Abstract
The objective of this study was to assess the use of Concarpus biochar as a soil amendment for reducing heavy metal accessibility and uptake by maize plants (Zea mays L.). The impacts of biochar rates (0.0, 1.0, 3.0, and 5.0% w/w) and two soil moisture levels (75% and 100% of field capacity, FC) on immobilization and availability of Fe, Mn, Zn, Cd, Cu and Pb to maize plants as well as its application effects on soil pH, EC, bulk density, and moisture content were evaluated using heavy metal-contaminated soil collected from mining area. The biochar addition significantly decreased the bulk density and increased moisture content of soil. Applying biochar significantly reduced NH4OAc- or AB-DTPA-extractable heavy metal concentrations of soils, indicating metal immobilization. Conocarpus biochar increased shoot dry biomass of maize plants by 54.5–102% at 75% FC and 133–266% at 100% FC. Moreover, applying biochar significantly reduced shoot heavy metal concentrations in maize plants (except for Fe at 75% FC) in response to increasing application rates, with a highest decrease of 51.3% and 60.5% for Mn, 28% and 21.2% for Zn, 60% and 29.5% for Cu, 53.2% and 47.2% for Cd at soil moisture levels of 75% FC and 100% FC, respectively. The results suggest that biochar may be effectively used as a soil amendment for heavy metal immobilization and in reducing its phytotoxicity.
Keywords: Biochar, Heavy metal immobilization, Heavy metal accessibility, Zea mays, Bulk density, Moisture content
1. Introduction
During the last decades, the concern for heavy metal contaminated soils has increased due to their stream seriously threatening the ecosystem (Friesl et al., 2003; Moon et al., 2013). The soils can be polluted with heavy metals by various human activities (Al-Farraj et al., 2013; Moon et al., 2013). Mining activity is one of the most important sources for heavy metals introduced into soils (Ok et al., 2011a,b; Reglero et al., 2008; Al-Farraj et al., 2013). Heavy metals are regarded as non-degradable or non-destroyable inorganic contaminants, changing their solubility and chemical forms and subsequently their availability to plants. The total contents of heavy metals in soils might not mirror their phytotoxicity and accessibility to plants. Phytoavailability refers to the readily available form of a heavy metal, which is uptaken by plants. Thus, it is very important to decrease heavy metal accessibility and availability to plants in contaminated soils.
Soil remediation from hazard contaminants is urgent to rehabilitate polluted soils for safe food production. The rehabilitation of heavy metal contaminated soils is usually dependent on in situ and ex-situ remediation techniques (Vangronsveld and Cunningham, 1998; Lim et al., 2013; Moon et al., 2013). The immobilization and phytoremediation techniques are currently considered as effective method to remediate heavy metal contaminated soils (Moon et al., 2013; Lim et al., 2013; Illera et al., 2004; Ok et al., 2010, 2011b; Usman et al., 2006). The soil amendments have to possess a high binding capacity as well as to be safe for the environment, and not to have adverse effects on soil structure, soil fertility, or the ecosystem. Recently, it has been reported that biochar produced from carbonization of organic wastes can be considered as an alternative additive, which may not only affect C sequestration of soil, but also change its physico-chemical and biological properties (Ibrahim et al., 2013; Lehmann et al., 2003; Chan et al., 2007; Glaser et al., 2002; Tryon, 1948). Additionally, due to its functional and sorptive characteristics, biochar has been suggested to be an effective sorbent for various hazardous inorganic and organic contaminants (Vithanage et al., 2014; Cao et al., 2009). It has been reported that the additions of biochars increased the soil capacity to retain and adsorb plant nutrients and decrease the nutrient losses by leaching (Uzoma et al., 2011; Chan et al., 2008, 2007; Lehmann et al., 2003; Glaser et al., 2002). Biochars have been also shown to decrease heavy metal mobility and bioavailability (Méndez et al., 2012). The addition of biochar to soils might modify some chemical soil properties such as cation exchange capacity and soil acidity, providing circumstances that are suitable for heavy metal immobilization and subsequently reducing their availability to plants (Park et al., 2011). The surface functional groups and adsorption sites on biochar could increase the soil cation exchange capacity and consequently increase metal exchange capacity of soil through the formation of complexes with cationic heavy metal (Paz-Ferreiro et al., 2014).
The use of biochar, depending on its quality, may affect chemical and biological soil properties, thus affecting heavy-metal redistribution among solid-phase components. Specifically, the effects of biochar application depend on various factors such as the soil type, the metal type, the type of feedstocks used for charring, pyrolysis conditions and the amount of biochar applied to the soil (Park et al., 2011; Debela et al., 2012). In Saudi Arabia, Conocarpus erectus L. is an evergreen tree planted around parking lots and along streets and its recycling is problematic due to its huge aboveground biomass and widespreadness. Conocarpus erectus L. wood is hard and durable and possesses high calorific value as fuel but it is most widely used for high-grade charcoal (Morton, 1981; Al-Wabel et al., 2013). Therefore, transforming conocarpus wastes into biochar may be considered as a mean for waste recycling (Al-Wabel et al., 2013). The main objective of the present study was to evaluate the efficacy of Concarpus biochar as a soil immobilizing agent for reducing heavy metal availability and uptake by maize plants as well as its application effects on soil pH, EC, bulk density, and soil moisture.
2. Materials and methods
2.1. Soil sampling
The heavy metal-contaminated soil for this study was collected from the mining area of Mahad AD’Dahab, Saudi Arabia. The soil samples were collected from the surface soil layer (0–20 cm). All soil samples were mixed thoroughly and then air-dried. Then, the soil samples were sieved passing through a 2-mm screen. Some chemical and physical properties of soil were estimated according to standard procedures (Sparks, 1996). The soil texture was determined by means of the hydrometer method. Soil pH and electrical conductivity (EC) were measured with a glass electrode using a soil-to-water ratio of 1:1. The total content of Fe, Mn, Zn, Cd, Cu and Pb was determined by EPA 3051 microwave digestion method. Heavy metal concentrations were determined by ICP (Perkin Elmer Optima 4300 DV ICP-OES). The total concentrations of heavy metals in soil samples are 26,118 mg kg−1 for Fe, 970 mg kg−1 for Mn, 5453 mg kg−1 for Zn, 1430 mg kg−1 for Cu, 8.12 mg kg−1 for Cd and 541 mg kg−1for Pb.
2.2. Biochar production and characteristics
The biochar applied in this study was produced by pyrolysis of conocarpus (Conocarpus species) trees wastes, which was collected from the campus of King Saud University, subjected to direct sunlight (moisture content was nearly 4.7%) and then cut down to small pieces (7–10 cm). After that, the pyrolysis process for conocarpus pieces was executed at a temperature of 400 °C ± 10 °C. The produced biochar had a pH of 9.85 measured with a glass electrode in a suspension of using a biochar-to-water ratio of 1:10; an EC of 1.23 dS m−1 measured with a glass electrode on a 1:10 extract of biochar-to-water. The percentage of ash content was amounted to 2.2%. The total contents of C, H, and N in biochar samples were measured by CHN analyzer (series II, Perkin Elmer, USA). The total contents of C, H, N and O were 76.18%, 2.53%, 0.42% and 18.67%, respectively.
2.3. Greenhouse pot experiment
The soil samples were air-dried and sieved through a 2-mm diameter stainless steel screen. The soil samples were treated with 0.0, 1.0, 3.0 and 5.0% (w/w) biochar. Soil samples (4 kg) of untreated soil and soil treated with biochar were placed in pots in 3 replicates for each treatment. Five corn (Zea mays L.) seeds were planted in the pots and then thinned to 2 plants after germination. Maize plants received the recommended rates of NPK. All pots were adjusted daily to water content of 75% and 100% field capacity (FC) by weight.
2.4. Soil and plant analysis
After 45 days from planting, shoots of Zea mays plants were cut at the soil surface and washed with DI water. Shoots were then oven-dried at 70 °C for 48 h, weighed for dry matter yield, and ground. Shoot concentrations of heavy metals were determined after digestion using H2SO4–H2O2 according to (Parkinson and Allen, 1975). The digestives were analyzed for Fe, Mn, Zn, Cd, Cu and Pb by ICP (Perkin Elmer Optima 4300 DV ICP-OES).
At the end of the experiment, soil samples from pots, were air-dried, and crushed to pass through a 2 mm sieve. Soil pH and electrical conductivity (EC) were measured with a glass electrode using a soil-to-water ratio of 1:1. The soil available fraction of Fe, Mn, Zn, Cd, Cu and Pb was measured after extraction with 1 M NH4OAc or ammonium bicarbonate-DTPA (AB-DTPA) (Soltanpour and Schwab, 1977). In addition, the bulk density (BD) and the soil moisture content (SMC) were also determined for each set of pots. The samples were taken at depths of 6.5 and 5 cm for BD and SMC, respectively. The soil BD was determined by the field core method, using soil cores of 5.3 cm ID and 6.5 cm length (Blake and Hartge, 1986); however, the SMC was determined by weighting 20 g of soil from each pot then disposed into a round aluminum container (5 cm i.d., previously weighed), weighed, and placed in a tray. The samples were then oven-dried at 105 °C until no change in weight was observed (approx. 24 h). After being oven-dried weight was measured, gravimetric water content was then determined for each sample (Cassel and Nielsen, 1986).
2.5. Statistical analysis
The means and standard deviations (±SD) of three replications are reported. Statistical analysis was performed using the software Statsoft for windows (Statsoft, 1995). Differences of means between treatments were tested by ANOVA and comparisons of means using LSD test, at p = 0.05.
3. Results
3.1. Changes in bulk density and soil moisture content
The results showed that the addition of biochar as a soil amendment improved the soil physical properties of bulk density and soil moisture content of heavy metal-contaminated soil planted with maize plants (Table 1). The BD decreased from 1.40 to 1.24 g cm−3 in control and 5.0% biochar rate, respectively, while maintaining the SMC at 75% of FC. In case of preserving the SMC at 100% FC, the BD decreased from 1.39 to 1.25 g cm−3 in control and 5.0% biochar rate, respectively (Table. 1). The results indicated that no significant difference of BD observed in case of changing moisture level. The results showed that the application of biochar increased the SMC from 0.06 g g−1 to 0.09 g g−1 in control and 5.0% biochar rate, respectively at 75% FC, and from 0.06 g g−1 to 0.16 g g−1 in control and 5.0% biochar rate, respectively at 100% FC.
Table 1.
Treatment effects on soil pH, EC, soil moisture content (SMC) and bulk density (BD).
| Moisture levels | Biochar rates (% w/w) | Soil property |
|||
|---|---|---|---|---|---|
| SMC (g g−1) | BD (g cm−3) | pH | EC (dS m−1) | ||
| 75% FC | 0.0 | 0.061 ± 0.007 | 1.40 ± 0.05 | 7.96 ± 0.04 | 1.97 ± 0.06 |
| 1.0 | 0.062 ± 0.01 | 1.38 ± 0.06 | 8.01 ± 0.01 | 2.03 ± 0.25 | |
| 3.0 | 0.064 ± 0.005 | 1.29 ± 0.07 | 8.05 ± 0.01 | 2.10 ± 0.10 | |
| 5.0 | 0.090 ± 0.01 | 1.24 ± 0.06 | 8.12 ± 0.03 | 2.60 ± 0.10 | |
| 100% FC | 0.0 | 0.059 ± 0.05 | 1.39 ± 0.01 | 7.98 ± 0.02 | 2.10 ± 0.10 |
| 1.0 | 0.075 ± 0.003 | 1.30 ± 0.03 | 8.00 ± 0.01 | 2.23 ± 0.15 | |
| 3.0 | 0.104 ± 0.04 | 1.29 ± 0.03 | 8.07 ± 0.02 | 2.60 ± 0.10 | |
| 5.0 | 0.161 ± 0.01 | 1.25 ± 0.04 | 8.15 ± 0.01 | 2.93 ± 0.23 | |
3.2. Changes in soil pH and EC
The impact of biochar application on soil pH and EC values is shown in Table 1. The statistical analysis showed a significant (p < 0.05) increase in pH and EC due to addition of biochar. The highest mean values of pH and EC were recorded in soils treated with 5.0% biochar, while the lowest values were found in the untreated soil. The values of soil pH increased by 0.16–0.17 unit at the highest application rate. Meanwhile, the EC values increased from 1.97–2.10 dS m−1 to 2.60–2.93 dS m−1 for 5.0% biochar.
3.3. Changes in soil available heavy metals
The concentrations of heavy metals measured from the soil at two different moisture levels (75% and 100%) using the two extractants of NH4OAc and AB-DTPA are mainly affected by the application of biochar (Table 2). Addition of biochar at 1.0%, 3.0% and 5.0% biochar significantly decreased both NH4OAc- and AB-DTPA-extractable heavy metals. However, there was little change among application rates (1.0–5.0%) of biochar, indicating no significant differences between the different application rates for the most studied metals. With the addition of biochar with soil moisture level of 75% FC, the concentrations of NH4OAc-extractable heavy metals was significantly reduced from 1.41 mg kg−1 to 0.79–0.84 mg kg−1 for Mn, from 7.36 mg kg−1 to 4.75–5.77 mg kg−1 for Zn, from 48.3 mg kg−1 to 35.2–37.7 mg kg−1 for Cu, from 0.62 mg kg−1 to 0.40–47 mg kg−1 for Cd and from 24.9 mg kg−1 to 17.6–19.4 mg kg−1 for Zn. However, in the case of 100% FC, applying biochar reduced the concentrations of NH4OAc-extractable heavy metals from 1.59 mg kg−1 to 0.82–0.99 mg kg−1 for Mn, from 28.2 mg kg−1 to 19.3–20.8 mg kg−1 for Zn, from 62.7 mg kg−1 to 37.0–45.2 mg kg−1 for Cu, from 0.68 to 0.52–0.57 for Cd and from 7.81 mg kg−1 to 5.56–5.99 mg kg−1 for Pb. It was generally observed that the concentrations of NH4OAc-extractable Fe were not detected in all the treatments.
Table 2.
Treatment effects on NH4OAc- and AB-DTPA-extractable heavy metals.
| Moisture levels | Biochar rates (%) | Fe | Mn | Zn | Cu | Cd | Pb |
|---|---|---|---|---|---|---|---|
| NH4OAc-extractable heavy metals (mg kg−1) | |||||||
| 75% FC | 0.0 | ND | 1.41 | 24.9 | 48.3 | 0.62 | 7.36 |
| 1.0 | ND | 0.84 | 18.9 | 36.7 | 0.47 | 5.77 | |
| 3.0 | ND | 0.80 | 19.4 | 37.7 | 0.45 | 5.11 | |
| 5.0 | ND | 0.79 | 17.6 | 35.2 | 0.40 | 4.75 | |
| LSD1 | – | 0.24 | 4.12 | 4.86 | 0.16 | 1.02 | |
| 100% FC | 0.0 | ND | 1.59 | 28.2 | 62.7 | 0.68 | 7.81 |
| 1.0 | ND | 0.91 | 20.8 | 45.2 | 0.50 | 5.99 | |
| 3.0 | ND | 0.99 | 19.3 | 44.0 | 0.57 | 5.92 | |
| 5.0 | ND | 0.82 | 20.8 | 37.0 | 0.52 | 5.56 | |
| LSD1 | – | 0.25 | 5.96 | 10.7 | 0.12 | 0.87 | |
| LSD2 | – | NS | NS | 7.45 | NS | NS | |
| AB-DTPA-extractable Heavy metals (mg kg−1) | |||||||
| 75% FC | 0.0 | 4.91 | 4.68 | 364 | 521 | 3.09 | 234 |
| 1.0 | 3.50 | 3.23 | 307 | 415 | 2.61 | 207 | |
| 3.0 | 2.44 | 2.08 | 234 | 293 | 2.01 | 149 | |
| 5.0 | 3.67 | 3.50 | 242 | 354 | 1.85 | 163 | |
| LSD1 | 0.44 | 0.41 | 58.5 | 51.4 | 0.41 | 34.3 | |
| 100% FC | 0.0 | 4.75 | 5.36 | 356 | 501 | 3.14 | 220 |
| 1.0 | 3.93 | 3.73 | 343 | 403 | 2.78 | 198 | |
| 3.0 | 3.97 | 3.82 | 324 | 387 | 2.63 | 194 | |
| 5.0 | 3.74 | 3.47 | 327 | 369 | 2.46 | 195 | |
| LSD1 | 0.57 | 0.61 | 23.4 | 54.5 | 0.12 | 16.0 | |
| LSD2 | NS | NS | 37.9 | NS | NS | NS | |
LSD1: least significance difference at p = 0.05 for biochar effect; LSD2: least significance difference at p = 0.05 for moisture effect; NS: not significant; ND: not detectable.
Similarly, the levels of all the heavy metals (Table 2) extracted with AB-DTPA were found significantly lower in the biochar-amended soil than control soil. In the case of 75% FC, applying biochar reduced the concentrations of AB-DTPA-extractable heavy metals from 4.91 mg kg−1 to 2.44–3.67 mg kg−1 for Fe, from 4.68 mg kg−1 to 2.08–3.50 mg kg−1 for Mn, from 364 mg kg−1 to 234–307 mg kg−1 for Zn, from 521 mg kg−1 to 293–415 mg kg−1 for Cu, from 3.09 mg kg−1 to 1.85–2.61 mg kg−1 for Cd and from 234 mg kg−1 to 149–207 mg kg−1. In the case of 100% FC, applying biochar reduced the concentrations of NH4OAc-extractable heavy metals from 4.75 mg kg−1 to 3.74–3.97 mg kg−1 for Fe, from 5.36 mg kg−1 to 3.47–3.82 mg kg−1 for Mn, from 356 mg kg−1 to 324–343 mg kg−1 for Zn, from 501 mg kg−1 to 369–403 mg kg−1 for Cu, from 3.14 mg kg−1 to 2.46–2.78 mg kg−1 for Cd and from 220 mg kg−1 to 194–198 mg kg−1 for Pb.
3.4. Changes in dry biomass and heavy metal content of maize shoots
Maize plants grown in the untreated soil showed lower biomass production relative to biochar-amended soils (Table 3). The dry matter of maize shoots in biochar amended soil increased to a highest weight with 5.0% biochar at 75% FC. Additionally, its highest weight at 100% FC was pronounced for 3.0% biochar, then decreased as biochar increased to 5.0%; however, all the biochar rates showed higher shoot dry matter than the control.
Table 3.
Treatment effects on the dry matter (DM) and heavy metal content of maize shoots.
| Moisture levels | Biochar rates (%) | DM mg pot−1 | Heavy metals |
||||
|---|---|---|---|---|---|---|---|
| Fe | Mn | Zn | Cu | Cd | |||
| Shoot heavy metal concentrations (mg kg−1) | |||||||
| 75% FC | 0.0 | 490 | 151 | 62.5 | 268 | 8.60 | 4.13 |
| 1.0 | 757 | 181 | 43.4 | 248 | 6.27 | 3.33 | |
| 3.0 | 857 | 168 | 34.9 | 207 | 5.00 | 2.40 | |
| 5.0 | 990 | 153 | 30.5 | 193 | 3.47 | 1.93 | |
| LSD1 | 307 | NS | 10.6 | 60.2 | 3.47 | NS | |
| 100% FC | 0.0 | 624 | 264 | 88.7 | 328 | 11.5 | 5.93 |
| 1.0 | 890 | 172 | 55.7 | 326 | 7.40 | 5.73 | |
| 3.0 | 957 | 219 | 44.4 | 277 | 7.60 | 4.27 | |
| 5.0 | 757 | 196 | 35.1 | 259 | 8.13 | 3.13 | |
| LSD1 | 260 | 81.2 | 21.6 | 68.7 | 3.08 | 2.55 | |
| LSD2 | NS | 41.7 | NS | 36.3 | 2.06 | 1.28 | |
| Metal uptake by shoots (μg plant−1) | |||||||
| 75% FC | 0.0 | 35.8 | 15.4 | 65.1 | 2.08 | 1.01 | |
| 1.0 | 63.1 | 16.7 | 99.9 | 2.21 | 1.46 | ||
| 3.0 | 71.9 | 14.7 | 87.3 | 2.17 | 0.99 | ||
| 5.0 | 75.9 | 15.0 | 95.4 | 1.71 | 0.95 | ||
| LSD1 | 27.7 | NS | NS | NS | NS | ||
| 100% FC | 0.0 | 80.2 | 27.4 | 102 | 3.50 | 1.79 | |
| 1.0 | 74.5 | 24.5 | 144 | 3.15 | 2.55 | ||
| 3.0 | 105 | 21.2 | 133 | 3.64 | 2.04 | ||
| 5.0 | 73.2 | 13.4 | 98.0 | 3.03 | 1.18 | ||
| LSD1 | 28.9 | 10.3 | NS | NS | 1.19 | ||
| LSD2 | 16.6 | 4.90 | 25.0 | 0.39 | 0.56 | ||
LSD1: least significance difference at p = 0.05 for biochar effect; LSD2: least significance difference at p = 0.05 for moisture effect; NS: not significant; ND: not detectable.
Among all investigated heavy metals, the Pb concentrations of shoots were below detection limit of ICP. The concentrations of Mn, Cu, Zn, Cd and Cu in shoots significantly decreased as a result of increasing biochar application rates compared with the control (no biochar) treatment (Table 3). These significant decreases were found in biochar-amended soil at both soil moisture levels of 75% and 100% FC. Heavy metal concentrations in maize shoots of untreated soil at 75% and 100% FC were 62.5 and 88.7 mg kg−1 for Mn, 268 and 328 mg kg−1 for Zn, 8.60 and 11.5 mg kg−1 for Cu and 4.13 and 5.93 mg kg−1 for Cd, respectively. The incorporation of biochar significantly reduced content of heavy metals (except for Fe at 75%) in maize shoots, with a highest decrease of 51.3% and 60.5% for Mn, 28.0% and 21.2% for Zn, 60.0% and 29.5% for Cu, 53.2% and 47.2% for Cd at soil moisture levels of 75% and 100% FC, respectively. It was observed that these highest reductions were pronounced for the highest biochar application rate. Moreover, applying biochar decreased significantly the concentrations of shoot Fe at 100% FC, with a highest reduction of 34.7% for Fe at 1.0% biochar.
When metal uptake was calculated based on biomass of maize shoot and its metal concentration, applying 5% biochar with moisture level of 100% FC decreased significantly the uptake amount of Cd, Cu and Mn, but its application with 75% FC did not show any significant differences in the uptake of these metals compared to untreated soil (Table 3). On the contrary, applying 3.0% and 5.0% biochar with moisture level of 75% FC increased significantly the uptake amount of Fe. Moreover, the uptake amount of Fe was increased significantly when biochar was added at 3.0% with moisture level of 100% FC.
Biochar application to the soil had a different effect on bio-concentration factor of heavy metals. With increasing application rate of biochar, the values of bio-concentration factor of Mn, Cd and Cu from soil available fraction extracted by NH4OAc were found lower than those of untreated soil. On the contrary, the highest application rate of biochar with moisture level of 100% FC increased bio-concentration values of Cu (see Fig. 1).
Figure 1.
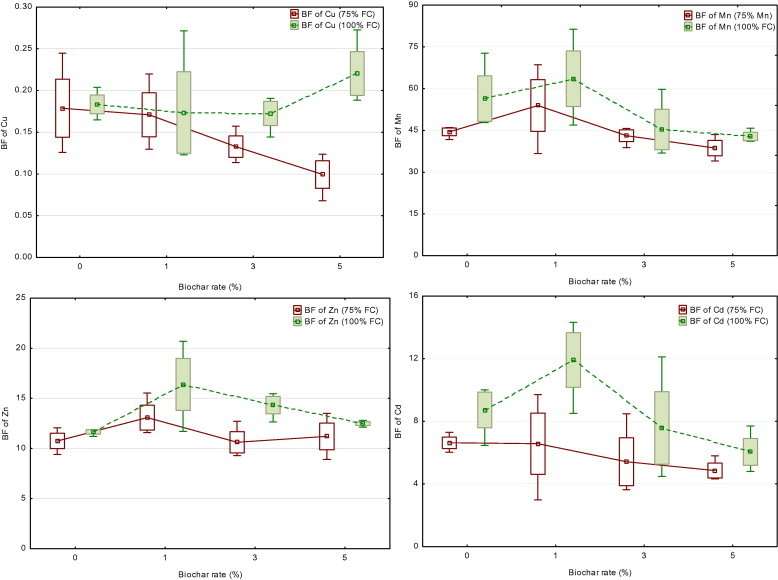
Treatment effects on bio-concentration factor (BF) of heavy metals to maize plants according to NH4OAc-extractable metal (shoot concentration/NH4OAc-extractable concentrations) (Mean; Box: Mean ± SE; Whisker: Min–Max).
Applying biochar amendment at moisture level of 75% FC generally increased the values of bio-concentration factor of Fe from soil available fraction extracted by AB-DTPA compared to the untreated soil (Fig. 2). Applying 5.0% biochar at 75% FC decreased significantly the bio-concentration factor of Cu and Mn from soil available fractions extracted by AB-DTPA compared to the untreated soil. Moreover, these values for Mn, Zn and Cd tended to decrease significantly with increasing application rate of biochar at 100% FC.
Figure 2.
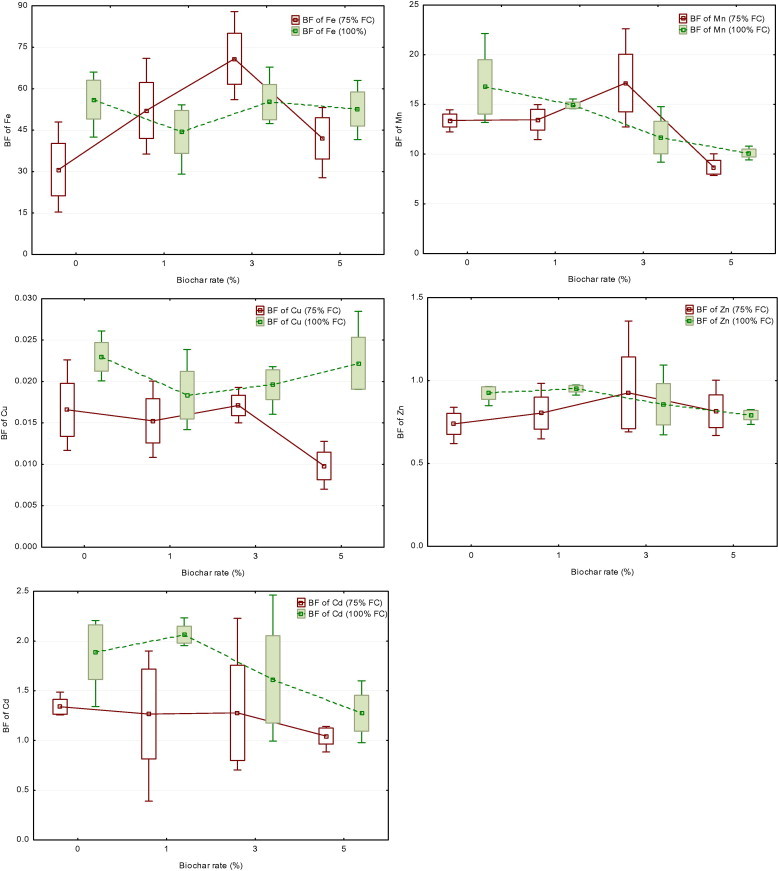
Treatment effects on bio-concentration factor (BF) of heavy metals to maize plants according to AB-DTPA-extractable metal (shoot concentration/AB-DTPA-extractable concentrations) (Mean; Box: Mean ± SE; Whisker: Min–Max).
In the present study, correlation analysis (r2) was performed in order to investigate the relationship between the extractable heavy metal content of soil and the total concentrations of metal in the shoots of maize plants (data not shown). Significant (p < 0.05) positive correlation (r2 = 0.57–0.80) was found between NH4OAc- or AB-DTPA-extractable Mn, Zn, Cu and Cd and heavy metal content of maize plants.
4. Discussion
In our study, the biochar addition significantly decreased the bulk density and increased moisture content of soil, similar to others in the literature (Novak et al., 2009; Ibrahim et al., 2013; Lei and Zhang, 2013). The impact of biochar addition on increasing the soil water retention is due to the large inner surface area and porous structure of biochar as well as to the increase in soil aggregation by biochar (Lei and Zhang, 2013). By applying biochar, the increase in soil aggregation might be also a possible reason to decrease soil bulk density (Harris et al., 1966; Masulili, 2010).
Applying biochar showed slight increase in soil pH, which can be due to ash accretion and dissolution of hydroxides and carbonates present in biochar (Lucchini et al., 2014). The alkalinity of the biochar and the release of basic cations into the soil could be responsible for increasing soil pH (Nguyen and Lehmann, 2009). In this context, the results of this study showed that the used biochar lie in the alkaline range (pH of 9.61). Numerous studies have found that biochar is an efficient material for increasing soil pH and induced liming effect (Ameloot et al., 2013; Nguyen and Lehmann, 2009; Lucchini et al., 2014).
According to the results of the present study, biochar increased shoot dry biomass by 54.5–102% at 75% FC and 133–266% at 100% FC. The enhanced impact of biochar on the dry matter of maize shoots is consistent with the results of other studies (Kimetu et al., 2008; Chan et al., 2007), where positive yield effects from biochar application. The response of dry matter yield to biochar is pronounced at increasing its application rate. This can be explained by improved effects on soil characteristics such as nutrients and water retention as well as cation exchange capacity. In this context, the results of this study showed that the addition of biochar to soil improved the physical properties of bulk density and soil moisture content. The effects of biochar on nutrient cycling and turnover are also possible, resulting in improved soil nutrients status subsequently reflecting on increasing plant growth. In a review of biochar and its use in soil, Sohi et al. (2010) revealed that the enhanced impact of biochar on plant growth and crop productivity may be due to non-nutrient improvement to soil function such as improved fertilizer use efficiency.
One of the most important advantages of single metal chemical extractions such as AB-DTPA and NH4OAc in the soils is to assess their bioavailability. The results clearly have shown that the concentrations of AB-DTPA-extractable heavy metals are higher than those extracted by NH4OAc-extractable, mainly due to their different strengths and affinities to extract different soil metal fractions. Among all heavy metals, NH4OAc-extractable Fe was not detectable, attributing to its low solubility and its presence in more non-labile solid phase of soil. The correlation study showed significant positive correlation between NH4OAc- or AB-DTPA-extractable Mn, Zn, Cu and Cd and their content in maize shoots, indicating that both extractants might be used to predict heavy metal availability to plants. The results clearly showed that the concentrations of all heavy metals in maize shoots (except for Mn) were significantly increased with increasing soil moisture levels. However, biochar application deceased significantly the shoot heavy metal content at soil moisture levels of 75% and 100% FC (except for Fe at 75% FC). Significant reductions in the concentration of heavy metals in maize shoots in biochar-amended soil can be attributed to the immobilization of available metals and dilution effect as a result of increasing plant biomass (Park et al., 2011). Namgay et al. (2010) found also that the application of biochar to soil has been shown to cause an apparent reduction in the concentrations of Cd, As and Pb in maize plants. According to the results of the present study, the soil available heavy metals extracted by NH4OAc and AB-DTPA decreased after biochar application, seeming to be a possible reason for the decreased plant content of heavy metals. Similarly, Park et al. (2011) reported that application of biochar significantly reduced the soil concentrations of Cd, Cu and Pb extracted by NH4NO3. Fellet et al. (2011) found also that applying biochar reduced the soil availability and mobility of heavy metals such as Cd, Cr, Pb, and Zn. Biochar might immobilize heavy metals by transforming the readily available fractions to geochemically more stable residual fractions, resulting in reducing the mobility and bioavailability of heavy metals (Ahmad et al., 2014a). Due to its particular characteristics, it has been suggested that biochar is more suitable than other materials to remediate different organic and inorganic contaminants (Paz-Ferreiro et al., 2014). There are several factors of biochar-amended soil that might decrease soil available heavy metals and alleviate their phytotoxicity (Kumpiene et al., 2008; Park et al., 2011). The heavy metal immobilization and sorption onto biochars might be ascribed to several combined processes of ion exchange, chemisorption, complexation and surface interaction (Ahmad et al., 2014b; Usman et al., 2013; Park et al., 2011; Cao et al., 2009). Indeed, biochars have large surface areas, implying a high metal affinity to form complexation onto biochar surface (Beesley and Marmiroli, 2011; Lu et al., 2012; Paz-Ferreiro et al., 2014). The complexation of the heavy metals with biochar functional groups can be attributed to physical adsorption or the metal exchange with biochar alkali and alkaline earth cations (Lu et al., 2012; Paz-Ferreiro et al., 2014). It is also possible that the metal immobilization might occur by the co-precipitation, mainly due to mineral components (carbonate, silicate and phosphate) of biochar and resulting in the precipitates formation of metal (hydr)oxide, carbonate, or phosphate (Usman et al., 2013; Park et al., 2011).
5. Conclusion
The results of this study clearly have shown that biochar application to heavy metal contaminated soil has the potential for heavy metal immobilization, reflecting on reducing metal accessibility to the aboveground compartment of maize plants. Moreover, the biochar addition significantly decreased the bulk density and increased soil moisture content. Therefore, biochar can be used to enhance metal phytostabilization of contaminated soils as well as improved plant growth and some soil physical properties.
Acknowledgement
This work was supported by NSTIP strategic technologies program number (ENV1592-11-02) in the Kingdom of Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad M., Lee S.S., Lim J.E., Lee S.-E., Cho J.S., Moon D.H., Hashimoto Y., Ok Y.S. Speciation and phytoavailability of lead and antimony in a small arms range soil amended with mussel shell, cow bone and biochar: EXAFS spectroscopy and chemical extractions. Chemosphere. 2014;95:433–441. doi: 10.1016/j.chemosphere.2013.09.077. [DOI] [PubMed] [Google Scholar]
- Ahmad M., Rajapaksha A.U., Lim J.E., Zhang M., Bolan N., Mohan D., Vithanage M., Lee S.S., Ok Y.S. Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere. 2014;99:19–33. doi: 10.1016/j.chemosphere.2013.10.071. [DOI] [PubMed] [Google Scholar]
- Al-Farraj A.S., Usman A.R.A., Al Otaibi S.H.M. Assessment of heavy metals contamination in soils surrounding a gold mine: comparison of two digestion methods. Chem. Ecol. 2013;29:329–339. [Google Scholar]
- Al-Wabel M.I., Al-Omran A., El-Naggar A.H., Nadeem M., Usman A.R.A. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013;131:374–379. doi: 10.1016/j.biortech.2012.12.165. [DOI] [PubMed] [Google Scholar]
- Ameloot N., De Neve S., Jegajeevagan K., Yildiz G., Buchan D., Funkuin Y.N., Prins W., Bouckaert L., Sleutel S. Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol. Biochem. 2013;57:401–410. [Google Scholar]
- Beesley L., Marmiroli M. The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar. Environ. Pollut. 2011;159:474–480. doi: 10.1016/j.envpol.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Blake G.R., Hartge K.H. Bulk Density. In: Klute A., editor. Methods of Soil Analysis. Part 1 - Physical and Mineralogical Methods. Second ed. American Society of Agronomy; Madison WI: 1986. [Google Scholar]
- Cao X.D., Ma L.N., Gao B., Harris W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol. 2009;43:3285–3291. doi: 10.1021/es803092k. [DOI] [PubMed] [Google Scholar]
- Cassel D.K., Nielsen D.R. Field capacity and available water capacity. In: Page A.L., Miller R.H., Keeney D.R., editors. Methods of Soil Analysis Part 2: Chemical and Microbiological Properties. Second ed. Agronomy Society of America; Madison, WI: 1986. [Google Scholar]
- Chan K.Y., Zwieten L.V., Meszaros I., Downie A., Joseph S. Agronomic values of greenwaste biochar as a soil amendment. Aust. J. Soil Res. 2007;45:629–634. [Google Scholar]
- Chan K.Y., Zwieten L.V., Meszaros I., Downie A., Joseph S. Using poultry litter biochars as soil amendments. Aust. J. Soil Res. 2008;46:437–444. [Google Scholar]
- Debela F., Thring R.W., Arocena J.M. Immobilization of heavy metals by co-pyrolysis of contaminated soil with woody biomass. Water Air Soil Pollut. 2012;223:1161–1170. [Google Scholar]
- Fellet G., Marchiol L., Delle Vedove G., Peressotti A. Application of biochar on mine tailings: Effects and perspectives for land reclamation. Chemosphere. 2011;83:1262–1267. doi: 10.1016/j.chemosphere.2011.03.053. [DOI] [PubMed] [Google Scholar]
- Friesl W., Lombi E., Horak O., Wenzel W. Immobilization of heavy metals in soils using inorganic amendments in a greenhouse study. J. Plant Nutr. Soil Sci. 2003;166:191–196. [Google Scholar]
- Glaser B., Lehmann J., Zech W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal-a review. Biol. Fertil. Soils. 2002;33:219–230. [Google Scholar]
- Harris R.F., Chesters G., Allen O.N. Dynamics of soil aggregation. Adv. Agron. 1966;18:108–169. [Google Scholar]
- Ibrahim H.M., Al-Wabel M.I., Usman A.R., Al-Omran A. Effect of Conocarpus biochar application on the hydraulic properties of a sandy loam soil. Soil Sci. 2013;178:165–173. [Google Scholar]
- Illera V., Garrido F., Vizcayno C., García-González M.T. Field application of industrial by-products as Al-toxicity amendments: chemical and mineralogical implications. Eur. J. Soil Sci. 2004;55:681–692. [Google Scholar]
- Kimetu J., Lehmann J., Ngoze S., Mugendi D., Kinyangi J., Riha S., Verchot L., Recha J., Pell A. Reversibility of soil productivity decline with organic matter of differing quality along a degradation gradient. Ecosystems. 2008;11:726–739. [Google Scholar]
- Kumpiene J., Lagerkvist A., Maurice C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments – a review. Waste Manage. 2008;28:215–225. doi: 10.1016/j.wasman.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Lehmann J., da Silva J.P., Steiner C., Nehls T., Zech W., Glaser B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments. Plant Soil. 2003;249:343. [Google Scholar]
- Lei Q., Zhang R. Effects of biochars derived from different feedstocks and pyrolysis temperatures on soil physical and hydraulic properties. J. Soils Sediments. 2013;13:1561–1572. [Google Scholar]
- Lim J.E., Ahmad M., Lee S.S., Shope C.L., Hashimoto Y., Kim K.-R., Usman A.R.A., Yang J.E., Ok Y.S. Effects of lime-based waste materials on immobilization and phytoavailability of cadmium and lead in contaminated soil. Clean – Soil Air Water. 2013;41:1235–1241. [Google Scholar]
- Lu H., Zhang Y.Y., Huang X., Wang S., Qiu R. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012;46:854–862. doi: 10.1016/j.watres.2011.11.058. [DOI] [PubMed] [Google Scholar]
- Lucchini P., Quilliam R.S., DeLuca T.H., Vamerali T., Jones D.L. Does biochar application alter heavy metal dynamics in agricultural soil? Agric. Ecosyst. Environ. 2014;184:149–157. [Google Scholar]
- Masulili A. Rice Husk Biochar for Rice Based Cropping System in Acid Soil 1. The Characteristics of Rice Husk Biochar and Its Influence on the Properties of Acid Sulfate Soils and Rice Growth in West Kalimantan. Indonesia J. Agric. Sci. 2010;2:39–45. [Google Scholar]
- Méndez A., Gómez A., Paz-Ferreiro J., Gascó G. Effects of sewage sludge biochar on plant metal availability after application to a Mediterranean soil. Chemosphere. 2012;89:1354–1359. doi: 10.1016/j.chemosphere.2012.05.092. [DOI] [PubMed] [Google Scholar]
- Moon D.H., Park J.W., Chang Y.Y., Ok Y.S., Lee S.S., Ahmad M. Immobilization of lead in contaminated firing range soil using biochar. Environ. Sci. Pollut. Res. 2013;20:8464–8471. doi: 10.1007/s11356-013-1964-7. [DOI] [PubMed] [Google Scholar]
- Morton J.F. Springer field; IL: 1981. Atlas of medicinal plants of middle America. Bahamas to Yucatan. C.C Thomas. [Google Scholar]
- Namgay T., Singh B., Singh B.P. Influence of biochar application to soil on the availability of As, Cd, Cu, Pb, and Zn to maize (Zea mays L.) J. Aust. Soil Res. 2010;48:638–647. [Google Scholar]
- Nguyen B.T., Lehmann J. Black carbon decomposition under varying water regimes. Org. Geochem. 2009;40:846–853. [Google Scholar]
- Novak J.M., Lima I., Xing B., Gaskin J.W., Steiner C., Das K.C., Ahmedna M., Rehrah D., Watts D.W., Busscher W.J., Schomberg H. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2009;3:195–206. [Google Scholar]
- Ok Y.S., Oh S.E., Ahmed M., Hyun S., Kim K.R., Moon D.H., Lee S.S., Lim K.J., Jeon W.T., Yang J.E. Effect of natural and calcined oyster shells on Cd and Pb immobilization in contaminated soils. Environ. Earth Sci. 2010;61:1301–1308. [Google Scholar]
- Ok Y.S., Kim S.C., Kim D.K., Skousen J.G., Lee J.S., Cheong Y.W., Kim S.J., Yang J.E. Ameliorants to immobilize Cd in rice paddy soils contaminated by abandoned metal mines in Korea. Environ. Geochem. Health. 2011;33:23–30. doi: 10.1007/s10653-010-9364-0. [DOI] [PubMed] [Google Scholar]
- Ok Y.S., Usman A.R.A., Lee S.S., Abd El-Azeem S.A.M., Choi B.S., Hashimoto Y., Yang J.E. Effect of rapeseed residue on cadmium and lead availability and uptake by rice plants in heavy metal contaminated paddy soil. Chemosphere. 2011;85:677–682. doi: 10.1016/j.chemosphere.2011.06.073. [DOI] [PubMed] [Google Scholar]
- Park J.H., Choppala G.H., Bolan N.S., Chung J.W., Chuasavathi T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil. 2011;348:439–451. [Google Scholar]
- Parkinson J.A., Allen S.E. A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Commun. Soil Sci. Plant Anal. 1975;6:1–11. [Google Scholar]
- Paz-Ferreiro J., Lu H., Fu S., Méndez A., Gasco G. Use of phytoremediation and biochar to remediate heavy metal polluted soils: a review. Solid Earth. 2014;5(65–75):2014. [Google Scholar]
- Reglero M.M., Gonzalez L.M., Taggart M.A., Mateo R. Transfer of metals to plants and red deer in an old lead mining area in Spain. Sci. Total Environ. 2008;406:287–297. doi: 10.1016/j.scitotenv.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Sohi S.P., Krull E., Lopez-Capel E., Bol R. Advances in Agronomy. Elsevier Academic Press Inc.; San Diego, CA-92101-4495, USA: 2010. A review of biochar and its use and function in soil; pp. 47–82. ISSN 0065–2213. [Google Scholar]
- Soltanpour P.N., Schwab A.P. A new soil test for macro- and micro-nutrients in alkaline soils. Commun. Soil Sci. Plant Anal. 1977;8:195–207. [Google Scholar]
- Sparks D.L. Soil Society of American; Madison, WI: 1996. Methods of soil analysis. [Google Scholar]
- StatSoft, Inc., 1995. Statistica for Windows (Computer Program Manual). StatSoft, Inc., Tulsa, OK.
- Tryon E.H. Effect of charcoal on certain physical, chemical, and biological properties of forest soils. Ecol. Monogr. 1948;18:81–115. [Google Scholar]
- Usman A.R., Kuzyakov Y., Lorenz K., Stahr K. Remediation of a soil contaminated with heavy metals by immobilizing compounds. J. Plant Nutr. Soil Sci. 2006;169:205–212. [Google Scholar]
- Usman A.R.A., Sallam A.S., Al-Omran A., El-Naggar A.H., Alenazi K.K.H., Nadeem M., Al-Wabel M.I. Chemically modified biochar produced from conocarpus wastes: an efficient sorbent for Fe(II) removal from acidic aqueous solutions. Adsorpt. Sci. Technol. 2013;31:625–640. [Google Scholar]
- Uzoma K.C., Inoue M., Andry H., Fujimaki H., Zahoor Z., Nishihara E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manage. 2011;27:205–212. [Google Scholar]
- Vangronsveld J.C.H.M., Cunningham S.D. Introduction to the concepts. In: Vangronsveld J., Cunningham S.D., editors. Metal-contaminated soils: In situ Inactivation and phytorestoration. R.G. Landes Co.; Georgetown, TX: 1998. [Google Scholar]
- Vithanage M., Rajapaksha A.U., Zhang M., Thiele-Bruhn S., Lee S.S., Ok Y.S. Acid-activated biochar increased sulfamethazine retention in soils. Environ. Sci. Pollut. Res. 2014 doi: 10.1007/s11356-014-3434-2. [DOI] [PubMed] [Google Scholar]


