for studies on a model more closely related to humans. In addition, the use of larger animals made it easier to monitor hemodynamic properties of the animals to control for confounding variables of anesthetic administration such as cardiovascular, respiratory or metabolic distress. They found that isoflurane, propofol, and ketamine could all induce increases in cell death in both fetal and neonatal rhesus monkey brains with careful control of hemodynamic properties [16-21]. They also went on to show that the toxic effects of isoflurane and propofol affected both the neuronal and oligodendrocyte populations, but did not appear to affect the astrocytes in the brain [18, 22]. This was an extremely important finding since oligodendrocytes are key supporting cells in the brain and are critical in neuronal myelination [23, 24]. Although astrocytes are important neuronal supporting cells, they are very low in number in the developing brain and increase rapidly throughout development [25]. The findings from these studies suggested that anesthetics could both directly and indirectly induce neuronal cell death in the developing brain. The extent of anesthetic-induced neurotoxicity may depend on the following variables:
Clinical significance and human studies
These and other studies conducted in animals led to a push for human epidemiologic studies and prompted the International Anesthesia Research Society (IARS) to partner with the US Food and Drug Administration to form SmartTots (www.smarttots.org), a research initiative aimed at evaluating the safety of anesthetic use in the pediatric population. In 2009, DiMaggio et al. found that children exposed to general anesthesia prior to the age of 3 were twice as likely to be diagnosed with behavioral or developmental disorders as unexposed children [29]. This was a retrospective study of more than 5,000 children enrolled in the New York Medicaid program and the results of this study raised significant safety concerns about the use of anesthetics in children. In 2010, Wilder and colleagues went on to evaluate the medical and educational records of over 5,000 children in a retrospective study aimed at identifying whether anesthetic administration early in life was linked to learning disabilities later on in humans. In this study, 593 of the children had received general anesthesia prior to the age of 4. They found that there was not a significant increase in learning disability diagnoses in children that had received a single administration of anesthesia, but that there was a significant increase in children that had received multiple administrations of anesthesia [30].
Several additional human retrospective studies found that there was a link between early exposure to anesthetics and later learning and behavioral abnormalities [31-33]. However, additional studies found no connection between early exposure to anesthetics and learning and behavioral abnormalities later in life. For example, in 2009 Bartels and colleagues assessed educational achievement and cognitive performance in over 1,100 monozygotic twin pairs from the Netherlands twin registry for which anesthetic exposure data was available. They found that there was a significant increase in learning disabilities in twins exposed to anesthesia when compared to unexposed twins. However, they found no difference between the unexposed and the exposed twin in discordant pairs suggesting that other factors may be responsible for the increases in learning disabilities rather than the anesthetic exposure [34]. In addition, in 2011 Hansen et al. assessed the academic performance of 2,689 Danish children that had undergone inguinal hernia repair surgery in infancy. An age-matched control group consisting of 14,575 children was also studied. They found that exposed children performed worse academically than their unexposed counterparts. However, they also found that once the data had been controlled for confounding variables, there was not a statistically significant difference between the groups [35].
Despite the large scale efforts of the SmartTots organization, the effect of anesthetics on the developing human brain remains uncertain. However, the number of laboratory studies over the last 7 years aimed at addressing this issue has increased nearly 5-fold [36]. Many human epidemiological studies are still ongoing as well in this field today including the Pediatric Anesthesia NeuroDevelopment Assessment (PANDA) study out of Columbia University, the General Anesthesia Safety (GAS) study from Children's Hospital Boston and the Mayo Safety in Kids (MASK) study being conducted at Mayo Clinic. Although the results of these human epidemiologic studies will be extremely useful, it will be difficult to properly dissect out the effects of anesthetic exposure from the effects of surgery, underlying medical conditions, socioeconomic status and other potentially confounding variables. At this point, there is not sufficient evidence in humans regarding the toxic effects of anesthetics on the developing brain, and as such, clinicians cannot be properly advised on the matter.
The human epidemiological studies have been unable to determine the safety of anesthetic use in the pediatric population and millions of children are exposed to anesthetics every year in the United States alone [37]. It is critical to develop a better human model by which to study anesthetic-induced developmental neurotoxicity and the mechanisms responsible for this toxicity in order to better guide clinicians and to develop possible preventative strategies. The emerging model of human embryonic stem cell-derived neurons has allowed us to directly assess the effects of anesthetics on developing human neurons and dissect out the mechanisms by which these anesthetics induce toxicity.
Human embryonic stem cell-derived neuron studies
Embryonic stem cells (ESCs) are cells derived from the inner cell mass of a blastocyst. The human embryo reaches the blastocyst stage (a pre-implantation stage) at day 4-5 post fertilization [38]. ESCs are inherently pluripotent, meaning that they can differentiate into cells from all three germ layers (ectoderm, endoderm, and mesoderm) and can replicate indefinitely. This, along with their high differentiation efficiency makes them more advantageous than other stem cell types such as adult stem cells. In 1981, Evans and Kaufman from the University of Cambridge and Martin from the University of California San Francisco discovered mouse ESCs and reported new techniques for culturing these cells in vitro [39, 40]. The establishment of these critical in vitro techniques opened the doors to many studies aimed at understanding development and disease. It wasn't until 1998 that James Thompson and colleagues at the University of Wisconsin-Madison developed a technique to isolate and culture human ESCs (hESCs) in vitro [38]. The seminal work of this group allowed for mechanistic based studies using a human cell line. This eliminated potential concerns regarding the relevancy of animal models to humans.
The generation of neurons from hESCs was first reported in 2001 by several groups. Carpenter and colleagues reported a method for deriving neurons from hESCs using embryoid body (EB) formation and immunoselection [41]. They found that the derived cells stained positive for neuron-specific markers, responded to neurotransmitter application, and displayed voltage-dependent channels on their cell surface. However, these initial protocols involved multi-step approaches and the immunoselection required to purify the populations could result in a mixed population of cells. In 2003, Schulz and colleagues reported a novel technique for the directed differentiation of neurons from hESCs [42]. This protocol involved EB and rosette formation.
Our group followed a similar approach and improved the efficiency of the differentiation by manual selection of rosettes. Neuronal differentiation was observed by morphological assessment in the culture after six days of culturing the hESC-derived neural stem cells (NSCs) in neuronal differentiation medium. Differentiated neurons exhibited round cell bodies with small projections. Two-week-old neurons expressed the neuron-specific marker β-tubulin III, the synaptic marker synapsin-1, the postsynaptic protein Homer 1 [43, 44], and immature neuron marker doublecortin [45]. In addition, differentiated neurons exhibited functional synapses [46]. Development of an in vitro neurogenesis system using human stem cells has opened up avenues of research for advancing our understanding of human brain development and the issues relevant to anesthetic-induced developmental toxicity in human neuronal lineages under controlled conditions. Recent studies from our and other groups, showed that isoflurane influenced human NSC proliferation and neurogenesis [47] and ketamine dose- and time-dependently induced hESC-derived neuron death [43]. Additionally, when cells underwent different lengths of exposure to propofol and were subjected to single and multiple exposures, propofol induced cell death in the hESC-derived neurons in a time, dose, and exposure number-dependent manner [45]. These findings in stem cell-derived human neurons have recapitulated the results of the animal and human epidemiologic studies [26, 27] [30].
Current mechanisms of anesthetic-induced developmental neurotoxicity
Despite many findings in animal models that anesthetics induce neurotoxicity in the developing brain, the mechanisms by which this toxicity occurs remain largely unknown. A possible role for calcium signaling, reactive oxygen species (ROS) production, mitochondrial abnormalities, neuroinflammation, and epigenetic changes in the mechanism of anesthetic-induced neurotoxicity have all been reported [43, 48-50]. Sinner et al. found that exposure of cultured rat hippocampal neurons to ketamine resulted in increases in intracellular calcium and neuronal apoptosis [51]. Intracellular calcium levels are tightly regulated under normal conditions. Persistent elevation of intracellular calcium, beyond normal levels, can induce apoptosis [52]. In response to ketamine, there was a significant increase in ROS production in the cytosol and superoxide generation within mitochondria in ketamine-treated hESC-derived neurons, indicating a mitochondrial origin of ROS. Trolox, a ROS scavenger, prevented ketamine-induced ROS production and apoptosis in differentiated human neurons [43]. Several animal studies have suggested that accumulation of ROS was associated with anesthetic-induced mitochondrial damage [53, 54]. Application of the antioxidant (7-nitroindazole, a nitric oxide synthase inhibitor) attenuated ketamine-induced rat forebrain-derived cultured neuronal cell death [55].
Although many different mechanisms and pathways have been implicated to play a role in anesthetic-induced neurotoxicity, the mitochondria appear to play key roles in this process through their crucial involvement in cellular processes and apoptosis [56]. Ketamine-induced apoptosis in stem cell-derived human neurons was accompanied by a significant decrease in mitochondrial membrane potential and an increase in cytochrome c release from mitochondria into the cytosol. In addition, most control neurons showed elongated and inter-connected, tubular mitochondria while much shorter and smaller mitochondria were prevalent in the ketamine-treated culture [43]. Dynamin-related protein 1 (Drp1) is a key regulator of mitochondrial fission and is primarily distributed in the cytoplasm of a healthy cell, but shuttles between the cytoplasm and the mitochondrial surface. It has also been shown that exposure of neonatal rat pups to general anesthetics induced significant decreases in the level of Drp1 in the cytosol and increases in mitochondrial Drp1 levels [57], leading to increases in mitochondrial fission. Inhibition of mitochondrial fission was shown to prevent mitochondrial cytochrome c release and apoptotic cell death [58]. Loss of mitochondrial membrane potential and release of cytochrome c from mitochondria are key events in initiating mitochondria-related apoptosis [59], indicating that the increased mitochondrial fission possibly plays an important role in the toxic effects of general anesthetics.
It was also reported that neuroinflammation was involved in anesthetic-induced neurotoxicity and cognitive impairment in the developing mouse brain. Exposure to 3% sevoflurane for 2 hours daily for 3 days induced cognitive impairment and neuroinflammation (e.g., increased interleukin-6 levels) in the developing mouse brain but not in adult mice. Anti-inflammatory treatment (ketorolac) attenuated the cognitive impairment, implicating neuroinflammation as a key mediator of anesthetic-induced neurotoxicity [49]. Additionally, alterations in the levels of a variety of neurotrophins have been implicated in anesthetic-induced developmental neurotoxicity. It was observed that exposure of rat pups to propofol induced a significant decrease in the level of nerve growth factor in the thalamus , a protein that is critical for the survival and growth of neurons. Propofol exposure was also shown to alter the expression levels of a variety of key neurotrophic factor receptors and downstream targets such as Akt and Erk [60]. It was recently reported by Han and colleagues that exposure of 7-day old mouse pups to 2 hours of 1.5% sevoflurane increased the phosphorylation of methyl-CpG island binding protein 2 in the hippocampus. The sevoflurane-induced increases of neuronal cell death and phosphorylation of methyl-CpG were reversed by pre-treatment with memantine, a partial antagonist of the NMDAR [48], suggesting that sevoflurane-induced epigenetic alterations might also play important roles in the neurotoxicity. Table 1 depicts example anesthetic-induced developmental neurotoxicity studies and the key findings from these studies.
Table 1.
The representative animal studies regarding anesthetic-induced developmental neurotoxicity
| Anesthetic | Dose/Duration | Model | Main findings | Reference |
|---|---|---|---|---|
| Sevoflurane | 3%, 6 hours | PD7 Sprague-Dawley rats | Sevoflurane elevated caspase-3 activation and ROS levels, decreased mitochondrial cardiolipin contents, altered cellular ultrastructure in the cerebral cortex and metabolic pathways of glucose and intracellular antioxidants. | Liu, et al.[109] |
| Ketamine | 25 μM, 24 hours | Hippocampal neuron cultures from 19-day-old Wistar rat embryos | Ketamine exposure significantly increased the number of apoptotic neurons and the cytosolic calcium concentration. Ketamine also led to a down-regulation of the CaMKII and a decrease in synapsin. | Sinner, et al.[51] |
| Sevoflurane | 1.5%, 2 hours | PD7 mouse pups | Sevoflurane increased the phosphorylation of methyl-CpG island binding protein 2 in the hippocampus and sevoflurane-induced increases of neuronal cell death and phosphorylation of methyl-CpG were reversed by pre-treatment with memantine, a partial antagonist of the NMDAR | Han, et al.[48] |
| Propofol | 25 mg/kg, 1 dose | PD14 Wistar rats | Propofol induced a significant decrease in the level of nerve growth factor in the thalamus and altered the expression levels of a variety of key neurotrophic factor receptors and downstream targets such as Akt and Erk. | Popic, et al.[60] |
| Midazolam/Nitrous Oxide/Isoflurane | 9 mg/kg midazolam, 75% NO, 0.75% iso, 6 hours | PD7 Sprague-Dawley rats | Anesthesia exposure up-regulated reactive oxygen species generation and down-regulated superoxide dismutase. Exposure to the anesthesia was also associated with increased mitochondrial fission. | Boscolo, et al.[57] |
| Ketamine | 100 μM, 24 hours | Human stem cell-derived neurons | Ketamine exposure increased neuronal apoptosis, ROS production, and mitochondrial fission, implicating mitochondrial dysfunction as a key mechanism by which ketamine induces neurotoxicity. | Bai, et al.[43] |
| Isoflurane | Surgical dose, 5 hours | Fetal rhesus macaques | Isoflurane induced a significant increase in apoptosis of neurons and oligodendrocytes in the fetal monkey brain. | Creeley, et al.[110] |
Despite these findings, the current neurotoxicity mechanisms are incomplete and work remains to be done to fully elucidate these pathways. A potential functional role for microRNAs in anesthetic-induced developmental neurotoxicity has recently emerged.
microRNAs and anesthetic-induced developmental neurotoxicity
MicroRNAs
Mature microRNAs are small non-coding RNA molecules that are approximately 22 nucleotides in length [61]. microRNAs are highly conserved and are believed to be critical components in evolution [62]. MicroRNAs can bind with perfect or imperfect complementary binding to target messager RNA (mRNA), leading to downregulation of protein expression through mRNA cleavage or translational repression [63, 64]. A single microRNA can have multiple mRNA targets and one mRNA can be regulated by one or multiple miRNAs.
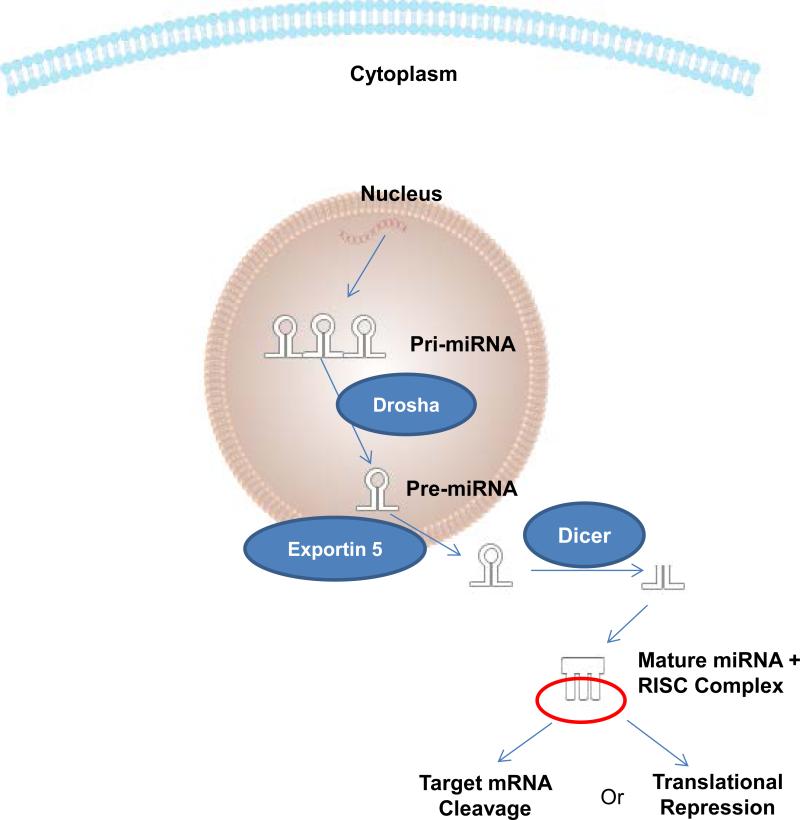
As depicted in Figure 1, microRNAs are transcribed in multiple hairpin structures in the nucleus by RNA polymerase II as large primary transcripts (pri-miRNAs). The pri-miRNAs are cleaved by the RNase III enzyme Drosha in the nucleus into hairpin loops called precursor microRNAs (pre-miRNAs). The resulting pre-miRNAs are approximately 70-nucleotides in length. The pre-miRNAs are exported out into the cytoplasm by Exportin 5. Once in the cytoplasm, the pre-miRNAs are further processed by the RNase III enzyme Dicer which removes the hairpin loop forming the mature miRNA strands. The 2 strands unwind and the more thermodynamically unstable strand typically degrades. The mature microRNA strand then incorporates into the RNA-Induced silencing complex (RISC) where it can act to induce silencing of its target mRNA [61, 64].
Figure 1. MicroRNA (miRNA) biogenesis and mechanisms of action.
miRNAs are transcribed in one or multiple hairpin structures in the nucleus by RNA polymerase II as large primary transcripts (pri-miRNAs). The pri-miRNAs are cleaved in the nucleus into shorter single hairpin loop called precursor miRNAs (pre-miRNAs) by the enzyme Drosha. The pre-miRNAs are exported out into the cytoplasm by Exportin 5. Once in the cytoplasm, the pre-miRNAs are further processed by the enzyme Dicer which removes the hairpin loop and forms the mature double-stranded miRNA. The two strands then unwind and incorporate into the RNA-Induced Silencing Complex (RISC), resulting in the mRNA cleavage or translational repression followed by downregulation of protein expression.
The first microRNA was discovered in 1993 by Lee and colleagues in C. Elegans [65]. However, it wasn't until 2000 that they were recognized as a distinctive group of RNA molecules responsible for mRNA regulation [66, 67]. In the decade following their initial discovery, microRNA research flourished and their importance in cancer and the heart was quickly discovered along with suitable approaches to manipulate their expression. Thousands of microRNAs have since been identified in various organisms through random cloning and sequencing or computational prediction and shown to be involved in the regulation of almost every cellular event in developmental and physiological processes [68]. Dysregulation of microRNAs has been reported to play a fundamental role in the onset, progression, and dissemination of many human diseases including neurodegeneration, and as such, they have become attractive therapeutic targets.
For example, microRNAs are highly enriched in the brain and have been implicated to play important roles in memory, neurogenesis, synaptic plasticity, and neuronal degeneration [69]. Several studies have cited dysregulation of microRNA expression in the postmortem brain tissue of neurodegenerative disease patients. One study found that there was a downregulation of the brain-specific microRNAs: miRs-9, -29b, and -181 in Alzheimer's disease patients [70] while another study revealed that similar and additional brain-specific microRNAs including miRs-9, -29b, -124a, and -132 were down-regulated in the brains of Huntington's disease patients [71]. However, the potential functions of microRNAs in anesthetic-induced neurotoxicity are just starting to be investigated. More recently, five studies from our and other laboratories pointed to important roles of several microRNAs (e.g., miR-21, miR-34a, miR-34c, miR-124, and miR-137) in anesthetic-induced developmental neurotoxicity using various experimental models [45, 72-75]. These studies have been summarized and are shown in Table 2.
Table 2.
Studies depicting a role of microRNAs in anesthetic-induced developmental neurotoxicity
| Anesthetic | Model | MicroRNA | Main Findings | Reference |
|---|---|---|---|---|
| Propofol | Human embryonic stem cell-derived neurons | miR-21 | The expression of miR-21 was downregulated following exposure to 6 hours of 20 μg/mL propofol. Overexpression of miR-21 attenuated the propofol-induced cell death. The toxicity occurred through a STAT3/miR-21/Sprouty 2/Akt-dependent mechanism. | Twaroski, et al. [45] |
| Ketamine | Neonatal mice | miR-34c | miR-34c was upregulated in the hippocampus of neonatal mice exposed to ketamine and downregulation of miR-34c attenuated the ketamine-induced neuronal cell death and cognitive impairment observed in the animals. | Cao, et al.[72] |
| Ketamine | Neonatal mice | miR-124 | miR-124 was upregulated in the hippocampus of neonatal mice exposed to ketamine and knockdown of miR-124 reduced ketamine-induced apoptosis in hippocampal CA1 neurons in vitro and activated the PKC-ERK pathway. miR-124 knockdown improved memory performance of mice treated with ketamine. | Xu, et al.[73] |
| Ketamine | One-month old C57/BL6 mice | miR-34a | Exposure to 50 mg/kg ketamine for 7 days induced apoptosis in hippocampal CA1 neurons and upregulated hippocampal miR-34a. Inhibition of miR-34a protected against anesthesia-induced neuroapoptosis and memory impairment while knockdown of its target, FGFR1 exacerbated the toxicity. | Jiang, et al.[74] |
| Ketamine | One-month old Sprague-Dawley rats | miR-137 | Exposure to 75 mg/kg ketamine for 3 days induced apoptosis in hippocampal CA1 neurons, downregulation of miR-137 in the hippocampus, and long-term memory impairment. Overexpression of miR-137 protected against hippocampal neurodegeneration and memory loss. | Huang, et al. [75] |
miR-21
MicroRNA-21 (miR-21) was one of the first microRNAs discovered in humans and its sequence was found to be highly conserved across species [76]. The human miR-21 gene is located within a coding gene known as vacuole membrane protein-1 on chromosome 17q23.2. Despite being located within a coding gene, the human miR-21 gene contains its own promoter and can be transcribed independently [77]. miR-21 has been identified to be involved in many cancers and is a well-established anti-apoptotic factor. Dysregulation of miR-21 has been shown to mediate hypoxia-induced neuroapoptosis [78] while overexpression of miR-21 decreased apoptosis in a rat model of traumatic brain injury [79]. In addition, exposure of fetal cerebral cortical-derived neuroepithelial cells to ethanol, an NMDA receptor antagonist and GABAA receptor agonist, was shown to suppress miR-21 [80].
Recently, Twaroski et al used hESC-derived neurons for the first time to study microRNA mechanisms governing anesthetic-induced neurotoxicity by exposing 2-week old neurons to 6 hours of 20 μg/mL propofol or the vehicle control, dimethyl sulfoxide. To examine whether microRNAs were playing a role in the observed propofol-induced toxicity, 84 of the most abundantly expressed microRNAs were screened using commercially available qRT-PCR arrays. They found that 20 microRNAs were significantly downregulated following exposure to propofol when compared to vehicle-treated cells [45]. Of these 20 microRNAs, several were of interest based upon their established roles in either physiological or pathological processes. For example, the let-7 family has been shown to be highly expressed in the brain and is important in stem cell differentiation and apoptosis [81]. In addition, miRs 9 and 124 have been shown to play a role in neuronal differentiation [82]. The target of greatest interest was miR-21 which is a well-established anti-apoptotic factor [83, 84].
To confirm that miR-21 was playing a role in the propofol-induced neurotoxicity, miR-21 was artificially up-regulated and knocked down in the stem cell-derived neurons using lipofectamine and a miR-21 mimic and antagomir, respectively. The results showed that miR-21 overexpression attenuated the propofol-induced cell death while miR-21 knockdown exacerbated the effects. There are many established upstream regulators of miR-21 including signal transducer and activator of transcription 3 (STAT3) [85-87]. All members of the STAT family translocate to the cell nucleus once activated by phosphorylation where they act as transcriptional activators [88, 89]. STAT3 was first discovered in 1994 and is activated when phosphorylated at the Tyrosine 705 position [90, 91]. Following propofol exposure, pSTAT3 expression in the neurons was significantly reduced but was not altered following manipulation of miR-21 expression, suggesting that STAT3 may be an important upstream regulator of miR-21 in propofol-induced neurotoxicity [45].
miR-21 also has thousands of established and predicted targets. Of these targets, programmed cell death protein 4 (PDCD4), Sprouty 1 and 2 and phosphatase and tensin homolog (PTEN) are, arguably, the most well studied [92]. There was no change in the expression of PTEN in hESC-derived neurons following propofol exposure, indicating that PTEN is not involved in this pathway. However, Sprouty 2 expression was significantly increased while the level of activated Akt, a serine/threonine kinase that is involved in many cell survival pathways through inhibition of apoptotic processes [93, 94], was reduced in the propofol-treated hESC-derived neurons. Sprouty 2 knockdown in the hESC-derived neurons using a small interfering RNA (siRNA)-mediated approach significantly attenuated the propofol-induced neuron death and the decrease in activated Akt expression. The authors concluded that Sprouty 2 is the direct target of miR-21 in the neurotoxicity and propofol induced toxicity in human stem cell-derived developing neurons possibly through a STAT3/miR-21/Sprouty 2/Akt dependent mechanism [45].
miR-34a
microRNA-34a (miR-34a) belongs to the miR-34 family of microRNAs comprising three processed microRNAs (miR-34 a/b/c) that are encoded by two different genes. miR-34a is encoded by its own transcript, whereas miR-34b and miR-34c share a common primary transcript [95]. The hippocampus is an area of the brain involved in learning and memory and has been shown to be a key site of neurotoxicity following exposure to anesthetics in developing animals. In the hippocampus, p53 targets the miR-34 family and this family of microRNAs is essential for cortical brain development [96]. One recent publication indicates the important role of miR-34a in ketamine-induced hippocampal apoptosis and memory impairment through fibroblast growth factor receptor 1 (FGFR1). In this study, one-month old C57/BL6 mice received daily intraperitoneal injections of anesthesia (ketamine, 50 mg/kg) for 7 days. Ketamine induced apoptosis of neurons in region I of the hippocampus [cornus ammonis (CA)1] and upregulated hippocampal miR-34a expression. Lentivirus-mediated inhibition of miR-34a protected against ketamine-induced neuroapoptosis and memory impairment. Luciferase assay demonstrated that FGFR1 was directly regulated by miR-34a in hippocampus and siRNA-induced FGFR1 down-regulation further exaggerated ketamine-induced neuroapoptosis in hippocampus [74]. These findings suggest an important role for miR-34a and FGFR1 in ketamine-induced neurotoxicity.
miR-34c
microRNA-34c (miR-34c) also belongs to the miR-34 family of microRNAs. miR-34c has been implicated to play a role in Alzheimer's disease (AD) [97, 98]. The expression level of miR-34c was increased in both cellular and plasma components of AD patients’ circulating blood samples compared to normal age-matched controls. Overexpression of miR-34c in cultures of human embryonic kidney cells (HEK 293) repressed the expression of targets such as Bcl2, SIRT1, Psen1, and Onecut2 that are involved in cell survival and oxidative defense pathways [98], suggesting that increased miR-34c may be one of many factors contributing to an overall systemic weakening of stress defense mechanisms and cell survival in AD patients.
Zhang and colleagues found that miR-34c was upregulated in the hippocampus of neonatal mice exposed to ketamine. They also showed that downregulation of miR-34c could attenuate the ketamine-induced neuronal cell death and cognitive impairment observed in the animals. Knocking down miR-34c activated the protein kinase C (PKC)/extracellular-signal regulated kinase (ERK) pathway, upregulated antiapoptotic protein BCL2, and ameliorated ketamine-induced apoptosis in the hippocampus. Cognitive examination with the Morris water maze test showed that ketamine-induced memory impairment was significantly improved in the animals by miR-34c downregulation [72]. Thus, miR-34c is appears to be important in regulating ketamine-induced developmental neurotoxicity in the hippocampus.
miR-124
MicroRNA-124 (miR-124) is the most abundant microRNA expressed in the vertebrate central nervous system (CNS). miR-124 is expressed in neurons but not astrocytes and the levels of miR-124 increase over time in the developing CNS. miR-124 is involved in neuronal maturation and differentiation and downregulation of miR-124 has been linked to apoptosis [99]. miR-124 was also required for hippocampal axogenesis and retinal cone survival through Lhx2 suppression [100, 101] and has been shown to induce neurite elongation by indirectly targeting Akt [102]. Xu and colleagues reported that miR-124 was upregulated in the hippocampus of neonatal mice exposed to high doses of ketamine. They found that lentivirus-mediated knockdown of miR-124 reduced ketamine-induced apoptosis in hippocampal CA1 neurons in vitro and upregulated the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor phosphorylation and activated PKC-ERK pathway [103]. Morris water maze test demonstrated that miR-124 knockdown improved memory performance of mice treated with ketamine, indicating that inhibiting miR-124 may provide a molecular target to attenuate the neurotoxicity.
miR-137
MicroRNA-137 (miR-137) has been shown to regulate neuronal maturation and dendritic morphogenesis during development [104]. Dysregulation of miR-137 expression may be associated with the pathogenesis and development of Alzheimer's disease [105]. Huang et al showed that upregulation of miR-137 protected against ketamine-induced hippocampal neurodegeneration in young rats [75]. In this study, 1 month old-Sprague-Dawley rats were systemically administrated ketamine (75 mg/kg) once per day for 3 days. Ketamine treatment resulted in neuroapoptosis in the hippocampal CA1 region, down-regulation of miR-137 in the hippocampus, and long-term memory dysfunction. Conversely, overexpression of miR-137 protected the brain against ketamine-induced neuroapoptosis and memory loss [75].
Conclusions and future directions
Mounting evidence from animal, epidemiology, and human stem cell-derived neuron models have shown that anesthetics can induce developmental neurotoxicity. The mechanisms governing the neurotoxicity are likely extremely complex and involve many converging or diverging pathways. Three recent studies from different groups began to clarify the role of microRNAs in anesthetic-induced developmental neurotoxicity using different models. These studies depicted altered profiles of microRNAs in developing human neurons and neonatal rodent brains in response to administration of the intravenous anesthetic drugs propofol or ketamine. Specifically, propofol downregulated miR-21 in stem cell-derived human neurons while ketamine upregulated miR-34a, miR-34c, and miR-124, and downregulated miR-137 in the mouse and rat hippocampus, respectively. Upregulation of miR-21 and miR-137 or downregulation of miR-34a, miR-34c, and miR-124 attenuated the neurotoxicity conferred by the anesthetics [45, 72-75], suggesting a functional role of these microRNAs in the neurotoxicity. However, the roles of microRNAs in anesthetic-induced developmental neurotoxicity are just beginning to be understood. Despite these findings, there are several important questions left unanswered and these are described below.
Role of other microRNAs in the neurotoxicity conferred by different anesthetic drugs
Since there are over one thousand microRNAs that have been found or predicted to be important in physiology and pathophysiology, it is very likely that other microRNAs could participate in the multiple facets of anesthetic-induced developmental neurotoxicity including neuroapoptosis and other brain developmental events (e.g., neurogenesis, NSC proliferation, and synaptogenesis). Future studies in this area may include expanding the focus of the current studies to identify additional microRNAs that may play a role in the observed anesthetic-induced toxicity. For example, Twaroski et al showed that 20 microRNAs were significantly down-regulated in stem cell-derived human neurons following exposure to propofol, many of which had been implicated in neurological development and disease. For instance, downregulation of miR-9 has been associated with several neurodegenerative diseases and it is known to be involved in neuronal differentiation [71, 106]. Interestingly, a recent study found that miR-9 is involved in neural lineage differentiation of mouse ESCs and this appears to be mediated by STAT3 [107]. Thus, miR-9 may be potential target to pursue in the future.
Role of microRNAs in the neurotoxicity conferred by different anesthetic drugs
As described above, the three published microRNA-based neurotoxicity studies were only focused on the effects of propofol and ketamine [45, 72-75]. However, many other anesthetic agents are used clinically such as sevoflurane and isofluranethat have been shown to induce developmental neurotoxicity either alone or in combination with other anesthetics in animal models. As such, it will also be critical to understand how microRNAs are altered when hESC-derived neurons or young animals are exposed to different anesthetic agents. Future studies focusing on these effects will be very important in understanding whether the mechanisms identified in the previous microRNA studies are relevant to all anesthetic agents or combinations of anesthetics or only to propofol or ketamine and will be critical for the future development of neuroprotective strategies.
Additional microRNA Targets
One microRNA can target many mRNAs. For instance, there are currently thousands of established and predicted targets of miR-21. The study by Twaroski et al focused on the role of Sprouty 2, a direct target of miR-21 in the propofol-induced neurotoxicity. Although Sprouty 2 did appear to play a key role in the propofol-induced neuronal cell death, the role of additional miR-21 targets cannot be ruled out since the knockdown of Sprouty 2 in this study only partially attenuated the propofol-induced cell death [45]. This could be due to the incomplete knockdown of Sprouty 2 or it could suggest a role for additional miR-21 targets. The expression of programmed cell death protein 4 (PDCD4), another direct target of miR-21 was upregulated following exposure to propofol as assessed by Western blot [108]. Nevertheless, future studies will need to include examination of the functional contribution to the observed toxicity of changes in PDCD4 expression and may focus on understanding the intricate balance between miR-21 expression and the expression of its many targets, and how shifts in that balance might be involved in anesthetic-induced neurotoxicity. The use of microRNA target arrays would allow for the assessment of changes in the expression of many predicted and validated targets of individual microRNAs of interest following exposure to anesthetics.
Taken together, the most recent studies suggest a novel microRNA-related mechanism by which propofol and ketamine, widely used anesthetic agents, induce cell death in developing human neurons and animal models, implicating an important role for microRNAs in anesthetic-induced neurotoxicity and further expanding the understanding of how anesthetic agents induce neuronal toxicity. Nevertheless, detailed mechanisms are still poorly understood. Millions of children are exposed to anesthetic agents every year and many of those procedures are unavoidable. Understanding the mechanisms by which anesthetics induce neurotoxicity is critical in order to prevent adverse neurological outcomes following anesthetic exposure in the developing brain. The microRNA findings might lead to the development of novel protective approaches aimed at mitigating the neurotoxic effects of anesthetics in young children.
Acknowledgement
This work was supported by R01GM112696 from the NIH (to Dr. Xiaowen Bai), by P01GM066730 and R01HL034708 from the NIH, Bethesda, MD, and by FP00003109 from Advancing a Healthier Wisconsin Research and Education Initiative Fund (to Dr. Zeljko J. Bosnjak).
References
- 1.Steen PA, Michenfelder JD. Neurotoxicity of anesthetics. Anesthesiology. 1979;50:437–453. doi: 10.1097/00000542-197905000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Bai X, Bosnjak ZJ. Emerging model in anesthetic developmental neurotoxicity: Human stem cells. Int J Clin Anesthesiol. 2013;1:1002. [PMC free article] [PubMed] [Google Scholar]
- 3.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, et al. Blockade of nmda receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 4.Jevtovic-Todorovic V. Developmental synaptogenesis and general anesthesia: A kiss of death? Curr Pharm Des. 2012;18:6225–6231. doi: 10.2174/138161212803832380. [DOI] [PubMed] [Google Scholar]
- 5.Jevtovic-Todorovic V. General anesthetics and the developing brain: Friends or foes? J Neurosurg Anesthesiol. 2005;17:204–206. doi: 10.1097/01.ana.0000178111.26972.16. [DOI] [PubMed] [Google Scholar]
- 6.Lunardi N, Ori C, Erisir A, Jevtovic-Todorovic V. General anesthesia causes long-lasting disturbances in the ultrastructural properties of developing synapses in young rats. Neurotox Res. 2010;17:179–188. doi: 10.1007/s12640-009-9088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 8.Dekaban AS. Changes in brain weights during the span of human life: Relation of brain weights to body heights and body weights. Ann Neurol. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- 9.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahraman S, Zup SL, McCarthy MM, Fiskum G. Gabaergic mechanism of propofol toxicity in immature neurons. J Neurosurg Anesthesiol. 2008;20:233–240. doi: 10.1097/ANA.0b013e31817ec34d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen X, Liu Y, Xu S, Zhao Q, Guo X, et al. Early life exposure to sevoflurane impairs adulthood spatial memory in the rat. Neurotoxicology. 2013;39C:45–56. doi: 10.1016/j.neuro.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, et al. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–848. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 13.Cheng B, Zhang Y, Wang A, Dong Y, Xie Z. Vitamin c attenuates isoflurane-induced caspase-3 activation and cognitive impairment. Mol Neurobiol. 2014 doi: 10.1007/s12035-014-8959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pesic V, Milanovic D, Tanic N, Popic J, Kanazir S, et al. Potential mechanism of cell death in the developing rat brain induced by propofol anesthesia. Int J Dev Neurosci. 2009;27:279–287. doi: 10.1016/j.ijdevneu.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu F, Paule MG, Ali S, Wang C. Ketamine-induced neurotoxicity and changes in gene expression in the developing rat brain. Curr Neuropharmacol. 2011;9:256–261. doi: 10.2174/157015911795017155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, et al. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012;116:372–384. doi: 10.1097/ALN.0b013e318242b2cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creeley C, Dikranian K, Dissen G, Martin L, Olney J, et al. Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth 110 Suppl. 2013;1:i29–38. doi: 10.1093/bja/aet173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paule MG, Li M, Allen RR, Liu F, Zou X, et al. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33:220–230. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slikker W, Jr., Zou X, Hotchkiss CE, Divine RL, Sadovova N, et al. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98:145–158. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- 21.Zou X, Patterson TA, Divine RL, Sadovova N, Zhang X, et al. Prolonged exposure to ketamine increases neurodegeneration in the developing monkey brain. Int J Dev Neurosci. 2009;27:727–731. doi: 10.1016/j.ijdevneu.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Brambrink AM, Back SA, Riddle A, Gong X, Moravec MD, et al. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol. 2012;72:525–535. doi: 10.1002/ana.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson ES, Bjartmar C. [oligodendrocytes have a key role in the development of cns function and in myelin related diseases]. Lakartidningen. 2000;97:3265–3268. [PubMed] [Google Scholar]
- 24.Schwab ME, Caroni P. Oligodendrocytes and cns myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J Neurosci. 1988;8:2381–2393. doi: 10.1523/JNEUROSCI.08-07-02381.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandeira F, Lent R, Herculano-Houzel S. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc Natl Acad Sci U S A. 2009;106:14108–14113. doi: 10.1073/pnas.0804650106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scallet AC, Schmued LC, Slikker W, Jr., Grunberg N, Faustino PJ, et al. Developmental neurotoxicity of ketamine: Morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol Sci. 2004;81:364–370. doi: 10.1093/toxsci/kfh224. [DOI] [PubMed] [Google Scholar]
- 27.Shen X, Dong Y, Xu Z, Wang H, Miao C, et al. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013;118:502–515. doi: 10.1097/ALN.0b013e3182834d77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fredriksson A, Ponten E, Gordh T, Eriksson P. Neonatal exposure to a combination of nmethyl-d-aspartate and gamma-aminobutyric acid type a receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107:427–436. doi: 10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- 29.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21:286–291. doi: 10.1097/ANA.0b013e3181a71f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, et al. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–1061. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ing C, DiMaggio C, Whitehouse A, Hegarty MK, Brady J, et al. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130:e476–485. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 33.Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, et al. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87:120–129. doi: 10.1016/j.mayocp.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartels M, Althoff RR, Boomsma DI. Anesthesia and cognitive performance in children: No evidence for a causal relationship. Twin Res Hum Genet. 2009;12:246–253. doi: 10.1375/twin.12.3.246. [DOI] [PubMed] [Google Scholar]
- 35.Hansen TG, Pedersen JK, Henneberg SW, Pedersen DA, Murray JC, et al. Academic performance in adolescence after inguinal hernia repair in infancy: A nationwide cohort study. Anesthesiology. 2011;114:1076–1085. doi: 10.1097/ALN.0b013e31820e77a0. [DOI] [PubMed] [Google Scholar]
- 36.Lin EP, Soriano SG, Loepke AW. Anesthetic neurotoxicity. Anesthesiol Clin. 2014;32:133–155. doi: 10.1016/j.anclin.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106:1681–1707. doi: 10.1213/ane.0b013e318167ad77. [DOI] [PubMed] [Google Scholar]
- 38.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 39.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 40.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP, et al. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001;172:383–397. doi: 10.1006/exnr.2001.7832. [DOI] [PubMed] [Google Scholar]
- 42.Schulz TC, Palmarini GM, Noggle SA, Weiler DA, Mitalipova MM, et al. Directed neuronal differentiation of human embryonic stem cells. BMC Neurosci. 2003;4:27. doi: 10.1186/1471-2202-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai X, Yan Y, Canfield S, Muravyeva MY, Kikuchi C, et al. Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway. Anesth Analg. 2013;116:869–880. doi: 10.1213/ANE.0b013e3182860fc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosnjak ZJ, Yan Y, Canfield S, Muravyeva MY, Kikuchi C, et al. Ketamine induces toxicity in human neurons differentiated from embryonic stem cells via mitochondrial apoptosis pathway. Curr Drug Saf. 2012;7:106–119. doi: 10.2174/157488612802715663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Twaroski DM, Yan Y, Olson JM, Bosnjak ZJ, Bai X. Down-regulation of microrna-21 is involved in the propofol-induced neurotoxicity observed in human stem cell-derived neurons. Anesthesiology. 2014;121:786–800. doi: 10.1097/ALN.0000000000000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson MA, Weick JP, Pearce RA, Zhang SC. Functional neural development from human embryonic stem cells: Accelerated synaptic activity via astrocyte coculture. J Neurosci. 2007;27:3069–3077. doi: 10.1523/JNEUROSCI.4562-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao X, Yang Z, Liang G, Wu Z, Peng Y, et al. Dual effects of isoflurane on proliferation, differentiation, and survival in human neuroprogenitor cells. Anesthesiology. 2013;118:537–549. doi: 10.1097/ALN.0b013e3182833fae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han XD, Li M, Zhang XG, Xue ZG, Cang J. Single sevoflurane exposure increases methylcpg island binding protein 2 phosphorylation in the hippocampus of developing mice. Mol Med Rep. 2015;11:226–230. doi: 10.3892/mmr.2014.2751. [DOI] [PubMed] [Google Scholar]
- 49.Cui Y, Ling-Shan G, Yi L, Xing-Qi W, Xue-Mei Z, et al. Repeated administration of propofol upregulated the expression of c-fos and cleaved-caspase-3 proteins in the developing mouse brain. Indian J Pharmacol. 2011;43:648–651. doi: 10.4103/0253-7613.89819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei H. The role of calcium dysregulation in anesthetic-mediated neurotoxicity. Anesth Analg. 2011;113:972–974. doi: 10.1213/ANE.0b013e3182323261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sinner B, Friedrich O, Zink W, Zausig Y, Graf BM. The toxic effects of s(+)-ketamine on differentiating neurons in vitro as a consequence of suppressed neuronal ca2+ oscillations. Anesth Analg. 2011;113:1161–1169. doi: 10.1213/ANE.0b013e31822747df. [DOI] [PubMed] [Google Scholar]
- 52.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: The calcium-apoptosis link. Nature Reviews Molecular Cell Biology. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, et al. The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. J Biol Chem. 2010;285:4025–4037. doi: 10.1074/jbc.M109.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C, Zhang X, Liu F, Paule MG, Slikker W., Jr. Anesthetic-induced oxidative stress and potential protection. ScientificWorldJournal. 2010;10:1473–1482. doi: 10.1100/tsw.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang C, Sadovova N, Patterson TA, Zou X, Fu X, et al. Protective effects of 7-nitroindazole on ketamine-induced neurotoxicity in rat forebrain culture. Neurotoxicology. 2008;29:613–620. doi: 10.1016/j.neuro.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Lei X, Guo Q, Zhang J. Mechanistic insights into neurotoxicity induced by anesthetics in the developing brain. Int J Mol Sci. 2012;13:6772–6799. doi: 10.3390/ijms13066772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boscolo A, Milanovic D, Starr JA, Sanchez V, Oklopcic A, et al. Early exposure to general anesthesia disturbs mitochondrial fission and fusion in the developing rat brain. Anesthesiology. 2013 doi: 10.1097/ALN.0b013e318289bc9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 59.Budd SL, Tenneti L, Lishnak T, Lipton SA. Mitochondrial and extramitochondrial apoptotic signaling pathways in cerebrocortical neurons. Proc Natl Acad Sci U S A. 2000;97:6161–6166. doi: 10.1073/pnas.100121097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Popic J, Pesic V, Milanovic D, Todorovic S, Kanazir S, et al. Propofol-induced changes in neurotrophic signaling in the developing nervous system in vivo. PLoS One. 2012;7:e34396. doi: 10.1371/journal.pone.0034396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartel DP. Micrornas: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 62.Lee CT, Risom T, Strauss WM. Evolutionary conservation of microrna regulatory circuits: An examination of microrna gene complexity and conserved microrna-target interactions through metazoan phylogeny. DNA Cell Biol. 2007;26:209–218. doi: 10.1089/dna.2006.0545. [DOI] [PubMed] [Google Scholar]
- 63.Bartel DP. Micrornas: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shukla GC, Singh J, Barik S. Micrornas: Processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3:83–92. [PMC free article] [PubMed] [Google Scholar]
- 65.Lee RC, Feinbaum RL, Ambros V. The c. Elegans heterochronic gene lin-4 encodes small rnas with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 66.Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny rnas with probable regulatory roles in caenorhabditis elegans. Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 67.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, et al. The 21-nucleotide let-7 rna regulates developmental timing in caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 68.Kozomara A, Griffiths-Jones S. Mirbase: Integrating microrna annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaur P, Armugam A, Jeyaseelan K. Micrornas in neurotoxicity. J Toxicol. 2012;2012:870150. doi: 10.1155/2012/870150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lau P, de Strooper B. Dysregulated micrornas in neurodegenerative disorders. Semin Cell Dev Biol. 2010;21:768–773. doi: 10.1016/j.semcdb.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 71.Packer AN, Xing Y, Harper SQ, Jones L, Davidson BL. The bifunctional microrna mir-9/mir-9* regulates rest and corest and is downregulated in huntington's disease. J Neurosci. 2008;28:14341–14346. doi: 10.1523/JNEUROSCI.2390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao SE, Tian J, Chen S, Zhang X, Zhang Y. Role of mir-34c in ketamine-induced neurotoxicity in neonatal mice hippocampus. Cell Biol Int. 2015;39:164–168. doi: 10.1002/cbin.10349. [DOI] [PubMed] [Google Scholar]
- 73.Xu H, Zhang J, Zhou W, Feng Y, Teng S, et al. The role of mir-124 in modulating hippocampal neurotoxicity induced by ketamine anesthesia. Int J Neurosci. 2014 doi: 10.3109/00207454.2014.919915. [DOI] [PubMed] [Google Scholar]
- 74.Jiang XL, Du BX, Chen J, Liu L, Shao WB, et al. Microrna-34a negatively regulates anesthesia-induced hippocampal apoptosis and memory impairment through fgfr1. Int J Clin Exp Pathol. 2014;7:6760–6767. [PMC free article] [PubMed] [Google Scholar]
- 75.Huang C, Zhang X, Zheng J, Chen C, Chen Y, et al. Upregulation of mir-137 protects anesthesia-induced hippocampal neurodegeneration. Int J Clin Exp Pathol. 2014;7:5000–5007. [PMC free article] [PubMed] [Google Scholar]
- 76.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed rnas. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 77.Garofalo M, Croce CM. Micrornas: Master regulators as potential therapeutics in cancer. Annu Rev Pharmacol Toxicol. 2011;51:25–43. doi: 10.1146/annurev-pharmtox-010510-100517. [DOI] [PubMed] [Google Scholar]
- 78.Zhang L, Dong LY, Li YJ, Hong Z, Wei WS. Mir-21 represses fasl in microglia and protects against microglia-mediated neuronal cell death following hypoxia/ischemia. Glia. 2012;60:1888–1895. doi: 10.1002/glia.22404. [DOI] [PubMed] [Google Scholar]
- 79.Ge XT, Lei P, Wang HC, Zhang AL, Han ZL, et al. Mir-21 improves the neurological outcome after traumatic brain injury in rats. Sci Rep. 2014;4:6718. doi: 10.1038/srep06718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sathyan P, Golden HB, Miranda RC. Competing interactions between micro-rnas determine neural progenitor survival and proliferation after ethanol exposure: Evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. J Neurosci. 2007;27:8546–8557. doi: 10.1523/JNEUROSCI.1269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roush S, Slack FJ. The let-7 family of micrornas. Trends Cell Biol. 2008;18:505–516. doi: 10.1016/j.tcb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 82.Roese-Koerner B, Stappert L, Koch P, Brustle O, Borghese L. Pluripotent stem cell-derived somatic stem cells as tool to study the role of micrornas in early human neural development. Curr Mol Med. 2013;13:707–722. doi: 10.2174/1566524011313050003. [DOI] [PubMed] [Google Scholar]
- 83.Chen Y, Liu W, Chao T, Zhang Y, Yan X, et al. Microrna-21 down-regulates the expression of tumor suppressor pdcd4 in human glioblastoma cell t98g. Cancer Lett. 2008;272:197–205. doi: 10.1016/j.canlet.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 84.Chan JA, Krichevsky AM, Kosik KS. Microrna-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 85.Han L, Yue X, Zhou X, Lan FM, You G, et al. Microrna-21 expression is regulated by beta-catenin/stat3 pathway and promotes glioma cell invasion by direct targeting reck. CNS Neurosci Ther. 2012;18:573–583. doi: 10.1111/j.1755-5949.2012.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. Stat3 activation of mir-21 and mir-181b-1 via pten and cyld are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shen XH, Han YJ, Zhang DX, Cui XS, Kim NH. A link between the interleukin-6/stat3 anti-apoptotic pathway and microrna-21 in preimplantation mouse embryos. Mol Reprod Dev. 2009;76:854–862. doi: 10.1002/mrd.21048. [DOI] [PubMed] [Google Scholar]
- 88.Darnell JE, Jr., Kerr IM, Stark GR. Jak-stat pathways and transcriptional activation in response to ifns and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 89.Levy DE. Physiological significance of stat proteins: Investigations through gene disruption in vivo. Cell Mol Life Sci. 1999;55:1559–1567. doi: 10.1007/s000180050395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Waitkus MS, Chandrasekharan UM, Willard B, Tee TL, Hsieh JK, et al. Signal integration and gene induction by a functionally distinct stat3 phosphoform. Mol Cell Biol. 2014;34:1800–1811. doi: 10.1128/MCB.00034-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhong Z, Wen Z, Darnell JE., Jr. Stat3: A stat family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 92.Buscaglia LE, Li Y. Apoptosis and the target genes of microrna-21. Chin J Cancer. 2011;30:371–380. doi: 10.5732/cjc.011.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brazil DP, Hemmings BA. Ten years of protein kinase b signalling: A hard akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 94.Song G, Ouyang G, Bao S. The activation of akt/pkb signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li XJ, Ren ZJ, Tang JH. Microrna-34a: A potential therapeutic target in human cancer. Cell Death Dis. 2014;5:e1327. doi: 10.1038/cddis.2014.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Agostini M, Tucci P, Steinert JR, Shalom-Feuerstein R, Rouleau M, et al. Microrna-34a regulates neurite outgrowth, spinal morphology, and function. Proc Natl Acad Sci U S A. 2011;108:21099–21104. doi: 10.1073/pnas.1112063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haramati S, Navon I, Issler O, Ezra-Nevo G, Gil S, et al. Microrna as repressors of stress-induced anxiety: The case of amygdalar mir-34. J Neurosci. 2011;31:14191–14203. doi: 10.1523/JNEUROSCI.1673-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bhatnagar S, Chertkow H, Schipper HM, Yuan Z, Shetty V, et al. Increased microrna-34c abundance in alzheimer's disease circulating blood plasma. Front Mol Neurosci. 2014;7:2. doi: 10.3389/fnmol.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang TC, Chang HY, Chen CY, Wu PY, Lee H, et al. Silencing of mir-124 induces neuroblastoma sk-n-sh cell differentiation, cell cycle arrest and apoptosis through promoting ahr. FEBS Lett. 2011;585:3582–3586. doi: 10.1016/j.febslet.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 100.Sanuki R, Onishi A, Koike C, Muramatsu R, Watanabe S, et al. Mir-124a is required for hippocampal axogenesis and retinal cone survival through lhx2 suppression. Nat Neurosci. 2011;14:1125–1134. doi: 10.1038/nn.2897. [DOI] [PubMed] [Google Scholar]
- 101.Miska EA, Alvarez-Saavedra E, Townsend M, Yoshii A, Sestan N, et al. Microarray analysis of microrna expression in the developing mammalian brain. Genome Biol. 2004;5:R68. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gu X, Meng S, Liu S, Jia C, Fang Y, et al. Mir-124 represses rock1 expression to promote neurite elongation through activation of the pi3k/akt signal pathway. J Mol Neurosci. 2014;52:156–165. doi: 10.1007/s12031-013-0190-6. [DOI] [PubMed] [Google Scholar]
- 103.Xu H, Zhang J, Zhou W, Feng Y, Teng S, et al. The role of mir-124 in modulating hippocampal neurotoxicity induced by ketamine anesthesia. Int J Neurosci. 2014:1–34. doi: 10.3109/00207454.2014.919915. [DOI] [PubMed] [Google Scholar]
- 104.Smrt RD, Szulwach KE, Pfeiffer RL, Li X, Guo W, et al. Microrna mir-137 regulates neuronal maturation by targeting ubiquitin ligase mind bomb-1. Stem Cells. 2010;28:1060–1070. doi: 10.1002/stem.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Geekiyanage H, Chan C. Microrna-137/181c regulates serine palmitoyltransferase and in turn amyloid beta, novel targets in sporadic alzheimer's disease. J Neurosci. 2011;31:14820–14830. doi: 10.1523/JNEUROSCI.3883-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dajas-Bailador F, Bonev B, Garcez P, Stanley P, Guillemot F, et al. Microrna-9 regulates axon extension and branching by targeting map1b in mouse cortical neurons. Nat Neurosci. 2012 doi: 10.1038/nn.3082. [DOI] [PubMed] [Google Scholar]
- 107.Krichevsky AM, Sonntag KC, Isacson O, Kosik KS. Specific micrornas modulate embryonic stem cell-derived neurogenesis. Stem Cells. 2006;24:857–864. doi: 10.1634/stemcells.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Twaroski D, Yan Y, Olson JM, Liang M, Bosnjak Z, et al. Analysis of micrornas and their potential targets in human embryonic stem cell-derived neurons treated with the anesthetic propofol. Method to study miRNAs in nervous system: 2015 in press. [Google Scholar]
- 109.Liu B, Gu Y, Xiao H, Lei X, Liang W, et al. Altered metabolomic profiles may be associated with sevoflurane-induced neurotoxicity in neonatal rats. Neurochem Res. 2015 doi: 10.1007/s11064-015-1529-x. In press. [DOI] [PubMed] [Google Scholar]
- 110.Creeley CE, Dikranian KT, Dissen GA, Back SA, Olney JW, et al. Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology. 2014;120:626–638. doi: 10.1097/ALN.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]