Abstract
The health and national security challenge of antibiotic resistance has led governments to adopt policies to stimulate new antibiotic R&D. Government programs that directly fund late-stage clinical development of antibiotics have emerged, including the Broad Spectrum Antimicrobial Program of the Biomedical Advanced Research and Development Authority in the United States, and the New Drugs for Bad Bugs program of the Innovative Medicines Initiative in the European Union. These efforts are collectively investing nearly $1 billion and are supporting nearly 20% of the global antibiotic pipeline. This article describes these programs, including the antibiotics and their targeted pathogens and clinical indications, as well as program mechanisms for project eligibility, selection, governance, funding, and IP management. Preliminary assessment of the impact of these mechanisms on the success of the programs is provided.
The challenge of antibiotic resistance has led governments to adopt policies to stimulate new antibiotic R&D. Two government programs that directly fund late-stage clinical development of antibiotics have emerged: the Broad Spectrum Antimicrobial Program of the Biomedical Advanced Research and Development Authority in the United States, and the New Drugs for Bad Bugs program of the Innovative Medicines Initiative in the European Union. This article describes these programs and offers a preliminary assessment of their success.
For much of the 20th century, the development and commercialization of antibiotics was a mainstay of the pharmaceutical industry. Beginning in the late 1990s, the industry began withdrawing from antibiotic drug development because of diminishing returns and the increasing financial attractiveness of other therapeutic areas.1-3 However, the medical need for new antibiotics remained and has grown more acute due to the emergence of resistance, generating widespread concern among medical practitioners and public health officials. In response, governments have sought mechanisms to stimulate private industry efforts by offsetting the risk to private capital invested in antibiotic development. Interventions have included “pull” mechanisms intended to incentivize development by increasing the commercial value of antibiotics, such as patent term extensions and advanced market commitments or prizes, as well as “push” mechanisms intended to reduce the amount of private capital needed for development, including regulatory reforms and public funding of antibiotic research and development.4-7
Public funding of research, especially basic research, such as bacterial pathogenesis and mechanisms of antimicrobial resistance, has been supported by government science funding agencies for many years and is widely recognized to provide an essential scientific foundation for the discovery of novel antibiotics. More recently, funding programs have emerged that support antibiotic discovery and preclinical and clinical development in pharmaceutical firms.8,9 The use of public funding for direct subsidization of late-stage development is particularly notable because the costs of development for any one product, including clinical trials and manufacturing design and scale-up, are substantial. In addition, the products under development are relatively close to commercialization and introduction into competitive market environments that are increasingly sensitive to cost. As a result, such programs are likely to test the boundaries of public-private partnerships with respect to issues such as shared risk, governance, transparency, intellectual property, and pricing.
This article compares 2 different approaches—one from the US and the other found in the European Union—to tackling the problem of incentivizing the development of needed antibiotics via public funding of late-stage development. While the final outcome of these projects remains to be seen, and therefore the efficacy of these mechanisms cannot yet be compared, the divergent strategies have already resulted in some notable differences in who and what receives subsidies and how projects are managed and overseen to maximize chances of success.
Late-Stage Antibiotic Development Funding Programs
Two government programs have emerged to support late-stage development of novel antibiotics: the US government's Broad Spectrum Antimicrobial Program in the Biomedical Advanced Research and Development Authority (BARDA), and the New Drugs for Bad Bugs program under the Innovative Medicines Initiative (IMI), a partnership between the European Union and the European pharmaceutical industry. Both of these programs were founded in just the past 5 years, and they collectively represent over $900 million in planned public funding.
Broad Spectrum Antimicrobial Program
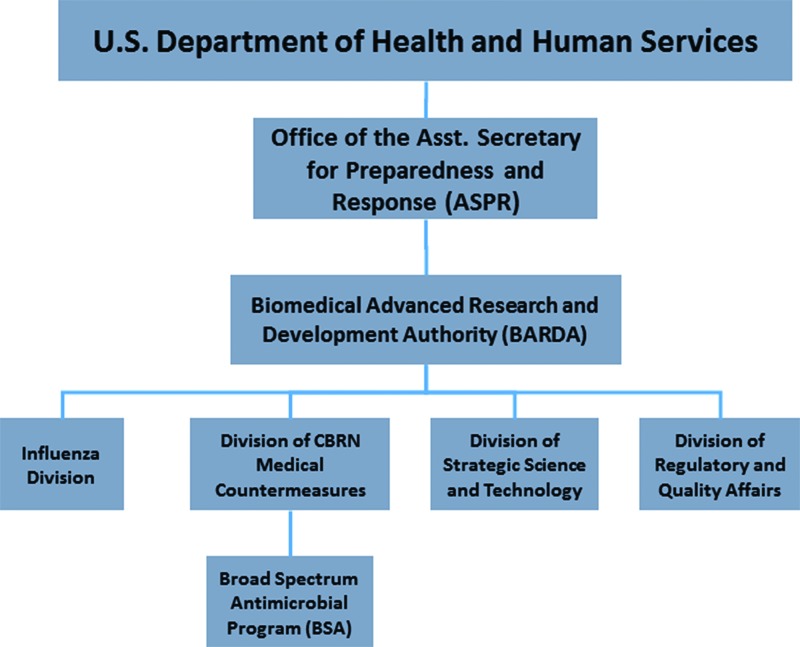
BARDA was established by the US Congress to provide federal investments in later stage development of novel countermeasures to bioterorrism and pandemic threats.10 Housed in the Department of Health and Human Services, the authority's structure reflects its foundational mission to address terrorist (CBRN Countermeasures Division) and pandemic (Influenza Division) threats (Figure 1). In 2010, BARDA established a broad spectrum antimicrobial (BSA) program in the CBRN division. The funding level for the BSA program proposed for the FY2015 budget is $79 million in an overall request of $415 million for BARDA.
Figure 1.
Organizational Structure of the Biomedical Advanced Research and Development Authority
To date, the broad spectrum antimicrobial program has initiated 7 projects with commercial firms that involve late stage development of novel antibiotics (Table 1). Although details of the activities under the projects are not available, the provided funding may be applied to virtually any technical aspect of development, including clinical trials, microbiology and animal studies, manufacturing development, and regulatory costs. In addition, each project involves development toward clinical and biodefense applications. All of the supported projects involve small molecule antibiotics being advanced as new chemical entities, although most are new derivatives of existing classes of drugs. Two have involved novel mechanisms of action, both from GSK. GSK is also the only large, multinational pharmaceutical company funded under the broad spectrum antimicrobial program to date. Other companies have included 2 mid-sized specialty pharma companies, Basilea, based in Switzerland, and The Medicines Company, based in the US. In addition, 3 US-based, small precommercial firms have been engaged: Achaogen, Cempra, and Tetraphase.
Table 1.
Investigational Antibiotics Supported by BARDA BSA Programa
| Innovator Company | Investigational Product | Drug Class | Targeted Indications | Targeted Pathogens | Targeted Biodefense Pathogens | Start Date | Total Contract (Base Period) Value in $Millions |
|---|---|---|---|---|---|---|---|
| The Medicines Companyb | Carbavance | Carbapenem/β-lactamase inhibitor | cUTI, HAP/VAP | Enterobacteriaceae, P. aeruginosa, A. baumannii | B. mallei, B. pseudomallei | 2014 | 89.8 (19.8) |
| Basilea | BAL 30072 | Monobactam | cUTI, cIAI, HAP/VAP | Enterobacteriaceae, P. aeruginosa, A. baumannii | B. mallei, B. pseudomallei | 2013 | 89 (16.8) |
| Cempra | Solithromycin | Macrolide | CABP | MRSA | B. anthracis, F. tularensis | 2013 | 58 (17.7) |
| GSK | GSK2140944, and others TBD | Topoisomerase inhibitorc | ASSSI, CABP | MRSA and others TBD | B. anthracis, Y. pestis, F. tularensis, and others TBD | 2013 | 196 (40) |
| Tetraphase | Eravacycline | Tetracycline | cUTI, cIAI | MRSA, Enterobacteriaceae | B. anthracis, Y. pestis, F. tularensis | 2012 | 67 (11.5) |
| GSKd | GSK 2251052 | Leucyl tRNA synthetase inhibitorc | cUTI, cIAI, HAP/VAP | Enterobacteriaceae | B. anthracis, Y. pestis | 2011 | 94.5 (38.5) |
| Achaogen | Plazomicin | Aminoglycoside | BSI, HAP/VAP | Enterobacteriaceae | Y. pestis, F. tularensis | 2010 | 103 (27) |
Abbreviations: ABSSI=acute bacterial skin and skin structure infections, BSI=blood stream infections, cIAI=complicated intraabdominal infections, cUTI=complicated urinary tract infections, HAP=hospital-acquired pneumonia, VAP=ventilator-associated pneumonia, GSK=GlaxoSmithKline, plc., TBD=to be determined.
From company and BARDA press releases.
The project is carried out by Rempex, a wholly owned subsidiary of The Medicines Company, Inc.
Denotes a novel mechanism of action.
The development program for GSK2251052 was suspended later in 2012, and the associated contract with BARDA was terminated. See O'Dwyer K, et al. Bacterial resistance to leucyl-tRNA synthetase inhibitor GSK2251052 develops during treatment of complicated urinary tract infections. Antimicrob Agents Chemother 2014;58: published online ahead of print.
New Drugs for Bad Bugs Program
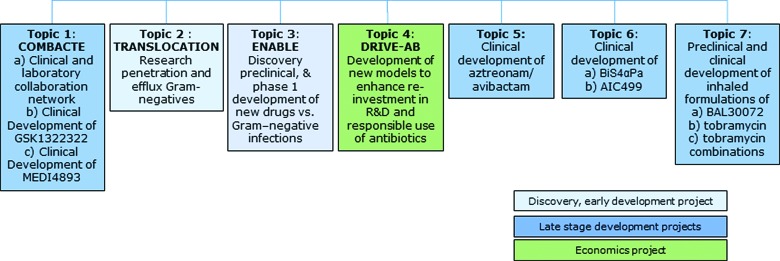
The Innovative Medicines Initiative is a public-private partnership between the EU and the European Federation of Pharmaceutical Industries and Associations (EFPIA), an industry association of European and multinational pharmaceutical companies. Established in 2008, the IMI uses joint public and private funds to support multiple projects across several therapeutic areas, as well as research questions in precompetitive fields such as predictive toxicology. In 2012, the New Drugs for Bad Bugs (ND4BB) program was established to focus specifically on antibiotic resistance, including the development of novel antibiotics, research topics in basic and translational science, and a project to examine and revise the underlying business model for commercial antibiotics (Figure 2). The effort arose from the formal determinations by Europe-wide government bodies that antimicrobial resistance posed a serious cost to European society and that a large-scale collaborative initiative with the EFPIA should be undertaken.11
Figure 2.
Overview of Projects Funded by the IMI's New Drugs for Bad Bugs Program. The late-stage development projects are the subject of this article.
The current set of ongoing or announced projects for late-stage antibiotic development and public and private funding (including in-kind contributions) levels are shown in Table 2. The projects include small molecules from known classes, as well as one molecule with a novel mechanism of action. In addition, 2 antibody projects are funded that represent novel treatment modalities and approaches for antibacterial treatment. In contrast to BARDA funding, which has supported multiple aspects of drug development, ND4BB funding focuses almost exclusively on clinical-trial and related expenses. The ND4BB has engaged primarily multinational pharmaceutical companies, concentrating on those based in Europe, along with US-based specialty pharma Cubist and Switzerland-based Basilea. AiCuris has been the only small, precommercial company engaged as a development leader thus far.
Table 2.
Antibiotic Development Projects Supported by the IMI ND4BB Programa
Ongoing or Planned Funding ( millions) millions) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Project Name | Contributing EFPIA Members | Investigational Product | Drug Class | Targeted Pathogens | Targeted Indicationsb | Start Date | Public | Private |
| COMBACTE | GSK, AstraZeneca, Janssen | GSK1322322c | Peptide deformylase inhibitord | MRSA | CABP, cSSSI | 2013 | 88 | 104 |
| MEDI4893 | Abd | MRSA | VAP, cSSSI | 26.4 | 25.4 | |||
| (Topic 5) | GSK, AstraZeneca, Basilea, Cubist | aztreonam/avibactam | Monobactam/β-lactamase inhibitor | Enterobacteriaceae, P. aeruginosa, Acinetobacter | cIAI, HAP/ VAP | TBD | 30.6 | 41.6 |
| (Topic 6) | GSK, AstraZeneca, Sanofi-Aventis, Novartis, AiCuris, Basilea | BiS4αPa | Abd | P. aeruginosa | VAP | TBD | 55.4 | 72.6 |
| AIC499 | β-lactam/β-lactamase inhibitor | Enterobacteriaceae, P. aeruginosa, Acinetobacter | cUTI, cIAI | 19.9 | 19.0 | |||
| (Topic 7) | Novartis, Basilea | BAL30072 (inhaled form) | Monobactam | P. aeruginosa, Acinetobacter, other non-fermenting gram-negativese | CF and non-CF BE | TBD | 27.0 | 31.0 |
| tobramycin inhaled powder (TIP) | Aminoglycoside | |||||||
Abbreviations: BE=bronchiectasis, cIAI=complicated intraabdominal infections, cSSSI=complicated skin and skin structure infections, cUTI=complicated urinary tract infections, CABP=community-acquired bacterial pneumonia, GSK=GlaxoSmithKline, plc. HAP=hospital-acquired pneumonia, Ab=antibody therapeutic, MRSA=methicillin-resistant S. aureus, TBD=to be determined, VAP=ventilator-associated pneumonia, CF=cystic fibrosis.
From the 6th, 8th, 9th, and 11th IMI Call for Proposals. http://www.imi.europa.eu/content/overview-imis-calls-how-participate.
In planned clinical studies, MEDI4893 and BiS4αPa are not to be used as therapeutics, but instead are to be administered prophylactically and reduction of incidence or severity of disease assessed.
The development program for GSK1322322 has reportedly been terminated. Pew Charitable Trusts, Antibiotics Currently in Clinical Development, September 2014. http://www.pewtrusts.org/∼/media/Assets/2014/10/AntibioticsInnovationProject_DataTableOct2014_v3.pdf?la=en. Accessed November 11, 2014.
Denotes a novel mechanism of action.
Stenotrophomonas maltophilia, Burkholderia cepacia, Achromobacter (or Alcaligenes) xylosoxidans, Ralstonia spp., and Pandoraea spp.
Comparison of BARDA-BSA and IMI-ND4BB
Project Eligibility
The BARDA and IMI initiatives differ substantially in terms of what entity may receive funding and where these funds may be expended. Funding from BARDA is open to both foreign and domestic companies, and several foreign companies have received awards. Moreover, funds may be spent in any geographic location, including at ex-US manufacturing subcontractors and clinical sites. In contrast, EU funding under IMI is limited to entities in the EU. For example, clinical sites outside of the EU cannot receive public funds. Within IMI, funding is further limited to small and medium-sized for-profit businesses, not-for-profits, and universities.12 Thus, the EFPIA pharmaceutical firms themselves are not eligible to receive public funds, although work done on behalf of these companies, such as the conduct of clinical trials at participating hospitals, universities, and service firms in the EU, is funded. In fact, for most IMI projects, not only can no public funds be spent outside of the EU, but private funds spent by EFPIA companies outside of the EU typically cannot be counted toward their cost share.13 However, for the ND4BB program, this rule has been relaxed to allow for some non-EU funding to be accounted for in the EFPIA-company share. Stated reasons for this exception include the recognized public health threat of resistance and because “the majority of drug development activities are being conducted outside of the E.U.”14(p12)
Project Selection
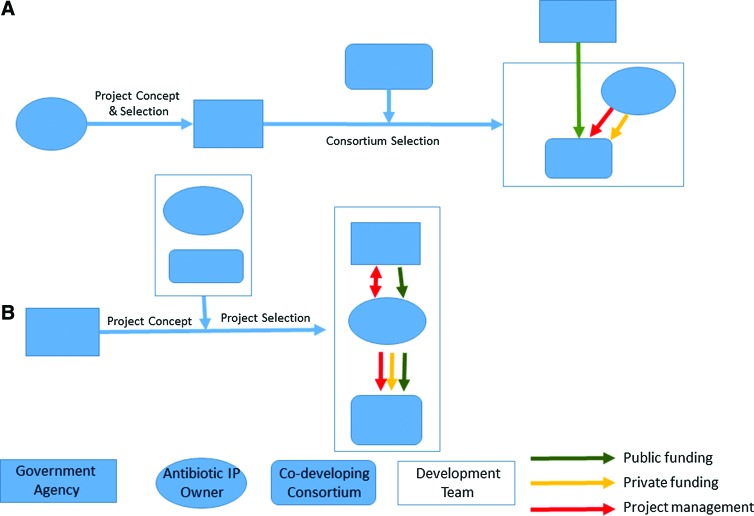
The origin and ultimate design of funded projects follow different paths in the US and EU (Figure 3). All BARDA-supported antibiotic projects to date have involved development toward both biodefense and resistant pathogens of public health and commercial interest and can thus be classified as “dual use.” This reflects the foundational mission and legislative authority of BARDA. The policy was explicitly stated in BARDA's 2011-2016 Strategic Plan, which noted, “BARDA will support the development of candidate antimicrobials for their commercial, clinically prevalent infectious disease indications, as long as our private sector partners concomitantly support the development of these products for biodefense threat agent indications.”15(p9) BARDA issues requests for proposals that provide high-level guidelines for projects including the stage of development, required biodefense spectrum of the agent, general nature of fundable activities (eg, manufacturing and clinical trials), and acceptable therapeutic modalities (eg, small molecule, monoclonal antibody), but otherwise leave it to innovators to propose a particular developmental antibiotic, describe the development path, and detail any collaborators and subcontractors slated for participation.16 The targeted nonthreat pathogen and commercial indication is not proscribed, and it is left to the innovator to propose a target product profile and to BARDA to accept its scientific, medical, and societal importance. Selection of the proposal is made following a technical evaluation by government personnel and nongovernment technical experts engaged for the purposes of the review following published criteria.
Figure 3.
IMI (A) and BARDA (B) Mechanisms for Project Selection, Financing, and Management
The second GSK project awarded in 2013 represents a departure from the approach described above and is unique among BARDA projects to date. Described by BARDA as a “strategic alliance” following a “portfolio approach,” it involves a “flexible” arrangement that includes development of GSK2140944 but also allows new drug candidates to be moved in or out of the project based on scientific progress.17 Projects in-licensed by GSK from third parties during the course of the agreement can also be considered. Key decisions surrounding the projects are to be decided at semi-annual portfolio reviews.
In contrast, the ND4BB program defines the antibiotic to be developed and its development path, including targeted indications at an early stage, prior to the request for proposals. In addition, biodefense applications are not pursued. The IMI describes the activities to be conducted, such as a clinical trial of a drug for treatment of lung infections, and seeks a consortium of organizations—typically universities, hospitals, other not-for-profits, and small and medium-sized businesses—to perform the project in collaboration with the developer. The selection of antibiotic to be developed is made by the EFPIA companies, with the detailed content developed by the companies and the IMI executive director's office.18 The research topics must then be approved by the IMI governing board, which consists of equal numbers of representatives of the EFPIA and European Commission.19
Funding Mechanism and Cost Sharing
In the US, contracts issued by federal agencies are mandated to follow contract terms and structures defined through the Federal Acquisition Regulation (FAR). BARDA usually issues contracts that follow a “cost-reimbursement” structure in which a “prime contractor,” typically the owner of the developmental antibiotic, executes the development program while being reimbursed for development costs incurred, up to a predefined limit or ceiling.20 In this approach, the antibiotic developer receives payment for costs even if the project eventually fails. Firms are also typically allowed a fee, or profit, under such contracts.21 Incurred costs are auditable, and payment back to the government can be required if the costs were not incurred under the contract's terms. Development milestones are integrated into projects in the form of contract options. In these models, the initial contract award starts a “base period” associated with a set cost ceiling and achievement of a certain stage of development. If the milestone is achieved, an option can then be exercised that increases the ceiling and allows reimbursement for costs for activities related to the next stage of development.
In addition to standard cost-reimbursement contracts, BARDA has applied Other Transactions Authority (OTA). OTA allows an agency to issue contracts outside of FAR rules, potentially permitting much more “commercial-like” partnerships to be established. An example of such an agreement is BARDA's second contract with GSK. The terms of this agreement are not currently publicly available, and the structure of the funding mechanisms cannot be described here. BARDA has also recently emphasized the use of cost-sharing arrangements, particularly when commercial application of the resultant product is likely.22,23 With cost-sharing, the antibiotic developer explicitly shares costs of contracted activities with BARDA and no fee can be collected. The extent and nature of sharing are not rigidly defined either in the FAR or by BARDA and are instead proposed by the developer and ultimately negotiated with the authority.
In the ND4BB program, as across all IMI programs, cost-sharing is strongly emphasized and well-defined. EFPIA companies commit to contributing funds and in-kind contributions up to a pre-agreed value. EU funds flow through a “managing entity” that itself is one of these eligible organizations, such as a research university, under the direction of the EFPIA company “coordinator.” Fundable entities can receive only up to 75% of research costs from EU funds, with the balance coming from the EFPIA participants. Profits, even by third-party contractors, are not allowed to be paid with EU funds. As with US government contracts, funds are paid as reimbursements for incurred costs and are subject to ceilings, as well as rules for cost allowance and auditing. However, in contrast to US programs, the expended EFPIA funds and in-kind contributions must be accounted for and reported to the IMI. Also, funding is not contractually linked to the achievement of technical milestones.
Project Governance
Under both BARDA and IMI regimes, the antibiotic developer retains primary control and ultimate responsibility for the conduct of the development program. The activities of the consortium of entities participating in the project are managed by the developer, including the disbursement of project funds, whether public or private. In turn, BARDA and IMI retain the rights to audit technical and cost aspects of the project at any time, as well as to cancel funding. However, the 2 programs differ substantially in the frequency and degree of government involvement in program oversight and decision making.
In the majority of its projects, BARDA contractually requires reporting regularly and with high frequency, including quarterly face-to-face meetings, monthly technical reporting, and bi-weekly teleconferences. BARDA also reviews and approves clinical protocols, regulatory submissions, and other important components of a development project. Further, BARDA has required contractors to implement Earned Value Management, a project management system that is not standard in the pharmaceutical industry and that requires expert assistance or training and specialized software. To manage the resultant workload, BARDA assigns a team of technical experts, typically consultants with former industry experience, to each project. For key milestone decisions, the use of contract options requires both the developer and the government to mutually agree on proceeding with a project prior to commitment for further funding. Critical points such as this often trigger an “in process review,” a formal assessment by experts from multiple government agencies, prior to any decision by the government.15
Within IMI there is very little involvement by government personnel in the day-to-day management of a project or in major milestone decisions. Written reports on technical progress are required infrequently, such as once a year. There is a process for formal interim evaluations performed by a set of independent experts, but the IMI website currently indicates these happen once, 2 years after a project starts. Key development milestone decisions are made by the coordinating EFPIA company.
Management of Intellectual Property and Data
Intellectual property (IP), particularly patents, plays a critical role in pharmaceutical development, as it protects an innovator from generic competition and allows recoupment of R&D investment. In the later stage of pharmaceutical development, IP usually plays a less central role as the most important IP, such as composition of matter of the active drug substance, is typically established during preclinical development. One possible exception is IP related to manufacturing and formulation technology, which can be developed later and, in some cases, be an important aspect of protection from generic competition during a product's lifecycle. The control of scientific data generated during development is also crucial for competitive and, during clinical trials, ethical reasons. Normally, if IP or data are developed at private expense, ownership and control remains with the innovator or license holder once brought into US and EU funding programs. However, the involvement of public funding may raise issues of public rights and openness.
In the US, IP rights are governed by the Bayh-Dole Act of 1980 and contractually implemented via the FAR.24 Under this regime, the developer may retain rights to the IP, while the government retains a nonexclusive license to utilize the IP for its own purposes. The government may also exercise “March-in-Rights” to acquire IP it has paid for.25 IP developed with federal funds is also subject to a “preference for US industry,” requiring commercialized derivative products to be manufactured in the US, although waivers can be obtained. With respect to collaborators, a contractor must specifically allow subawardees that are not-for-profits or small businesses to retain license to the IP they develop, subject to Bayh-Dole terms. More generally, the FAR directs that contractors cannot “use their ability to award subcontracts as economic leverage to acquire rights for themselves in inventions.”26
Data, in contrast, is co-owned by the government and the developer under BARDA contracts. However, companies do have an expectation of confidentiality from government officials, since release of private confidential information by such officials is prohibited.27 For contractors working for the government, separate confidentiality agreements are generated. Within a consortia assembled by the antibiotic developer, confidentiality may be imposed as is standard business practice. Notably, because an OTA contract is not subject to the FAR, these IP and data rights terms may be changed or waived.28
The EU does not seek any rights to IP developed with public funding, and there are no restrictions on withholding IP rights to third parties, such as subcontractors. However, management within the development consortia plays a greater role. As awards are given to consortia of self-assembled performers, these groups may not all be familiar or comfortable with the business practices that are standard in the pharmaceutical industry. Here, IMI lays out broad principles for the management of IP, such as the sharing of new IP among all participants that contributed to the invention, as well as an expectation of confidentiality among participants and a right of prepublication review.29 However, all of these terms can be modified under the consortium agreement governing a particular project. Conflicts in IMI consortia over data access have been reported. For example, one not-for-profit group withdrew from the consortium for GSK1322322, citing in an open letter concerns regarding transparency including a requirement in the project agreement that GSK provide written approval for any publications that included project data.30
Postcommercialization Commitments
Consideration for postmarketing returns on investment, such as royalties or milestone payments based on sales targets, is common in private precommercial product licensing agreements, as well as in product development agreements with industry issued by not-for-profit agencies such as the Bill and Melinda Gates Foundation or the Wellcome Trust. However, neither BARDA nor the IMI have policies that place explicit postcommercialization commitments or requirements on the owners of the antibiotic projects they have supported. Such requirements could counteract the core public policy's intent to incentivize industry to pursue antibiotic drug development.
Discussion
Given the recent emergence of the ND4BB and BSA programs, it is too early to judge their impact on improving the number of new antibiotics approved or in development. But by other measures, the programs already appear to be having a significant impact. Nearly 1 in 5 antibiotics in clinical development globally, including half of those directed to gram-negative infections, are affiliated with BARDA and/or IMI.31 The majority of the projects supported are addressing serious infections caused by multi-drug resistant gram-negative bacteria, an area recognized as having the highest unmet medical need.32-34 In addition, the efforts include antibiotics with novel mechanisms of action and, in the case of the IMI, antibody approaches that represent new modalities for antibacterial treatment. Both programs are also supporting initiatives to enhance the antibiotic development process, including key epidemiologic studies to enhance disease progression and clinical outcome understandings, novel clinical trial designs, limited population regulatory approaches, and development and integration of rapid diagnostics and biomarkers. Thus, BARDA and IMI have taken on high-value programs and are positioned at the forefront of antibiotic development.
The BSA and ND4BB programs have made this progress despite certain limitations on their scope. Thus far, BARDA has required all funded projects to address biodefense pathogens as well as the more clinically prevalent targets. This policy may limit the participation of companies that do not wish to invest in developing the capability for drug development in this specialty area. It may also account for the high number of small businesses participating in the BARDA program, which have higher barriers to accessing risk capital from traditional sources. Small firms are also adaptable and may be more willing to take on compliance with the FAR and BARDA's project governance requirements than larger companies. It is notable in this regard that BARDA's use of OTA authority and application of more flexible program management structures have been applied in collaborations with GSK, a large multinational pharmaceutical firm. These trends may change, as BARDA's program is rapidly evolving. A recent presidential executive order, issued as part of an executive branch initiative addressing antimicrobial resistance, appears to decouple the need for BARDA-funded antibiotic programs to address both public health and biodefense indications.35 The impact of this change on BARDA's support for antibiotic projects has yet to be seen, but it could result in engagement of a greater breadth of industry, support for new classes of drugs such as vaccines and narrow-spectrum therapeutics, and expansion of the program as resources previously set aside for biodefense-directed development become available.
The IMI imposes severe restrictions on any EU funding spent outside of Europe, as well as requiring EFPIA membership to participate. This limits access to development projects that happen to be owned by companies without operations in the region, as well as to antibiotic projects that may have all or substantially all development components conducted on other continents. To date, all of the developmental antibiotics advanced by ND4BB are derived from EFPIA member company pipelines. None are owned by companies based outside of Europe, even though several multinational and specialty pharmaceutical firms based in the US and Japan are members of the EFPIA. Moreover, neither the mechanisms for obtaining EFPIA membership nor the method for selecting a development program from within the consortium for funding are transparent. As interest in IMI increases, EFPIA may change its procedures. However, it is not likely that the EU will change its funding policies without substantial pressure. Thus, promising projects that happen to be owned or have clinical programs outside of Europe will be shut out of IMI for the foreseeable future.
The European and US efforts have established public-private partnership models that appear to balance both the business and public interest with some success. However, the balance remains uneasy. The challenge of intellectual property and data rights are managed within well-defined regimes acceptable to both parties, although not without some controversy.36 A shared financial risk model has been adopted, with the costs of development divided between government and the private partner. Here, the primary difference lies with transparency. Under IMI the size of the private share is public and carefully accounted. Under US programs, details of the contribution of the private partner remain confidential. Perhaps most profoundly, BARDA takes an active role in the selection of antibiotic projects and oversight of the project. In contrast, for the late-stage clinical development programs under ND4BB, it is largely left to the EFPIA companies to determine which antibiotic project to advance within the program, and both large and small decisions are left to the developer. While BARDA's active role may be uncomfortable for some companies and thus a factor in limiting participation, it is not yet clear which of these 2 models (if either) supports a more enriched antibiotic armamentarium. However, another important test of the success of these programs will be public acceptance, particularly when the new antibiotics from these programs are introduced commercially with premium prices. In the US, for example, protests have formed over the prices charged by companies for medicines originally discovered with NIH grant funding even though the private sector was responsible for clinical development.37 One can easily imagine a similar controversy developing around a high-priced antibiotic that has received substantial public subsidies for costs immediately preceding market introduction. Outcomes here are also in the future, but both companies and governments alike may wish to begin to evaluate now the public reaction to the partnership models they have established.
Conclusion
The need for new antibiotics has grown acute as bacterial drug resistance has become a global health concern. The US and EU are leading the world in establishing policies to incentivize pharmaceutical industry firms to enter a field they have abandoned, or have threatened to abandon. The new BARDA and IMI funding programs are among the most visible of these efforts, and they are already showing signs of having the potential to make a significant impact on this challenge. But the policies differ substantially in their approaches to cost sharing, project governance, and project selection. Determination of optimal strategies for these public-private partnerships remain to be seen and will ultimately be judged not only on the basis of productivity, but also broad acceptance of the balance among public investment, private gain, and public health reward.
References
- 1.Kinch MS, Patridge E, Plummer M, Hoyer D. An analysis of FDA-approved drugs for infectious disease: antibacterial agents. Drug Discov Today 2014;19(9):1283-1287 [DOI] [PubMed] [Google Scholar]
- 2.Katz ML, Mueller LV, Polyakov M, Weinstock SF. Where have all the antibiotic patents gone? Nat Biotechnol 2006;24(12):1529-1531 [DOI] [PubMed] [Google Scholar]
- 3.Projan S. Why is big pharma getting out of antibacterial drug discovery? Curr Opin Microbiol 2003;6(5):427-430 [DOI] [PubMed] [Google Scholar]
- 4.Sertkaya A, Eyraud J, Birkenbach A, et al. Analytical Framework for Examining the Value of Antibacterial Products. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health & Human Services; April 2014. http://aspe.hhs.gov/sp/reports/2014/antibacterials/rpt_antibacterials.cfm Accessed October29, 2014 [Google Scholar]
- 5.Laxminarayan R, Powers JH. Antibacterial R&D incentives. Nat Rev Drug Discov 2011;10(10):727-728 [DOI] [PubMed] [Google Scholar]
- 6.Matheny J, Mair M, Mulcahy A, Smith BT. Incentives for biodefense countermeasures development. Biosecur Bioterror 2007;5(3):228-238 [DOI] [PubMed] [Google Scholar]
- 7.Nathan C, Goldberg FM. The profit problem in antibiotic R&D. Nat Rev Drug Discov 2005;4(11):887-891 [DOI] [PubMed] [Google Scholar]
- 8.Rex JH. ND4BB: addressing the antimicrobial resistance crisis. Nat Rev Microbiol 2014;12:231-232 [Google Scholar]
- 9.Peters NK, Dixon DM, Holland SM, Fauci AS. The research agenda of the National Institute of Allergy and Infectious Diseases for antimicrobial resistance. J Infect Dis 2008;197(8):1087-1093 [DOI] [PubMed] [Google Scholar]
- 10.42 U.S.C. § 247d–7e, enacted via Title IV of the Pandemic and All-Hazards Preparedness Act (Public Law 109-417)
- 11.Directorate-General for Health & Consumers, European Commission. Communication from the Commission to the European Parliament and the Council: Action Plan Against the Rising Threats from Antimicrobial Resistance. COM (2011) 748. November 2011. http://ec.europa.eu/dgs/health_consumer/docs/communication_amr_2011_748_en.pdf Accessed November9, 2014
- 12.Innovative Medicines Initiative. Rules for Participation in the IMI Joint Undertaking Collaborative Projects. 2012. http://www.imi.europa.eu/webfm_send/486 Accessed November6, 2014
- 13.Innovative Medicines Initiative. IMI Financial Guidelines. Version 2, June 2013. jhttp://www.imi.europa.eu/sites/default/files/uploads/documents/FinancialGuidelines_June2013.pdf Accessed November6, 2014
- 14.Innovative Medicines Initiative. 6th Call for Proposals 2012. http://www.imi.europa.eu/sites/default/files/uploads/documents/6th_Call/IMI_6thCall_v1.0_20120508.pdf Accessed November6, 2014
- 15.Biomedical Advanced Research and Development Authority. BARDA Strategic Plan 2011-2016. October 2011. http://www.phe.gov/about/barda/Documents/barda-strategic-plan.pdf Accessed October15, 2014
- 16.BARDA. Broad Agency Announcement for the Advances Research and Development of Chemical, Biological, Radiological, and Nuclear (CBRN) Medical Countermeasures for BARDA, CBRN-BAA-13-SOL-0013, July 31, 2013
- 17.HHS forms strategic alliance to develop new antibiotics; approach provides a pipeline of new drugs rather than a single medical countermeasure [news release], May 22, 2013. US Department of Health and Human Services; http://www.phe.gov/Preparedness/news/Pages/strategic-alliance-130522.aspx Accessed October16, 2014 [Google Scholar]
- 18.Innovative Medicines Initiative. Overview of IMI's calls for proposals. http://www.imi.europa.eu/content/overview-imis-calls-how-participate Accessed November11, 2014
- 19.Council Regulation (EC) No 73/2008 of December 20, 2007 Setting up the Joint Undertaking for the Implementation of the Joint Technology Initiative on Innovative Medicines
- 20.FAR 16.301
- 21.FAR 15.404-4(c)(4)(i)(A)
- 22.Using innovative business models to enhance antibiotic development. US Department of Health and Human Services website; ASPR Blog Post, July 9, 2014. http://www.phe.gov/ASPRBlog/Lists/Posts/Post.aspx?ID=98 Accessed October, 16, 2014 [Google Scholar]
- 23.The Medicines Company. BARDA awards contract worth up to $90 million to The Medicines Company/Rempex for development of gram-negative antibiotic [news release]. February 5, 2014. http://ir.themedicinescompany.com/phoenix.zhtml?c=122204&p=irol-newsArticle&ID=1897071 Accessed October16, 2014
- 24.FAR 52.227-11
- 25.35 U.S.C. 203 and 210(c)
- 26.FAR 27.304-3(c)
- 27.18 U.S.C. 1905.
- 28.American Bar Association, Section of Public Contract Law, Ad Hoc Working Group on Other Transactions, Department of Defense “Other Transactions”: An Analysis of Applicable Laws, 2000 [Google Scholar]
- 29.IMI Intellectual Property Policy. 2007. http://www.imi.europa.eu/sites/default/files/uploads/documents/imi-ipr-policy01august2007_en.pdf Accessed November11, 2014
- 30.Garattini S, Bertelé V, Bertolini G. A failed attempt at collaboration. BMJ 2013;347:f5354. [DOI] [PubMed] [Google Scholar]
- 31.Pew Charitable Trusts. Antibiotics currently in clinical development. September 2014. http://www.pewtrusts.org/∼/media/Assets/2014/10/AntibioticsInnovationProject_DataTableOct2014_v3.pdf?la=en Accessed November11, 2014
- 32.World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. April 2014. http://apps.who.int/iris/bitstream/10665/112647/1/WHO_HSE_PED_AIP_2014.2_eng.pdf?ua=1 Accessed November14, 2014
- 33.Boucher HW, Talbot GH, Benjamin DK Jr, et al. 10×’20 progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis 2013;56(12):1685-1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control. Antibiotic Resistance Threats in the United States, 2013. April 2013. http://www.cdc.gov/drugresistance/threat-report-2013/index.html Accessed April6, 2015
- 35.E.O. 13676. Combating Antibiotic-Resistant Bacteria. September 18, 2014, Section 8
- 36.Jack A. Compound interests: how a partnership between academics and a drug company came unstuck. BMJ 2013;347:f5356. [DOI] [PubMed] [Google Scholar]
- 37.Connolly C. NIH declines to enter AIDS drug price battle. Washington Post August 5, 2004:A4 [Google Scholar]