Abstract
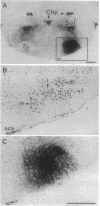
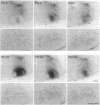
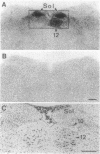
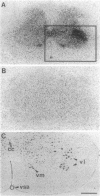
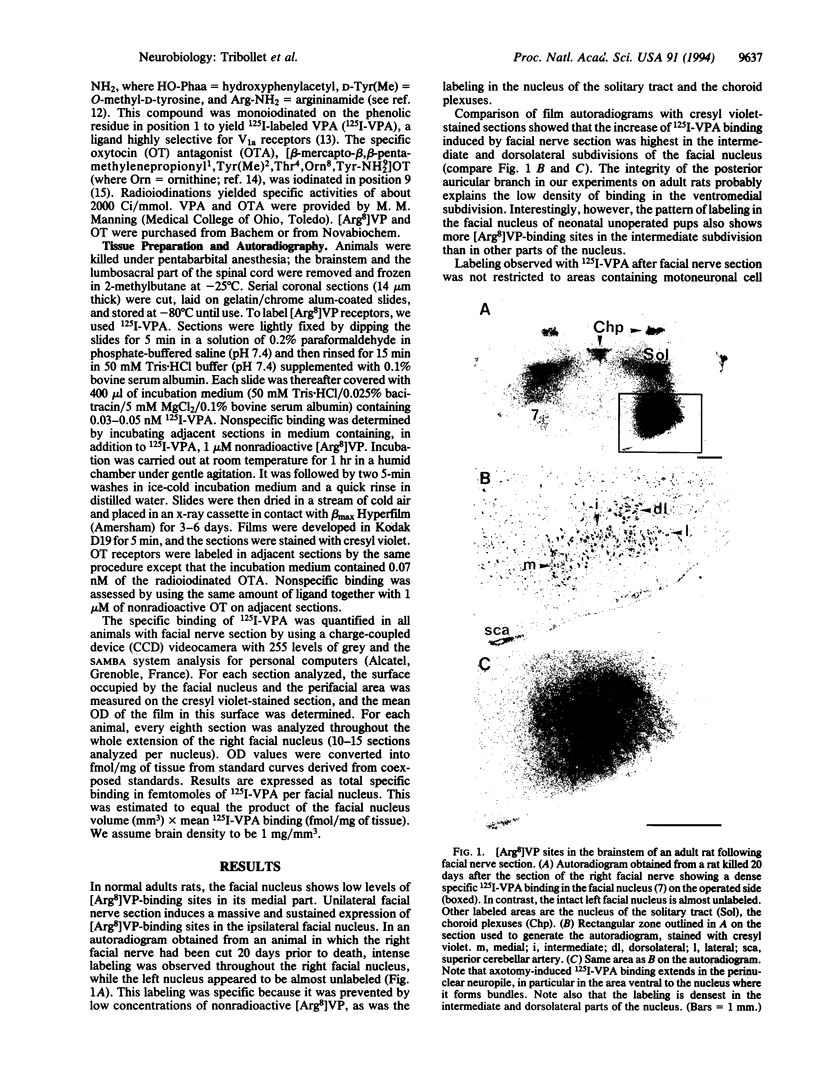
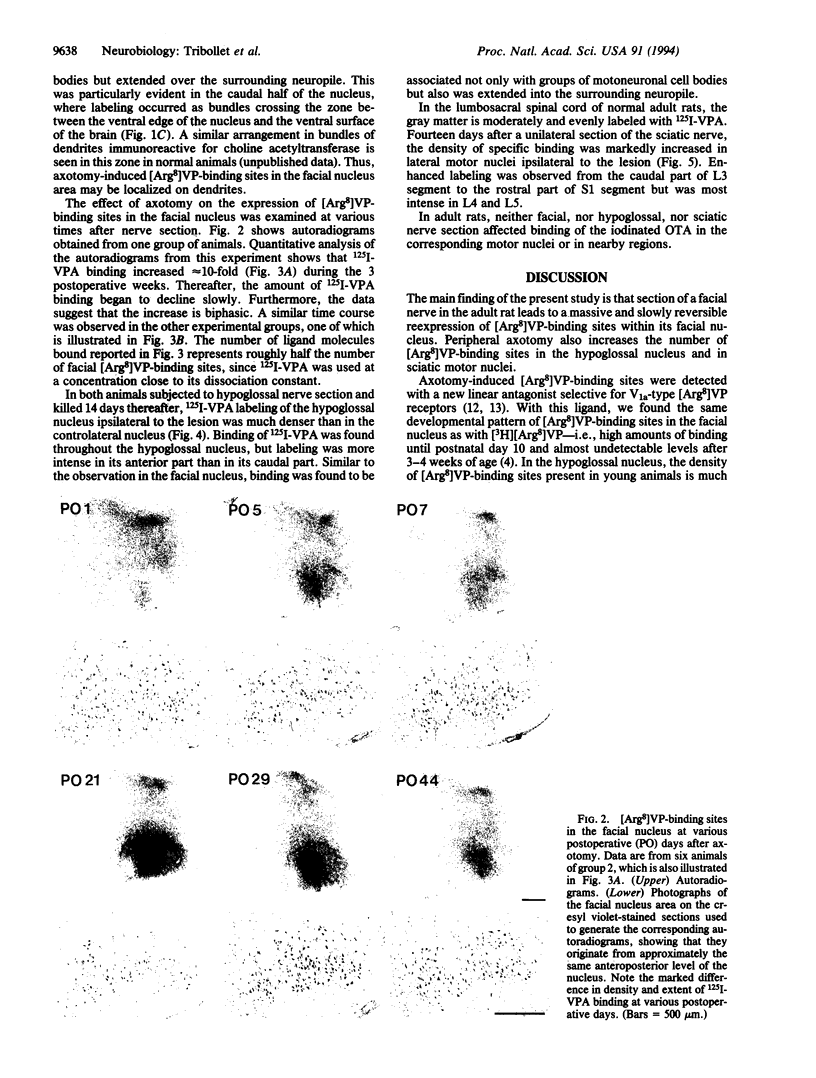
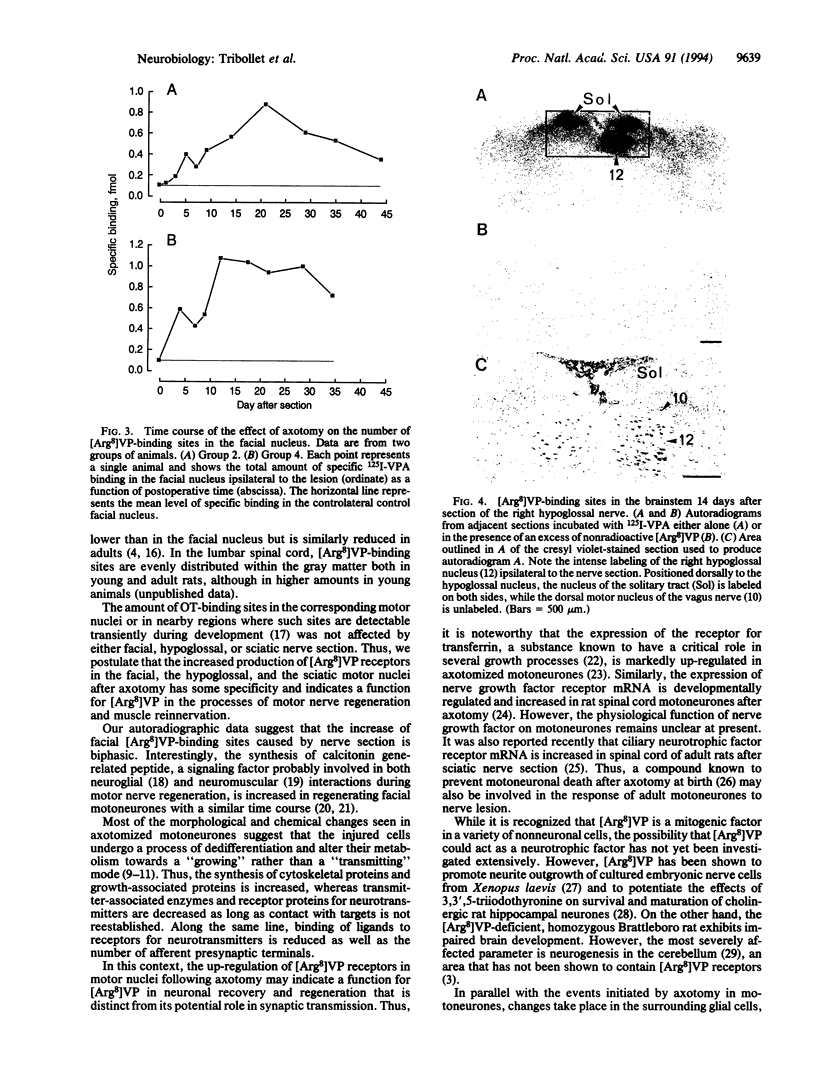
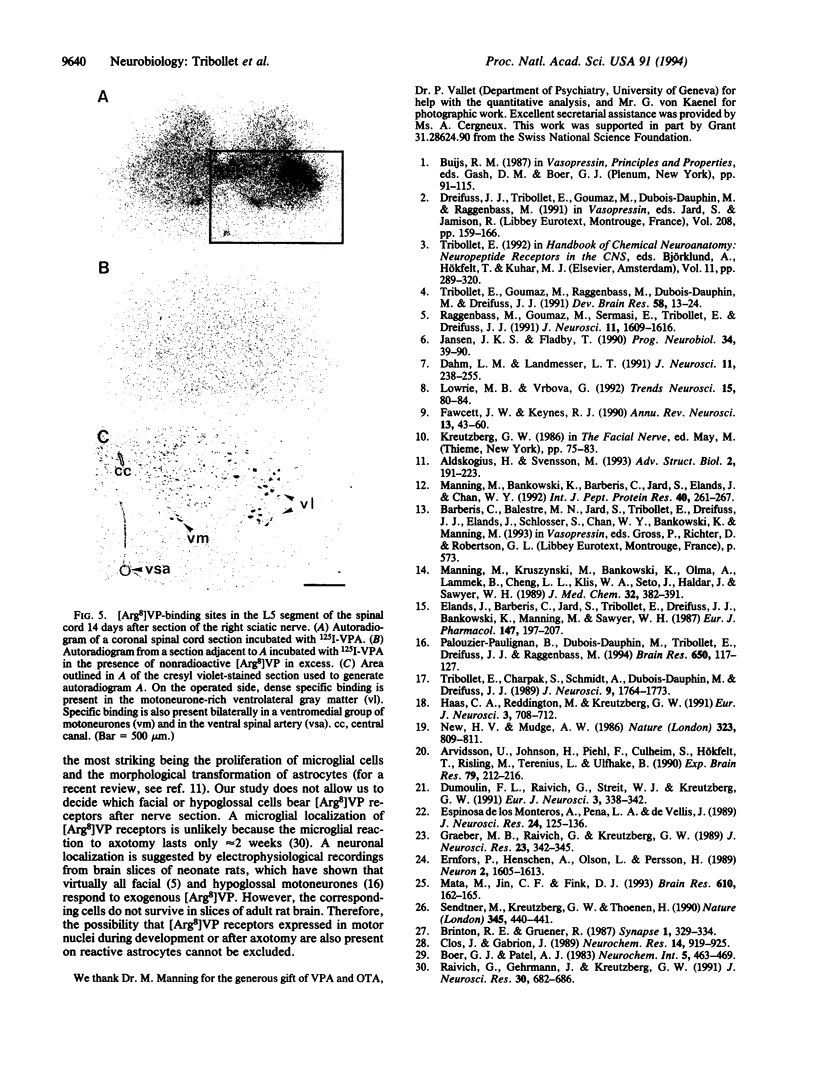
8-L-Arginine vasopressin ([Arg8]VP) receptors are expressed transiently in the rat facial nucleus during the perinatal period. Electrophysiological studies suggest that at least part of these receptors is located on facial motoneurones. In the present study we report that, in the adult rat, unilateral section of a facial nerve results in a massive and transient reexpression of [Arg8]VP receptors in the deeferented facial nucleus. Data were obtained by quantitative film autoradiography. During the first 2 postoperative weeks, binding of an iodinated ligand selective for V1a-type receptors increased about 10-fold. Maximal levels of binding were maintained for 1-2 weeks and then started to decrease. Binding was not strictly restricted to the facial nucleus but included the neuropile between motoneuronal pools and the perifacial area, which may indicate a dendritic localization of [Arg8]VP receptors. To investigate whether other motor nuclei also react to axotomy by up-regulating [Arg8]VP receptors, we sectioned either a hypoglossal nerve or a sciatic nerve. Two weeks after surgery, the hypoglossal nucleus or sciatic motoneuronal pools ipsilateral to the lesion were intensely labeled with the iodinated ligand. In contrast, nerve section had no effect on oxytocin binding sites in facial, hypoglossal, or sciatic motor nuclei. The results suggest that [Arg8]VP receptor expression in motor nuclei may depend upon neuromuscular contacts and, thus, that [Arg8]VP may be involved in the establishment of neuromuscular connections during development and in their reestablishment after nerve injury.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvidsson U., Johnson H., Piehl F., Cullheim S., Hökfelt T., Risling M., Terenius L., Ulfhake B. Peripheral nerve section induces increased levels of calcitonin gene-related peptide (CGRP)-like immunoreactivity in axotomized motoneurons. Exp Brain Res. 1990;79(1):212–216. doi: 10.1007/BF00228891. [DOI] [PubMed] [Google Scholar]
- Brinton R. E., Gruener R. Vasopressin promotes neurite growth in cultured embryonic neurons. Synapse. 1987;1(4):329–334. doi: 10.1002/syn.890010406. [DOI] [PubMed] [Google Scholar]
- Clos J., Gabrion J. A thyroid hormone-vasopressin interaction promotes survival and maturation of hippocampal neurons dissociated postnatally. Neurochem Res. 1989 Oct;14(10):919–925. doi: 10.1007/BF00965924. [DOI] [PubMed] [Google Scholar]
- Dahm L. M., Landmesser L. T. The regulation of synaptogenesis during normal development and following activity blockade. J Neurosci. 1991 Jan;11(1):238–255. doi: 10.1523/JNEUROSCI.11-01-00238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin F. L., Raivich G., Streit W. J., Kreutzberg G. W. Differential Regulation of Calcitonin Gene-related Peptide (CGRP) in Regenerating Rat Facial Nucleus and Dorsal Root Ganglion. Eur J Neurosci. 1991;3(4):338–342. doi: 10.1111/j.1460-9568.1991.tb00820.x. [DOI] [PubMed] [Google Scholar]
- Elands J., Barberis C., Jard S., Tribollet E., Dreifuss J. J., Bankowski K., Manning M., Sawyer W. H. 125I-labelled d(CH2)5[Tyr(Me)2,Thr4,Tyr-NH2(9)]OVT: a selective oxytocin receptor ligand. Eur J Pharmacol. 1988 Mar 1;147(2):197–207. doi: 10.1016/0014-2999(88)90778-9. [DOI] [PubMed] [Google Scholar]
- Ernfors P., Henschen A., Olson L., Persson H. Expression of nerve growth factor receptor mRNA is developmentally regulated and increased after axotomy in rat spinal cord motoneurons. Neuron. 1989 Jun;2(6):1605–1613. doi: 10.1016/0896-6273(89)90049-4. [DOI] [PubMed] [Google Scholar]
- Espinosa de los Monteros A., Peña L. A., de Vellis J. Does transferrin have a special role in the nervous system? J Neurosci Res. 1989 Oct;24(2):125–136. doi: 10.1002/jnr.490240202. [DOI] [PubMed] [Google Scholar]
- Fawcett J. W., Keynes R. J. Peripheral nerve regeneration. Annu Rev Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- Graeber M. B., Raivich G., Kreutzberg G. W. Increase of transferrin receptors and iron uptake in regenerating motor neurons. J Neurosci Res. 1989 Jul;23(3):342–345. doi: 10.1002/jnr.490230315. [DOI] [PubMed] [Google Scholar]
- Haas Carola A., Reddington Martin, Kreutzberg Georg W. Calcitonin Gene-related Peptide Stimulates the Induction of c-fos Gene Expression in Rat Astrocyte Cultures. Eur J Neurosci. 1991 Jul;3(7):708–712. doi: 10.1111/j.1460-9568.1991.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Jansen J. K., Fladby T. The perinatal reorganization of the innervation of skeletal muscle in mammals. Prog Neurobiol. 1990;34(1):39–90. doi: 10.1016/0301-0082(90)90025-c. [DOI] [PubMed] [Google Scholar]
- Lowrie M. B., Vrbová G. Dependence of postnatal motoneurones on their targets: review and hypothesis. Trends Neurosci. 1992 Mar;15(3):80–84. doi: 10.1016/0166-2236(92)90014-y. [DOI] [PubMed] [Google Scholar]
- Manning M., Bankowski K., Barberis C., Jard S., Elands J., Chan W. Y. Novel approach to the design of synthetic radioiodinated linear V1A receptor antagonists of vasopressin. Int J Pept Protein Res. 1992 Sep-Oct;40(3-4):261–267. doi: 10.1111/j.1399-3011.1992.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Manning M., Kruszynski M., Bankowski K., Olma A., Lammek B., Cheng L. L., Klis W. A., Seto J., Haldar J., Sawyer W. H. Solid-phase synthesis of 16 potent (selective and nonselective) in vivo antagonists of oxytocin. J Med Chem. 1989 Feb;32(2):382–391. doi: 10.1021/jm00122a016. [DOI] [PubMed] [Google Scholar]
- Mata M., Jin C. F., Fink D. J. Axotomy increases CNTF receptor mRNA in rat spinal cord. Brain Res. 1993 Apr 30;610(1):162–165. doi: 10.1016/0006-8993(93)91231-g. [DOI] [PubMed] [Google Scholar]
- New H. V., Mudge A. W. Calcitonin gene-related peptide regulates muscle acetylcholine receptor synthesis. 1986 Oct 30-Nov 5Nature. 323(6091):809–811. doi: 10.1038/323809a0. [DOI] [PubMed] [Google Scholar]
- Palouzier-Paulignan B., Dubois-Dauphin M., Tribollet E., Dreifuss J. J., Raggenbass M. Action of vasopressin on hypoglossal motoneurones of the rat: presynaptic and postsynaptic effects. Brain Res. 1994 Jul 4;650(1):117–126. doi: 10.1016/0006-8993(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Raggenbass M., Goumaz M., Sermasi E., Tribollet E., Dreifuss J. J. Vasopressin generates a persistent voltage-dependent sodium current in a mammalian motoneuron. J Neurosci. 1991 Jun;11(6):1609–1616. doi: 10.1523/JNEUROSCI.11-06-01609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivich G., Gehrmann J., Kreutzberg G. W. Increase of macrophage colony-stimulating factor and granulocyte-macrophage colony-stimulating factor receptors in the regenerating rat facial nucleus. J Neurosci Res. 1991 Dec;30(4):682–686. doi: 10.1002/jnr.490300412. [DOI] [PubMed] [Google Scholar]
- Sendtner M., Kreutzberg G. W., Thoenen H. Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature. 1990 May 31;345(6274):440–441. doi: 10.1038/345440a0. [DOI] [PubMed] [Google Scholar]
- Tribollet E., Charpak S., Schmidt A., Dubois-Dauphin M., Dreifuss J. J. Appearance and transient expression of oxytocin receptors in fetal, infant, and peripubertal rat brain studied by autoradiography and electrophysiology. J Neurosci. 1989 May;9(5):1764–1773. doi: 10.1523/JNEUROSCI.09-05-01764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E., Goumaz M., Raggenbass M., Dubois-Dauphin M., Dreifuss J. J. Early appearance and transient expression of vasopressin receptors in the brain of rat fetus and infant. An autoradiographical and electrophysiological study. Brain Res Dev Brain Res. 1991 Jan 15;58(1):13–24. doi: 10.1016/0165-3806(91)90232-8. [DOI] [PubMed] [Google Scholar]