Abstract
Stem cell therapy on acute myocardial infarction (AMI) has been performed for over a decade. In the present study, cardiac perfusion, metabolism and function in dogs with AMI treated by intracoronary injection of bone marrow-derived mesenchymal stem cells (MSCs) were evaluated by dual isotope simultaneous acquisition (DISA) of single positron emission computed tomography (SPECT). Dogs (n=12, 20–30 kg) were randomly assigned to two groups: A graft study (n=6) and control group (n=6). Bone marrow mesenchymal aspirate was collected 3 weeks before surgical procedure. Stem cells were induced by 5-azacytidine for differentiation into myocytes. The dog AMI model was produced by blocking the blood stream at 1/3 of the distinct left anterior descending coronary artery for 90 min. For dogs in the grafting group, MSCs were transplanted by intracoronary injection, and for the control group, 0.9% NaCl was injected instead. At 1 and 10 weeks after MSCs were grafted, respectively, SPECT DISA was performed for each dog in the two groups with 99mTc-SPECT MIBI (925 MBq) and 18F-FDG (222 MBq) for evaluation of myocardial perfusion and metabolism. After the dogs were sacrificed, heart tissue was stained by myocyte-specific antibodies for newborn vessels, troponin T and bromodeoxyuridine (BrdU). Following induction by 5-azacytidine, the morphological features with colony formation, microfilament, as well as atrial granules and positive stainings of α-actinin, myosin and troponin I demonstrated strongly that the MSCs differentiated into myocytes. The number of viable myocardial segments was 10 in the grafting group, which was significantly greater compared with the control group. The ejection fraction of the infarcted left ventricle (LVEF,%) increased from 53.80±9.58 to 70.00±7.52 (change, 16.20±2.93) at 1 and 10 weeks after MSCs engraftment, whilst in the control group, LVEF was 50.50±8.02 and 56.50±7.24 (change, 5.50±2.69), respectively. The LVEF difference was statistically significant (P<0.05) between the graft and control groups. Furthermore, immunostaining of all the myocyte-specific antibodies (for newly born vessels, troponin T and BrdU) was positive. In conclusion, direct intracoronary injection of bone marrow MSCs into injured myocardium in the experimental dog AMI model can significantly improve cardiac function with new vessel formation and myocyte-specific biomarker expression, and in particular, the present study further shows that DISA SPECT can be used for the assessment of stem cell transplantation in the heart.
Keywords: perfusion, metabolism, stem cell, acute myocardial infarction
Introduction
Acute myocardial infarction (AMI) remains the leading cause of morbidity, mortality and heart failure worldwide. Over the past few decades, significant efforts have been made in the treatment of AMI based on early reperfusion of the culprit artery, including pharmacological and mechanical therapies, resulting in significantly reducing mortality (1). However, the lack of repair of a substantial amount of damaged cardiac tissue may lead to congestive heart failure. Necrosis or scarred tissue following AMI in the heart will lead to significant ventricular remodeling characterized by dilation of the left ventricular cavity, thinning of the infarcted tissue and electrical remodeling, resulting in the increase of the risk of sudden cardiac fatality. The use of stem cells with angiogenic properties for therapy of AMI has been widely investigated as a feasible strategy for repairing injured myocardial muscle tissue (2,3). Mesenchymal stem cells (MSCs), as a subpopulation of bone marrow cells, were found to differentiate into various cell types, including functional cardiomyocytes. MSCs are easily isolated, have a proclivity for ex vivo expansion and a potential for allogeneic use (4). MSC therapy for AMI has been investigated widely in animals and humans (5,6).
Positron emission tomography (PET) has been applied to investigate the improvement of myocardial function following stem cell therapy for AMI in humans and animals as a non-invasive imaging tool, such as imaging of 11C-acetate, 13N-ammonia and 18F-FDG (7). These PET tracers are not always available for numerous hospitals as their generation requires the on-site cyclotron (except 18F-FDG) (8,9). Compared with PET, single proton emission computed tomography (SPECT) is much more available in practices. In particular, SPECT with dual isotope simultaneous acquisition (DISA) allowed the assessment of myocardial perfusion (with 99mTc-MIBI) and metabolism (with 18F-FDG) in a single study by Slart et al (10,11). Therefore, DISA SPECT provides an easy procedure for stem cell study to assess myocardial function for humans and animals. Thus, the present study was designed to evaluate the engraftment in myocardium and the improvement of cardiac function following intracoronary injection of pre-differentiated MSCs in a canine model with induced AMI using DISA SPECT, to quantify myocardial perfusion and metabolism, respectively.
Materials and methods
Animal model of myocardial infarction
All the surgical procedures and postoperative care were performed in accordance with the guidelines of the Beijing Fuwai Hospital Animal Care and Use Committee (Beijing, China). All the study protocols were approved by the Ethics Committee of Beijing Fuwai Hospital. A total of 12 dogs (male and female, 2–3 years old and 20–30 kg) were included in the experiment: 6 were the control group and another 6 were the graft group. All the dogs in the two groups were anesthetized by venous injection of ketamine (50 mg/kg) and diazepam (0.05 mg/kg). Following the induction of anesthesia, the animals were intubated and mechanically ventilated through a small-animal ventilator with a minute volume of 1.5–2.5 ml. Respiratory rate and tidal volume were adjusted according to body weight and other physiological parameters. To avoid heat loss, the body temperature was maintained by a heating lamp and the animals were covered with drapes, leaving only the necessary surgical area uncovered. The electrocardiogram was monitored throughout the surgery. Subsequent to the opening of the thoracic cavity and pericardium, four silk suture strings were placed underneath the vessel and the surrounding myocardium at the distal left anterior descending (LAD) coronary artery. LAD was ligated for 5 min and the perfusion was restored for 10 min. After this, ligation was maintained for 90 min, and LAD perfusion was restored again. Duration ligation, a small piece of a polyethylene tube was used to avoid damage to the vessel.
Bone marrow harvest, stem cells isolation, expansion and labeling
Four weeks before surgery, 10–15 ml bone marrow aspirate was collected in a syringe with 1 ml heparin from the iliac crest from each dog after anesthesia was administrated. Bone marrow mononuclear cells were isolated using Ficoll-Hypaque solution (1.077 g/ml; Sigma-Aldrich, St. Louis, MI, USA) and plated in Dulbeccos modified Eagles medium (DMEM) containing 10% fetal bovine serum (FBS) and antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin; Invitrogen, Carlsbad, CA, USA). The media were changed and maintained in DMEM-F12 medium supplemented with 10% FBS and antibiotics for several weeks. To induce cell differentiation, cells were incubated in DMEM-F12 medium supplemented with 5% FBS and antibiotics 24 h before treatment with 5-azacytidine (20 µmol/l; Sigma-Aldrich) from the third passage. Following incubation, media were changed and maintained in DMEM-F12 supplemented with 10% FBS and antibiotics for several weeks.
Cell differentiation was evaluated morphologically and immunohistochemically. Briefly, after 4 weeks of culture, differentiated cells were evaluated by phase contrast microscopy and electron microscopy, respectively. Furthermore, cultured cells were immunostained by monoclonal antibodies to sarcomeric α-actine, cardiac myosin heavy chain and troponin I (Sigma-Aldrich).
Cellular transplant
For the graft group, at 2 or 3 h after perfusion was restored following 90 min ligation of LAD, 2–3 ml (107 cells) of in vitro culture-expanded MSCs were implanted to the AMI area by intracoronary injection using a thin syringe. To identify the grafted cells in the host myocardium, one-third of the implanted MSCs were labeled with bromodeoxyuridine (BrdU) prior to implantation.
For dogs in the control group, 0.9% NaCl was injected in the same way. Electrocardiography (ECG) was performed during surgical procedure and continued to 24–48 h.
Myocardial perfusion and metabolism assessment by SPECT
DISA SPECT with 18F-FDG and 99mTc-MIBI was employed to assess myocardial perfusion and metabolism simultaneously, using a double-head γ-camera equipped with ultra-high-energy collimators (VariCam; GE Healthcare, Cleveland, OH, USA). The imaging protocol was as previously described [Slart et al (11) and De Boer et al (12)]. Briefly, at week 10 after implantation, 99mTc-MIBI (925 MBq) and 18F-FDG (222 MBq) were injected into the ear vein of a dog after administration of anesthesia, and scanning started at 60–90 min after injection. The acquisition was set to continuous mode with a 64×64 word matrix. Dual isotope acquisition was obtained with two separate energy windows: 140 keV with 20% window and 511 keV with 20% window. Following this, a standard gated SPECT myocardial perfusion scan was performed for LVEF measurement.
Image analysis
Myocardial perfusion and metabolic imaging data were analyzed semi-quantitatively by two experienced nuclear cardiologists independently, with a five-point scoring system: 0, normal uptake; 1, mild hypoperfusion; 2, moderate hypoperfusion; 3, severe hypoperfusion; and 4, no uptake. Two cardiologists were blinded to the experimental groups.
Histology, pathology and immunohistochemistry
All the dogs were sacrificed following SPECT imaging. The heart was arrested by infusion of cadmium chloride (0.1 M) into the left atrium. The heart and circulation were perfused with phosphate-buffered saline [PBS (pH 7.4) 50 nM sodium nitroprusside] and 5% formalin in PBS at a physiological pressure for 10 min. Subsequently, the hearts were dissected and immersion-fixed in 10% formalin in PBS for 24 h, processed and embedded in paraffin for further morphological, histological and immunohistochemical analyses. The heart was cut longitudinally, perpendicular to the infarcted area, resulting in an anterior and posterior section. From the two sections, 10 µm slices were cut.
Statistical analysis
Differences among groups were compared using one-way analysis of variance with Students t-test using SPSS v.12 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Mesenchymal stem cell culture and differentiation
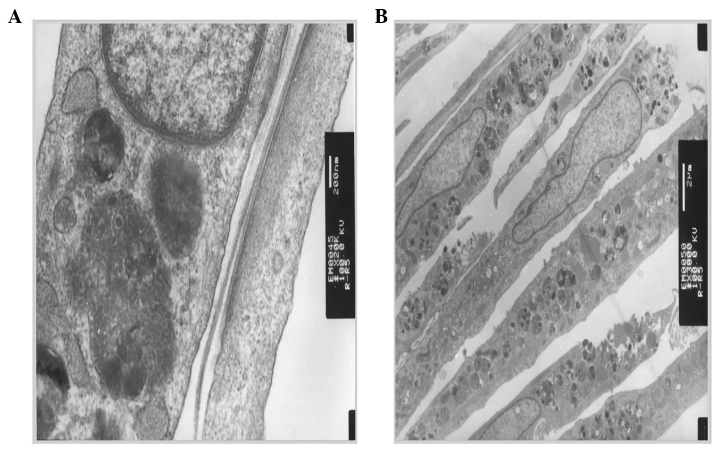
Differentiation of mesenchymal stem cells to myocytes induced by 5-azacytidine was evaluated by microscopic and electron microscopic observations, as well as immunohistochemical staining. Fig. 1 shows the morphological changes following induction, from rod-like (Fig. 1A) to colony formation (Fig. 1B) and spindle-like cells (Fig. 1C). Using an electron microscope, microfilaments and atrial granules around the nucleus were identified (Fig. 2). Furthermore, by immunohistochemical staining, sarcomeric α-actinin, cardiac myosin heavy head and troponin I were positive (Fig. 3). These observations confirmed that bone marrow mesenchymal stem cells have differentiated into cardiomyocytes.
Figure 1.

Photomicrographs of cultured mesenchymal stem cells (MSCs) with characteristics of MSCs in morphology and proliferation. (A) A single stem cell. (B) Colony formation of stem cells. (C) Spindle-like stem cell arrangement.
Figure 2.
Electron micrographs of differentiated mesenchymal stem cells (MSCs) characterized with (A) microfilaments and (B) atrial granules around the nucleus.
Figure 3.
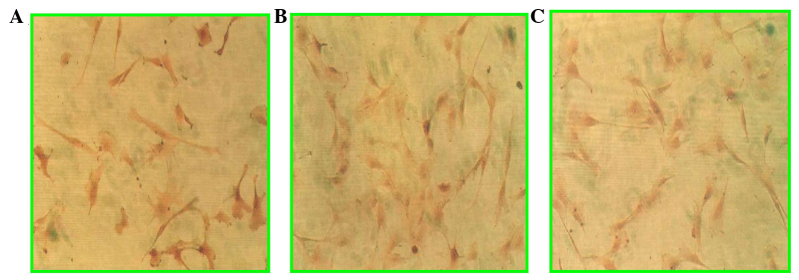
Photomicrographs of cultured mesenchymal stem cells (MSCs) immunohistochemically stained for cardiac sarcomeric (A) α-actinin, (B) myosin heavy head and (C) troponin I.
Serum enzyme increase following ligation of LAD
The levels of serum enzymes after 9–12 h following LAD ligation, including creatine kinase (CK), isoenzymes of creatine kinase (CK-MB), glutamic oxaloacetic transaminase (GOT), glutamate-pyruvate transaminase (GPT) and troponine T (TNT), were significantly increased, as shown in Table I, in the two groups. High levels of cardiomyocytes enzyme in the serum indicated that myocardial ischemia or AMI occurred in the dogs of the two groups.
Table I.
Levels of serum enzymes at 9–12 h after LAD ligation.
| Group | CK, IU/l | CK-MB, IU/l | GOT, IU/l | GPT, IU/l | TNT |
|---|---|---|---|---|---|
| Control | 4,998.6±198.4 | 423.7±67.9 | 494.8±36.7 | 231.3±45.6 | +++ |
| Graft | 4,987.5±158.3 | 428.3±58.2 | 487.6±40.4 | 235.4±41.8 | +++ |
No statistically significant difference between the control and graft groups. LAD, left anterior descending; CK, creatine kinase; CK-MB, isoenzymes of creatine kinase; GOT, glutamic oxaloacetic transaminase; GPT, glutamate-pyruvate transaminase; TNT, troponin T.
Myocardial function changes following MSCs implantation
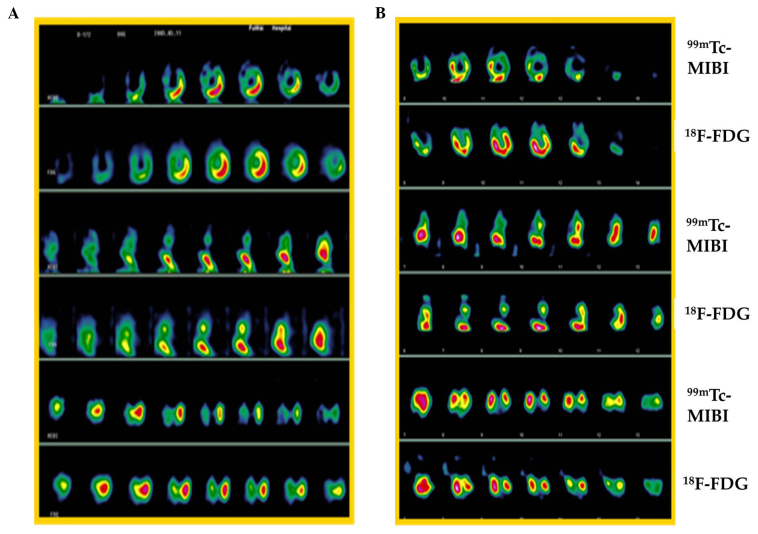
One myocardial viability case is shown in Fig. 4 at different time points [1 (Fig. 4A) and 4 weeks (Fig. 4B)] after ligation of LAD. The change in the number of viable/infarcted segments in myocardium is presented as a summary in Table II. The infarcted number of myocardial segments was decreased significantly in the graft group, from 25 (1 week) to 15 (10 weeks), following MSCs implantation, indicating that myocardial regeneration occurred in the infarction area. Cardiac function was also improved significantly in the graft group, as LVEF increased from 53.80% (1 week) to 70.00% (10 weeks), but in the control group, LVEF had a small increase (from 50.5 to 56.5%) at the same time-points, as shown in Table III.
Figure 4.
Myocardial perfusion and metabolic images from DISP single positron emission computed tomography (SPECT). Imaging was performed at (A) 1 and (B) 10 weeks after ligation of descending coronary artery (LAD). 99mTc-MIBI represents myocardial perfusion imaging and 18F-FDG represents myocardial metabolic imaging.
Table II.
Infarcted segment number change at different time points after mesenchymal stem cell implantation.
| Group | 1 week | 10 weeks | Change |
|---|---|---|---|
| Control | 20 | 18 | 2 |
| Graft | 25 | 15 | 10 |
Table III.
Change of LVEF (%) following mesenchymal stem cell implantation.
| Group | 1 week | 10 weeks | Diffference |
|---|---|---|---|
| Control | 50.50±8.02 | 56.50±7.24 | 5.50±2.69 |
| Graft | 53.80±9.58 | 70.00±7.52a | 16.20±2.93a |
Indicates significant difference between the two groups (control and graft). LVEF, left ventricle.
Immunohistochemical staining
Evidence from immunohistochemical staining demonstrated the engraftment of MSCs in the infarcted myocardial area (Fig. 5). In the antibody staining of BrdU (Fig. 5A and B), angiogenesis and troponin I were found, and similarly, lymphocytic infiltration was present in the infarcted myocardium (Fig. 5C).
Figure 5.

Photomicrographs of infarcted myocardium implanted with mesenchymal stem cells (MSCs) at 10 weeks after cell transplantation. Tissue sections were positively immunohistochemically stained for (A) BrdU (angiogenesis), (B) troponin I and (C) H&E (lymphocytic infiltration).
Discussion
Using the dog AMI model in the present study, intracoronary injection of bone marrow-derived MSCs into injured myocardium significantly improved cardiac functions, including reduction of the infarcted area and increase of left ventricle (LVEF). Notably, DISA and gated SPECT can be used for assessment of viability of newly-formed myocardium following MSCs transplantation, and this is of importance in practice, as SPECT is significantly less expensive with longer-lived more easily-obtained radioisotopes, that that of PET (11,13).
Previously, stem cell-based treatments of AMI have been a focus of investigation. Autologous bone marrow-derived MSCs have been widely utilized as a result of plasticity, availability and lack of immunological rejection or ethical issues (14,15). However, poor survival of transplanted MSCs in the injured myocardial area is one barrier to the success of stem cell therapy for AMI (16). Previous studies have shown that improvement of the periinfarct milieu with pharmacological treatment, such as simvastatin and atorvastatin, is beneficial for the survival of implanted stem cells (17–19). In the present study, pharmacological treatment was not used for improvement of the microenvironment for implanted stem cells, but a suitable injection time was selected; MSCs were injected after 2 h of myocardial reperfusion with 90 min of LAD ligation. Immediate injection of MSCs may be better that that of implantation within a week after AMI.
DISA SPECT offers the advantage of obtaining information on myocardial perfusion using 99mTc-MIBI and metabolism using 18F-FDG in a single study, with shortened duration of the procedure and an identical geometric registration of different isotope images (9). Direct comparison of the perfusion tracer 99mTc-MIBI with metabolic tracer 18F-FDG can determine the scarce and viable myocardium [Slarts et al (10)]. Comparing with PET perfusion tracers, such as 15O-water, 13N-ammonia (which requires on-site cyclotron for radionuclide production) and 82Rb-rubidium (requires on-site generation for radionuclide production), DISA SPECT with 99mTc-MIBI/18F-FDG is much more widely used in hospital routine practice. Therefore, in the future, with rapid development of stem cell implantation-based therapy for treatment of AMI, DISP SPECT and gated SPECT will play more roles in assessment of cardiomyogenesis, angiogenesis, cardiac function and viable myocardial change following stem cell implantation for the injured heart.
In conclusion, direct intracoronary injection of bone marrow MSCs into injured myocardium in the experimental dog AMI model can significantly improve cardiac function with new vessel formation and myocyte-specific biomarker expression. In particular, the study further shows that DISA SPECT can be used for assessment of stem cells transplantation in the heart.
Acknowledgements
The present study was funded by a grant from the Fujian Provincial Youth Personnel of Scientific and Technology innovation item (no. 2005J070) and from the Affiliated Hospital of Inner Mongolia Medical College (no. nmgfy 200802). The authors thank Professors Xiejie Liu, Rongfang Shi and Zuoxiang He at Beijing Fuwai Hospital.
References
- 1.Keeley EC, Hillis LD. Primary PCI for myocardial infarction with ST-segment elevation. N Engl J Med. 2007;356:47–54. doi: 10.1056/NEJMct063503. [DOI] [PubMed] [Google Scholar]
- 2.Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: A systematic review. Eur Heart J. 2008;29:1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 3.Penn MS, editor. Stem Cells and Myocardial Regeneration. 1st. Humana Press; Totowa: 2007. p. 277. [Google Scholar]
- 4.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigo SF, van Ramshorst J, Hoogslag GE, Boden H, Velders MA, Cannegieter SC, Roelofs H, Al Younis I, Dibbets-Schneider P, Fibbe WE, et al. Intramyocardial injection of autologous bone marrow-derived ex vivo expanded mesenchymal stem cells in acute myocardial infarction patients is feasible and safe up to 5 years of follow-up. J Cardiovasc Transl Res. 2013;6:816–825. doi: 10.1007/s12265-013-9507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuda K, Fujita J. Mesenchymal, but not hematopoietic, stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction in mice. Kidney Int. 2005;68:1940–1943. doi: 10.1111/j.1523-1755.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- 7.Castellani M, Colombo A, Giordano R, Pusineri E, Canzi C, Longari V, Piccaluga E, Palatresi S, Dellavedova L, Soligo D, et al. The role of PET with 13N-ammonia and 18F-FDG in the assessment of myocardial perfusion and metabolism in patients with recent AMI and intracoronary stem cell injection. J Nucl Med. 2010;51:1908–1916. doi: 10.2967/jnumed.110.078469. [DOI] [PubMed] [Google Scholar]
- 8.Saraste A, Kajander S, Han C, Nesterov SV, Knuuti J. PET: Is myocardial flow quantification a clinical reality? J Nucl Cardiol. 2012;19:1044–1059. doi: 10.1007/s12350-012-9588-8. [DOI] [PubMed] [Google Scholar]
- 9.Rahmim A, Zaidi H. PET versus SPECT: Strengths, limitations and challenges. Nucl Med Commun. 2008;29:193–207. doi: 10.1097/MNM.0b013e3282f3a515. [DOI] [PubMed] [Google Scholar]
- 10.Slart RH, Bax JJ, van Veldhuisen DJ, van der Wall EE, Dierckx RA, Jager PL. Imaging techniques in nuclear cardiology for the assessment of myocardial viability. Int J Cardiovasc Imaging. 2006;22:63–80. doi: 10.1007/s10554-005-7514-8. [DOI] [PubMed] [Google Scholar]
- 11.Slart RH, Bax JJ, van Veldhuisen DJ, van der Wall EE, Irwan R, Sluiter WJ, Dierckx RA, de Boer J, Jager PL. Prediction of functional recovery after revascularization in patients with chronic ischaemic left ventricular dysfunction: Head-to-head comparison between 99mTc-sestamibi/18F-FDG DISA SPECT and 13N-ammonia/18F-FDG PET. Eur J Nucl Med Mol Imaging. 2006;33:716–723. doi: 10.1007/s00259-005-0016-z. [DOI] [PubMed] [Google Scholar]
- 12.De Boer J, Slart RH, Blanksma PK, Willemsen AT, Jager PL, Paans AM, Vaalburg W, Piers DA. Comparison of 99mTc-sestamibi-18F-fluorodeoxyglucose dual isotope simultaneous acquisition and rest-stress 99mTc-sestamibi single photon emission computed tomography for the assessment of myocardial viability. Nucl Med Commun. 2003;24:251–257. doi: 10.1097/00006231-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Hutton BF. The origins of SPECT and SPECT/CT. Eur J Nucl Med Mol Imaging. 2014;41(Suppl 1):S3–S16. doi: 10.1007/s00259-013-2606-5. [DOI] [PubMed] [Google Scholar]
- 14.Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. Concise review: Mesenchymal stromal cells: Potential for cardiovascular repair. Stem Cells. 2008;26:2201–2210. doi: 10.1634/stemcells.2008-0428. [DOI] [PubMed] [Google Scholar]
- 15.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 16.Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song L, Yang YJ, Dong QT, Qian HY, Gao RL, Qiao SB, Shen R, He ZX, Lu MJ, Zhao SH, et al. Atorvastatin enhance efficacy of mesenchymal stem cells treatment for swine myocardial infarction via activation of nitric oxide synthase. PLoS One. 2013;8:e65702. doi: 10.1371/journal.pone.0065702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang YJ, Qian HY, Huang J, Li JJ, Gao RL, Dou KF, Yang GS, Willerson JT, Geng YJ. Combined therapy with simvastatin and bone marrow-derived mesenchymal stem cells increases benefits in infarcted swine hearts. Arterioscler Thromb Vasc Biol. 2009;29:2076–2082. doi: 10.1161/ATVBAHA.109.189662. [DOI] [PubMed] [Google Scholar]
- 19.Soboleva EL, Gabbasov ZA, Agapov AA, Akchurin RS, Saburova OS, Romanov YA, Smirnov VN. Circulating bone marrow stem/progenitor cells in vascular atherogenesis and in the noninvasive diagnosis of coronary stenosis. Exp Clin Cardiol. 2005;10:184–188. [PMC free article] [PubMed] [Google Scholar]