Abstract
Dendritic cells (DCs) play a significant role in establishing self-tolerance through their ability to present self-antigens to developing T cells in the thymus. DCs are predominantly localized in the medullary region of thymus and present a broad range of self-antigens, which include tissue-restricted antigens expressed and transferred from medullary thymic epithelial cells, circulating antigens directly captured by thymic DCs through coticomedullary junction blood vessels, and peripheral tissue antigens captured and transported by peripheral tissue DCs homing to the thymus. When antigen-presenting DCs make a high affinity interaction with antigen-specific thymocytes, this interaction drives the interacting thymocytes to death, a process often referred to as negative selection, which fundamentally blocks the self-reactive thymocytes from differentiating into mature T cells. Alternatively, the interacting thymocytes differentiate into the regulatory T (Treg) cells, a distinct T cell subset with potent immune suppressive activities. The specific mechanisms by which thymic DCs differentiate Treg cells have been proposed by several laboratories. Here, we review the literatures that elucidate the contribution of thymic DCs to negative selection and Treg cell differentiation, and discusses its potential mechanisms and future directions.
Keywords: Dendritic cell, Central tolerance, Thymus, Clonal deletion, Negative selection, Regulatory T cell
INTRODUCTION
Dendritic cells (DCs) play an important role in inducing antigen-specific immunity by presenting antigens to naïve T cells and differentiating the antigen-specific T cells into effector T cells (1,2). Upon recognition of microbes, DCs internalize and digest them into small peptides, which are subsequently loaded onto the antigen-presenting molecules major histocompatibility complex (MHC) classes I and II and displayed at cell surface. When the surface MHCI-peptide complexes are recognized by the CD8+ naïve T cells that express the specific T cell receptor (TCR), this recognition results in the development of CD8+ cytotoxic T effector cells capable of killing infected cells in the host. On the other hand, the surface MHCII-peptide complexes are recognized by the specific TCR-bearing CD4+ naïve T cells, and this recognition leads to the development of CD4+ helper T effector cells capable of assisting other immune cells clearing the infecting microbes.
While immunity is beneficial when it develops against foreign antigens, it is detrimental when it develops against self-antigens as it results in unnecessary and potentially harmful host cell damages. Not surprisingly, our immune system has evolved to ensure such harmful autoimmunity not to arise. One fundamental system created is to interfere the development of self-reactive T cells in the thymus. Thymus is a primary lymphoid organ where T cells of both CD4+ and CD8+ lineages develop from hematopoietic precursor cells originated from bone marrows. Each of developing T cells (thymocytes) expresses a TCR molecule by a process of random gene rearrangement, which creates a diverse repertoire of T cell compartment that can recognize pathogens of an enormous diversity. However, this random process unwantedly but inevitably accompanies a generation of TCRs that react to self-antigens at a high affinity. Thymocytes expressing such self-specific TCRs, if they develop into mature T cells, are likely to cause the harmful autoimmunity. To prevent this from happening, self-specific thymocytes are either routed to cell death, a process named negative selection or clonal deletion, or differentiated into regulatory T (Treg) cells, a distinct T cell subset equipped with immune suppressive activities (3,4,5). These two processes will be referred to as central tolerance in this review.
Central tolerance is crucially dependent on thymic antigen presenting cells (APCs) including cortical thymic epithelial cells (cTECs) and medullary thymic epithelial cells (mTECs). cTECs play a vital role in positively selecting thymocytes, but they can also negatively select thymocytes of strong self-reactivity, and can also support Treg cell differentiation (6,7,8). Similarly, mTECs negatively select thymocytes of strong self-reactivity, and also mediate positive selection of Treg cells. Notably, mTECs uniquely express a transcription factor named AIRE (AutoImmune REgulator), which causes transcription of a wide selection of tissue-restricted genes that are usually only expressed in peripheral tissues. Accordingly, a broad array of tissue-restricted antigens are expressed and presented in mTECs, and this presentation mediates negative selection and Treg cell differentiation of thymocytes specific for the tissue-restricted antigens (9,10,11,12). However, not every tissue-restricted antigen is expressed in mTECs (13), and even among those expressed, some are not effectively presented by mTECs (14). Thus, additional mechanisms are likely to operate that complement mTECs, and fulfill central tolerance.
In addition to cTECs and mTECs, DCs occupy a significant fraction of thymic antigen presenting cells. While the role of DCs in immunity has been well recognized, their role in central tolerance has not been firmly established until recently. Here, we will review the literatures that addressed the role of DCs in central tolerance following a brief description of the subset and origin of thymic DCs.
SUBSET AND ORIGIN OF THYMIC DCs
Thymic DCs consist of three distinct subsets: CD8+ conventional DCs (cDCs), Sirpα+ cDCs, and plasmacytoid DCs (pDCs). CD8+ DCs occupy approximately 50% of thymic DC pool, Sirpα+ DCs 20%, and pDCs the rest (15).
CD8+ DCs develop from intrathymic precursor cells, but the exact identity of these precursors has not been clearly defined. Early studies have suggested that there are common T-myeloid precursors that can differentiate into either T cells or myeloid cells including DCs. For example, thymic lymphoid precursor cells, when transferred into irradiated thymus, formed progenies of both DCs and T cells (16,17). Furthermore, T cells and DCs were generated in the thymus by an identical kinetics (17). In addition, human thymic cells that differentiated into DCs in vitro expressed a transcript that encodes the α subunit of the pre TCR complex (18). However, several recent studies have shown that CD8+ thymic DCs originate from a distinct precursor that does not differentiate into T cells but does differentiate into DCs. One study performed a fate mapping study using the reporter of IL-7 receptor, a key marker of lymphoid lineages, and showed that the reporter was never expressed in thymic cDCs while it was expressed in T cells (19). Another study used the reporter of CD207, a marker of CD8+ DCs, and showed that this reporter was not expressed in T cells, but expressed in CD8+ thymic DCs (20). More recently, a new strategy named retroviral barcoding was used to determine lineage relationship between thymic DCs and T cells (21). This study revealed a high similarity between thymic DCs, splenic DCs, and bone marrow-derived progenitors, but a marked difference between thymic DCs and mature T cells. Notably, this study also showed that T-lineage progenitors differentiate into DCs under certain circumstances such as lymphopenic or DC-depleted condition. Thus, DC development in the thymus appears to entail plasticity, which is likely to help homeostatic maintenance of the cells.
Unlike CD8+ DCs, Sirpα+ DCs and pDCs develop extrathymically and home to the thymus at steady state (22,23,24). Thymic homing of Sirpα+ DCs is dependent on a CCR2-mediated chemotaxis (25,26) and an α4 integrin-dependent adhesion (27) while pDC homing is dependent on CCR9 and α4 integrin (28). Both DC subsets home to the thymus through blood vessels, but the specific tissues that they originated from has not been comprehensively determined.
ANTIGEN ACQUISITION AND PRESENTATION BY THYMIC DCs
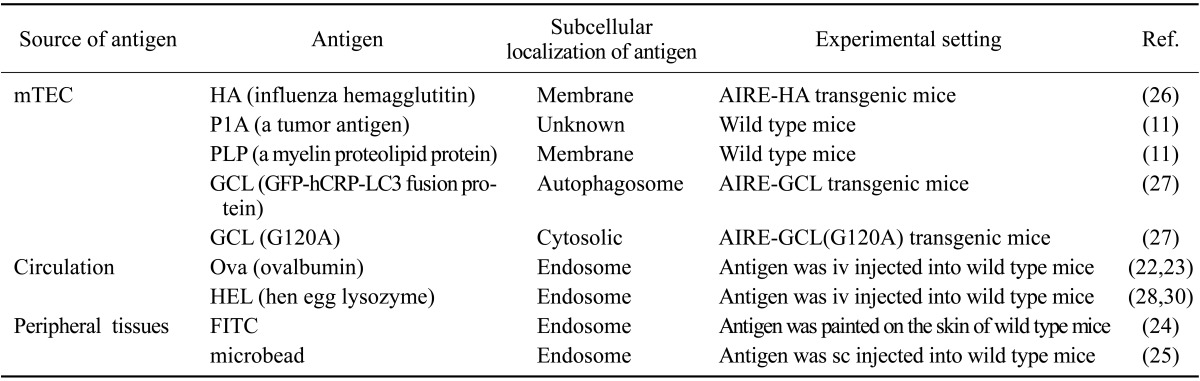
Thymic DCs acquire antigens through at least three independent pathways (Table I). First, they acquire antigens from mTECs. These antigens include tissue-restricted self-antigens expressed under the control of AIRE, and span a broad range of subcellular origins such as the membrane, nucleus, and cytosol (14,29,30). Secondly, thymic DCs acquire antigens from the blood. Sirpα+ DCs are enriched in cortico-medullary perivascular space and also around small vessels (26), which are freely permeable to circulating antigens. Through this strategic localization, Sirpα+ DCs effectively capture and present blood-borne antigens (26,31,32,33). Lastly, Sirpα+ DCs and pDCs bring the antigens they acquired in peripheral tissues as they home to the thymus, and present them to the thymocytes. When mice were intravenously (iv) injected with beads that are too large to penetrate the thymic blood vessels, the beads were found in Sirpα+ DCs and pDCs, but not in CD8+ DCs, in the thymus (28). In addition, when mice were subcutaneously injected with the beads, the beads were also found in Sirpα+ DCs and pDCs in the thymus (28). Similarly, when mice were painted on the skin with a small molecule fluorophore, the fluorophore was found in thymic DCs (27). In addition, an antigen expressed exclusively in cardiac myocytes was presented to the antigen-specific thymocytes in an α4 integrin-dependent manner (27). These findings suggest that DCs in the circulation, skin, heart, and possibly other peripheral tissues, migrate to the thymus and present the peripheral tissue antigens.
Table I. Specific antigens acquired and/or presented by thymic DCs.
ROLE OF THYMIC DCs IN NEGATIVE SELECTION
Thymic DC presentation of self-antigens significantly contributes to the negative selection of CD4+ thymocytes. When MHCII expression was specifically ablated in bone marrow-derived APCs in mice, CD4+ thymocytes accumulated in the thymus (34,35). CD4+ thymocytes also accumulated when thymi of wild type mice were engrafted into mice deficient in MHCII (29). These findings indicate that MHCII-mediated antigen presentation by bone marrow-derived APCs plays an important role in the negative selection of CD4+ thymocytes. More recently, mice that lacked MHCII specifically in DCs were also found to accumulate CD4+ thymocytes (8). Furthermore, mice that lacked DCs also showed a marked accumulation of CD4+ thymocytes (36), demonstrating the important role of DCs in the negative selection of CD4+ thymocytes.
The role of DCs in CD4+ T cell negative selection was also examined by using transgenic mice that express a specific MHCII, a specific TCR, or a specific antigen (Table II). When the MHCII I-E molecule was specifically expressed on DCs, the I-E reactive CD4+ T cells were negatively selected (37). When ovalbumin (ova) or the influenza antigen hemagglutinin (HA) was specifically expressed on DCs, the ova- or HA-reactive TCR-expressing CD4+ thymocytes were deleted in a large fraction (23,29). Also, when hen egg lysozyme (HEL) or ova was injected to the blood, they were taken up by DCs, and the CD4+ thymocytes that expressed the HEL- or ova-specific TCR were effectively deleted (26,32,33). In addition, when ova was expressed in mTEC through AIRE, the CD4+ thymocytes that expressed ova-reactive TCR were mostly deleted, which was completely dependent on bone marrow-derived APCs (38).
Table II. Specific TCR T cell clones negatively selected or differentiated into Treg cells by thymic DCs.
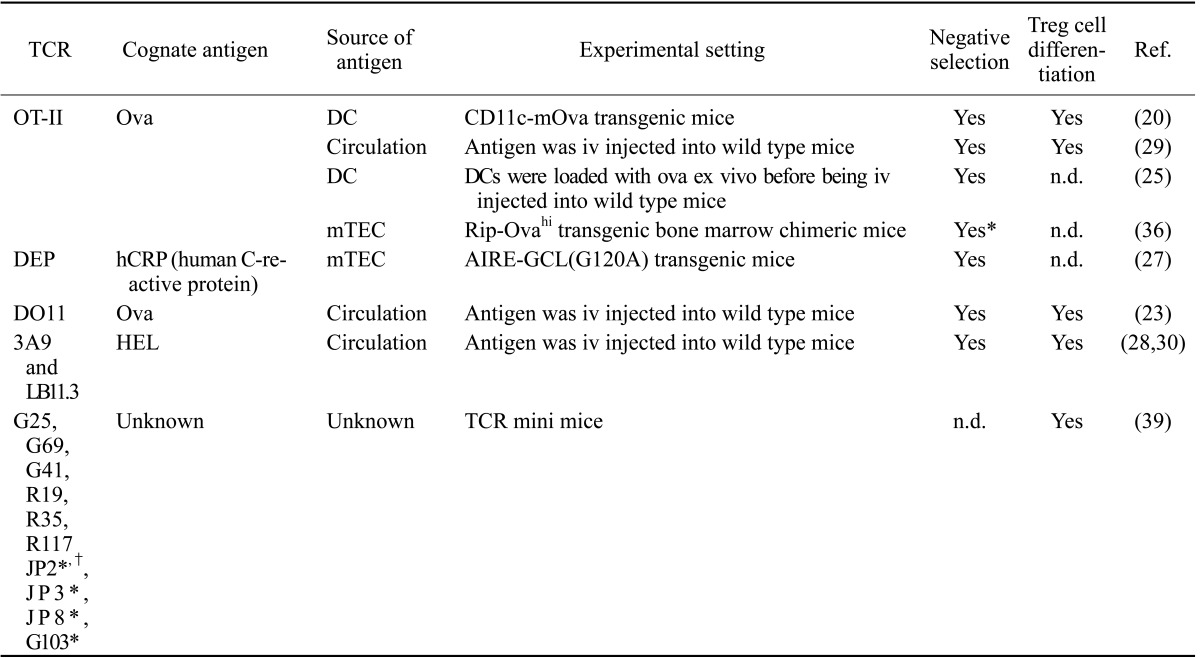
n.d.; not determined. *Dependency of bone marrow-derived APCs instead of DCs was determined. †Partially dependent of mTECs
In some occasions, DCs hardly contribute to the negative selection of antigen-specific CD4+ thymocytes even if the antigens are presented by DCs. For example, in transgenic mice where HA or ova was expressed by mTECs under the control of AIRE, DCs presented both HA and ova at detectable levels, this presentation was not necessary for deleting the antigen-specific CD4+ thymocytes (29). In contrast, mTECs presented the antigens at much high levels and played a sufficient role in deleting those thymocytes (29,35). It is not clear understood what determines DC-dependency vs. mTEC-dependency for clonal deletion, particularly when antigens are expressed in mTECs. Interestingly, Aichinger et al. has recently reported that a model antigen expressed under the control of AIRE induced efficient deletion of specific CD4+ thymocytes by mTECs when targeted to autophagosomes, whereas interference with autophagosomal routing of this antigen through exchange of a single amino acid or ablation of an essential autophagy gene induced CD4+ thymocyte deletion by DCs (30). This finding suggests that mTEC may focus presentation of antigens processed by autophagosomes while the rest of antigens are given to DCs to share the burden.
The role of DCs in CD8+ T cell deletion is less clearly understood. Ablation of MHCI expression in bone marrow-derived APCs resulted in a slight increase in CD8+ thymocytes (34), but DC ablation did not result in such increases (36). The ova-specific OT-I CD8+ thymocytes were effectively deleted when ova was expressed under the control of AIRE. However this deletion did not require MHCI expression by bone marrow-derived APCs (39). Interestingly, the mTEC-expressed tumor antigen P1A was not presented by mTECs but presented by DCs (14), suggesting that DCs may delete the P1A-specific CD8+ thymocytes. However, this possibility has not been tested.
ROLE OF THYMIC DCs IN TREG CELL DEVELOPMENT
The role of thymic DCs in Treg cell development was a long-standing subject with debate. Mice that are deficient in DCs from birth or in adult life had the number of Treg cells similar to mice sufficient in DCs (36,40). Mice that are deficient in MHCII in bone marrow-derived APCs or DCs also had a normal number of Treg cells (8,29). These findings led to a claim that DCs are not necessary for Treg development. However, this claim was not readily consented in the field because DCs could positively select Treg cells specific for the antigens that they present (Table II). For example, mice that expressed ova-specific OT-II TCR produced ova-specific Treg cells when they expressed ova in DCs (23). The same mice also produced ova-specific Treg cells when they were injected with ova iv, the route by which injected antigen is exclusively taken up and presented by DCs among thymic APCs (32). Similar observation was made when the HEL-specific TCR transgenic mice were iv injected with HEL (33).
Recently, Perry et al. has taken a completely new approach to address the role of DCs in Treg cell development, namely a systematic analysis of the TCR clones (41). Because the vast diversity in polyclonal T cells precludes experimental analysis at the level of individual TCRs, they utilized mice in which TCR diversity is limited by a transgenic fixed TCRβ chain. This allowed high-throughput analysis of the TCR repertoire at the individual TCR levels through sequencing of the variable region of a TCRα chain. First, they assessed the contribution of bone marrow-derived APCs to Treg cell development by injecting MHCII-deficient bone marrows into irradiated wild type mice and determining Treg cell TCR repertoire in comparison to that of control mice. Interestingly, not only a loss of certain TCRs but also an enrichment of distinct TCRs was found by MHCII ablation on bone marrow-derived APCs, suggesting that bone-marrow-derived APCs mediate negative as well as positive selection of Treg cells. This finding provide potential explanation of why ablation of MHCII in bone marrow-derived APCs did not reduce the number of Treg cells in previous studies. Next, they closely examined 15 TCR clones that are most abundant in wild type mice for their dependency on bone marrow-derived APCs. Remarkably, there was a reduction of ten TCR clones in mice where MHCII was ablated in bone-marrow-derived APCs, indicating a substantial contribution of bone marrow-derived APCs to the development of these TCR Treg cell clones. Finally, they examined the contribution of DCs to the selection of these frequent TCR Treg cell clones. For this examination, thymocytes were transduced with the retrovirus that encode each of the frequent TCR clones, injected into the thymus of the mice that lacked DCs, and examined whether they differentiated into Treg cells. Many of the clones failed to differentiate into Treg cells, indicating that these clones are completely dependent on DCs for Treg cell development (Table II). It is notable that this study also revealed that approximately half of AIRE-dependent Treg cell selection utilizes a pathway dependent on DCs, particularly CD8+ DCs, suggesting that CD8+ DCs play an important role in acquiring and presenting AIRE-derived antigens.
THE MECHANISMS BY WHICH THYMIC DCs MEDIATE TREG CELL DEVELOPMENT
It has been extensively investigated what molecules and signaling pathways are required for thymocytes to become Treg cells. For example, thymocytes should receive strong TCR-signaling to differentiate into Treg cells (42). They also should receive signaling from costimulatory receptors such as CD28 (43) and tumor necrosis factor receptor super family (TNFRSF) (44). In addition, they need signaling from common cytokine receptor γ-chain receptors such as the receptors for IL-2, IL-15, and IL-7 (45,46). However, relatively little is known what molecules need to be expressed and what cellular events have to occur in DCs for them to select Treg cells.
An early study suggested that thymic stromal lymphopoietin (TSLP)-mediated maturation of thymic DCs plays an important role in Treg cell development (47). TSLP is expressed by Hassall's corpuscles, groups of epithelial cells within the thymic medulla, in human. Treatment of human thymic cDCs with TSLP resulted in an increased expression of the costimulatory molecules CD80 and CD86. These TSLP-conditioned thymic cDCs were then able to induce Treg cell differentiation in vitro. More recently, human pDCs were also found to express TSLP receptor upon activation and respond to TSLP (48). TSLP-conditioned pDCs expressed high levels of CD80 and CD86, and efficiently induced the generation of Treg cells in vitro (48). These studies suggest that TSLP mediates maturation of thymic DCs, and this maturation equips DCs with costimulatory molecules that promote Treg cell differentiation. However, the role of TSLP signaling in Treg cell development in vivo has not been established. Mice that lack TSLP receptor produced a normal number of Treg cells in the thymus (49), suggesting that TSLP signaling is not essential in Treg cell development at least in mice.
CD70 in DCs has been suggested to play an important role in Treg development (50). CD70, a member of TNF super family (TNFSF) is expressed in mTECs and thymic DCs, and its receptor CD27 is expressed in thymocytes. Genetic ablation of either CD70 or CD27 reduced the number of Treg cells in the thymus, while CD27 signaling rescued developing Treg cells from apoptosis. Furthermore, CD70 on CD8+ DCs promoted Treg cell differentiation in vitro. These findings suggest that DC interaction with thymocytes through CD70-CD27 costimulatory molecules plays a significant role in Treg development. More recently, additional TNFSF members such as GITRL, TNF, and OX40L were also found to contribute to Treg development (44). These TNFSF members are expressed in mTECs and/or thymic DCs and bind to corresponding receptors expressed in Treg cell precursors. This binding enhances the ability of the Treg cell progenitors to compete for limiting amounts of IL-2 and secure the developmental niche for Treg cells in the thymus.
We have recently found that MARCH1-mediated MHCII ubiquitination promotes DC selection of Treg cells (32). MARCH1 is a membrane-associated ubiquitin ligase that mediates ubiquitination of MHCII and CD86 in DCs, and induces endocytosis and lysosomal degradation of these molecules (51,52,53,54,55). Interestingly, mice deficient in either MARCH1 or the ubiquitin-acceptor residue of MHCII failed to produce a proper number of Treg cells in the thymus. These mice also failed to produce ova-specific Treg cells when ova was injected iv or expressed in mTECs. Moreover, DCs from these mice poorly generated antigen-specific Treg cells in vitro. These findings suggest that MARCH1-mediated MHCII ubiquitination conditions DCs to effectively generate Treg cells. However, the specific mechanism by which MHCII ubiquitination confers this function to DCs is not clear. One possibility is that MHCII ubiquitination reduces the surface levels of MHCII in DCs, reducing the amount of self-antigens presented by DCs. This reduction may rescue some of the high affinity self-reactive T cells from being negatively selected and help them differentiate into Treg cells. However, we found this hypothesis unlikely to be true because ablation of MHCII ubiquitination did not enhance negative selection of CD4+ thymocytes (32). Furthermore, lowering the amount of antigens did not help the MHCII ubiquitination-deficient DCs generate Treg cells (32). Thus, a more likely hypothesis is that MHCII ubiquitination controls DC antigen presentation not quantitatively but qualitatively so that the ubiquitination regulates the repertoire of peptides presented via MHCII or regulates some accessory cellular events accompanied by the antigen presentation, thus controlling the repertoire and/or functional properties of differentiating Treg cells.
CONCLUSION AND FUTURE DIRECTION
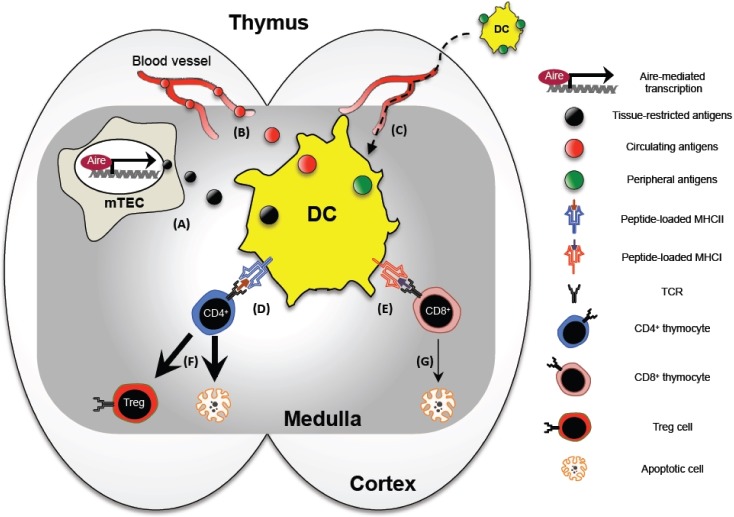
In conclusion, DCs are tactically organized in the thymus to acquire and present a vast array of self-antigens, and delete the self-reactive thymocytes or differentiate them into Treg cells thus constructing a useful and safe repertoire of T cells (summarized in Fig. 1). Although there are numerous questions that remain to be answered, here we picked three outstanding questions that we believe warrant the highest priority.
Figure 1. The role of dendritic cells in the thymus. DCs are positioned in the medullary region of the thymus, and acquire tissue-restricted antigens from mTECs (A) and circulating antigens from blood vessels (B). Some thymic DCs originate from periphery carrying antigens acquired from the peripheral tissue sites (C). The acquired antigens are presented to CD4+ thymocytes via MHCII (D) and CD8+ thymocytes via MHCI (E), and this presentation results in thymocyte apoptosis or Treg cell differentiation (F and G).
First, it will be important to identify the specific mechanisms by which DCs acquire antigens from mTECs because such mechanism is likely to be an important means by which AIRE-driven tissue-restricted antigens, which are expressed only in 10~15% of mTECs (56), are disseminated in the thymus and recognized by thymocytes with the maximal frequency. One possibility is that mTECs release their intracellular materials either constitutively or in a regulated manner, and these materials are then actively endocytosed by DCs. Indeed, human TECs have been shown to release exosomes in culture that carried tissue-restricted antigens (57). Another possibility is that mTECs undergoing homeostatic turnover release their materials through apoptosis, and these apoptotic bodies are captured and presented by DCs. In line with this possibility, AIRE has been shown to promote apoptosis by mediating nuclear translocation of stress sensor protein GAPDH (58). Alternatively, mTECs may make a direct interaction with neighboring or even remote DCs via some intercellular connection devices, and transfer their materials through such devices. A recent study has shown that intestinal DCs and macrophages establish gap junctions, a protein channel that directly connects the cytoplasm of two cells (59). This channel is utilized by macrophages to transfer food antigens that they captured from intestinal lumen to the neighboring DCs. Another study has shown that DCs form a network of tunneling nanotube-like structures upon activation and that this network facilitates intercellular transfer of intracellular vesicles and antigens between DCs (60). Similar structures may be formed between mTECs and thymic DCs, and mediate antigen transfer.
Secondly, the specific mechanisms by which thymic DCs mediate negative selection and Treg cell differentiation will need to be better defined. One of the prevailing hypotheses is that the strong affinity interaction between thymic APCs and thymocytes results in negative selection while the intermediate affinity interaction results in Treg cell differentiation (5). However, it is not difficult to find the TCR clones that are deleted as well as differentiated into Treg cells at the same time. Thus, there must be a mechanism(s) extrinsic to TCR that directs thymocytes to one fate vs. the other. As we reviewed early, several mechanisms have been proposed by which thymic DCs are conditioned to induce Treg cell differentiation. Perhaps, this mechanism could operate in a regulated manner; thymocytes that make a cognate interaction with DCs become Treg cells only if the mechanism has turned on; otherwise they undergo apoptosis.
Lastly, very little is known on the specific contribution that thymic DCs make to peripheral immune homeostasis. Humans and mice both develop multiple organ-specific autoimmune diseases in the absence of functional AIRE (61,62). As some of AIRE-driven tissue-restricted antigens are presented by thymic DCs, DCs may make a significant contribution to the prevention of organ-specific autoimmunity. In addition, thymic DCs may play a unique role in preventing allergic diseases or commensal-associated auto-inflammatory diseases. Sirpα+ DCs and pDCs in the thymus are originated from periphery, potentially carrying peripheral antigens to the thymus and presenting them. Thus, it is conceivable that these DCs may present environmental antigens that accessed to the respiratory tracts, food antigens that entered the intestine, and commensal antigens living in mucosal surfaces. This presentation may play an important role in establishing tolerance to allergens and other innocuous foreign antigens, thus serving for mucosal immune homeostasis. We envision that advanced knowledge on thymic DC functions and underlying mechanisms will lay the groundwork for development of novel therapeutics for autoimmune and allergic disorders.
ACKNOWLEDGEMENTS
This work is supported by the UCSF Sandler Asthma Basic Research Center and the National Institute of Health, USA, Grant R01 GM105800.
Abbreviations
- DC
dendritic cell
- mTEC
medullary thymic epithelial cell
- AIRE
autoimmune regulator
- pDC
plasmacytoid dendritic cell
- Treg
regulatory T
- MARCH1
membrane-associated RING-CH1
- TSLP
thymic stromal lymphopoietin
Footnotes
CONFLICTS OF INTEREST: The authors have no financial conflict of interest.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 3.Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9:833–844. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- 4.Mathis D, Benoist C. Back to central tolerance. Immunity. 2004;20:509–516. doi: 10.1016/s1074-7613(04)00111-6. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol. 2012;12:157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 6.Goldman KP, Park CS, Kim M, Matzinger P, Anderson CC. Thymic cortical epithelium induces self tolerance. Eur J Immunol. 2005;35:709–717. doi: 10.1002/eji.200425675. [DOI] [PubMed] [Google Scholar]
- 7.Ahn S, Lee G, Yang SJ, Lee D, Lee S, Shin HS, Kim MC, Lee KN, Palmer DC, Theoret MR, Jenkinson EJ, Anderson G, Restifo NP, Kim MG. TSCOT+ thymic epithelial cell-mediated sensitive CD4 tolerance by direct presentation. PLoS Biol. 2008;6:e191. doi: 10.1371/journal.pbio.0060191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liston A, Nutsch KM, Farr AG, Lund JM, Rasmussen JP, Koni PA, Rudensky AY. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc Natl Acad Sci U S A. 2008;105:11903–11908. doi: 10.1073/pnas.0801506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng MH, Shum AK, Anderson MS. What's new in the Aire? Trends Immunol. 2007;28:321–327. doi: 10.1016/j.it.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, Kang C, Geddes JE, Allison JP, Socci ND, Savage PA. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathis D, Benoist C. A decade of AIRE. Nat Rev Immunol. 2007;7:645–650. doi: 10.1038/nri2136. [DOI] [PubMed] [Google Scholar]
- 12.Wirnsberger G, Hinterberger M, Klein L. Regulatory T-cell differentiation versus clonal deletion of autoreactive thymocytes. Immunol Cell Biol. 2011;89:45–53. doi: 10.1038/icb.2010.123. [DOI] [PubMed] [Google Scholar]
- 13.Lv H, Havari E, Pinto S, Gottumukkala RV, Cornivelli L, Raddassi K, Matsui T, Rosenzweig A, Bronson RT, Smith R, Fletcher AL, Turley SJ, Wucherpfennig K, Kyewski B, Lipes MA. Impaired thymic tolerance to alpha-myosin directs autoimmunity to the heart in mice and humans. J Clin Invest. 2011;121:1561–1573. doi: 10.1172/JCI44583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med. 2009;206:1505–1513. doi: 10.1084/jem.20082449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu L, Shortman K. Heterogeneity of thymic dendritic cells. Semin Immunol. 2005;17:304–312. doi: 10.1016/j.smim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Ardavin C, Wu L, Li CL, Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 17.Wu L, Li CL, Shortman K. Thymic dendritic cell precursors: relationship to the T lymphocyte lineage and phenotype of the dendritic cell progeny. J Exp Med. 1996;184:903–911. doi: 10.1084/jem.184.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Res PC, Couwenberg F, Vyth-Dreese FF, Spits H. Expression of pTalpha mRNA in a committed dendritic cell precursor in the human thymus. Blood. 1999;94:2647–2657. [PubMed] [Google Scholar]
- 19.Schlenner SM, Madan V, Busch K, Tietz A, Laufle C, Costa C, Blum C, Fehling HJ, Rodewald HR. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity. 2010;32:426–436. doi: 10.1016/j.immuni.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Luche H, Ardouin L, Teo P, See P, Henri S, Merad M, Ginhoux F, Malissen B. The earliest intrathymic precursors of CD8alpha(+) thymic dendritic cells correspond to myeloid-type double-negative 1c cells. Eur J Immunol. 2011;41:2165–2175. doi: 10.1002/eji.201141728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyszkiewicz M, Zietara N, Fohse L, Puchalka J, Diestelhorst J, Witzlau K, Prinz I, Schambach A, Krueger A. Limited niche availability suppresses murine intrathymic dendritic-cell development from noncommitted progenitors. Blood. 2015;125:457–464. doi: 10.1182/blood-2014-07-592667. [DOI] [PubMed] [Google Scholar]
- Li J, Park J, Foss D, Goldschneider I. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J Exp Med. 2009;206:607–622. doi: 10.1084/jem.20082232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Proietto AI, van DS, Zhou P, Rizzitelli A, D'Amico A, Steptoe RJ, Naik SH, Lahoud MH, Liu Y, Zheng P, Shortman K, Wu L. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci U S A. 2008;105:19869–19874. doi: 10.1073/pnas.0810268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donskoy E, Goldschneider I. Two developmentally distinct populations of dendritic cells inhabit the adult mouse thymus: demonstration by differential importation of hematogenous precursors under steady state conditions. J Immunol. 2003;170:3514–3521. doi: 10.4049/jimmunol.170.7.3514. [DOI] [PubMed] [Google Scholar]
- 25.Baba T, Badr MS, Tomaru U, Ishizu A, Mukaida N. Novel process of intrathymic tumor-immune tolerance through CCR2-mediated recruitment of Sirpalpha+ dendritic cells: a murine model. PLoS One. 2012;7:e41154. doi: 10.1371/journal.pone.0041154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baba T, Nakamoto Y, Mukaida N. Crucial contribution of thymic Sirp alpha+ conventional dendritic cells to central tolerance against blood-borne antigens in a CCR2-dependent manner. J Immunol. 2009;183:3053–3063. doi: 10.4049/jimmunol.0900438. [DOI] [PubMed] [Google Scholar]
- 27.Bonasio R, Scimone ML, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 28.Hadeiba H, Lahl K, Edalati A, Oderup C, Habtezion A, Pachynski R, Nguyen L, Ghodsi A, Adler S, Butcher EC. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36:438–450. doi: 10.1016/j.immuni.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 30.Aichinger M, Wu C, Nedjic J, Klein L. Macroautophagy substrates are loaded onto MHC class II of medullary thymic epithelial cells for central tolerance. J Exp Med. 2013;210:287–300. doi: 10.1084/jem.20122149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atibalentja DF, Murphy KM, Unanue ER. Functional redundancy between thymic CD8alpha+ and Sirpalpha+ conventional dendritic cells in presentation of blood-derived lysozyme by MHC class II proteins. J Immunol. 2011;186:1421–1431. doi: 10.4049/jimmunol.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh J, Wu N, Baravalle G, Cohn B, Ma J, Lo B, Mellman I, Ishido S, Anderson M, Shin JS. MARCH1-mediated MHCII ubiquitination promotes dendritic cell selection of natural regulatory T cells. J Exp Med. 2013;210:1069–1077. doi: 10.1084/jem.20122695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atibalentja DF, Byersdorfer CA, Unanue ER. Thymus-blood protein interactions are highly effective in negative selection and regulatory T cell induction. J Immunol. 2009;183:7909–7918. doi: 10.4049/jimmunol.0902632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Meerwijk JP, Marguerat S, Lees RK, Germain RN, Fowlkes BJ, MacDonald HR. Quantitative impact of thymic clonal deletion on the T cell repertoire. J Exp Med. 1997;185:377–383. doi: 10.1084/jem.185.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinterberger M, Aichinger M, Prazeres da CO, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol. 2010;11:512–519. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- 36.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brocker T. The role of dendritic cells in T cell selection and survival. J Leukoc Biol. 1999;66:331–335. doi: 10.1002/jlb.66.2.331. [DOI] [PubMed] [Google Scholar]
- 38.Hubert FX, Kinkel SA, Davey GM, Phipson B, Mueller SN, Liston A, Proietto AI, Cannon PZ, Forehan S, Smyth GK, Wu L, Goodnow CC, Carbone FR, Scott HS, Heath WR. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood. 2011;118:2462–2472. doi: 10.1182/blood-2010-06-286393. [DOI] [PubMed] [Google Scholar]
- 39.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darrasse-Jeze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, Masilamani RF, Dustin ML, Rudensky A, Liu K, Nussenzweig MC. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry JS, Lio CW, Kau AL, Nutsch K, Yang Z, Gordon JI, Murphy KM, Hsieh CS. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity. 2014;41:414–426. doi: 10.1016/j.immuni.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 43.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 44.Mahmud SA, Manlove LS, Schmitz HM, Xing Y, Wang Y, Owen DL, Schenkel JM, Boomer JS, Green JM, Yagita H, Chi H, Hogquist KA, Farrar MA. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat Immunol. 2014;15:473–481. doi: 10.1038/ni.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 46.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W, Liu YJ. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 48.Hanabuchi S, Ito T, Park WR, Watanabe N, Shaw JL, Roman E, Arima K, Wang YH, Voo KS, Cao W, Liu YJ. Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J Immunol. 2010;184:2999–3007. doi: 10.4049/jimmunol.0804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mazzucchelli R, Hixon JA, Spolski R, Chen X, Li WQ, Hall VL, Willette-Brown J, Hurwitz AA, Leonard WJ, Durum SK. Development of regulatory T cells requires IL-7Ralpha stimulation by IL-7 or TSLP. Blood. 2008;112:3283–3292. doi: 10.1182/blood-2008-02-137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coquet JM, Ribot JC, Babala N, Middendorp S, van der Horst G, Xiao Y, Neves JF, Fonseca-Pereira D, Jacobs H, Pennington DJ, Silva-Santos B, Borst J. Epithelial and dendritic cells in the thymic medulla promote CD4+Foxp3+ regulatory T cell development via the CD27-CD70 pathway. J Exp Med. 2013;210:715–728. doi: 10.1084/jem.20112061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsuki Y, Ohmura-Hoshino M, Goto E, Aoki M, Mito-Yoshida M, Uematsu M, Hasegawa T, Koseki H, Ohara O, Nakayama M, Toyooka K, Matsuoka K, Hotta H, Yamamoto A, Ishido S. Novel regulation of MHC class II function in B cells. EMBO J. 2007;26:846–854. doi: 10.1038/sj.emboj.7601556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shin JS, Ebersold M, Pypaert M, Delamarre L, Hartley A, Mellman I. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature. 2006;444:115–118. doi: 10.1038/nature05261. [DOI] [PubMed] [Google Scholar]
- 53.Baravalle G, Park H, McSweeney M, Ohmura-Hoshino M, Matsuki Y, Ishido S, Shin JS. Ubiquitination of CD86 is a key mechanism in regulating antigen presentation by dendritic cells. J Immunol. 2011;187:2966–2973. doi: 10.4049/jimmunol.1101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Niel G, Wubbolts R, Ten Broeke T, Buschow SI, Ossendorp FA, Melief CJ, Raposo G, van Balkom BW, Stoorvogel W. Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity. 2006;25:885–894. doi: 10.1016/j.immuni.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 55.De Gassart A, Camosseto V, Thibodeau J, Ceppi M, Catalan N, Pierre P, Gatti E. MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I down-regulation. Proc Natl Acad Sci U S A. 2008;105:3491–3496. doi: 10.1073/pnas.0708874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Derbinski J, Gäbler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202:33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skogberg G, Lundberg V, Berglund M, Gudmundsdottir J, Telemo E, Lindgren S, Ekwall O. Human thymic epithelial primary cells produce exosomes carrying tissue-restricted antigens. Immunol Cell Biol. 2015 doi: 10.1038/icb.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liiv I, Haljasorg U, Kisand K, Maslovskaja J, Laan M, Peterson P. AIRE-induced apoptosis is associated with nuclear translocation of stress sensor protein GAPDH. Biochem Biophys Res Commun. 2012;423:32–37. doi: 10.1016/j.bbrc.2012.05.057. [DOI] [PubMed] [Google Scholar]
- 59.Mazzini E, Massimiliano L, Penna G, Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1(+) macrophages to CD103(+) dendritic cells. Immunity. 2014;40:248–261. doi: 10.1016/j.immuni.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 60.Zaccard CR, Watkins SC, Kalinski P, Fecek RJ, Yates AL, Salter RD, Ayyavoo V, Rinaldo CR, Mailliard RB. CD40L induces functional tunneling nanotube networks exclusively in dendritic cells programmed by mediators of type 1 immunity. J Immunol. 2015;194:1047–1056. doi: 10.4049/jimmunol.1401832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Finnish-German APECED Consortium. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 62.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]