Abstract
Significance: This article discusses the history and developments of silicone gel sheeting (SGS) scar therapy. Furthermore, we review a breadth of literature to gain an insight into how and why topical silicone gels remain the favored treatment of medical experts in scar management. We also analyze an ever increasing number of alternative therapies claiming to provide enhanced scar reduction performance.
Recent Advances: Topical silicone gel treatments seem to remain the first point of clinical recommendation in scar management. SGS has been used in scar therapy for over 30 years, during which its efficacy has been the subject of numerous clinical evaluations.
Critical Issues: While the exact mechanisms by which SGS improves hypertrophic scars, keloid development and recovery are yet to be fully agreed upon, its ability to do so remains largely undisputed at present. However, there still is ongoing deliberation over the exact mechanism of action of silicone in improving a scar. At present it is likely that through occlusion of the scar site and hydration of the wound bed, the overactivity of scar-related cells is suppressed, and their activity normalized.
Future Direction: The clinical support of topical silicone gel products, relative to all alternative scar therapies, is considered the internationally recommended first-line form of scar management, and favored by consensus among healthcare professionals. However, there still remains the need for further clinical evidence and a better understanding of the mechanism behind the benefit of silicone gel for use in the prevention of abnormal scarring.

Benjamin Bleasdale, PhD
Scope and Significance
Silicone gel sheeting (SGS) has been used in scar therapy for over 30 years, during which its efficacy has been the subject of numerous clinical evaluations. While the exact mechanisms by which SGS improves hypertrophic scars, keloid development and recovery are yet to be fully agreed upon, its ability to do so remains largely undisputed. As such, topical silicone gel treatments seem to remain the first point of clinical recommendation in scar management. This article aims to review a breadth of literature to gain an insight not only into how, but also why topical silicone gels remain the favored treatment of medical experts in scar management. We also analyze an ever increasing number of alternative therapies claiming to provide enhanced scar reduction performance as a comparison.
Translational Relevance
Silicone has been used for more than 30 years in the treatment of cutaneous scars.1 New formulations and formats of silicone gel products are becoming available for use in the treatment of scarring resulting from surgery, burns, and other skin injuries requiring hospital treatment. Current gel sheet products have been designed to be worn for up to 24 h, washed and reused, however, this approach can be inconvenient and may put some patients at risk of skin infection.2 Single-use silicone gel formats aim to be more hygienic and convenient while remaining cost effective. Reduced cost and improved formulation also improves access to the home healthcare market providing consumers with products that can be used in the home. First aid products using silicone gel could improve the outcome of scarring following accidents in the home as well as providing protection to newly healed wounds. Treatment of acne scars with silicone gel products has also demonstrated positive outcomes and so consumer products for this application may provide additional benefits.3
Clinical Relevance
The repair of injured skin tissue, whether as a result of an acute injury or from a more chronic disease process can often result in the formation of a permanent scar.4 The formation of scar tissue is generally characterized by thick levels of collagen. Large areas of scar tissue and scars on more visible areas of the body are undesirable and can impact on an individual's self esteem and, therefore, quality of life. Some scars, particularly those resulting from deep wounds such as burns, can restrict a patient physically, due to the large amount of skin contracture. These contractures will increase the skins tension and also decrease its mobility. Treatments that prevent and reduce the formation and appearance of scars have continued to gain interest with plastic surgeons and patients alike. The increasing trend for cosmetic surgery has been a key driver to identify new and improved solutions for scar reduction.5 Current treatments include noninvasive treatments such as pressure therapy and application of silicone sheets6 and invasive treatments such as steroid injections and surgical revision (Table 1).
Table 1.
Treatment of scars
| Treatment | Description | Advantages | Disadvantages |
|---|---|---|---|
| Pressure therapy | Application of elastic bandages or pressure garments to apply pressure to scar sites. | Noninvasive. Can be applied at home by the patient. | Often cause discomfort to the wearer which affects compliance. To achieve optimum results from pressure therapy require 6–12 months constant wear. |
| Silicone gel therapy | Application of silicone gel sheets or gel formulated in a tube. | Noninvasive. Can be applied at home by the patient. | To achieve optimum results from silicone gel therapy require 6–12 months constant wear. |
| Steroid injections | Injection of corticosteroids directly into the scar tissue which inhibits fibrosis and reduces the number of contractile myofibroblasts. | Can inhibit the formation of hypertrophic scarring. | Requires multiple injections over a period of time to be administered by a clinician. |
| Dermal fillers | Fillers such as collagen can be injected at the scar site. | Can be used to improve the contours of pitted scars. | Must be carried out by a cosmetic/healthcare professional. Results are temporary. |
| Dermabrasion | Controlled abrasion or planing of upper to mid skin layers. This technique has largely been replaced with advanced methods such as laser resurfacing. | Can smooth raised scars and reduce shallow/mid-depth acne scars. | Invasive procedure which usually requires an anesthetic. The resultant wounds also carry the risk of further scar formation. |
| Microdermabrasion | A cosmetic procedure involving the exfoliation of the skin epidermis. | Noninvasive, nonsurgical and usually pain-free technique. | Usually only effective on shallow scars such as those caused by acne. |
| Laser resurfacing | The use of intense pulsed light typically with an erbium or CO2 laser. | Can reduce the elevation of scars and also soften scar tissue. | Must be carried out by a cosmetic/healthcare professional. Depending on the laser used, results can be short or long term. |
| Skin grafts (punch grafts) | Small skin grafts are taken from unscarred skin and used to cover a scar. | Most commonly used for deeper acne scarring. | An invasive technique which requires additional wounds to be created to harvest skin tissue. |
| Surgical revision | Can be used to improve the appearance of prominent, irregular-shaped scars. | An invasive surgical technique that requires often deep wounds to be created and which will form a further scar. | |
| Ablative fractional resurfacing | A series of patients who experienced rapid and sustained healing of long-standing erosions and ulcers associated with traumatic scars and split-thickness skin grafts after initiating a course of AFR. | Advantages include the novel concept of photomicrodebridement, including biofilm disruption and the stimulation of de novo growth factor secretion and collagen remodeling. |
AFR, ablative fractional resurfacing.
Background
Unlike fetal wounds, which can heal through a process of complete regeneration,7 skin injury is resolved typically through a process of repair. This repair process initially involves hemostasis and the restoration of the skins protective function through reepithelialization and also provides immunological protection against contaminants such as bacteria through an inflammatory response.8 As the inflammatory phase resolves, the body initiates mechanisms to complete the repair process beneath the healed skin epithelium. Dermal cell proliferation and migration from the skin tissue adjacent to the wound occurs to replace the fibrin clot formed during hemostasis with new granulation tissue. Dermal fibroblasts in the dermis adjacent to the wound are activated and are referred to as myofibroblasts. These cells migrate into the granulation tissue where they produce collagen and cause the contraction of the replacement dermis.4 Wound contraction brings wound edges together and fibroblast remodeling replace granulation tissue with a mature collagen matrix to fortify the repaired tissue site. It is this remodeling phase that involves the formation and maturation of the visible scar tissue and can last a year or more.
Discussion
Types of scars
Scars form following acute injury, such as a surgical incision or from a burn and also can arise from a chronic disease state such as acne.9 The process of skin repair and subsequent scar formation is impacted by a number of factors, including the size and position of the wound on the body, whether the initial injury is incisional or excisional, and also a patient's age, ethnicity, and skin type which can predispose individuals to more severe scars.10 Scars can vary in appearance from flat, hypopigmented to red, raised, uneven areas of tissue and can also feel painful, itchy, or hypersensitive.11
Keloid scars are formed when the scar tissue extends beyond the margin of the original wound and is the most extreme type of scarring. When this scar type occurs, a small wound such as an insect bite or piercing can result in the formation of a much larger elevated area of tissue which even after surgical revision can reform. Keloid scars are not to be confused with hypertrophic scars which are raised and often discolored, but do not extend beyond the boundary of the initial wound. Hypertrophic scarring typically develops in wounds at locations on the body which are under tension, such as shoulders, ankles, knees, and the neck.
Skin injury which extends deep in to the dermal and subdermal tissue such as burns, abrasions, and highly invasive surgical procedures can lead to the formation of highly contracted scar tissue which over time can lead to physical restrictions. This has a pronounced effect when the injury occurs in children as restriction caused by contracted scar tissue worsens as the child grows and can require repeated surgical intervention to resolve.
Pitted scarring is frequently associated with the occurrence of acne and results in the formation of depressions and pits on the skin surface. These often occur on the face, which can affect an individual's psychological wellbeing long after the resolution of the acne.
Treatment/prevention of scars
A list of present methods used to treat scars can be found in Table 1.
Role of silicone in scar reduction
The epidermis may take around 2 weeks to sufficiently regenerate after a full-thickness wound has occurred due to it penetrating through the epidermis and into the dermis layers. During this time, intervention with scar development will have little to no effect as high collagen levels are required at this early wound healing stage.12
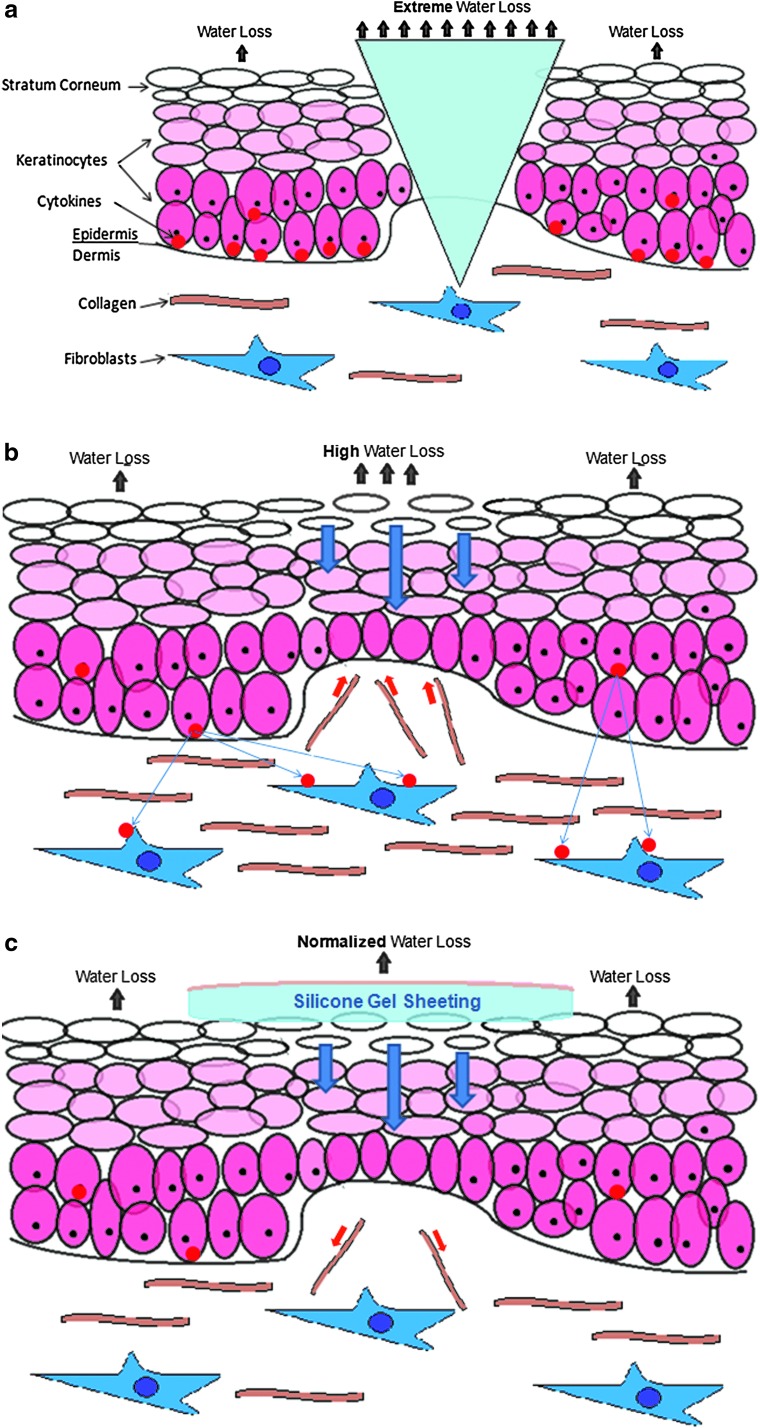
After this period, the new immature stratum corneum allows abnormally high levels of transepidermal water loss (Fig. 1a–c). This dehydration of the stratum corneum signals the keratinoctyes to produce cytokines, which signals to fibroblasts to synthesize and release collagen. The newly formed collagen rushes to the scar site and is the cause of many undesirable physical and aesthetic properties associated with scarring. The application of SGS replicates the stratum corneum's occlusion properties, normalizing hydration of the scar site to that of healthy skin, perhaps inhibiting the instruction sent to the fibroblasts to produce excess collagen cells.13
Figure 1.
Water loss under extreme, high, and normalized conditions. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound.
Mechanisms of action of SGS
Although the mechanism of action of silicone-based products in scar management have not been completely determined, there are many mechanisms by which clinical studies have shown them to significantly impact the improvement of both hypertrophic and keloid scars.
The logical mechanism of scar therapy treatments is to counteract the phylogenetic process by which scars are formed. It is hypothesized that wound healing has been optimized for speed as opposed to quality of healing under far less sanitary conditions to prevent infection.12 This speed optimization is now less of a necessity, with higher demand for aesthetically pleasing and fully-functioning skin becoming an ever increasing focus in the medical and consumer industries. The application of SGS addresses the physiological processes associated with this speed optimization by way of numerous reported mechanisms.
SGS's ability to provide improved occlusion and hydration to the wound bed has been cited as its key physical mode of action in numerous studies. The physiological impact of improved occlusion and hydration on a developing scar is to provide the newly formed, underdeveloped stratum corneum aid in retaining optimum water levels. If the stratum corneum is dehydrated, it will signal to the keratinocytes in the epidermal skin layer to produce cytokines, which in turn signal fibroblasts to produce excessive amounts of collagen to aid water retention of the stratum corneum. It is by this process that the undesirable attributes of a hypertrophic scar are developed, rather than that of normal skin. Whereas it was once considered that higher occlusion is better, newer studies have shown that too strong a moisture barrier can have a detrimental effect,13 SGS is known to simulate the homeostasis of the stratum corneum.14,15 SGS, unlike other dressings, provide a level of occlusion similar to normal skin, which is thought to explain why cytokine and fibroblast activity and collagen formation are significantly reduced in SGS-treated scars16–18 and stratum corneum hydration is normalized.19 It is worth noting that the gentle removal of SGS compared with alternative adhesive sheeting also minimizes skin stripping of the newly formed stratum corneum, further contributing to its treatment potential.20,21
Another physical mode of action provided by SGS is transferring tension from the lateral edges of the wound bed to the silicone gel sheet. The gentle reduction in tension which can be provided by an adhesive SGS provides the ideal environment for normal scar development and can significantly reduce the rate of abnormal and keloid scarring.22 Other treatments have the potential to apply too much or misplaced tension reduction, resulting in a poorer performance.23
SGS has also been found to inhibit the body's natural reaction to increase skin capillaries through hyperemia.24 This reduces the blood supply to the scar site and the exaggeration of the healing process, along with the intensity of the fully formed scar's appearance and physical properties. The mechanism by which SGS has this effect has not yet been eluded, but research has prompted the idea that a significant temperature increase of the scar site is involved with the alteration of localized blood flow.25,26
A final proposed mechanism of action is that SGS generates a negatively charged static electric field through the creation of friction between itself and the skin. This static electricity is thought to aid the alignment of collagen cells, thus resulting in the involution of raised scars.25,27,28
The findings surrounding therapeutic mechanisms support the hypothesis that there are multiple combined processes by which SGS create homeostasis of the skin's barrier function beyond straightforward hydration and occlusion of the scar site. Many suggested factors are regarded as having a significant influence in the overall efficacy of SGS, although some lack thorough clinical support. However, through decades of clinical research and practical evidence, silicone-based products are widely regarded as an effective scar therapy solution.
Advantages of SGS
The international clinical recommendations on Scar Management (written for the International Advisory Panel on Scar Management) recommend SGS as the first-line therapy with intralesional corticosteroids being the secondary recommendation based on a systematic review of the clinical literature currently available.29
SGS has remained the favored clinical scar therapy since its efficacy in the management of hypertrophic and keloid scars became more widely recognized throughout the 1980s. Since these early studies,1,30,31 the subject of scar therapy has advanced dramatically along with clinical evidence, commercial engagement, and consumer interest.32–36 This progression has been driven by the ongoing clinical support which continues to raise interest and awareness of the efficacy of SGS in scar management in comparison to the alternatives.32,37,38
In fact, it is proposed that topical SGS and intralesional steroids are the only evidence-based recommendable forms of treatment to control the quality of a scar.39 SGS not only prevails in terms of performance but in terms of its ease of use; its noninvasive application and relatively low cost give it a distinct practical advantage over the competition.40 There are three general areas by which scar improvement is measured, size reduction, appearance, and calming effect; SGS is considered to be at the forefront of therapies addressing each of these requirements.
Although there is no universal scar scoring system, a measurement commonly used to indicate scar improvement is the scar elevation index (SEI), which is essentially a measure of the height of the scar tissue in relation to the normal surrounding skin. The epidermal thickness index can be used as a supporting indication of how well the wounded skin has regenerated. Many studies have proven the effectiveness of SGS in minimizing the SEI, returning it closer to normal skin in both hypertrophic scars13,14,21,41,42 and keloids.43 Another physical measure is pliability of the scar tissue, also found to be improved.34,44,45
Alternative measures often relate to aesthetic appearance rather than physical/practical improvements. Such measures tend to focus on color amelioration through altered pigmentation. Scarred skin often appears darker or paler than the surrounding areas mainly due to a buildup of new tissue with an excess or deficiency of melanin, respectively. Here too, SGS is backed by numerous clinical studies showing benefits in improving scar coloration for both hypertrophic scars34,46 and keloids.31 Another such aesthetic appearance factor particularly associated with keloid scarring is the texture of the scar tissue also found to be improved by SGS application.31 Other typically used measures are those relating to pain, discomfort, irritation, and itching associated with developing scar tissue. These side effects of scar development are thought to relate to several possible cellular mechanisms during the wound healing processes and can be prevalent to widely varying degrees at each stage—inflammation, proliferation, and remodeling—and as such can last months or even years, becoming a source of long-term discomfort and a problem in need of addressing. Hypertrophic and keloid scar-related physical discomfort ranging from itching to pain have been seen to improve dramatically upon SGS treatment.34,38,43,47,48
Silicones possess many skin-friendly properties—biocompatibility, atraumatic removal, extended wear time, repositionable, resistant to microbial growth, and hydrophobicity.49 The rapid uptake of silicone gel adhesive into the healthcare industry has driven it to be the favored adhesive in several healthcare sectors, particularly those applications with direct skin adhesion—such as advanced wound care, scar therapy, device fixation, surgical draping, ostomy, drug delivery, and consumer healthcare.49
Two main forms of silicone adhesive technologies exist, namely pressure sensitive adhesive (PSA) silicones and soft skin adhesive (SSA) silicone. Although both provide ideal solutions to many healthcare applications, the latter displays distinct advantageous properties in scar therapy which sets it apart from other less suited adhesives. As described previously, maintaining the stratum corneum plays a vital role in the improved development of scar tissue, which is a factor addressed by SSA on two levels. Not only do SSA silicones have the advantage of atraumatic skin removal, no skin stripping, and no painful skin or hair pulling; but another advantage also lies in the fact that SSAs, unlike alternatives, have a low viscous component that limits their flow and consequently their readiness to absorb materials at the surface of the skin such as stratum corneum cells and lipids. The adhesive surface of SSAs remains relatively clean and can be removed, reused and cleaned repeatedly without diminishing its integrity.
Although they have a gel-like consistency and are commonly referred to as a silicone gel, the absence of reinforcing silica filler means SSAs are in fact cross-linked polydimethylsiloxanes with low amounts of free extractable molecules. Despite low consistency and some compressibility, SSAs show resilience and quick recovery under cyclic deformation. The PSA property of SSA is mainly based on the capacity of the surface to quickly wet the skin and conform to its relief with only minimal flow. This minimized flow is accounted for by the low level of viscous component and results in only a small dissipation of the energy occurring when deformation pressure is applied, ultimately resulting in an immediate debonding from the skin at low peel or shear force.
This adhesive platform has been extensively used in scar treatment for more than 30 years, demonstrating safety and efficacy recognized by wound care professionals. Tweaks in formulation variants have been made throughout this time period, but the mechanics by which it takes effect have remained essentially the same.
Limitations of SGS
Although outside of the remit of this article, it is important to note that despite the weight of research in support of SGS usage in scar therapy, some studies have highlighted limitations of its use under certain conditions. The main limitations are related to patient compliance; topical SGS can be cumbersome to keep on the scar, with some patients showing an aversion to wearing SGS in visible areas.29 Whereas another study draws attention to certain SGS products leading to possible skin irritation in hot climates.50 A limitation of SGS's practical application is with burn cases, in which the affected area may be very large. In these situations SGS can prove impractical.
Summary
Results from clinical trials, now brought together through numerous reviews have strongly suggested that SGS are an effective preventative and reduction therapy for excessive scarring.46 However, a recent article by O'Brien et al. 2013 indicated in their review that at present there still remains weak clinical evidence of the benefits of SGS for use in the prevention of abnormal scarring in high-risk individuals.46 However, they also concluded that the clinical trials, which have been used to evaluate silicone gel for the treatment of hypertrophic and keloid scarring, demonstrate that there are improvements in scar thickness and scar color. Although there is still some deliberation over the exact mechanism of action, it is likely that through occlusion of the scar site and hydration of the wound bed, the overactivity of scar-related cells is suppressed, and their activity normalized. Although this is seen as the main mechanism of action, several others are seen to be offered by the unique properties of silicone-based products, a potential explanation for the lack of efficacy offered by alternative occlusive treatments. The clinical support of topical silicone gel products relative to all alternative scar therapies is what has preserved its position as the internationally recommended first-line form of scar management, favored by consensus among healthcare professionals. Although steroid injections offer significant positive impact on scarring, they remain a supporting treatment, in part, due to the associated expense and impracticality. Other existing treatments such as topical creams/gels and dressings containing active additives such as onion extract, vitamins C and E, and moisturizing agents are often seen to deliver little to no extra benefit than SGS itself.
Take-Home Messages.
• Topical silicone gel treatments seem to remain the first-line therapy for clinical recommendation in scar management.
• The exact mechanisms by which SGS improves hypertrophic scar and keloid development and recovery are yet to be fully eluded and agreed upon.
• There still remains the need for good clinical evidence to demonstrate the benefit of silicone gel for use in the prevention of abnormal scarring.
Abbreviations and Acronyms
- AFR
ablative fractional resurfacing
- PSA
pressure sensitive adhesive
- SEI
scar elevation index
- SGS
silicone gel sheeting
- SSA
soft skin adhesive
Acknowledgments and Funding Sources
No funding sources were obtained for this review article.
Author Disclosure and Ghostwriting
B.B. is employed by Scapa Group plc., by whom the content of this article was expressly written. No ghostwriters were used to write this article.
About the Authors
Benjamin Bleasdale has held multiple positions at Scapa Healthcare; starting in Business Development before shifting to his current position in a cross-departmental Product Management function, working closely with marketing, R&D, and commercial groups. Leading research projects into various areas of adhesive use in healthcare, he has developed a broad specialized knowledge of the therapeutic use of adhesives in skin-contact applications. Ben holds a degree in Business Management. Sean Kelly has 14 years of experience in medical device R&D, project management, manufacturing, quality and regulations. He was Global Development Manager at Scapa Healthcare, part of the Scapa Group Plc. Before Scapa, Sean was employed as a Medical Device Regulator at the Notified Body (BSi) working with leading wound care and other medical device manufacturers. Before BSi, Sean worked at Advanced Medical Solutions, Bristol Myers Squibb, and ConvaTec Ltd. Sean is a qualified Project Manager with a degree in Chemistry. Simon Finnegan obtained his MCHEM in Biological Chemistry in 2010 from the University of Sheffield, United Kingdom. Since then, Simon has undertaken a DTC-TERM PhD position at the University of Sheffield, United Kingdom, designing novel silicones. Kathryn Murray is presently doing a PhD in the Department of Chemistry at the University of Sheffield, United Kingdom. Before this, she held numerous scientific positions at Broughton Laboratories Ltd. and WOUNDCHEK Laboratories (a spin-out company from Systagenix Wound Management Ltd.). Professor Steven L. Percival holds a PhD in Microbiology and Biofilms, a BSc in Applied Biological Sciences, Postgraduate Certificate in Education, Diploma in Business Administration, and MSc's in Public Health and Medical Microbiology and Molecular Microbiology. Early in his career, Steven held commercial operations and R&D positions in Phenomenex Ltd. and the Department of Biotechnology, British Textile Technology Group (BTTG), followed then by 6 years as a Senior University Lecturer in Medical Microbiology and Head of the Biofilm Research Group. Later he held positions of Global Director of R&D, Chief Scientific Officer (CSO) at Aseptica, Inc., and senior clinical fellowships at the Centers for Disease Control, Atlanta and Leeds Teaching Hospitals Trust. More recently, Steven held senior R&D positions at Bristol Myers Squibb, ConvaTec, and Advanced Medical Solutions Plc. In 2011, Steven joined Scapa Healthcare Plc as Vice President of Global Healthcare R&D and in 2013 was awarded the position of Honorary Professor in the Institute of Ageing and Chronic Disease and the Surface Science Research Centre at the University of Liverpool, United Kingdom. He is a globally recognized key opinion leader in biofilms, wound microbiology, infection control, and antimicrobials and has chaired many meetings and conferences globally on these subject areas. He has written over 340 scientific publications and conference abstracts and has authored or edited seven textbooks. Steven has also provided over 100 presentations globally at conferences and hospitals.
References
- 1.Perkins K, Davey RB, Wallis KA. Silicone gel: a new treatment for burn scars and contractures. Burns 1983;3:201–204 [DOI] [PubMed] [Google Scholar]
- 2.McCarty M. An evaluation of evidence regarding application of silicone gel sheeting for the management of hypertrophic scars and keloids. J Clin Aesthet Dermatol 2010;3:39. [PMC free article] [PubMed] [Google Scholar]
- 3.Jemec GB, Jemec B. Acne: treatment of scars. Clin Dermatol 2004;22:434–438 [DOI] [PubMed] [Google Scholar]
- 4.Weedon D, Strutton G. Skin Pathology, 2nd ed. New York, NY: Churchill Livingstone, 2002 [Google Scholar]
- 5.Uebelhoer NS, Ross EV, Shumaker PR. Ablative fractional resurfacing for the treatment of traumatic scars and contractures. Semin Cutan Med Surg 2012;31:110–120 [DOI] [PubMed] [Google Scholar]
- 6.Meaume S, Le Pillouer-Prost A, Richert B, Roseeuw D, Vadoud J. Management of scars: updated practical guidelines and use of silicones. Eur J Dermatol 2001;1:1–1 [DOI] [PubMed] [Google Scholar]
- 7.Metcalfe AD, Ferguson MW. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface 2007;4:413–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucalo B, Eaglstein WH, Falanga V. Inhibition of cell proliferation by chronic wound fluid. Wound Repair Regen 1993;1:181–186 [DOI] [PubMed] [Google Scholar]
- 9.Garg S, Baveja S. Combination therapy in the management of atrophic acne scars. J Cutan Aesthet Surg 2014;7:18–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res 2012;49:35–43 [DOI] [PubMed] [Google Scholar]
- 11.Bae SH, Bae YC. Analysis of frequency of use of different scar assessment scales based on the scar condition and treatment method. Arch Plast Surg 2014;41:111–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayat A, McGrouther DA, Ferguson MWJ. Skin scarring. Br Med J 2003;326:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mustoe TA. Evolution of silicone therapy and mechanism of action in scar management. Aesthetic Plast Surg 2008;32:82–92 [DOI] [PubMed] [Google Scholar]
- 14.Tandara AA, Mustoe TA. The role of the epidermis in the control of scarring: evidence for mechanism of action for silicone gel. J Plast Reconstr Aesthet Surg 2008;61:1219–1225 [DOI] [PubMed] [Google Scholar]
- 15.Kloeters O, Tandara A, Mustoe TA. Hypertrophic scar model in the rabbit ear: a reproducible model for studying scar tissue behavior with new observations on silicone gel sheeting for scar reduction. Wound Repair Regen 2007;15:S40–S45 [DOI] [PubMed] [Google Scholar]
- 16.Kelemen O, Hegedűs G, Kollár L, Menyhei G, Seress L. Morphological analysis of the connective tissue reaction in linear hypertrophic scars treated with intralesional steroid or silicone-gel sheeting. A light and electron microscopic study. Acta Biol Hung 2008;59:129–145 [DOI] [PubMed] [Google Scholar]
- 17.Hanasono MM, Lum J, Carroll LA, Mikulec AA, Koch RJ. The effect of silicone gel on basic fibroblast growth factor levels in fibroblast cell culture. Arch Facial Plast Surg 2004;6:88–93 [DOI] [PubMed] [Google Scholar]
- 18.Kuhn MA, Moffit MR, Smith PD, et al. Silicone sheeting decreases fibroblast activity and downregulates TGFbeta2 in hypertrophic scar model. Int J Surg Investig 2000;2:467–474 [PubMed] [Google Scholar]
- 19.Suetak T, Sasai S, Zhen YX. Effects of silicone gel sheet on the stratum comeum hydration. Br J Plast Surg 2000;53:503–507 [DOI] [PubMed] [Google Scholar]
- 20.Nickoloff BJ, Naidu Y. Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. J Am Acad Dermatol 1994;30:535–546 [DOI] [PubMed] [Google Scholar]
- 21.O'Shaughnessy KD, De La Garza M, Roy NK, Mustoe TA. Homeostasis of the epidermal barrier layer: a theory of how occlusion reduces hypertrophic scarring. Wound Repair Regen 2009;17:700–708 [DOI] [PubMed] [Google Scholar]
- 22.Akaishi S, Akimoto M, Hyakusoku H, Ogawa R. 142B: the relationship between keloid growth pattern and stretching tension-visual analysis using the finite element method. Plast Reconstr Surg 2010;125:96. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa R, Akaishi S, Hyakusoku H. Differential and exclusive diagnosis of diseases that resemble keloids and hypertrophic scars. Ann Plast Surg 2009;62:660–664 [DOI] [PubMed] [Google Scholar]
- 24.Rabello FB, Souza CD, Farina Júnior JA. Update on hypertrophic scar treatment. Clinics 2014;69:565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borgognoni L. Biological effects of silicone gel sheeting. Wound Repair Regen 2002;10:118–121 [DOI] [PubMed] [Google Scholar]
- 26.Musgrave MA, Umraw N, Fish JS, Gomez M, Cartotto RC. The effect of silicone gel sheets on perfusion of hypertrophic burn scars. J Burn Care Rehabil 2002;23:208–214 [DOI] [PubMed] [Google Scholar]
- 27.Har-Shai Y, Lindenbaum ES, Gamliel-Lazarovich A, Beach D, Hirshowitz B. An integrated approach for increasing the survival of autologous fat grafts in the treatment of contour defects. Plast Reconstr Surg 1999;104:945–954 [DOI] [PubMed] [Google Scholar]
- 28.Hirshowitz B, Lindenbaum E, Har-Shai Y, Feitelberg L, Tendler M, Katz D. Static-electric field induction by a silicone cushion for the treatment of hypertrophic and keloid scars. Plast Reconstr Surg 1998;101:1173–1183 [DOI] [PubMed] [Google Scholar]
- 29.Mustoe TA, Cooter RD, Gold MH, et al. International Advisory Panel on Scar Management. International clinical recommendations on scar management. Plast Reconstr Surg 2002;110:560–571 [DOI] [PubMed] [Google Scholar]
- 30.Quinn KJ. Silicone gel in scar treatment. Burns 1987;13:S33–S40 [DOI] [PubMed] [Google Scholar]
- 31.Mercer NSG. Silicone gel in the treatment of keloid scars. Br J Plast Surg 1989;42:83–87 [DOI] [PubMed] [Google Scholar]
- 32.O'Brien L, Pandit A. Silicon gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev 2006;CD003826. [DOI] [PubMed] [Google Scholar]
- 33.Momeni M, Hafezi F, Rahbar H, Karimi H. Effects of silicone gel on burn scars. Burns 2009;35:70–74 [DOI] [PubMed] [Google Scholar]
- 34.Li-Tsang CW, Lau J, Choi J, Chan CC, Jianan L. A prospective randomized clinical trial to investigate the effect of silicone gel sheeting (Cica-Care) on post-traumatic hypertrophic scar among the Chinese population. Burns 2006;32:678–683 [DOI] [PubMed] [Google Scholar]
- 35.Gold MH, Foster TD, Adair MA, Burlison K, Lewis T. Prevention of hypertrophic scars and keloids by the prophylactic use of topical silicone gel sheets following a surgical procedure in an office setting. Dermatol Surg 2001;27:641–644 [DOI] [PubMed] [Google Scholar]
- 36.Carney SA, Cason CG, Gowar JP, et al. Cica-Care gel sheeting in the management of hypertrophic scarring. Burns 1994;20:163–167 [DOI] [PubMed] [Google Scholar]
- 37.Morganroth P, Wilmot AC, Miller C. Over-the-counter scar products for postsurgical patients: disparities between online advertised benefits and evidence regarding efficacy. J Am Acad Dermatol 2009;61:e31–e47 [DOI] [PubMed] [Google Scholar]
- 38.Sproat JE, Dalcin A, Weitauer N, Roberts RS. Hypertrophic sternal scars: Silicone gel sheet versus Kenalog injection treatment. Plast Reconstr Surg 1992;90:988–992 [PubMed] [Google Scholar]
- 39.Signorini M, Clementoni MT. Clinical evaluation of a new self-drying silicone gel in the treatment of scars: a preliminary report. Aesthetic Plast Surg 2007;31:183–187 [DOI] [PubMed] [Google Scholar]
- 40.Berman B, Perez OA, Konda S, et al. A review of the biologic effects, clinical efficacy, and safety of silicone elastomer sheeting for hypertrophic and keloid scar treatment and management. Dermatol Surg 2007;33:1291–1303 [DOI] [PubMed] [Google Scholar]
- 41.Saulis AS, Mogford JH, Mustoe TA, Tredget EE, Anzarut A. Effect of Mederma on hypertrophic scarring in the rabbit ear model. Plast Reconstr Surg 2002;110:177–183 [DOI] [PubMed] [Google Scholar]
- 42.Tollefson TT, Kamangar F, Aminpour S, Lee A, Durbin-Johnson B, Tinling S. Comparison of effectiveness of silicone gel sheeting with microporous paper tape in the prevention of hypertrophic scarring in a rabbit model. Arch Facial Plast Surg 2012;14:45–51 [DOI] [PubMed] [Google Scholar]
- 43.Eishi K, Bae SJ, Ogawa F, Hamasaki Y, Shimizu K, Katayama I. Silicone gel sheets relieve pain and pruritus with clinical improvement of keloid: possible target of mast cells. J Dermatolog Treat 2003;14:248–252 [DOI] [PubMed] [Google Scholar]
- 44.Maher SF, Dorko L, Saliga S. Linear scar reduction using silicone gel sheets in individuals with normal healing. J Wound Care 2012;21:602–609 [DOI] [PubMed] [Google Scholar]
- 45.Ahn ST, Monafo WW, Mustoe TA. Topical silicone gel: a new treatment for hypertrophic scars. Surgery 1989;106:781–786 [PubMed] [Google Scholar]
- 46.O'Brien L, Jones DJ. Silicone gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev 2013;9:CD003826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakuraba M, Takahashi N, Akahoshi T, Miyasaka Y, Suzuki K. Use of silicone gel sheets for prevention of keloid scars after median sternotomy. Surg Today 2011;41:496–499 [DOI] [PubMed] [Google Scholar]
- 48.Amicucci G, Schietroma M, Rossi M, Mazzotta C. Silicone occlusive sheeting vs silicone cushion for the treatment of hypertrophic and keloid scars. A prospective-randomized study. Ann Ital Chir 2004;76:79–83 [PubMed] [Google Scholar]
- 49.Sandhofer M, Schauer P. The safety, efficacy, and tolerability of a novel silicone gel dressing following dermatological surgery. Skinmed 2011;10:S1–S7 [PubMed] [Google Scholar]
- 50.Puri N, Talwar A. The efficacy of silicone gel for the treatment of hypertrophic scars and keloids. J Cutan Aesthet Surg 2009;2:104–106 [DOI] [PMC free article] [PubMed] [Google Scholar]