Abstract
Background
Non-alcoholic steatoheaptitis (NASH), the critical stage of non-alcoholic fatty liver disease (NAFLD), is of chronic progression and can develop cirrhosis even hepatocellular carcinoma (HCC). However, non-invasive biomarkers for NASH diagnosis remain poorly applied in clinical practice. Our study aims at testing the accuracy of the combination of cytokeratin-18 M30 fragment (CK-18-M30), fibroblast growth factor 21 (FGF-21), interleukin 1 receptor antagonist (IL-1Ra), pigment epithelium-derived factor (PEDF) and osteoprotegerin (OPG) in diagnosing NAFLD and NASH.
Methods
179 patients with biopsy-proven NAFLD were enrolled as training group, 91 age- and gender-matched healthy subjects were recruited at the same time as controls. 63 other NAFLD patients were separately collected as validation group. 45 alcoholic fatty liver disease (AFLD) patients, 50 hepatitis B virus (HBV) patients, 52 hepatitis C virus (HCV) patients were also included. Serum biomarker levels were measured by enzyme-linked immunosorbent assay.
Results
Serum levels of CK-18-M30, FGF-21, IL-1Ra and PEDF increased, while OPG decreased in a stepwise fashion in controls, non-NASH NAFLD patients and NASH patients (P < 0.01). The area under receiver-operating characteristics curve to diagnose NASH was 0.86 for CK-18-M30, 0.89 for FGF-21, 0.89 for IL-1Ra, 0.89 for PEDF and 0.89 for OPG. CK-18-M30 had 70% negative predictive value (NPV) and 79% positive predictive value (PPV) to diagnose NASH. A 5-step approach measuring CK-18-M30 followed by FGF21, IL-1Ra, PEDF and OPG gradually improved the NPV to 76% and PPV to 85%, which reached 80% and 76% respectively in the validation cohort.
Conclusion
Compared to single biomarker, stepwise combination of CK-18-M30, FGF-21, IL-1Ra, PEDF and OPG can further improve the accuracy in diagnosing NASH.
Introduction
Non-alcoholic fatty liver disease (NAFLD) has become the comonest form of chronic liver disease in European and Americn populations [1–3]. Recent studies show that the prevalence of NAFLD in adult population is 30–40% in the United States [4], 11–45% in northern Asia and 9–45% in South Asia as well as Southeast Asia during the past decade, and theses percentages continue to grow with the increase of obesity population worldwide [5]. NAFLD patients are more likely to suffer from liver fibrosis, liver cancer, type-2 diabetes, cardiovascular diseases, chronic glomerulo nephritis and other diseases [6–8]. NAFLD has become one of the most important public health issues in 21st century.
NAFLD includes a spectrum of diseases from simple fatty liver disease, to non-alcoholic steatohepatitis (NASH), liver cirrhosis and hepatocellular carcinoma (HCC). NASH, the critical stage in NAFLD progression, is the only bridge between NAFLD and liver cirrhosis [9–12], and its persistent existence has become one of the leading causes of liver cirrhosis and even HCC [13]. Statistical data show that 15%-20% of NASH patients will develop liver cirrhosis in 10 to 20 years [14–15], suggesting the risk of disease progression is much higher. Therefore, clinical diagnosis of NASH has gained more importance.
Although liver biopsy remains the ‘Gold Standard’ for the diagnosis of NASH [16], it is invasive and not easily accepted by patients. Non-invasive diagnosis by serum biomarkers are eagerly needed. Previous studies demonstrated that several serum biomarkers exhibit potential in diagnosing NAFLD and NASH. Cytokeratin-18 M30 fragment (CK-18-M30) could indicate hepatocyte apoptosis in NASH and was closely associated with NASH inflammation and fibrosis stage [17–21]. Fibroblast growth factor 21 (FGF-21) had the function of reducing blood glucose, lipid and insulin levels, reversing liver steatosis and improving insulin sensitivity [22, 23]. Interleukin 1 receptor antagonist (IL-1Ra), the only natural antagonist in cytokines, could suppress the activity of IL-1 and relieve the inflammation, pimelosis and damage of liver [24]. Pigment epithelium-derived factor (PEDF) is an independent risk factor of NAFL [25–27]. PEDF had the ability of anti-fibrosis and was closely related to the development and progression of fibrosis [28]. Osteoprotegerin (OPG) was closely associated with NASH steatosis and the OPG level in NASH patients was clearly lower than that in healthy subjects [29].
Neverthless, the feasibility and accuracy of these biomarkers have not been tested in large corhot, and the sensativity and specificity of the single biomarker in diagnosing NASH are relatively low according to previous reports. Moreover, single serum biomarker can only reflect one aspect of disease progression instead of the overall situation [30, 31]. Therefore, it raises the question whether the combination of these serum biomarkers could improve the accuracy in diagnosing NASH and what form of combination could achieve better diagnostic accuracy.
Our study aims at testing the accuracy of CK-18-M30, FGF-21, IL-1Ra, PEDF and OPG in non-invasive diagnosis of NAFLD and NASH. Meanwhile, our study will for the first time, combine these biomarkers and investigate what kind of combination could yield better performance in diagnosing NASH.
Materials and Methods
Study Participants
179 patients with biopsy-proven NAFLD from 2006 to 2013 were enrolled as training group, and 91 age- and gender-matched healthy subjects were recruited at the same time as controls. 45 alcoholic fatty liver disease (AFLD) patients, 50 hepatitis B virus (HBV) patients, 52 hepatitis C virus (HCV) patients were also included to differentiate NAFLD from other liver diseases. 63 other NAFLD patients were separately collected to validate the diagnosis efficacy. All patients have undergone liver biopsy, the criterion standard, and clinical examinations to ensure definite diagnosis of the disease.
Consecutive adult patients who underwent liver biopsy for suspected NAFLD at Beijing 302 Hospital, Beijing, China were retrospectively and prospectively screened into this study. Patients with history of excessive acloholic consumption (more than 140 g/week for male and 70 g/week for female; Acloholic Consumption = Intake volume × Alcoholic Strength × 0.8) [32] were excluded. Patients with liver diseases which can influence serum biomarker level including viral hepatitis, drug-induced liver disease, total parenteral nutrition, hepatolenticular degeneration and primary liver cancer were also excluded. Histological changes of the enrolled subjects should meet the pathological criteria for fatty liver diseases.
Age (±2 yrs) and gender-matched healthy cases from physical examination population also at Beijing 302 Hospital, Beijing, China were recruited at the same time as controls. All these cases had passed comprehensive liver and metabolic assessment and the recruitment standards included: 1) in good health; 2) with normal liver function; 3) B-ultrasound examination showed no symptoms of fatty liver; 4) without history of excessive alcoholic consumption; 5) without history of diabetes, hypertension and normal metabolic parameters.
All AFLD, HBV and HCV cases had clear evidence of liver injury based on pathological diagnosis and clinical examinations made according to clinical practice guidelines [33–35]. Other enrollment criteria were as follows: 1) history of excessive alcoholic consumption (>140 g/week for male and 70 g/week for female) for AFLD group; 2) Clinical history of chronic hepatitis B or HBsAg (+) for more than 6 months for HBV cases; 3) Clinical history of chronic hepatitis C and no HCV/HIV co-infection for HCV group; 4) The biochemical indicators of the enrolled cases have met with the specific standard of each liver disease. All these three groups of patients should have no history of HCC, liver transplant or decompensated liver diseases.
The study was conducted according to the guidelines of the Declaration of Helsinki. The protocol was approved by the Ethics Committee of Beijing 302 Hospital and all subjects gave their written informed consent to participate in this study.
Clinical and Biochemical Characterization
All subjects were given physical examination as well as physiological and biochemical detection. Body mass index (BMI) was calculated by measurement of height and weight (BMI = weight (kg) / height2 (m2)).
Fasting venous blood of all subjects (NAFLD, AFLD, HBV and HCV patients one day before the liver biopsy) were collected to evaluate liver function and related biochemical indicators, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), AST / ALT, γ-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), cholinesterase, total cholesterol (TC), triglyceride (TG), high density lipoprotein (HDL), low-density lipoprotein (LDL).
Liver Histology
Percutaneous liver biopsy was performed by experienced physicians with a 16-gauge Hepafix needle under the guidance of B- ultrasound [36]. The liver biopsy specimens were immediately fixed in 10% formalin and embedded in paraffin. The histological slides were read and semi-quantitatively scored by two experienced pathologists. Histological grading and staging of NAFLD were scored according to NAFLD activity score (NAS), a semi-quantitative scoring system reported by Kleiner and his colleagues [37]. NAS, ranging from 0–8, was the sum of steatosis, lobular inflammation and hepatocellular ballooning scores. Steatosis was scored from 0 to 3: S0: no steatosis or less than 5%, S1: 5–33%, S2: 34–66%, S3: >66%. Lobular inflammation was graded as follows: stage 0, no foci; stage 1: < 2 foci per 200 × field; stage 2: 2–4 foci per 200 × field; stage 3: > 4 foci per 200 × field. Ballooning degeneration of liver cells was evaluated as: grade 0, absent; grade 1, few; grade 2, a lot. For analysis in this study, NAFLD cases with NAS≥5 were considered as NASH patients and NAFLD cases with NAS≤4 were grouped as non-NASH patients including simple fatty liver cases (NAS 0–2) and borderline NASH cases (NAS 3–4) [38, 39].
Serum CK-18-M30, FGF-21, IL-1Ra, PEDF and OPG
3–4 ml antecubital vein blood was collected from each subject after an overnight fasting. Blood samples were centrifuged at 3000 r/min for 10 min and serum aliquots were stored at −80°C for detection. Serum biomarkers levels were quantified by commercially available ELISA kits (Human CK-18-M30, FGF-21, IL-1Ra, PEDF, OPG, R&D, USA) according to the manufacturer's protocol.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation. Clinical data between groups were compared by Chi-squared test; quantitative variables were analyzed using t-test and one-way analysis of variance for normal distributional data. Intergroup comparisons were performed using ANOVA. Spearman’s correlation coefficient was used to estimate the correlation between serum biomarkers levels and related factors. LSD test was used for multiple pairwise comparison. Multiple linear regression was used to determine the independent factors associated with biomarker levels. Receiver operating characteristic (ROC) curve analysis was conducted to assess the performance of biomarkers in the diagnosis of NAFLD/NASH by calculating the area under receiver operating characteristic curve (AUROC). The maximum sensitivity and specificity of biomarker combination were determined by Youden index. Rank correlation was used to handle variables that were not normally distributed. The statistics were analyzed using the SPSS 18.0 for windows (SPSS, Inc, Chicago, IL, USA) and SAS 9.0 (SAS, Inc, Cary NC, USA). A two-tailed p value of < 0.05 showed the differences were statistically significant.
Results
Patients’ Characteristics
179 NAFLD patients in training group included 86 males and 93 females with a mean age of 30.3±11.8 yrs. Among them, 52 had NAS 0–2, 59 had NAS 3–4 and 68 had NAS≥5, making a 68 NASH cases and 111 non-NASH cases. The validation group was consisted of 33 males and 30 females (mean age, 31.6±9.2yrs), including 20 NAS 0–2, 18 NAS3-4 and 25 NAS≥5. 30–45% of the NAFLD patients were accompanied with type 2 diabetes and hypertension. No significant differences existed in the clinical biochemical indicators between training group and validation group. 91 healthy controls (42 males, 49 females), 45 AFLD patients (24 males, 21 females), 50 HBV patients (29 males, 21 female) and 52 HCV patients (23 males, 29 females) were also enrolled at the same time.
All groups did not differ in terms of age and gender. The disease groups (NAFLD, AFLD, HBV and HCV) and healthy cases had significant differences in clinical biochemical indexes (Table 1). Significant differences existed in the levels of ALT, AST, TG and BMI between non-NASH and NASH cases (P < 0.01). NAFLD group, HBV group and HCV group had differences in TC and TG (P < 0.05) and significant difference in AST/ALT (P < 0.01). Compared with other diseases, cholinesterase was obviously higher in NAFLD (P < 0.01). BMI levels showed obvious differences among NAFLD, HBV and HCV group, but not between NAFLD and AFLD cases.
Table 1. Clinical characteristics of all subjects.
| Control | NAS0~2 | NAS3~4 | NASH | AFLD | HBV | HCV | |
|---|---|---|---|---|---|---|---|
| All | 91 | 52 | 59 | 68 | 45 | 50 | 52 |
| Age (yr) | 29.40±7.00 | 28.30±9.70 | 30.90±14.00 | 31.70±13.50 | 38.00±7.52 | 30.36±15.51 | 35.33±19.31 |
| Gender (male/female) | 42/49 | 29/23 | 26/33 | 31/37 | 24/21 | 29/21 | 23/29 |
| BMI (kg/m 2 )** | 19.80±2.10 | 25.60±2.90 | 26.90±3.60 | 28.70±3.60 | 26.74±1.66 | 22.11±4.39 | 20.37±2.06 |
| ALT(U/L)** | 27.20±10.30 | 65.90±26.10 | 108.80±36.70 | 160.60±28.50 | 122.88±120.51 | 81.55±67.09 | 48.67±51.00 |
| AST(U/L)** | 29.60±5.90 | 33.80±16.50 | 50.10±20.30 | 89.60±25.20 | 47.63±28.55 | 87.55±88.24 | 52.00±44.13 |
| AST/ALT$$ | 0.89±0.26 | 0.53±0.15 | 0.65±0.18 | 0.64±0.21 | 0.60±0.33 | 1.29±1.24 | 1.26±0.49 |
| ALP(U/L) | 93.30±16.20 | 100.80±18.60 | 115.40±17.30 | 157.50±13.70 | 96.00±23.53 | 129.55±94.80 | 117.78±106.18 |
| GGT(U/L) | 36.30±17.60 | 150.30±20.50 | 90.10±16.70 | 87.50±18.60 | 63.88±53.19 | 69.91±152.10 | 30.67±21.12 |
| Cholinesterase (U/L) | 9025.80 ±1231.20 | 8651.40 ±1253.20 | 9239.00 ±1478.20 | 9742.80 ±1576.30 | 8522.89 ±2279.93 | 6576.40 ±1516.10 | 7303.89 ±1411.10 |
| TC(mmol/L)## | 4.75±0.86 | 4.87±0.94 | 4.69±0.78 | 4.78±1.13 | 4.43±0.84 | 3.78±0.42 | 3.61±0.92 |
| TG(mmol/L)## | 1.83±0.49 | 2.31±0.67 | 2.01±0.56 | 1.95±0.36 | 1.79±0.68 | 1.35±1.34 | 1.03±0.35 |
| HDL(mmol/L) | 0.91±0.34 | 1.06±0.22 | 0.95±0.17 | 1.06±0.25 | 0.95±0.13 | 1.19±0.32 | 1.17±0.27 |
| LDL(mmol/L) | 2.88±0.31 | 3.61±0.67 | 3.35±0.63 | 3.24±0.59 | 3.10±0.64 | 2.55±0.40 | 2.36±0.62 |
NAFLD, AFLD, HBV, HCV patients and age-and gender-matched control subjects were enrolled from 2006 to 2013. Significant differences existed in the levels of ALT, AST, TG and BMI between non-NASH and NASH cases.
** Between Non-NASH and NASH groups, significant at P < 0.01.
## Among NAFLD, HBV, HCV group, significant at P < 0.05.
$$ Among NAFLD, HBV, HCV group, significant at P < 0.01
All NASH patients had steatosis and lobular inflammation, while 89.71% of them (n = 61) had ballooning and 92.65% (n = 63) had fibrosis. Significant differences existed in histological features of NAFLD, such as steatosis, ballooning, fibrosis and lobular inflammation, between non-NASH and NASH groups. Steatosis, lobular inflammation, balloon degeneration of hepatocytes, and fibrosis in NASH patients were more serious than those of non-NASH cases (P < 0.01) (S1 Table).
Levels of Serum Biomarkers
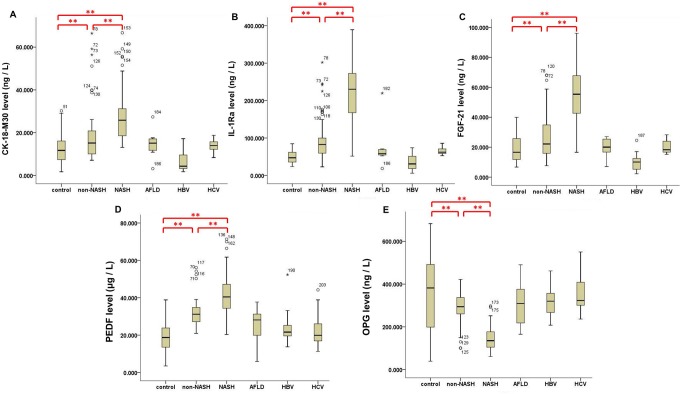
After analyzing the serum biomarker levels in training group and controls, we found that CK-18-M30, IL-1Ra, FGF-21 and PEDF were significantly higher in NAFLD training group (median 18.30 ng/L, 104.80 ng/L, 39.30 ng/L, and 33.50 μg/L respectively) than controls (median 14.10 ng/L, 47.10 ng/L, 16.60 ng/L and 18.70 μg/L respectively). On the contrary, OPG was significantly lower in training group (median 234.10 ng/L) than in controls (median 381.90 ng/L). There were no statistically significant differences among the serum levels of CK-18-M30, IL-1Ra, FGF-21, PEDF and OPG between healthy controls and AFLD, HBV and HCV groups (P > 0.05) (Fig 1; S2 Table).
Fig 1. Serum levels of five biomarkers in control, non-NASH, NASH and other disease groups.
Levels of CK-18-M30, FGF-21, IL-1Ra and PEDF were significantly higher in NAFLD training group (A-D) whereas OPG was significantly lower (E), significant at **P < 0.01. There were no statistically significant differences among the serum biomarker levels between healthy controls and AFLD, HBV and HCV groups.
Also the levels of CK-18-M30, IL-1Ra, FGF-21 and PEDF in NAFLD cases in the training group increased with the elevation of NAS scores while OPG level decreased conversely (S1 Fig). Interestingly, we noted that serum level of some biomarkers specifically differed in certain groups. For example, Serum OPG level could distinguish simple fatty liver patients form the controls (P = 0.01). Serum PEDF level in the borderline NASH patients significantly differed from other NAFLD cases (P < 0.05). Borderline NASH patients and simple fatty liver patients were distinguished significantly by PEDF level. All serum biomarkers levels were highly correlated with NAS (P < 0.01) (S3 Table).
Correlation between Serum and Clinical Biomarkers in Diagnosing NASH
Serum levels of CK-18-M30, IL-1Ra, FGF-21, PEDF and OPG were highly correlated with clinical characteristics and pathological features (S3 Table). Multiple linear regression analysis was conducted to study the association between serum biomarkers levels and pathological features of NASH in NAFLD patients after adjusting for age and gender. We found that steatosis, severe lobular inflammation, ballooning and fibrosis were independent factors of all serum biomarkers.
ROC of Serum Biomarkers in Diagnosing NASH
To test the effectiveness of these serum biomarkers in diagnosing NASH, we performed ROC curve analysis on both non-NASH and NASH cases. All five biomarkers showed good diagnostic performance, and the AUROC were calculated to determine best cut-off value, sensitivity and specificity of each biomarker in diagnosing NASH (Table 2, S2 Fig).
Table 2. AUROC and OR analysis of serum biomarkers to determine effectiveness in diagnosing NASH.
| Biomarker | AUROC | Cut-off Value | Sensitivity | Specificity | OR | 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|
| CK-18-M30 | 0.86 | 17.75 ng/L | 80.30% | 79.60% | 2.25 | 0.68 | 7.49 |
| IL-1Ra | 0.89 | 98.47 ng/L | 80.10% | 76.30% | 10.06 | 2.92 | 34.69 |
| FGF-21 | 0.89 | 40.64 ng/L | 79.30% | 77.40% | 24.76 | 6.82 | 89.86 |
| PEDF | 0.89 | 23.96 μg/L | 79.60% | 78.40% | 1.21 | 0.37 | 3.94 |
| OPG | 0.89 | 252.18 ng/L | 81.30% | 74.60% | 1.38 | 0.36 | 5.26 |
AUROC were calculated to determine best cut-off value, sensitivity and specificity of serum CK-18-M30, IL-1Ra, FGF-21, PEDF and OPG in diagnosing NASH.Logistic regression analysis was performed by using cut-off value of serum biomarkers in diagnosing NASH. OR of each serum biomarker and 95% confidence intervals were also determined.
Multivariate Logistic Regression Analysis
After adjusting some confounding factors such as age and gender, we performed Logistic regression analysis using cut-off value of each biomarker in NASH diagnosis, and obtained the following two regression equations.
The first one involved only the five biomarkers:
And the second one was added other clinical biochemical indicators:
Relative risk (OR) of each serum biomarker and 95% confidence intervals (95% CI) were also determined (Table 2).
Diagnostic Accuracy of Logistic Models
The diagnostic performance of Logistic models was firstly evaluated in training group by ROC curves. When Logistic Y1 was applied, the AUROC of NASH diagnosis was 0.53. Under the condition of maximum sensibility and specificity, the cut-off value and 95% CI were 1.94 and 0.24–0.36, respectively. The diagnostic sensibility and specificity also reached 43% and 56%. For Logistic Y2, the AUROC was 0.44, and the sensibility and specificity for diagnosis was 38% and 44%, respectively.
Then we accessed the diagnostic performances of two Logistic models in validation group. The AUROC was 0.56 for Logistic Y1, while the sensibility and specificity were 42% and 51%, respectively. For Logistic Y2, the AUROC was 0.48, and the sensibility and specificity for diagnosis was 40% and 46%, respectively. There was no statistical differences between training group and validation group (P > 0.05) (S4 Table).
5-step Layered Combination of Serum Biomarkers in Diagnosing NASH
Since the regression equation did not demonstrate better effect in diagnosing NASH than single biomarker did, we decided to use another form of biomarker combination, a 5-step approach measuring CK-18-M30 successively followed by FGF21, IL-1Ra, PEDF and OPG. When FGF21, IL-1Ra, PEDF and OPG were added in proper sequence on the basis of CK-18-M30, the negative predictive value (NPV) and positive predictive value (PPV) in diagnosing NASH both gradually increased (Table 3).
Table 3. Stepwise combination of serum biomarkers increased diagnosis performance for NASH in NAFLD patients.
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|
| CK-18-M30 | 87.00 | 90.00 | 79.00 | 71.00 |
| IL-1Ra | 84.00 | 91.00 | 80.00 | 73.00 |
| FGF-21 | 79.00 | 88.00 | 84.00 | 69.00 |
| PEDF | 83.00 | 75.00 | 78.00 | 70.00 |
| OPG | 85.00 | 82.00 | 83.00 | 64.00 |
| Combined Model | 94.00 | 96.00 | 85.00 | 76.00 |
Combined model demonstrated better effect in diagnosing NASH than single serum biomarker.
Combined Model: Stepwise combination of CK-18-M30, IL-1Ra, FGF-21, PEDF, and OPG in NASH diagnosis.
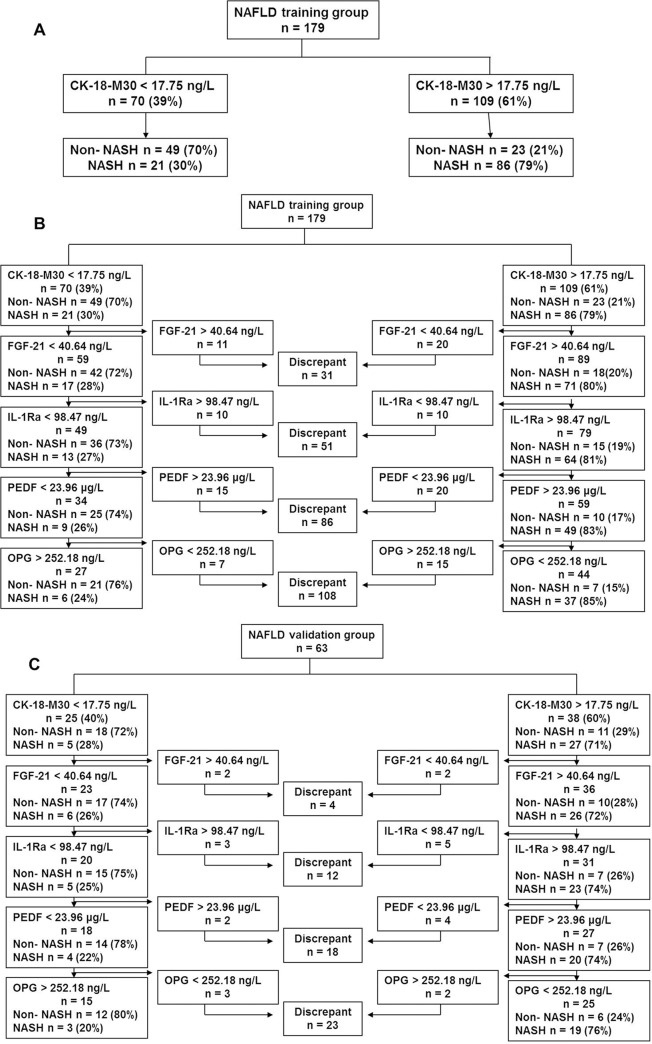
When CK-18-M30 was used alone, 70 patients (39%) had a CK-18-M30 level below 17.75 ng/L. 49 of these 70 patients were non-NASH patients, yielding a NPV of 70% and a sensitivity of 87%. In contrast, 109 subjects had a CK-18-M30 level above 17.75 ng/L, in which 86 subjects were NASH patients, yielding a PPV of 79% and a specificity of 90% (Fig 2A).
Fig 2. NASH diagnosis by serum biomarkers in NAFLD training group and validation group.
(A) NASH diagnosis solely by CK-18-M30. (B) 5-step combination of serum biomarkers in diagnosing NASH in training group. (C) 5-step combination of serum biomarkers in diagnosing NASH in validation group.
FGF-21, IL-1Ra, PEDF and OPG were successively added and the accuracy in diagnosing NASH was further improved. Among the 70 patients with CK-18-M30 level below 17.75 ng/L, 59 had a FGF21 level below 40.64 ng/L. 42 patients did not have NASH, yielding a NPV of 72% and a sensitivity of 88%. Among 109 patients with CK-18-M30 level above 17.75 ng/L, 89 had FGF-21 above 40.64 ng/L, yielding a PPV of 80% and a specificity of 91% (Fig 2B). Finally, when IL-1Ra (cut-off valve 98.47 ng/L), PEDF (cut-off value 23.96 μg/L) and OPG (cut-off value of 252.18 ng/L) were added sequentially, the NPV was elevated to 76%, sensitivity to 94%, PPV to 85%, and specificity to 96% (Fig 2B).
The 5-step approach was then validated in 63 NAFLD patients with paired liver biopsies, in which the proportion of NASH patients was similar to that in training group. 15 out of the 63 validation cases had twice follow–up biopsies and the comparison of two biopsy results showed the 15 cases had progressed from non-NASH to NASH. In these prospective follow–up cases, levels of serum CK-18-M30, FGF-21, IL-1Ra and PEDF were increased, while OPG decreased in cases in non-NASH stage (first biopsy) compared to NASH stage (second biopsy). In 63 NAFLD validation patients, 25 cases had a CK-18-M30 level below the cut-off value, and 38 cases had a CK-18-M30 level above the cut-off value. Then we continued to diagnose NASH with FGF-21, IL-1Ra, PEDF and OPG on this basis, and found 12 patients were non-NASH and the NPV was up to 80%, sensitivity to 95%. 19 NASH cases were predicted by the established noninvasive diagnostic models, the PPV was 76% and the specificity was 97% (Fig 2C).
Discussion
Previous studies demonstrated that the serum levels of CK-18-M30, FGF-21, IL-1Ra, PEDF and OPG exhibited significant differences in patients with NAFLD [40–43, 26]. Growing evidence suggest that these markers play an important role in the pathogenesis of NAFLD. Meanwhile, these markers involve in NAFLD development and are related to steatosis, inflammation, hepatocyte apoptosis and fibrosis during disease progression [44–51]. Our study showed the serum levels of CK-18-M30, FGF-21, IL-1Ra and PEDF were significantly higher in NAFLD patients compared with healthy subjects, positively correlated with NAS scores and pathological characteristics of NAFLD, while the serum level of OPG was the opposite. These findings and the previous reports showed these factors were correlated with NAFLD, leading to the preliminary conclusion that the five biomarkers could be noninvasive diagnostic markers for NAFLD.
To further clarify whether these biomarkers were specific to NAFLD or exhibited differences in all kinds of liver diseases, we tested the serum levels of the biomarkers in AFLD, HBV and HCV groups simultaneously. Interestingly, we found that the statistical differences between NAFLD and healthy controls were more significant than those of other diseases. This further confirmed our presupposition that the five biomarkers had higher specificity for NAFLD and potential in diagnosing NAFLD and NASH.
In addition, we also discovered that the levels of these biomarkers could serve as evaluation indicators of NAFLD degree. An interesting finding during our study was that some biomarkers were specifically differed in cases with certain degree of NAFLD, which meant that they could be used to differentiate different degrees of NAFLD. For example, OPG could differentiate control group and simple fatty liver patients, while PEDF could clearly distinguish simple liver patients from NASH patients. The serum levels of biomarkers in follow-up cases changed with disease progression from non-NASH to NASH. Thus, we could come to the first conclusion that CK-18-M30, FGF-21, IL-1Ra, PEDF and OPG were noninvasive diagnostic serum biomarkers for NAFLD and NASH.
The logistic regression analysis revealed these five biomarkers were independent risk factors of NASH progression. Population with abnormal levels of CK-18-M30, FGF-21, IL-1Ra, PEDF and OPG seemed more possible to suffer from NASH, indicating these biomarkers have a crucial place in NASH prediction.
Two noninvasive diagnosis models were established based on logistic regression analysis of the five serum biomarkers and the other clinical biochemical indicators. However, the ROC curve analysis of the two models in NASH diagnosis showed that the sensibility, specificity, PPV and NPV of two models were lower than those of single index. These findings were in accordance with the previous studies in which they found the diagnostic efficiency of the combined model might be lower than that of the single index. A group of Spanish researchers found that Forns index, a mathematic model, could only distinguish slight hepatic fibrosis, in contrast, the positive rate of diagnosis for significant hepatic fibrosis was lower [52]. The report indicated that this model couldn’t predict and diagnose for hepatic fibrosis accurately [52] and the ideal noninvasive model should possess the characteristics of high accuracy (sensibility, specificity, NPV and PPV values). Nevertheless, Shen et al [53] concluded that the 2-step combination of serum biomarkers could improve the sensibility and specificity of NASH diagnosis. Therefore, our study turned to try the stepwise combination of these five biomarkers for NASH diagnosis.
Combination of these five biomarkers in different sequences had been tried and we finally found the 5-step approach measuring CK-18-M30 successively followed by FGF21, IL-1Ra, PEDF and OPG could achieve better diagnostic performance. The NPV and PPV increased to 76% and 85% respectively and the noninvasive test efficiency was improved. Cases whose non-invasive diagnosis results were not consistent with the biopsy results were put into discrepant zones. The 5-step approach measuring biomarkers increased the noninvasive test efficiency compared to the partial combination of biomarkers. The validation experiment showed that this noninvasive step-by-step test exhibited good sensibility and accuracy in diagnosing NASH.
Our study has several limitations. Firstly, sampling bias may occur as liver biopsy was used as the gold standard. However, liver biopsy is currently the only reference standard for NASH diagnosis. Besides, the biopsy specimens were assessed by two experienced pathologists, which could eliminate the influence of human factors. Secondly, liver biopsies were not performed in control subjects. It is unethical to perform biopsy in cases without liver diseases. Instead, the selection of healthy population was based on physical examination, strict laboratory tests and B-ultrasonic examination, which could exclude the interference of other diseases. Thirdly, the validation cohort was an independently collected and could accurately evaluate the model. Fourthly, the samples were all selected from Chinese population, whether our results could be extrapolated to populations with different ethnic background needs further research. The limitations are common in some genetic and ethnically homogeneous researches.
In conclusion, the serum levels of CK-18-M30, FGF21, IL-1Ra, PEDF and OPG were strongly associated with NASH progression and may serve as biomarkers of NASH identification. The stepwise combination of these biomarkers could further enhance NASH diagnosis accuracy and played an important role in NAFLD clinical diagnosis, therapeutic effect evaluation as well as prevention.
Supporting Information
(TIF) (A~D) The serum levels of CK-18-M30, IL-1Ra, FGF-21 and PEDF in NAFLD training group increased with the elevation of NAS scores. (E) The serum levels of OPG level decreased in NAFLD training group with the elevation of NAS score.
(TIF)
(TIF) The five biomarkers showed good diagnostic performance for NASH. (A) NASH diagnosis by CK-18-M30. (B) NASH diagnosis by FGF-21. (C) NASH diagnosis by IL-1Ra. (D) NASH diagnosis by PEDF. (E) NASH diagnosis by OPG.
(TIF)
(S2 Table, DOC)
(DOC)
(S3 Table, DOC)
(DOC)
(S4 Table, DOC)
(DOC)
Acknowledgments
We thank all the patients and healthy subjects participating in this study. We appreciate Prof. Panyong Mao, Dr. Guangde Zhou and Dr. Fangli Zhang for their instructive advices and useful suggestions.
Data Availability
All relevant data are within the paper and its Supporting Information files
Funding Statement
The work was supported by: 1. Clinical High-tech Key Project (Grant No. 2010jxgs099); 2. Research Found of Capital Medical Development (Grant No. 2014-2-5032), website: http://www.bjhbkj.com/; 3. Chinese Foundation for Hepatitis Prevention and Control—WBE Liver Fibrosis Foundation (Grant No. CFHPC20131020), website: http://www.cfhpc.org/home/Details/787. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003; 98: 960–967. [DOI] [PubMed] [Google Scholar]
- 2. Kim WR, Brown RSJ, Terrault NA, EI-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002; 36: 227–242. [DOI] [PubMed] [Google Scholar]
- 3. Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004; 126: 586–597. [DOI] [PubMed] [Google Scholar]
- 4. Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohep-atitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011; 140: 124–131. 10.1053/j.gastro.2010.09.038 [DOI] [PubMed] [Google Scholar]
- 5. Farrell GC, Wong VW, Chitturi S. NAFLD in Asia—as common and important as in the West. Nat Rev Gastroenterol Hepatol. 2013; 10: 307–318. 10.1038/nrgastro.2013.34 [DOI] [PubMed] [Google Scholar]
- 6. Lonardo A, Sookoian S, Chonchol M, Loria P, Targher G. Cardiovascular and Systemic Risk in Nonalcoholic Fatty Liver Disease-Atherosclerosis As a Major Player in the Natural Course of NAFLD. Curr Pharm Des. 2013; 19: 5177–5192. [PubMed] [Google Scholar]
- 7. Musso G, Anty R, Petta S. Antioxidant Therapy and Drugs Interfering with Lipid Metabolism: Could They Be Effective in NAFLD Patients? Curr Pharm Des. 2013; 19: 5297–5313. [PubMed] [Google Scholar]
- 8. Ahmed MH, Barakat S, Almobarak AO. Nonalcoholic fatty liver disease and cardiovascular disease: has the time come for cardiologists to be hepatologists? J Obes. 2012: 483135 10.1155/2012/483135 Epub 2012 Dec 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998; 114: 842–845. [DOI] [PubMed] [Google Scholar]
- 10. Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006; 43: S99–S112. [DOI] [PubMed] [Google Scholar]
- 11. Kumar KS, Malet PF. Nonalcoholic steatohepatitis. Mayo Clin Proc. 2000; 75: 733–739. [DOI] [PubMed] [Google Scholar]
- 12. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999; 116: 1413–1419. [DOI] [PubMed] [Google Scholar]
- 13. Polyzos SA, Kountouras J, Zavos C. Nonalcoholic fatty liver disease: the pathogenetic roles of insulin resistance and adipocytokines. Curr Mol Med. 2009; 9: 299–314. [DOI] [PubMed] [Google Scholar]
- 14. Greenfield V, Cheung O, Sanyal AJ. Recent advances in nonalcholic fatty liver disease. Curr Opin Gastroenterol. 2008; 24: 320–327. 10.1097/MOG.0b013e3282fbccf2 [DOI] [PubMed] [Google Scholar]
- 15. Kashi MR, Torres DM, Harrison SA. Current and emerging therapies in nonalcoholic fatty liver disease. Semin Liver Dis. 2008; 28: 396–406. 10.1055/s-0028-1091984 [DOI] [PubMed] [Google Scholar]
- 16. Bianchi L. Liver biopsy in elevated liver function tests? An old question revisited. J Hepatol. 2001; 35: 290–294. [DOI] [PubMed] [Google Scholar]
- 17. Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009; 50: 1072–1078. 10.1002/hep.23050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lebensztejn DM, Wierzbicka A, Socha P, Pronicki M, Skiba E, Werpachowska I, et al. Cytokeratin-18 and hyaluronic acid levels predict liver fibrosis in children with non-alcoholic fatty liver disease. Acta Biochim Pol. 2011; 58: 563–566. [PubMed] [Google Scholar]
- 19. Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006; 44: 27–33. [DOI] [PubMed] [Google Scholar]
- 20. Fitzpatrick E, Mitry RR, Quaglia A, Hussain MJ, DeBruyne R, Dhawan A. Serum levels of CK18 M30 and leptin are useful predictors of steatohepatitis and fibrosis in paediatric NAFLD. J Pediatr Gastroenterol Nutr. 2010; 51: 500–506. 10.1097/MPG.0b013e3181e376be [DOI] [PubMed] [Google Scholar]
- 21. Vos MB, Barve S, Joshi-Barver S, Carew JD, Whitington PF, McClain CJ. Cytokeratin 18, a marker of cell death,is increased in children with suspected nonalcoholic fatty liver disease. J Pediater Gastroenterol Nutr. 2008; 47: 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008; 149: 6018–6027. 10.1210/en.2008-0816 [DOI] [PubMed] [Google Scholar]
- 23. Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009; 58: 250–259. 10.2337/db08-0392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012; 122: 3476–3489. 10.1172/JCI60777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen C, Tso AW, Law LS, Cheung BM, Ong KL, Wat NM, et al. Plasma Level of Pigment Epithelium-Derived Factor Is Independently Associatedwith the Development of the Metabolic Syndrome in Chinese Men: A 10-YearProspective Study. J Clin Endocrinol Metab. 2010; 95: 5074–5081. 10.1210/jc.2010-0727 [DOI] [PubMed] [Google Scholar]
- 26. Yilmaz Y, Eren F, Ayyildia T, Colak Y, Kurt R, Senates E, et al. Serum pigment epithelium-derived factor levels are increased in patients with biopsy-proven nonalcoholic fatty liver disease and independently associated with liver steatosis. Clinica Chimica Acta. 2011; 412: 2296–2299. [DOI] [PubMed] [Google Scholar]
- 27. Yilmaz Y, Eren F. Identification of a support vector machine-based biomarker panel with high sensitivity and specificity for nonalcoholic steatohepatitis. Clin Chim Acta. 2012; 414:154–157. 10.1016/j.cca.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 28. Mao T, Gao L, Li H, Li J. Pigment epithelium-derived factor inhibits high glucose induced oxidative stress and fibrosis of cultured human glomerular mesangial cells. Saudi Med J. 2011; 32: 769–777. [PubMed] [Google Scholar]
- 29. An JJ, Han DH, Kim DM, Kim SH, Rhee Y, Lee EJ, et al. Expression and regulation of osteoprotegerin in adipose tissue. Yonsei Med J. 2007; 48: 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tamimi TI, Elgouhari HM, Alkhouri N, Yerian LM, Berk MP, Lopez R, et al. An apoptosis panel for nonalcoholic steatohepatitis diagnosis. J Hepatol. 2011; 54: 1224–1229. 10.1016/j.jhep.2010.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bell LN, Theodorakis JL, Vuppalanchi R, Saxena R, Bemis KG, Wang M, et al. Serum proteomics and biomarker discovery across the spectrum of nonal-coholic fatty liver disease. Hepatology. 2010; 51: 111–120. 10.1002/hep.23271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liver Disease and Metabolic Disorders Research Group (preparing), Chinese Society of Endocrinology. Consensus for diagnosis and treatment of nonalcoholic fatty liver diseases and metabolic disorders. Clin J Endocrinol Metab. 2010; 26: 531–534. (in Chinese) [Google Scholar]
- 33. Fatty Liver and Alcoholic Liver Disease Study Group of the Chinese Liver Disease Association. Guidelines for diagnosis and treatment of alcoholic liver disease. Zhonghua Ganzangbing Zazhi. 2006; 14:164–166. (in Chinese) [PubMed] [Google Scholar]
- 34. Sherman M, Shafran S, Burak K, Doucette K, Wong W, Girgrah N, et al. Management of chronic hepatitis B: consensus guidelines. Can J Gastroenterol. 2007; 21: 5C–24C. [PMC free article] [PubMed] [Google Scholar]
- 35. Alberti A, Benvegnu L. Management of hepatitis C. J Hepatol. 2003; 38: S1014–S118. [DOI] [PubMed] [Google Scholar]
- 36. Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010; 59: 969–974. 10.1136/gut.2009.205088 [DOI] [PubMed] [Google Scholar]
- 37. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005; 41: 1313–1321. [DOI] [PubMed] [Google Scholar]
- 38. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999; 116: 1413–1419. [DOI] [PubMed] [Google Scholar]
- 39. Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011; 54: 344–353. 10.1002/hep.24376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009; 50: 1072–1078. 10.1002/hep.23050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010; 139: 456–463. 10.1053/j.gastro.2010.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yilmaz Y, Yonal O, Kurt R, Oral AY, Eren F, Ozdogan O, et al. Serum levels of osteoprotegerin in the spectrum of nonalcoholic fatty liver disease. Scand J Clin Lab Invest. 2010; 70: 541–546. 10.3109/00365513.2010.524933 [DOI] [PubMed] [Google Scholar]
- 43. Pihlajamäki J, Kuulasmaa T, Kaminska D, Dimonen M, Kärjä V, Grönlund S, et al. Serum interleukin 1 receptor antagonist as an independent marker of non-alcoholic steatohepatitis in humans. J Hepatol. 2012; 56: 663–670. 10.1016/j.jhep.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 44.Gürgen S, Yücel A, Karakuş A, Ceçen D, Ozen G, Koçtürk S. Usage of whey protein may cause liver damage via inflammatory and apoptotic responses. Hum Exp Toxicol. 2014 Oct 28. pii: 0960327114556787. [DOI] [PubMed]
- 45. Tabuchi M, Tomioka K, Kawakami T, Murakami Y, Hiramatsu M,Itoshima T, et al. Serum cytokeratin 18 M30 antigen level and its correlation with nutritional parameters in middle-aged Japanese males with nonalcoholic fatty liver disease (NAFLD). J Nutr Sci Vitaminol. 2010; 56: 271–278. [DOI] [PubMed] [Google Scholar]
- 46. Younossi ZM, Jarrar M, Nugent C, Randhawa M, Afendy M, Stepanova M, et al. A novel diagnostic biomarker panel for obesity-related nonalcoholic steatohepatitis (NASH). Obes Surg. 2008; 18: 1430–1437. 10.1007/s11695-008-9506-y [DOI] [PubMed] [Google Scholar]
- 47. Szalay F, Hegedus D, Lakatos PL, Tornai I, Bajnok E, Dunkel K, et al. High serum osteoprotegerin and low RANKL in primary biliary cirrhosis. J Hepatol. 2003; 38: 395–400. [DOI] [PubMed] [Google Scholar]
- 48. Yamagishi SI, Matsui T, Kawaguchi T, Sata M. Pathophysiological role of pigment epithelium-derived factor (PEDF) in hepatic disorders. Curr Med Chem. 2010; 17: 1995–2000. [DOI] [PubMed] [Google Scholar]
- 49. Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010; 139: 456–463. 10.1053/j.gastro.2010.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yilmaz Y. non-alcoholic fatty liver disease and osteoporosis—clinical and molecular crosstalk. Aliment Pharmacol Ther. 2012; 36: 345–52. 10.1111/j.1365-2036.2012.05196.x [DOI] [PubMed] [Google Scholar]
- 51. Meier CA, Bobbioni E, Gabay C, Assimacopoulos-Jeannet F, Golay A, Dayer JM. IL-1 receptor antagonist serum levels are increased in human obesity: a possible link to the resistance to leptin? J Clin Endocrinol Metab. 2002; 87: 1184–1188. [DOI] [PubMed] [Google Scholar]
- 52. Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002; 36: 986–992. [DOI] [PubMed] [Google Scholar]
- 53. Shen J, Chan HL, Wong GL, Choi PC, Chan AW, Chan HY, et al. Non-invasive diagnosis of non-alcoholic steatohepatitis by combined serum biomarkers. J Hepatol. 2012; 56: 1363–1370. 10.1016/j.jhep.2011.12.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF) (A~D) The serum levels of CK-18-M30, IL-1Ra, FGF-21 and PEDF in NAFLD training group increased with the elevation of NAS scores. (E) The serum levels of OPG level decreased in NAFLD training group with the elevation of NAS score.
(TIF)
(TIF) The five biomarkers showed good diagnostic performance for NASH. (A) NASH diagnosis by CK-18-M30. (B) NASH diagnosis by FGF-21. (C) NASH diagnosis by IL-1Ra. (D) NASH diagnosis by PEDF. (E) NASH diagnosis by OPG.
(TIF)
(S2 Table, DOC)
(DOC)
(S3 Table, DOC)
(DOC)
(S4 Table, DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files