Abstract
Liver fibrosis is a major disease that is primarily caused by hepatitis virus infections, toxins, and alcohol abuse. Diagnosing and staging liver fibrosis are critical in guiding the treatment of chronic liver diseases, according to several international and Chinese guidelines. Liver biopsy is the gold standard for diagnosing and staging liver fibrosis, but it is invasive and suffers from several limitations. Consequently, much research has focused on the search for a noninvasive serum biomarker of fibrosis. In this study, we determined that Chitinase 3-like 1 (CHI3L1) is an abundantly expressed liver gene whose expression is highly enriched in the liver. We then compared serum levels of CHI3L1 among patients with various stages of liver fibrosis, as determined by liver biopsies, and found that the CHI3L1 levels were able to differentiate early stages of liver fibrosis (S0–S2) from late stages of liver fibrosis (S3–S4). We further showed that CHI3L1 is a good marker of substantial fibrosis, with areas under the ROC curves (AUCs) of 0.94 for substantial (S2, S3, S4) fibrosis and 0.96 for advanced (S3, S4) fibrosis. Finally, we showed that CHI3L1 is superior to hyaluronic acid (HA), type III procollagen (PCIII), laminin (LN), and type IV collagen (CIV), which are also serum biomarkers of liver fibrosis, in identifying advanced liver fibrosis in patients with HBV-related liver fibrosis in China.
Introduction
Liver fibrosis is a wound-healing response of liver cells to chronic injuries caused by viral infections, toxins, alcohol abuse, and other causes. Liver fibrosis is accompanied by a constant process of destruction and repair of the hepatic parenchyma that is caused by inflammation, and it often results in serious complications, including portal hypertension and liver failure. It can also give rise to hepatocellular carcinoma (HCC). Liver fibrosis can lead to cirrhosis, which is defined as the end stage of liver fibrosis (Pellicoro et al., 2014).
In China, hepatitis B is the major cause of injuries leading to liver fibrosis and cirrhosis (Liao et al., 2013; Xu et al., 2003). Cirrhosis is a important factor in the development of HCC because the cumulative 5-year risk of developing HCC in patients with cirrhosis ranges from 5% to 30%, depending on several factors, including the presence and stage of underlying liver disease, ethnicity, age, gender, and the duration of exposure to primary hepatotropic viruses. Therefore, staging liver fibrosis before cirrhosis develops could allow early-stage liver fibrosis to be detected soon enough for potentially curative treatments to be administered.
According to several international and Chinese guidelines for the treatment of chronic liver diseases, including hepatitis B virus (HBV) infection, accurate determination of fibrosis stages is critical for optimizing the timing of antiviral treatment (Asia-Pacific Consensus on Hepatitis B and C, 2000; National Institutes of Health, 2002). Elevated alanine transaminase (ALT) levels equal to or greater than the upper limit of normal (ULN) have been used as a major factor in deciding to initiate antiviral therapy (Chao et al., 2014). Approximately one-fifth of patients with ALT levels less than the ULN have substantial liver fibrosis; these patients would be missed using ALT alone as the determining factor for initiating treatment (Chao et al., 2014). Therefore, additional staging markers are needed.
Liver biopsy is the gold standard for detecting and staging liver fibrosis (Papatheodoridis and Manolakopoulos, 2009). However, liver biopsy is a complicated procedure that includes sampling and staining tissue and having the resulting slides read by a pathologist. Liver biopsies may also cause complications, including post-procedure pain or bleeding, sampling error (as only 1/50,000th of the liver is sampled), and inter- and intra-pathologist variability (Afdhal, 2004). Over the past decades, many noninvasive techniques have been developed with the aim of either replacing liver biopsies or conducting pre-screening for liver biopsies.
These techniques rely on either of two distinct but complementary approaches: a non-biomarker-based approach, which relies on the measurement of liver stiffness using elastography-based technologies, such as the widely proposed FibroScan method; or a serum marker-based approach, which relies on the quantification of biomarkers of fibrosis in serum.
The FibroScan method, which uses transient elastography, reliably detects cirrhosis in most HBV and HCV patients; however, it cannot be used in approximately 20% of HBV and HCV patients, particularly those with ascites and obesity, and its performance varies with operator experience (Degos et al., 2010). For serum biomarkers, the most common test platforms are the FibroTest (Biopredictive, Houilles, France) and the ActiTest (Biopredictive), both of which use a combination of levels of alpha-2-macroglobulin, alpha-2 globulin (or haptoglobin), gamma globulin, apolipoprotein A1, gamma-glutamyl transpeptidase (GGT), or total bilirubin, and age and sex information to generate their results. Together, these tests are marketed as the HCV-FibroSure Test (LabCorp, Burlington, NC); this test is the most widely used test for the assessment of fibrosis.
However, this test does not stage liver fibrosis well. Rossi et al. (2003) investigated FibroTest scores of 125 patients with hepatitis C and found that 57 of these patients had FibroTest scores either less than 0.1 (indicating no fibrosis) or greater than 0.6 (indicating substantial fibrosis). They found that 6 (18%) of 33 patients who had FibroTest scores less than 0.1 and were therefore deemed unlikely to have fibrosis in fact had substantial fibrosis. Conversely, five (21%) of the 24 patients with scores greater than 0.6 who were thus predicted to be likely to have substantial fibrosis instead had mild fibrosis. The investigators found large discrepancies between the test results and the biopsy results in approximately 19% of the patients. The discordance between the FibroTest and liver biopsy results was similarly reported to be 28.7% (154 of 537 patients) by Poynard et al. (2012). Therefore, serum markers that can be used to stage fibrosis with greater accuracy are needed.
Chitinase 3-like 1 (CHI3L1, also known as YKL-40) is a member of the chitinase family but lacks chitinase activity; it encodes a glycoprotein that is a member of the 18-glycosyl hydrolase family (Libreros et al., 2013). The function of this glycoprotein is unclear, but it has been hypothesized that CHI3L1 plays a role in both inflammation and tissue remodeling (Libreros et al., 2013). Immunohistochemical analysis demonstrated positive staining for CHI3L1 antigens in areas with fibrosis, particularly areas with active fibrogenesis. Several studies have established that CHI3L1 is a biomarker for alcoholic cirrhosis (Johansen et al., 1997) and HCV-induced liver fibrosis (Johansen et al., 2000; Tran et al., 2000; Nojgaard et al., 2003). However, to our knowledge, the performance of CHI3L1 in staging or diagnosing HBV-related liver fibrosis has not been systematically analyzed. Our laboratory seeks to identify novel biomarkers of liver fibrosis in the Chinese population. Therefore, we sought to determine whether CHI3L1 is a good biomarker for staging or diagnosing liver fibrosis in HBV-related chronic liver disease in the Chinese population.
Materials and Methods
Patients
Ninety-eight consecutive treatment-naive chronic hepatitis B (CHB) patients who had undergone percutaneous liver biopsies were prospectively enrolled in this study in the Department of Infectious Diseases of the Zhejiang Provincial People's Hospital from June 2012 through December 2013. The inclusion criteria for the study were age greater than 20 years, positive HBsAg for more than 6 months, HBV DNA levels ≥103 copies/mL, and ALT levels ≤2 ULN (ULN=50 U/L); ALT and HBV DNA levels were monitored monthly for 6 months prior to enrollment to ensure that ALT levels ≤2 ULN and HBV DNA levels ≥103 copies/mL were maintained. Exclusion criteria for the study included co-infection with human immunodeficiency virus (HIV) or hepatitis C virus (HCV), compensated or decompensated liver cirrhosis, alcoholic liver diseases, non-alcoholic fatty liver disease (NAFLD), autoimmune liver diseases, chronic liver diseases due to other causes, renal insufficiency, inadequate biopsy samples, and incomplete clinical data. In addition, 146 serum samples from stage S3 and S4 hepatic fibrosis patients were collected at three other major hospitals in Hangzhou, China, including the First Affiliated Hospital of College of Medicine, the Second Affiliated Hospital of College of Medicine of Zhejiang University, and Sir Run Run Shaw Hospital. Informed consent was obtained from each patient, and the study protocol complied with the ethical guidelines of the 1975 Declaration of Helsinki. The study was approved by the institutional review board of each hospital.
Enzyme-linked immunosorbent assay (ELISA)
CHI3L1 ELISA kits (Hangzhou Proprium Biotech Co. Ltd, Hangzhou, Zhejiang, China) were used to quantify the serum CHI3L1 levels.
Liver biopsies and the staging of fibrosis
The staging of fibrosis was confirmed by liver biopsies. Percutaneous liver biopsies were conducted using an 18G biopsy needle guided by ultrasound. The specimens were then fixed, paraffin-embedded, and stained with hematoxylin and eosin (HE). For the diagnosis of fibrosis, 1.5–2.5 cm of liver tissue containing at least six portal tracts was used in analyses. Liver fibrosis stages (S0–S4) were determined using Scheuer's classification system by a single pathologist who was blinded to the patients' clinical data.
Statistical analysis
All statistical analyses were performed using MedCalc software (Version 13.0.0.0). Differences between groups were tested using the Mann-Whitney U-test (for continuous variables and for nonparametric analyses for independent samples). Comparative ROC analyses were conducted using a nonparametric approach previously described by Delong et al. (1988).
Results
CHI3L1 is an abundantly expressed liver gene whose expression is highly enriched in the liver
Under normal physiological conditions, CHI3L1 expression is low or absent in many tissues (Johansen, 2006). For example, CHI3L1 expression is absent in normal human monocytes but is strongly induced during the late stages of human macrophage differentiation (Krause et al., 1996). However, a systematic analysis of CHI3L1 expression in multiple tissues was not conducted before the arrival of high-throughput technologies. In 2008, Dezso et al. performed a microarray analysis of 32 human tissues and found that the highest levels of expression of CHI3L1 were observed in the liver, out of all of the 32 tissues that were tested (data not shown). However, because the dynamic range of microarrays is limited, we did not initially appreciate that CHI3L1 is, in fact, highly expressed in liver tissue.
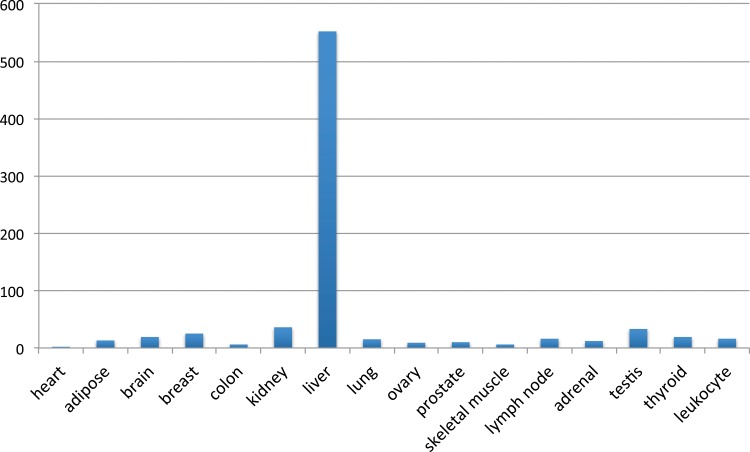
RNA sequencing (RNA-seq), which is capable of detecting expression levels over a much greater dynamic range than is possible using older technologies, such as cDNA arrays, allows CHI3L1 expression levels to be determined over a large dynamic range in many normal human tissues. The data from the Illumina Human Body Map 2.0 (http://genomicdbdemo.bxgenomics.com/) show that CHI3L1 is expressed at a level of 552 FPKM (fragments per kb of exon per million fragments mapped) in the liver, whereas it is expressed at very low levels (median 15, with a maximum of 36 in the kidney) in all of the other 15 tissues for which data are available, including all of the major organs: the heart, brain, breast, colon, kidney, lung, muscle, lymph node, and thyroid, and leukocytes (Fig. 1).
FIG. 1.
CHI3L1 expression levels among 16 normal human tissues, as determined using the Illumina Human Body Map 2.0 (http://genomicdbdemo.bxgenomics.com/). Y-axis: FPKM (fragments per kb of exon per million fragments mapped) values. Each column represents a different tissue.
The level of expression of CHI3L1 in the liver is 15.3-fold (compared with that in the kidney) to 276-fold higher (compared with that in the heart) than its level of expression in other tissues. These data suggest that CHI3L1 is a liver-specific or a highly liver-enriched gene and that it is also abundantly expressed. At 552 FPKM, the level of expression of CHI3L1 is even higher than that of PSA (KLK3), at 349 FPKM in the Illumina Human Body Map 2.0 database, and PSA is a prostate-specific gene and a marker of prostate cancer. The establishment of CHI3L1 as an abundantly expressed gene whose expression is enriched in the liver is important because this corrects the misconception that CHI3L1 is expressed at similar levels in many tissues and thus should alleviate concerns that it might not be a good marker of liver disease (Johansen, 2006).
CHI3L1 is able to differentiate early stages of liver fibrosis (S0-S2) from late stages of liver fibrosis (S3-S4)
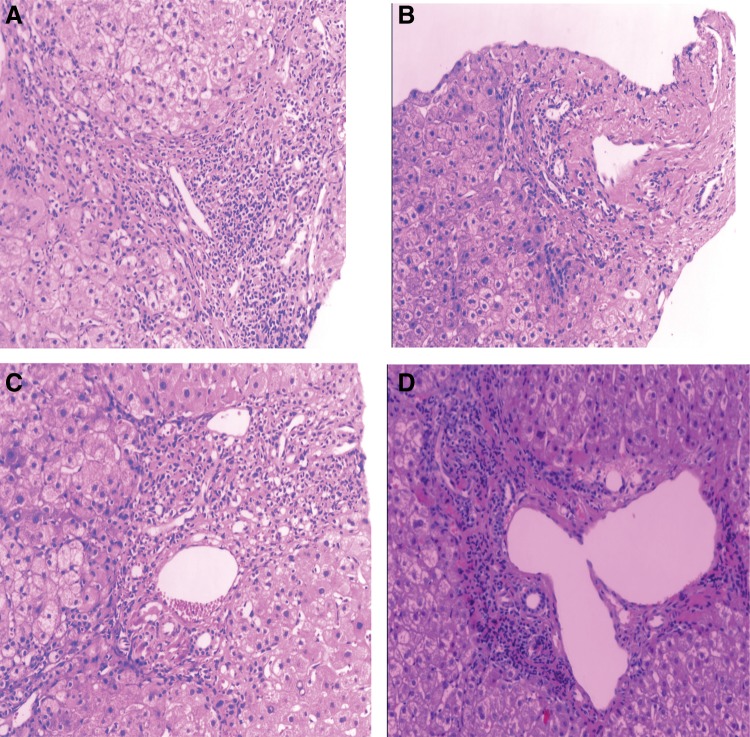
To investigate whether CHI3L1 was able to differentiate early stages of liver fibrosis from late stages of liver fibrosis, we compared serum levels of CHI3L1 and stages of liver fibrosis determined from liver biopsies from 39 patients with stage S0 liver fibrosis, 36 patients with stage S1 liver fibrosis, 16 patients with stage S2 liver fibrosis, and 153 patients with stage S3 or S4 liver fibrosis. Representative images of staining of liver biopsy tissue for different stages of fibrosis are shown in Figure 2. All of the raw data used in this analysis are shown in Supplementary Table S1 (supplementary material is available online at www.liebertpub.com/omi). We calculated the median levels of CHI3L1 in serum samples from patients with different pathological stages of fibrosis (Table 1). We found little difference in the expression level of CHI3L1 between patients with no fibrosis (S0) and those with the earliest stage of fibrosis (S1); therefore, we grouped patients with early-stage fibrosis (S0–S1) together. The median expression level of CHI3L1 was 46.51 ng/mL, and the mean expression level of CHI3L1 was 64.79 ng/mL in the S0–S1 group of patients.
FIG. 2.
Pathology staining of liver biopsies at different stages of fibrosis. Representative images of liver fibrosis at stages S0, S1, S2, and S4, clockwise from the top left.
Table 1.
Median Levels of CHI3L1 Expression in Patients with Different Stages of Liver Fibrosis
| Stage | N | Median | 95% CI |
|---|---|---|---|
| S0 | 39 | 46.150 | 38.692–55.790 |
| S1 | 36 | 47.050 | 35.963–55.396 |
| S2 | 16 | 69.475 | 57.165–125.007 |
| S3–S4 | 153 | 188.800 | 169.408–228.196 |
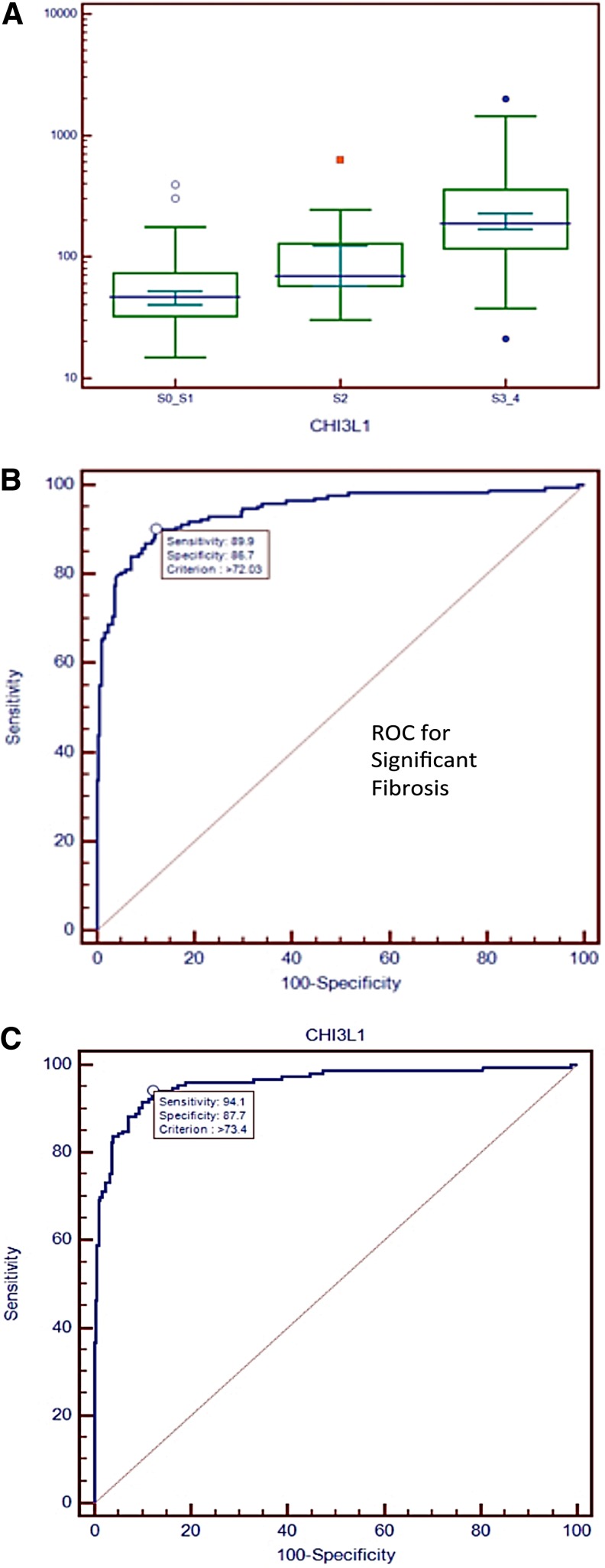
In patients with stage S2 fibrosis, the median and mean CHI3L1 levels increased to 69.48 ng/mL and 130.04 ng/mL, respectively. In patients with stage S3–S4, the median and mean CHI3L1 levels further increased to 188.88 ng/mL and 277.46 ng/mL, respectively. A box-and-whisker plot for the three groups of patients with different stages of liver fibrosis is shown in Figure 3A. We found that the difference in CHI3L1 levels between the group of patients with stage S0-S1 fibrosis and the group of patients with stage S2 fibrosis patients is highly statistically significant (p=0.0015, Mann-Whitney U-test, two-tailed). We also found a highly statistically significant difference (p=0.0002, the Mann-Whitney U-test, two-tailed) between the group of patients with stage S2 liver fibrosis and those with stage S3–S4 liver fibrosis. Thus, we found that serum CHI3L1 levels could differentiate between early-stage (S0–S1), middle-stage (S2), and late-stage (S3–4) liver fibrosis in patients with HBV-related liver fibrosis in China.
FIG. 3.
Analysis of CHI3L1 as a staging and diagnostic marker for liver fibrosis. (A) Box-and-whisker plots of CHI3L1 in different groups of patients with various stages of fibrosis. (B) ROC curve analysis for substantial fibrosis (S2, S3, S4). (C) ROC curve analysis for advanced (S3, S4) fibrosis.
CHI3L1 is a diagnostic marker of substantial or advanced liver fibrosis
Determining whether substantial fibrosis, defined as fibrosis at stages greater than or equal to S2 (i.e., stage S2, S3, or S4 fibrosis), in chronic HBV patients is critical for guiding the prognosis and treatment of patients with hepatitis B (Asia-Pacific Consensus on Hepatitis B and C, 2000; National Institutes of Health, 2002). Encouraged by our finding that CHI3L1 is a good marker for staging fibrosis, we sought to determine whether CHI3L1 is a good marker for identifying substantial fibrosis. ROC curve analysis produced areas under ROC curves (AUCs) of 0.94 and 0.96 for substantial (S2, S3, S4) fibrosis and advanced (S3, S4) fibrosis, respectively (Fig. 3B–C). CHI3L1 levels differentiated between substantial and advanced fibrosis with a sensitivity of 94.1% and a specificity of 87.7% when a criterion of CHI3L1 level>73.4 ng/mL was used to diagnose advanced fibrosis. We next recruited patients from Sir Run Run Shaw Hospital (Hangzhou) as a validation set for testing predictions made using serum CHI3L1 levels. We recruited 168 normal individuals and 85 advanced (S3, S4) fibrosis patients (Supplementary Table S2). The area under the ROC curve (AUC) for advanced fibrosis for the validation set is 0.96. The sensitivity and specificity were 91.8% and 91.7%, respectively, when a cutoff value of 78.48 ng/mL was used. When using a cutoff value of 73.4 ng/mL as determined previously, the sensitivity was 91.76% for the validation set, and the specificity was 87.06%.
Comparison of CHI3L1 and several commonly used serum markers for diagnosing advanced liver fibrosis
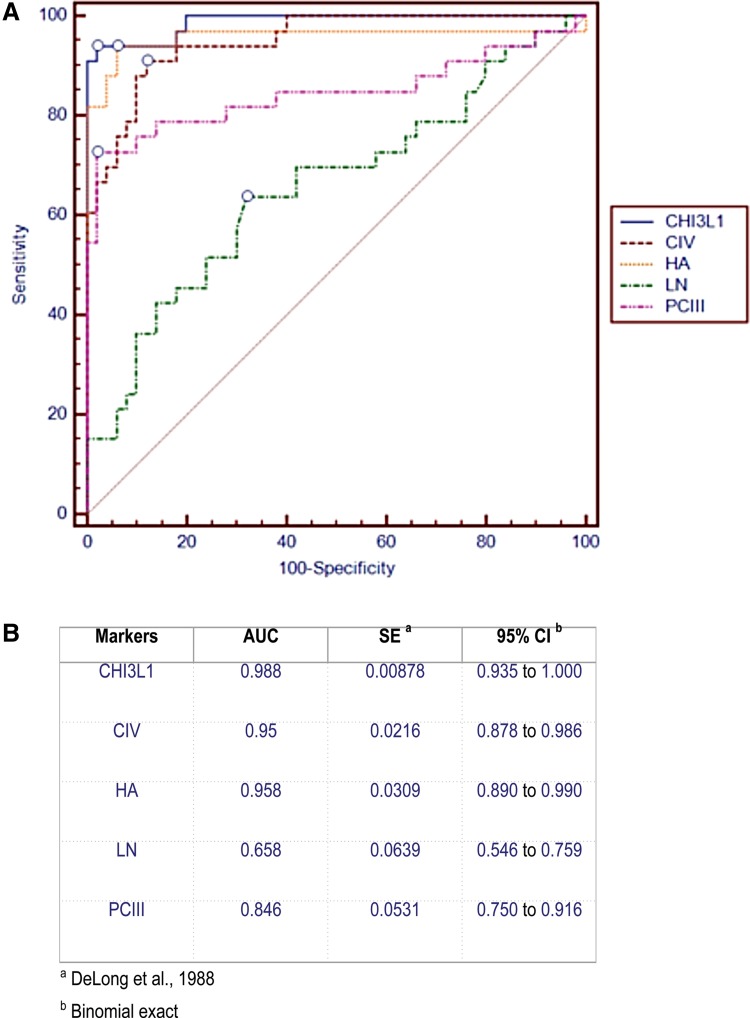
Traditionally, the serum markers hyaluronic acid (HA), type III procollagen (PCIII), laminin (LN), and type IV collagen (CIV) have been used to diagnose liver fibrosis or cirrhosis (Rossi et al., 2007). We compared the performance of CHI3L1 to the performance of these serum fibrosis markers for the detection of advanced liver fibrosis. We measured the levels of CHI3L1 side-by-side with the levels of these older four markers—HA, PCIII, LN, and CIV—in 36 patients with advanced-stage liver fibrosis and 50 healthy individuals. All data are presented in Supplementary Table S3. We conduced a comparative ROC analysis for these 5 markers individually for diagnosing advanced liver fibrosis (Fig. 4A). CHI3L1 performed the best among the five markers, with an AUC of 0.99 (Fig. 4B).
FIG. 4.
Comparison of CHI3L1 with four other serum markers for the detection of liver fibrosis. (A) Comparative ROC analysis of CHI3L1 and four other serum markers, hyaluronic acid (HA), type III procollagen (PCIII), laminin (LN), and type IV collagen (CIV), for the detection of advanced liver fibrosis. (B) AUC (area under the curve) values for the five serum markers.
Discussion
The correct staging of liver fibrosis is critical for guiding the treatment of chronic hepatitis. The gold standard for staging liver fibrosis, the liver biopsy, is an invasive procedure and has many limitations (Motola et al., 2014). First, only approximately 1/50,000 the volume of the liver is sampled in a liver biopsy; therefore, a biopsy is unable to reflect fibrotic changes occurring throughout the entire liver and hence does not detect cirrhosis in 10%–30% of patients (Motola et al., 2014). Additional disadvantages include disagreements between pathologists and a risk of complications that range from mild abdominal pain to severe hemorrhage and injury to the biliary system (Motola et al., 2014). Therefore, many investigators are pursuing the development of noninvasive procedures or tests for staging liver fibrosis or diagnosing substantial liver fibrosis.
In this study, we showed that CHI3L1 is a marker that is able to differentiate early-stage fibrosis from late-stage fibrosis (Fig. 3A) in HBV-related liver fibrosis patients in China. Such determinations are critical for guiding the clinical treatment of chronic HBV carriers (Asia-Pacific Consensus on Hepatitis B and C, 2000; National Institutes of Health, 2002). Adams et al. (2005) sought to create an algorithm that accurately and reliably predicts liver fibrosis stages among hepatitis C patients based on the levels of several serum markers and developed a model (HepaScore) based on bilirubin levels, gamma-glutamyl transferase levels, hyaluronic acid (HA) levels, alpha-2-macroglobulin levels, age, and gender that produced areas under the ROC curves (AUCs) of 0.85, 0.96, and 0.94 for substantial (S2, S3, S4) fibrosis, advanced (S3, S4) fibrosis, and cirrhosis (S4), respectively.
We further showed that CHI3L1 is capable of identifying substantial liver fibrosis (≥S2) or advanced liver fibrosis (>S3; Fig. 3B, C). We showed that CHI3L1 identifies advanced liver fibrosis in patients with HBV-related liver fibrosis in China better than hyaluronic acid (HA), type III procollagen (PCIII), laminin (LN), and type IV collagen (CIV), all of which are other serum markers of liver fibrosis (Fig. 4A, B). Our observations in Chinese patients with HBV-related liver fibrosis are similar to previous observations in HCV-related liver fibrosis. Rath et al. (2011) tested the abilities of many biomarkers, including CHI3L1 (YKL-40), hyaluronic acid (HA), laminin, C-terminal procollagen I peptide, MMP-9, TIMP-1, TIMP-2, and a complex of MMP-9 and TIMP-1, to detect HCV-related liver fibrosis and found that CHI3L1 performed the best among the biomarkers tested.
Conclusions
We have shown that CHI3L1 is a liver-enriched gene that may aid in the staging of liver fibrosis and in the diagnosis of advanced liver fibrosis in chronic HBV patients in China.
Supplementary Material
Acknowledgments
This work was supported by grant 2012AA022705 (B.L.) from the Ministry of Science and Technology of China.
Author Disclosure Statement
This work was supported by a grant from Hangzhou Proprium Biotech Co. Ltd.
References
- Adams LA, Bulsara M, Rossi E, et al. (2005). Hepascore: An accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem 51, 1867–1873 [DOI] [PubMed] [Google Scholar]
- Afdhal NH. (2004). Biopsy or biomarkers: Is there a gold standard for diagnosis of liver fibrosis? Clin Chem 50, 1299–1300 [DOI] [PubMed] [Google Scholar]
- Asia-Pacific Consensus on Hepatitis B and C; Core Working party. (2000). Consensus statements on the prevention and management of hepatitis B and hepatitis C in the Asia-Pacific region. J Gastroenterol Hepatol 15, 825–841 [DOI] [PubMed] [Google Scholar]
- Chao DT, Lim JK, Ayoub WS, Nguyen LH, and Nguyen MH. (2014). Systematic review with meta-analysis: The proportion of chronic hepatitis B patients with normal alanine transaminase </=40 IU/L and significant hepatic fibrosis. Aliment Pharmacol Ther 39, 349–358 [DOI] [PubMed] [Google Scholar]
- Degos F, Perez P, Roche B, et al. (2010). Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: A multicenter prospective study (the FIBROSTIC study). J Hepatol 53, 1013–1021 [DOI] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, and Clarke-Pearson DL. (1988). Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 44, 837–845 [PubMed] [Google Scholar]
- Dezso Z, Nikolsky Y, Sviridov E, et al. (2008). A comprehensive functional analysis of tissue specificity of human gene expression. BMC Biol 6, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JS, Moller S, Price PA, et al. (1997). Plasma YKL-40: A new potential marker of fibrosis in patients with alcoholic cirrhosis? Scand J Gastroenterol 32, 582–590 [DOI] [PubMed] [Google Scholar]
- Johansen JS, Christoffersen P, Moller S, et al. (2000). Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol 32, 911–920 [DOI] [PubMed] [Google Scholar]
- Johansen JS. (2006). Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull 53, 172–209 [PubMed] [Google Scholar]
- Krause SW, Rehli M, Kreutz M, Schwarzfischer L, Paulauskis JD, and Andreesen R. (1996). Differential screening identifies genetic markers of monocyte to macrophage maturation. J Leukoc Biol 60, 540–545 [DOI] [PubMed] [Google Scholar]
- Liao B, Wang Z, Lin S, et al. (2013). Significant fibrosis is not rare in Chinese chronic hepatitis B patients with persistent normal ALT. PLoS One 8, e78672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libreros S, Garcia-Areas R, and Iragavarapu-Charyulu V. (2013). CHI3L1 plays a role in cancer through enhanced production of pro-inflammatory/pro-tumorigenic and angiogenic factors. Immunol Res 57, 99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motola DL, Caravan P, Chung RT, and Fuchs BC. (2014). Noninvasive biomarkers of liver fibrosis: Clinical applications and future directions. Curr Pathobiol Rep 2, 245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health Consensus Development Conference. (2002). Statement: Management of hepatitis C 2002. Gastroenterology 123, 2082–209912454863 [Google Scholar]
- Nojgaard C, Johansen JS, Christensen E, Skovgaard LT, Price PA, and Becker U. (2003). Serum levels of YKL-40 and PIIINP as prognostic markers in patients with alcoholic liver disease. J Hepatol 39, 179–186 [DOI] [PubMed] [Google Scholar]
- Papatheodoridis GV, and Manolakopoulos S. (2009). EASL clinical practice guidelines on the management of chronic hepatitis B: The need for liver biopsy. J Hepatol 51, 226–227 [DOI] [PubMed] [Google Scholar]
- Pellicoro A, Ramachandran P, Iredale JP, and Fallowfield JA. (2014). Liver fibrosis and repair: Immune regulation of wound healing in a solid organ. Nat Rev Immunol 14, 181–194 [DOI] [PubMed] [Google Scholar]
- Poynard T, Munteanu M, Deckmyn O, et al. (2012). Validation of liver fibrosis biomarker (FibroTest) for assessing liver fibrosis progression: Proof of concept and first application in a large population. J Hepatol 57, 541–548 [DOI] [PubMed] [Google Scholar]
- Rath T, Roderfeld M, Guler C, et al. (2011). YKL-40 and transient elastography, a powerful team to assess hepatic fibrosis. Scand J Gastroenterol 46, 1369–1380 [DOI] [PubMed] [Google Scholar]
- Rossi E, Adams L, Prins A, et al. (2003). Validation of the FibroTest biochemical markers score in assessing liver fibrosis in hepatitis C patients. Clin Chem 49, 450–454 [DOI] [PubMed] [Google Scholar]
- Rossi E, Adams LA, Bulsara M, and Jeffrey GP. (2007). Assessing liver fibrosis with serum marker models. Clin Biochem Rev 28, 3–10 [PMC free article] [PubMed] [Google Scholar]
- Tran A, Benzaken S, Saint-Paul MC, et al. (2000). Chondrex (YKL-40), a potential new serum fibrosis marker in patients with alcoholic liver disease. Eur J Gastroenterol Hepatol 12, 989–993 [DOI] [PubMed] [Google Scholar]
- Xu J, Wang QX, Jiang D, et al. (2003). [Relationship between the genotypes of hepatitis B virus and the severity of liver diseases]. Zhonghua Gan Zang Bing Za Zhi 11, 11–13 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.