Abstract
The plasma membrane aquaglyceroporin Fps1 is responsible for glycerol transport in yeast in response to changes in extracellular osmolarity. Fps1 functions as a homotetramer, and control of its channel activity in response to hyperosmotic shock involves a redundant pair of fungus-specific regulators, Rgc1 and Rgc2 (regulators of the glycerol channel), and the mitogen-activatd protein kinase (MAPK) Hog1 (high-osmolarity glycerol response). Rgc1 and Rgc2 maintain Fps1 in an open-channel state by binding to its C-terminal cytoplasmic domain. Phosphorylation of Rgc1 and Rgc2 by Hog1 induces their eviction from Fps1 and consequent channel closure. In the absence of Fps1 channel function, cells experience chronic cell wall stress, which may be exploited for antifungal drug development. We show here that Rgc1 and Rgc2 form homodimers and heterodimers with each other and that dimer formation of Rgc2 is mediated by its N-terminal domain. Mutations that prevent Rgc2 dimerization block its ability to open Fps1. Therefore, the Rgc-Rgc dimer interface might be an attractive drug target.
INTRODUCTION
Under conditions of high-osmolarity stress, many fungal species, including Saccharomyces cerevisiae, maintain osmotic equilibrium by producing and retaining high concentrations of glycerol as a compatible solute (1). Intracellular glycerol concentration is regulated in S. cerevisiae in part by the Fps1 plasma membrane glycerol channel (2–4). Increased external osmolarity induces Fps1 closure, whereas decreased osmolarity causes channel opening, both within seconds of the change in external osmolarity (4). This channel is required for survival of a hypo-osmotic shock, when yeast cells must export glycerol rapidly to prevent bursting (2, 4). Fps1 is also required for controlling turgor pressure during fusion of mating yeast cells (5).
Fps1 is a member of the major intrinsic protein (MIP) family of channel proteins. The MIP family is subdivided into members that are selectively permeable to water (aquaporins) and those permeated by glycerol and to a lesser extent by water, called aquaglyceroporins, or glycerol facilitators (6, 7). Numerous aquaporins have been shown to function as homotetramers, with each monomer possessing its own channel (8–13). Fps1 similarly functions as a homotetramer (14).
Relative to nonfungal aquaglyceroporins, Fps1 possesses N-terminal and C-terminal cytoplasmic extensions that are important for its regulation (15, 16). Fps1 activity is controlled by a pair of redundant positive regulators, named Rgc1 and Rgc2 (for regulator of the glycerol channel; encoded by YPR115W and YGR097W, respectively) (17), which maintain Fps1 in an open-channel conformation through binding to its C-terminal cytoplasmic domain (18). Aside from centrally located pleckstrin homology (PH) domains, which are critical for their interaction with Fps1, Rgc1 and Rgc2 do not possess any other identifiable domains. The RGC2 gene was suggested previously to play a role in cell wall biogenesis through its identification in a genetic screen for activators of the Skn7 transcriptional regulator and hence named ASK10 (activator of Skn7) (19). Additionally, Ask10 has been reported to bind to RNA polymerase II and its associated C-type cyclin Ssn8 and has been implicated in the oxidative stress-induced destruction of Ssn8 (20). We have not established any connection between the function of Rgc2 in the regulation of Fps1 and its potential roles in transcriptional control (17).
Regulation of Fps1 in response to changes in osmolarity involves the mitogen-activated protein kinase (MAPK) Hog1 (high-osmolarity glycerol response) (4, 21, 22), a homolog of the mammalian p38 MAPK, which binds to the N-terminal cytoplasmic domain of Fps1 (23). Hog1 activated in response to hyperosmotic stress is recruited to a docking site within the Fps1 N-terminal domain, which it uses as a platform from which to phosphorylate Rgc2 (and Rgc1), thereby causing its eviction from the Fps1 C-terminal domain and consequent closure of the channel (18).
Loss of either FPS1 or RGC1 and RGC2 function results in excess turgor pressure and consequent cell wall stress (17). Additional cell wall stress imposed upon these mutants causes cell lysis. Although the fungal kingdom is replete with Rgc orthologs, they are not represented in metazoans, suggesting that the Rgc-Fps pathway may be an attractive target for antifungal drug development. Indeed, loss of the Fps glycerol channels in the fungal pathogen Candida glabrata sensitizes cells to antifungal agents that target the cell wall (24). In this study, we show that that Rgc2 functions as a homodimer and as heterodimers with Rgc1. A dimerization domain was identified within the N-terminal region of Rgc2.
MATERIALS AND METHODS
Strains, growth conditions, and transformations.
The S. cerevisiae strains used in this study were all derived from the Research Genetics background S288c (Research Genetics, Inc., Huntsville, AL) and are listed in Table 1. Yeast cultures were grown in YPD (1% Bacto yeast extract, 2% Bacto peptone, 2% glucose) or SD (0.67% yeast nitrogen base, 2% glucose) supplemented with the appropriate nutrients to select for plasmids. Yeast transformations and dilution spot assays for arsenite sensitivity were conducted as described previously (18).
TABLE 1.
Yeast strains
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| DL3187 | MATa S288c (BY4741) his3Δ leu2Δ ura3Δ lys2Δ | Research Genetics |
| DL3207 | MATa S288c rgc1Δ::KanMX rgc2Δ::KanMX | 17 |
| DL4062 | MATα S288c rgc1::KanMX rgc2::RGC2-S344A T808A S948A S75A S827A S1021A S1035A-Flag-HPH (RGC2-7A) | 18 |
| DL4070 | MATα S288c rgc1Δ::KanMX rgc2::RGC2-Flag-HPH | 18 |
| DL4110 | MATa S288c rgc1Δ::KanMX rgc2::RGC2-Flag-HPH | This study |
| DL4111 | MATa S288c rgc1::KanMX rgc2::RGC2-S344A T808A S948A S75A S827A S1021A S1035A-Flag-HPH (RGC2-7A) | This study |
| DL4112 | MATa/α S288c rgc1Δ::KanMX/rgc1Δ::KanMX rgc2::RGC2-Flag-HPH/rgc2::RGC2-Flag-HPH | This study |
| DL4113 | MATa/α S288c rgc1Δ::KanMX/rgc1Δ::KanMX rgc2::RGC2-Flag-HPH/rgc2::RGC2-S344A T808A S948A S75A S827A S1021A S1035A-Flag-HPH (RGC2-7A) | This study |
| DL4114 | MATa/α S288c rgc1Δ::KanMX/rgc1Δ::KanMX rgc2::RGC2-S344A T808A S948A S75A S827A S1021A S1035A-Flag-HPH (RGC2-7A)/rgc2::RGC2-S344A T808A S948A S75A S827A S1021A S1035A-Flag-HPH (RGC2-7A) | This study |
Plasmid construction.
The plasmids used in this study are listed in Table 2. All forms of Rgc2 were expressed under the control of the MET25 promoter. N-terminal deletions of Rgc2 were generated by double-overlap PCR mutagenesis (25) using as the template pRS316-MET25P-RGC2-3HA (p3151) (18). Plasmid pRS315-MET25P-RGC2-3HA p3391) was generated by subcloning MET25P-RGC2 into pRS315-3HA-ADH1T (p3147) (18) digested with SalI (upstream) and NotI (downstream). To generate RGC2 alleles tagged with the sequence for a Myc epitope, MET25P-RGC2 was prepared from pRS316-MET25P-RGC2-3HA or its deletion variants (p3221 to p3384), by PCR with primers that include a SalI (upstream) and a SmaI (downstream) site. PCR products were cloned into YEp181-MET25P-FPS1-Myc vector (p3121) (14) digested with SalI and SmaI (replacing MET25P-FPS1), to yield YEp181-MET25P-RGC2-Myc (p3392) or its deletion variants (p3385 to p3389).
TABLE 2.
Plasmids
| Plasmid | Description | Source or reference |
|---|---|---|
| p115 | pRS316 | 26 |
| p2501 | pUT36-MET25P-RGC2-HIS6 | 17 |
| p3121 | YEp181-MET25P-FPS1-Myc | 14 |
| p3147 | pRS315-3HA-ADH1T | 18 |
| p3148 | pRS316-3HA-ADH1T | 18 |
| p3151 | pRS316-MET25P-RGC2-3HA | 18 |
| p3221 | pRS316-MET25P-rgc2-Δ480-3HA | This study |
| p3230 | pRS316-MET25P-rgc2-PHD123Δ-3HA | 18 |
| p3301 | pRS316-MET25P-rgc2-Δ360-3HA | 18 |
| p3378 | pRS316-MET25P-rgc2-Δ240-3HA | This study |
| p3379 | pRS316-MET25P-rgc2-Δ120-3HA | This study |
| p3380 | pRS316-MET25P-rgc2-Δ86-3HA | This study |
| p3381 | pRS316-MET25P-rgc2-Δ60-3HA | This study |
| p3382 | pRS316-MET25P-rgc2-Δ28-3HA | This study |
| p3383 | pRS316-MET25P-rgc2-Δ111-7-3HA | This study |
| p3384 | pRS316-MET25P-rgc2-Δ123-9-3HA | This study |
| p3385 | YEp181-MET25P-rgc2-Δ120-Myc | This study |
| p3386 | YEp181-MET25P-rgc2-Δ111-7-Myc | This study |
| p3387 | YEp181-MET25P-rgc2-Δ123-9-Myc | This study |
| p3388 | YEp181-MET25P-rgc2-CΔ-Myc | This study |
| p3389 | YEp181-MET25P-rgc2-PHD123Δ-Myc | This study |
| p3390 | YEp181-MET25P-RGC1-Myc | This study |
| p3391 | pRS315-MET25P-RGC2-3HA | This study |
| p3392 | YEp181-MET25P-RGC2-Myc | This study |
| p3395 | pRS316-MET25P-RGC1-3HA | This study |
RGC1 was tagged so as to add either a Myc or triple-hemagglutinin (3HA) epitope to the C terminus of the protein and expressed under the control of the MET25 promoter. MET25P-RGC1 was prepared by double-overlap PCR in two steps. First, for the Myc-tagged construction, the RGC1 coding sequence was amplified from the genome with primers that included an overlapping region with MET25P (upstream) and a SmaI site (downstream). The MET25P sequence was amplified from YEp181-MET25P-FPS1-Myc (p3121). Next, these fragments were amplified together using primers with a SalI site (upstream of MET25P) and a SmaI site (downstream of RGC1) and cloned into YEp181-MET25P-FPS1-Myc vector (p3121) digested with SalI and SmaI (replacing MET25P-FPS1), to yield YEp181-MET25P-RGC1-Myc (p3390). For the 3HA-tagged construct, MET25P-RGC1 was amplified by PCR from p3390 with primers that include a SalI (upstream) and NotI (downstream) site and cloned into pRS316-3HA-ADH1T (p3148) (18) digested with SalI and NotI to yield pRS316-MET25P-RGC1-3HA (p3395). The DNA sequences of all mutant alleles were confirmed.
Coimmunoprecipitation and immunoblot analysis.
Cultures for coimmunoprecipitation (co-IP) experiments with were grown to mid-log phase in selective medium and starved for methionine for 2 h to induce expression of Rgc2 or Rgc1. For hyperosmotic stress experiments, cultures were shocked by adding 3 M sorbitol to a final concentration of 1.8 M. Protein extraction and coimmunoprecipitations were carried out as described previously (18). Extracts (100 μg of protein) were exposed to mouse monoclonal anti-Myc antibody (1 μg, 9E10; Pierce) or anti-HA antibody (1 μg, 16B12; Covance) for 1 h at 4°C and precipitated with protein A affinity beads for 1 h at 4°C. Samples were washed with IP buffer three times and boiled in SDS-PAGE buffer. Proteins were separated by SDS-PAGE (7.5% gels) followed by immunoblot analysis using anti-Myc antibody (9E10; Pierce), anti-HA (16B12; Covance), or anti-tetra-His antibody (Qiagen) at a dilution of 1:10,000. Secondary antibodies (goat anti-mouse IgG; Amersham) were used at a dilution of 1:10,000.
Cross-linking.
For dimethyl suberimidate (DMS) cross-linking, Rgc2-Myc immunoprecipitates from extracts were treated with various concentrations of DMS in 50 mM HEPES buffer (pH 7.0) for 2 h at room temperature prior to separation of proteins by SDS-PAGE.
RESULTS AND DISCUSSION
In the absence of stress, Rgc1 and Rgc2 are associated with Fps1 and maintain the channel in the open state (18). In response to hyperosmotic shock, Rgc1 and Rgc2 are phosphorylated on multiple sites by the MAP kinase Hog1. These phosphorylations cause the eviction of Rgc1 and Rgc2 from Fps1, thereby allowing closure of the channel. A mutant form of Rgc2 that lacks seven Hog1 phosphorylation sites (RGC2-7A) is stabilized on the C-terminal cytoplasmic domain of Fps1. This gain-of-function RGC2 allele renders Fps1 in a constitutively open state, as judged both by diminished capacity to retain glycerol in response to hyperosmotic shock and by increased sensitivity to arsenite, which enters the cell through Fps1 (18).
Rgc2 and Rgc1 form homodimers and heterodimers.
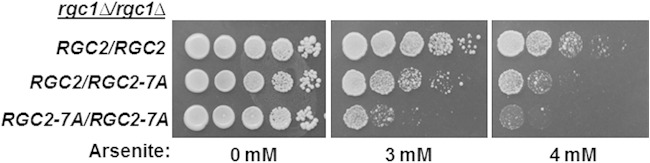
Assessment of the behavior of gain-of-function mutations in the presence of the wild-type allele can offer clues about protein-protein interactions. To determine if the RGC2-7A mutation is dominant to RGC2, we constructed a heterozygous diploid that is also homozygous for the rgc1Δ mutation. The heterozygote displayed intermediate sensitivity to arsenite between the homozygous RGC2 and RGC2-7A strains (Fig. 1). One possible explanation for this behavior is that, because Fps1 forms a homotetramer (14), an Rgc2 molecule may bind to each channel subunit such that channel activity would be impacted by competition for binding between the two Rgc2 forms. However, in this case, it would be expected that wild-type Rgc2 monomers would be supplanted on Fps1 by the mutant Rgc2 form as Hog1 specifically displaces wild-type Rgc2. Alternatively, Rgc2 may self-associate, and mixed Rgc2 multimers (Rgc2 and Rgc2-7A) may respond to phosphorylation by Hog1 in a manner intermediate between those of multimers comprised of just one form or the other. Therefore, we tested by coimmunoprecipitation the ability of Rgc2 to self-associate. Differentially epitope-tagged forms of Rgc2 (Rgc2-3HA and Rgc2-6×His) were coexpressed in yeast and found to coimmunoprecipitate (Fig. 2A). To rule out the possibility that the observed association between Rgc2 molecules is indirect through their association with Fps1, we exposed cells to hyperosmotic shock, a condition shown previously to evict Rgc2 from Fps1 (18). The Rgc2-Rgc2 association was not diminished in response to hyperosmotic shock (Fig. 2B), indicating that the apparent self-association is not mediated indirectly by Rgc2-Fps1 binding. The observed Rgc2 band shift in response to hyperosmotic shock is the consequence of phosphorylation by Hog1 and other protein kinases (17, 18). We next tested Rgc1 for self-association by coexpression of differentially epitope-tagged forms of this protein (Rgc1-Myc and Rgc1-3HA). Like Rgc2, Rgc1 coimmunoprecipitated with itself (Fig. 2C).
FIG 1.
Mixed dominance of the constitutive RGC2-7A mutant. Arsenite sensitivity of the RGC2-7A mutation. The RGC2-7A allele was integrated into the yeast genome at the RGC2 locus of a strain bearing an rgc1Δ mutation. Diploids homozygous for the rgc1Δ mutation were tested for arsenite sensitivity. Equivalent numbers of cells in 10-fold serial dilutions of each strain were spotted onto YEPD plates with or without the indicated concentration of arsenite and incubated for 3 days at 30°C. Yeast strain genotypes were RGC2/RGC2 (DL4112), RGC2/RGC2-7A (DL4113), and RGC2-7A/RGC2-7A (DL4114).
FIG 2.
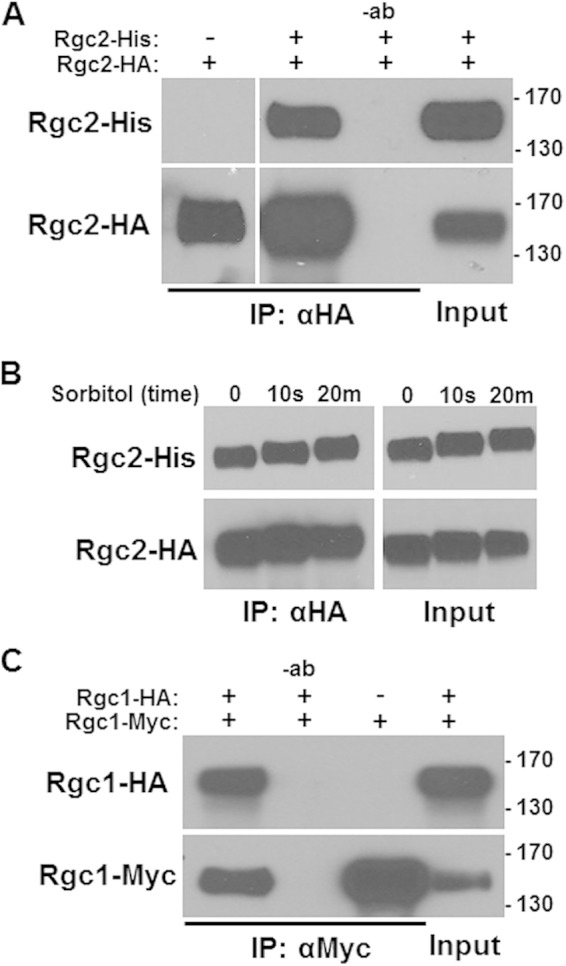
Self-association of Rgc1 and Rgc2. (A) Coimmunoprecipitation of Rgc2-His with Rgc2-HA. Rgc2-His was tested for coimmunoprecipitation (IP) with Rgc2-HA from extracts (input) of wild-type cells (DL3187) coexpressing both forms from plasmids p2501 and p3391, respectively. Immunoprecipitates were separated by SDS-PAGE and subjected to immunoblot analysis. Controls were from cells that did not express one of the tagged proteins, or in the absence of the IP antibody (−ab). Molecular mass markers (in kilodaltons) are shown on the right. (B) Rgc2 self-association is not affected by hyperosmotic shock. Sorbitol was added to a final concentration of 1.8 M to cultures of wild-type cells (DL3187) coexpressing Rgc2-HA and Rgc2-His and incubated for the indicated times prior to co-IP analysis. (C) Coimmunoprecipitation of Rgc1-HA with Rgc1-Myc. Rgc1-HA was tested for co-IP with Rgc1-Myc from extracts (input) of wild-type cells (DL3187) coexpressing both forms from plasmids p3395 and p3390, respectively. Controls are as in panel A.
We next used chemical cross-linking to determine the number of Rgc2 monomers that form a complex with each other. Epitope-tagged Rgc2 (Rgc2-Myc), expressed in an rgc1Δ rgc2Δ strain, was immunoprecipitated and subjected to cross-linking by DMS. Monomers of Rgc2-Myc migrate by SDS-PAGE with an apparent molecular mass of 150 kDa (Fig. 3A). In the presence of increasing concentrations of DMS, a single additional band appeared at approximately 300 kDa, suggesting that Rgc2 forms a homodimer. No additional bands were evident even on longer exposure (data not shown).
FIG 3.
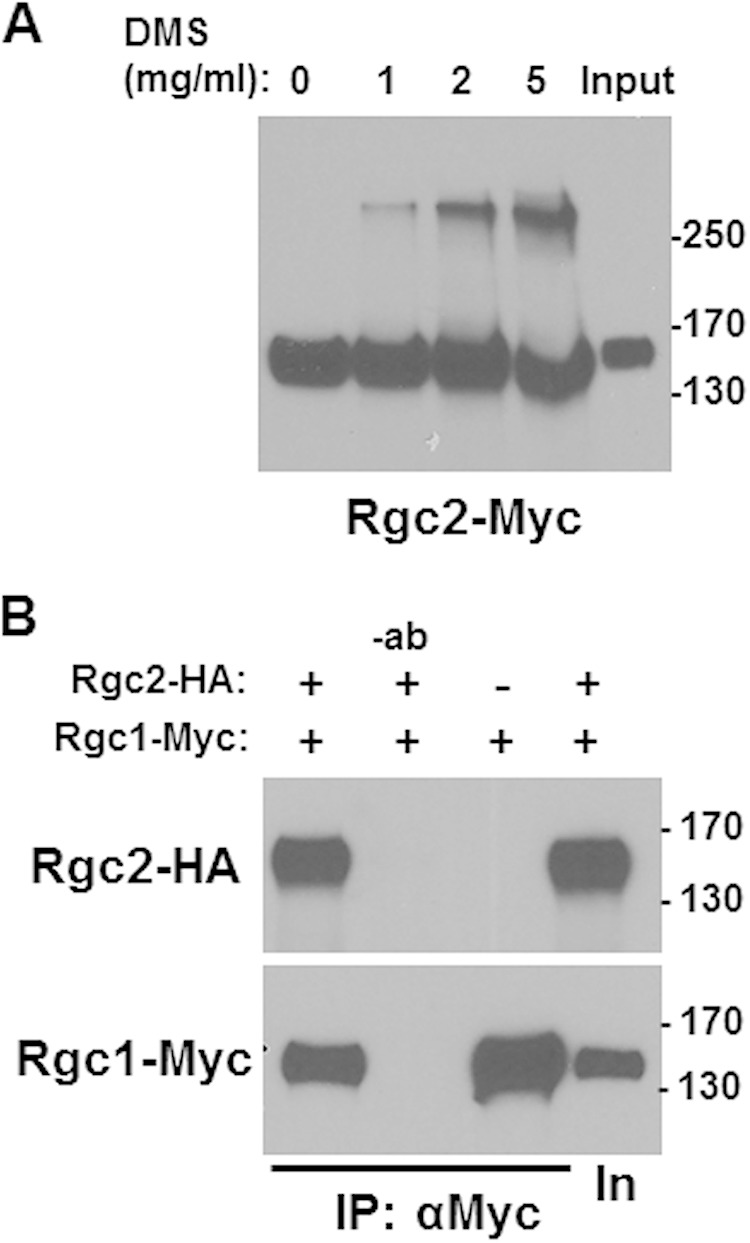
Rgc2 exists as a homodimer in vivo. (A) Rgc2-Myc was immunoprecipitated from an extract of rgc1Δ rgc2Δ cells (DL3207) transformed with YEp181-MET25-RGC2-Myc (p3392), and immunoprecipitates were treated with the indicated concentration of DMS to introduce intersubunit cross-links. Samples were subjected to SDS-PAGE separation and immunoblot analysis. Untreated extract (input) control is in the rightmost lane. (B) Rgc2 associates with Rgc1. Rgc1-Myc was immunoprecipitated from an extract of wild-type cells (DL3187) cotransformed with YEp181-MET25-RGC1-Myc (p3390) and pRS316-MET25-RGC2-HA (p3151) and tested for co-IP with Rgc2-HA. Molecular mass markers (in kilodaltons) are shown on the right. Immunoprecipitates were separated by SDS-PAGE and subjected to immunoblot analysis.
Because Rgc2 evidently forms a homodimer and it is functionally redundant with Rgc1, we next asked if Rgc2 associates with Rgc1. Differentially epitope-tagged Rgc2 (Rgc2-3HA) and Rgc1 (Rgc1-Myc) were coexpressed in yeast and found to coimmunoprecipitate (Fig. 3B), supporting the conclusion that Rgc1 and Rgc2 can form either homodimers or heterodimers in vivo.
The N terminus of Rgc2 possesses a dimerization domain.
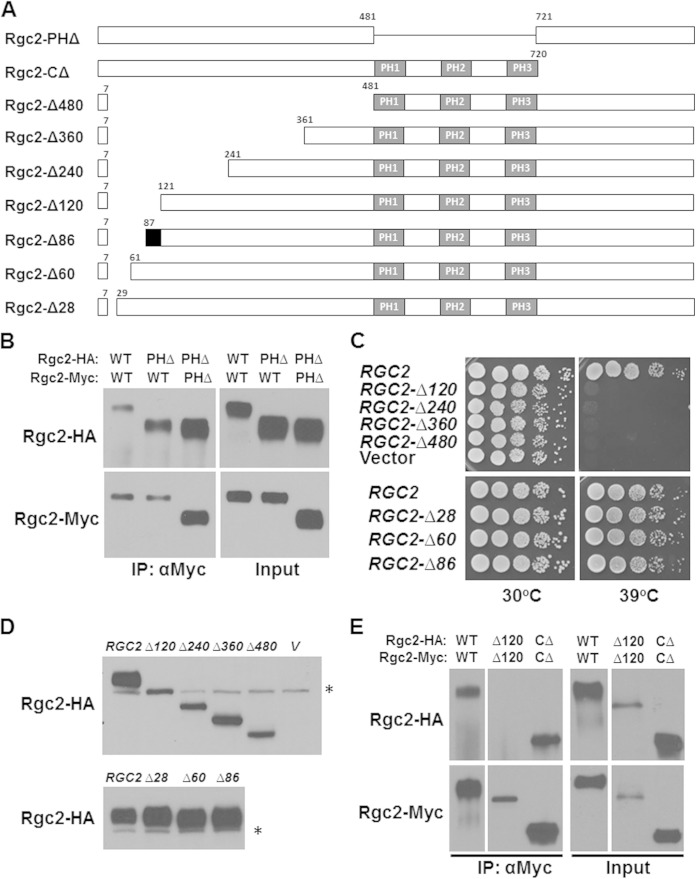
Rgc2 interacts with Fps1 through a tripartite PH domain, which is required for association with Fps1, evidently through interaction with a partial PH domain within the Fps1 C-terminal domain (18). To test the possibility that this domain is required for Rgc2 dimerization, we asked if a form of Rgc2 that lacks the PH domain can self-associate (Fig. 4A). Differentially epitope-tagged forms of Rgc2 were coexpressed in a strain lacking endogenous Rgc1 and Rgc2. Figure 4B shows that Rgc2-PHΔ is capable of association both with wild-type Rgc2 and with another molecule of Rgc2-PHΔ, indicating that the PH domain is not required for Rgc2 dimerization. Additionally, this result supports our earlier conclusion that the importance of the Rgc2 PH domain is its association with a partial PH domain in Fps1 (18).
FIG 4.
The N-terminal domain of Rgc2 is important for self-association and function. (A) Schematic of Rgc2 showing various deletions within its N-terminal domain, a pleckstrin homology (PH) domain deletion and a C-terminal truncation. The region between residues 87 and 120 (black) is essential for Rgc2 self-association. (B) The Rgc2 PH domain is dispensable for self-association. Extracts from rgc1Δ rgc2Δ cells (DL3207) cotransformed with plasmids expressing the HA-tagged wild-type or PH domain deletion form of Rgc2 from pRS316-MET25-RGC2-HA (p3151 or p3230, respectively) and the corresponding Myc-tagged forms from YEp181-MET25-RGC2-Myc (p3392 or p3389, respectively) were tested for co-IP. Immunoprecipitates were separated by SDS-PAGE and subjected to immunoblot analysis. (C) N-terminal deletion mutants of RGC2 expressed from centromeric plasmids were tested for the ability to complement the temperature-sensitive cell lysis defect of an rgc1Δ rgc2Δ mutant. Equivalent numbers of cells in 10-fold serial dilutions of each strain were spotted onto YEPD plates and incubated for 3 days at the indicated temperature. Cell lysis was confirmed by the microscopic appearance of nonrefractile “ghosts.” The vector control was pRS316. (D) Detection of deletion forms of Rgc2-HA. The strains expressing the various deletion forms of Rgc2 were tested by immunoblot for steady-state levels of Rgc2-HA. The asterisks indicate nonspecific bands. (E) The N-terminal region of Rgc2 is essential for self-association. Extracts from rgc1Δ rgc2Δ cells (DL3207) cotransformed with plasmids expressing the indicated form of Rgc2-HA or Rgc2-Myc were tested for co-IP by immunoprecipitation with anti-Myc antibodies. Immunoprecipitates were separated by SDS-PAGE and subjected to immunoblot analysis.
We have shown previously that the C-terminal domain of Rgc2 is dispensable for its function (17). Therefore, we focused our attention on the Rgc2 N-terminal domain, constructing a set of nested N-terminal deletion forms that retain the first seven amino acids (Fig. 4A). A deletion that removed residues 8 to 120 (Rgc2-Δ120) was unable to complement the temperature-sensitive cell lysis defect of an rgc1Δ rgc2Δ mutant (Fig. 4C), despite its being expressed, albeit at a somewhat reduced level than the wild type (Fig. 4D). This phenotype results from a loss of Fps1 channel activity (17, 18). Similarly, more extensive N-terminal deletions were nonfunctional (Fig. 4C). Therefore, we tested Rgc2-Δ120, as well as the functional C-terminal truncation form (Rgc2-CΔ) of Rgc2, for its ability to self-associate. Although the C-terminal truncation form of Rgc2 coimmunoprecipitated with itself, the Rgc2-Δ120 N-terminal deletion form failed to self-associate (Fig. 4E). In contrast to the above result, a deletion mutant of Rgc2 that removed the N-terminal region from residues 8 to 86 was functional (Rgc2-Δ86) (Fig. 4C and D), suggesting that a region of Rgc2 between residues 87 and 120 (but perhaps extending beyond 120) is critical for self-association. These results also suggest that the ability of Rgc2 to self-associate through its N-terminal domain is critical to its function. We showed previously that the Rgc2-Δ360 N-terminal deletion form is able to bind Fps1 (18), but this mutant form was unable to open the channel (Fig. 4C), suggesting that the ability of Rgc2 to bind Fps1 is not sufficient for its action and that it must interact as a dimer. We observed additionally that deletion mutant forms of Rgc2 that were nonfunctional and unable to self-associate were maintained at lower steady-state levels than either wild-type Rgc2 or functional deletion mutants (Fig. 4D, top versus bottom). This may reflect instability of monomeric Rgc2.
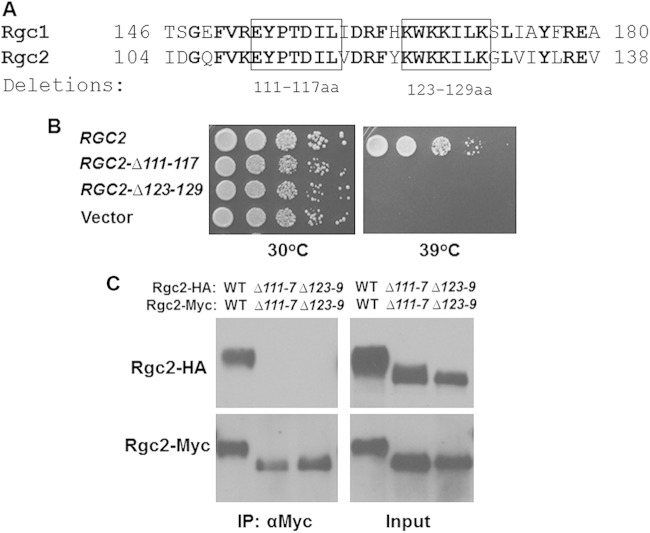
Alignment of Rgc2 with Rgc1 revealed a region of very high conservation between residues 108 and 129 of Rgc2 (Fig. 5A). Therefore, we constructed two short internal deletion mutations in Rgc2 that removed residues 111 to 117 and 123 to 129. We chose these regions because one (residues 123 to 129) is highly basic and the other possesses some acidic residues, suggesting the possibility that Rgc2 self-associates through electrostatic interactions across this region. Both mutant forms failed to complement an rgc1Δ rgc2Δ mutant for Fps1 channel function (Fig. 5B). Therefore, we tested by coimmunoprecipitation the ability of these mutant forms to self-associate. Neither of these deletion mutants of Rgc2 was capable of dimer formation (Fig. 5C), supporting the dual conclusions that this region is critical for Rgc2 self-association and that self-association is critical for Rgc2 function. There is considerable sequence similarity between the Rgc1 and Rgc2 N-terminal domains extending up to the PH domain, and our deletion analysis does not rule out a role for additional sequences in dimerization. However, we have not investigated any role these sequences might have in Rgc2 dimerization.
FIG 5.
A region within the N-terminal domain of Rgc2 is essential for self-association and function. (A) Sequence alignment of highly conserved region within the N-terminal domains of Rgc1 and Rgc2. Boxes indicate the short regions targeted for deletion. (B) N-terminal deletion mutants of RGC2 were tested for the ability to complement the temperature sensitive cell lysis defect of an rgc1Δ rgc2Δ mutant as in Fig. 4. (C) Extracts from rgc1Δ rgc2Δ cells (DL3207) cotransformed with plasmids expressing the indicated form of Rgc2-HA or Rgc2-Myc were tested for co-IP by immunoprecipitation with anti-Myc antibodies. Immunoprecipitates were separated by SDS-PAGE and subjected to immunoblot analysis.
We have presented evidence that Rgc1 and Rgc2 form homodimers and heterodimers with each other. However, we do not know the orientation of Rgc monomers with respect to each other. It is possible that the highly conserved region near the N termini of Rgc1 and Rgc2 allows dimerization in an antiparallel manner, which would leave the PH domain of each monomer free to interact with Fps1. However, the symmetry of arrangement of the Fps1 tetramer suggests an organization mirrored in the binding of Rgc1 and Rgc2. Specifically, the Rgc1 and Rgc2 PH domains interact with the C-terminal cytoplasmic domains of Fps1. Therefore, it seems most likely that the orientation of the PH domains would be uniformly arranged in parallel, rather than antiparallel, dimers of Rgc1 and Rgc2.
Additionally, we do not know the stoichiometric relationship of Rgc1 and -2 subunits with Fps1. It is possible that two Rgc dimers bind to each Fps1 tetramer, providing a single PH domain for interaction with each Fps1 molecule. Alternatively, four dimers may bind to each Fps1 tetramer. Further studies will be required to address these questions. In any case, because we regard Rgc1 and Rgc2 as attractive antifungal targets, and their ability to dimerize is critical to their function, it may be possible to identify small molecules that either interfere with their binding to Fps1 or interfere with their ability to self-associate.
ACKNOWLEDGMENT
This work was supported by a grant from the NIH (GM48533) to D.E.L.
REFERENCES
- 1.Nevoigt E, Stahl U. 1997. Osmoregulation and glycerol metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev 21:231–241. doi: 10.1111/j.1574-6976.1997.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 2.Luyten K, Albertyn J, Skibbe WF, Prior BA, Ramos J, Thevelein JM, Hohmann S. 1995. Fps1, a yeast member of the MIP family of channel proteins, is a facilitator for glycerol uptake and efflux and is inactive under osmotic stress. EMBO J 7:1360–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutherland FC, Lages F, Lucas C, Luyten K, Albertyn J, Hohmann S, Prior BA, Kilian SG. 1997. Characteristics of Fps1-dependent and -independent glycerol transport in Saccharomyces cerevisiae. J Bacteriol 179:7790–7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamás MJ, Luyten K, Sutherland FC, Hernandez A, Albertyn J, Valadi H, Li H, Prior BA, Kilian SG, Ramos J, Gustafsson L, Thevelein JM, Hohmann S. 1999. Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol Microbiol 31:1087–1104. doi: 10.1046/j.1365-2958.1999.01248.x. [DOI] [PubMed] [Google Scholar]
- 5.Philips J, Herskowitz I. 1997. Osmotic balance regulates cell fusion during mating in Saccharomyces cerevisiae. J Cell Biol 138:961–974. doi: 10.1083/jcb.138.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S. 2002. Aquaporin water channels—from atomic structure to clinical medicine. J Physiol 542:3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borgnia MJ, Agre P. 2001. Reconstruction and functional comparison of purified GlpF and AqpZ, the glycerol and water channels from Escherichia coli. Proc Natl Acad Sci U S A 98:2888–2893. doi: 10.1073/pnas.051628098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aerts T, Xia J-Z, Slegers H, de Block J, Clauwaert J. 1990. Hydrodynamic characterization of the major intrinsic protein from the bovine lens fiber membranes. J Biol Chem 265:8675–8680. [PubMed] [Google Scholar]
- 9.Beuron F, Le Cahérec F, Guillam MT, Cavalier A, Garret A, Tassan JP, Delamarche C, Schultz P, Mallouh V, Rolland JP, Hubert J-F, Gouranton J, Thomas D. 1995. Structural analysis of a MIP family protein from the digestive tract of Cicadella virtridis. J Biol Chem 270:17414–17422. doi: 10.1074/jbc.270.29.17414. [DOI] [PubMed] [Google Scholar]
- 10.König N, Zampighi GA, Butler PJG. 1997. Characterization of the major intrinsic protein (MIP) from bovine lens fibre membranes by electron microscopy and hydrodynamics. J Mol Biol 265:590–602. doi: 10.1006/jmbi.1996.0763. [DOI] [PubMed] [Google Scholar]
- 11.Shi L, Skach WR, Verkman AS. 1994. Functional independence of monomeric CHIP28 water channels revealed by expression of wild-type mutant heterodimers. J Biol Chem 269:10417–10422. [PubMed] [Google Scholar]
- 12.Smith B, Agre P. 1991. Erythrocyte Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J Biol Chem 266:6407–6415. [PubMed] [Google Scholar]
- 13.Verbavatz JM, Brown D, Sabolić I, Valenti G, Ausiello DA, Van Hoek AN, Ma T, Verkman AS. 1993. Tetrameric assembly of CHIP28 water channels in liposomes and cell membranes: a freeze-fracture study. J Cell Biol 123:605–618. doi: 10.1083/jcb.123.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beese-Sims SE, Lee J, Levin DE. 2011. Yeast Fps1 glycerol facilitator functions as a homotetramer. Yeast 28:815–819. doi: 10.1002/yea.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedfalk K, Bill RM, Mullins JG, Karlgren S, Filipsson C, Bergstrom J, Tamás MJ, Rydström J, Hohmann S. 2004. A regulatory domain in the C-terminal extension of the yeast glycerol channel Fps1p. J Biol Chem 279:14954–14960. doi: 10.1074/jbc.M313126200. [DOI] [PubMed] [Google Scholar]
- 16.Tamás MJ, Karlgren S, Bill RM, Hedfalk K, Allegri L, Ferreira M, Thevelein JM, Rydström J, Mullins JG, Hohmann S. 2003. A short regulatory domain restricts glycerol transport through yeast Fps1p. J Biol Chem 278:6337–6345. doi: 10.1074/jbc.M209792200. [DOI] [PubMed] [Google Scholar]
- 17.Beese SE, Negishi T, Levin DE. 2009. Identification of positive regulators of the yeast fps1 glycerol channel. PLoS Genet 5:e1000738. doi: 10.1371/journal.pgen.1000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Reiter W, Dohnal I, Gregori C, Beese-Sims S, Kuchler K, Ammerer G, Levin DE. 2013. MAPK Hog1 closes the S. cerevisiae glycerol channel Fps1 by phosphorylating and displacing its positive regulators. Genes Dev 27:2590–2601. doi: 10.1101/gad.229310.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page N, Sheraton J, Brown JL, Stewart RC, Bussey H. 1996. Identification of ASKl0 as a multicopy activator of Skn7p-dependent transcription of a HIS3 reporter gene. Yeast 12:267–272. doi:. [DOI] [PubMed] [Google Scholar]
- 20.Cohen TJ, Lee K, Rutkowski LH, Strich R. 2003. Ask10p mediates the oxidative stress-induced destruction of the Saccharomyces cerevisiae C-type cyclin Ume3p/Srb11p. Eukaryot Cell 2:962–970. doi: 10.1128/EC.2.5.962-970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmadpour D, Geijer C, Tamas MJ, Lindkvist-Petersson K, Hohmann S. 2014. Yeast reveals unexpected roles and regulatory features of aquaporins and aquaglyceroporins. Biochim Biophys Acta 1840:1482–1491. doi: 10.1016/j.bbagen.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 22.Hohmann S. 2009. Control of high osmolarity signaling in the yeast Saccharomyces cerevisiae. FEBS Lett 583:4025–4029. doi: 10.1016/j.febslet.2009.10.069. [DOI] [PubMed] [Google Scholar]
- 23.Mollapour M, Piper PW. 2007. Hog1 mitogen-activated protein kinase phosphorylation targets the yeast Fps1 aquaglyceroporin for endocytosis, thereby rendering cells resistant to acetic acid. Mol Cell Biol 27:6446–6456. doi: 10.1128/MCB.02205-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beese-Sims SE, Pan S-J, Lee J, Hwang-Wong E, Cormack BP, Levin DE. 2012. Mutants in the Candida glabrata glycerol channels are sensitized to cell wall stress. Eukaryot Cell 11:1512–1519. doi: 10.1128/EC.00231-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho S-N, Hunt HD, Horton RM, Pullen JK, Pease LR. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 26.Sikorski RS, Hieter P. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]