Abstract
Vascular calcification (VC), in which high serum phosphate plays a critical role, is one major problem in patients with chronic kidney disease. Clinical studies report that magnesium has a protective effect on VC. However, the studies regarding the impact of high serum magnesium on VC at a cellular level are few and require further investigation. Therefore, the present study explored the effect of magnesium on calcification induced by β-glycerophosphate (BGP) in rat aortic vascular smooth muscle cells (RAVSMCs). In the present study, the addition of magnesium decreased calcium deposition, which was increased by BGP. Higher magnesium levels inhibited BGP-induced alkaline phosphatase (ALP) activity and decreased the expression of core-binding factor α-1 (Cbfα1). In conclusion, higher magnesium levels prevented BGP-induced calcification in RAVSMCs and inhibited the expression of Cbfα1 and ALP. Thus, magnesium is influencing the expression of Cbfα1 and ALP associated with VC and may have the potential to serve as a role for VC in clinical situations.
Keywords: magnesium, phosphate, vascular calcification, vascular smooth muscle cells
Introduction
Chronic kidney disease (CKD) is becoming a major public health concern and is also highly prevalent in developing countries (1,2). Recently, based on a report published in the Lancet in 2012, China had a higher incidence and prevalence rate of CKD, estimating the overall prevalence of CKD to be 10.8% (3). The number of patients with CKD in China is estimated to be ~119.5 million (4). The incidence of complications in the CKD patients is increasing with the changes of lifestyle. Vascular calcification (VC) is a frequent complication in patients with CKD and directly or indirectly contributes to cardiovascular disease and increased mortality rates (5,6). It is reported that VC is an actively regulated progress. Apart from the passive precipitation of calcium phosphate, the transdifferentiation of vascular smooth muscle cells (VSMCs) into osteoblast-like cells, as well as changes in the expression of bone-associated and mineralization regulating proteins, and apoptosis play critical roles in VC (7). However, the underlying pathophysiological mechanisms are not well understood. It is proposed that along with traditional cardiovascular risk factors, non-traditional risk factors, such as those associated with uremic status and a disturbed bone and mineral metabolism, are likely to control whether or not VC occurs in patients with CKD (8).
Magnesium is involved in numerous important enzymatic processes and also plays a role in skeletal and mineral metabolism and vascular tone (9,10). Data from observational clinical studies showed an inverse association between serum magnesium concentrations and the presence of VC or atherosclerosis (11,12). However, to date, only a limited number of experimental studies performed in animals have confirmed these findings, whereas the majority investigated the role of lowering dietary magnesium on VC (13,14). Thus far, only 2 studies have reported that magnesium has a favorable effect on bone matrix mineralization in an in vitro model (15,16). Therefore, the present study aimed to investigate whether increasing magnesium concentrations influence calcification of rat aortic vascular smooth muscle cells (RAVSMCs).
Materials and methods
Cell culture of RAVSMC
RAVSMCs were prepared as previously described with minor modifications (17–19). Briefly, RAVSMCs were isolated from the thoracic aorta of adult male Sprague-Dawley rats (80–100 g). After the rats had been anaesthetized with chloral hydrate (400 mg/kg), the thoracic aorta was obtained under aseptic conditions and cut into small pieces following the removal of residual blood. Small pieces of tissue (1–2 mm2) were placed in a culture dish in Dulbecco's modified Eagle's medium (DMEM) supplemented with 15% fetal bovine serum (FBS; HyClone Corp., Logan, UT, USA), 4.5 g glucose, 100 U/ml penicillin and 100 µg/ml streptomycin in a 5% CO2 incubator at 37°C. Cells that migrated from explants were collected when confluent. The cells were maintained in DMEM supplemented with 15% FBS, and the medium was replaced twice a week. RAVSMCs were identified by the SMC typical hill and valley appearance, and immunocytochemistry using a monoclonal antibody against the α-smooth muscle actin protein 1A4 (Acta 2) (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) further confirmed the purity of the primary cell culture (20). The cells between passages 6 and 12 were used in all the experiments.
Calcification assays
RAVSMC calcification was induced as previously described (21). VSMCs were randomly divided into a negative control group, high phosphorus and magnesium intervention groups, a negative control group using low glucose DMEM medium containing 10% FBS and a high phosphorus group in which 10 mM β-glycerophosphate (BGP) was added to normal medium to generate high phosphorus medium. Magnesium chloride was added to the medium for the magnesium intervention groups on the basis of high phosphorus; the final concentrations of magnesium ions (Mg2+) were 11, 2 and 3 mM (magnesium intervention groups 1, 2 and 3, respectively), and the stimulating time was 7 days. Control medium was prepared equally but without BGP. The medium was replaced twice a week and Mg2+ was added from the beginning and at each medium renewal of the experiment. Supernatants were collected for further investigations.
For precise biochemical Ca2+ measurements, calcium content in the supernatant was extracted with 6 N hydrochloric acid overnight. The o-cresolphthalein complexone method was conducted using a Calcium Assay kit (BioSino Biotechnology, Beijing, China) and normalized to the protein content of the same culture.
For alizarin red staining (AR), cells were washed twice with phosphate-buffered saline (PBS) and fixed with ethanol 95%. Subsequently, the samples were exposed to 40 mM AR (pH 4.2). Following two washing steps, images were captured of the wells to show the presence of induced mineralization.
Assay of alkaline phosphatase (ALP) activity
Cells were plated at a density of 2×105 cells/well into 24-well plates. The cells were cultured for 7 days after intervention and rinsed 3 times with PBS. Subsequently, 500 µl of 0.1% Triton X-100 was added to each well and incubated for 12–24 h at 4°C. The protein assay was performed with the bicinchoninic acid protein assay reagent (Sigma, St. Louis, MO, USA). ALP activity was assayed by a method modified from that of Lowry et al (22). In brief, the assay mixtures contained 0.1 M 2-amino-2-methyl-1-propanol, 1 mM MgCl2, 8 mM p-nitrophenyl phosphate disodium and the cell homogenates. After incubation for 4 min at 37°C, the reaction was stopped with 0.1 N NaOH and the absorbance was read at 405 nm. Each value was expressed as p-nitrophenol produced in nanomoles/min/microgram of protein.
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR)
RT-PCR was performed to determine the expression levels of the target gene, Cbfα1. Total RNA was extracted from cultured cells using TRIzol reagent (Invitrogen Life Technologies, Grand Island, NY, USA) according to the manufacturer's instructions. cDNA was synthesized using the advantage RT-for-PCR kit (Clontech, Palo Alto, CA, USA) with oligo (dT) priming according to the manufacturer's instructions. The GAPDH gene was used as the endogenous control. The primers for Cbfα1 were: 5′-CCGCACGACAACCGCACCAT-3′ (sense) and 5′-CGCTCCGGCCCACAAATCTC-3′ (antisense). The primers for GAPDH were 5′-CAAGGTCATCCATGACAACTTTG-3′ (sense), 5′-GTCCACCACCCTGTTGCTGTAG-3 (antisense). The generated PCR products were 289-bp for Cbfα1 and 496-bp for GAPDH. The PCR condition consisted of one incubation for 2 min at 95°C, followed by 35 cycles of a 30-sec denaturation at 95°C, a 30-sec annealing at 55°C and a 45-sec extension at 72°C, and a final extension at 72°C for 5 min. The PCR products were tested in 1% agarose gel in electrophoresis, visualized with ethidium bromide staining and quantified using an image analysis system (Gel work-2ID; Cell Signaling Technology, Shanghai, China). The optical density value of the mRNA expression was calculated.
Statistical analysis
Each experiment was repeated independently 3 times and all the data are expressed as mean ± standard deviation. Means were compared by the Student's t-test. Means between multiple groups were performed using the analysis of variance (ANOVA). Intergroup comparison was made using ANOVA and the Student-Newman-Keuls test. All the statistical analyses were performed using the SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). For all the statistical tests, P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of BGP on calcium content of cultured RAVSMCs
Biochemical Ca2+ measurements were performed on day 7 after the intervention with various media conditions. The calcium content of the BGP group was significantly higher than those of the control group (Fig. 1A).
Figure 1.
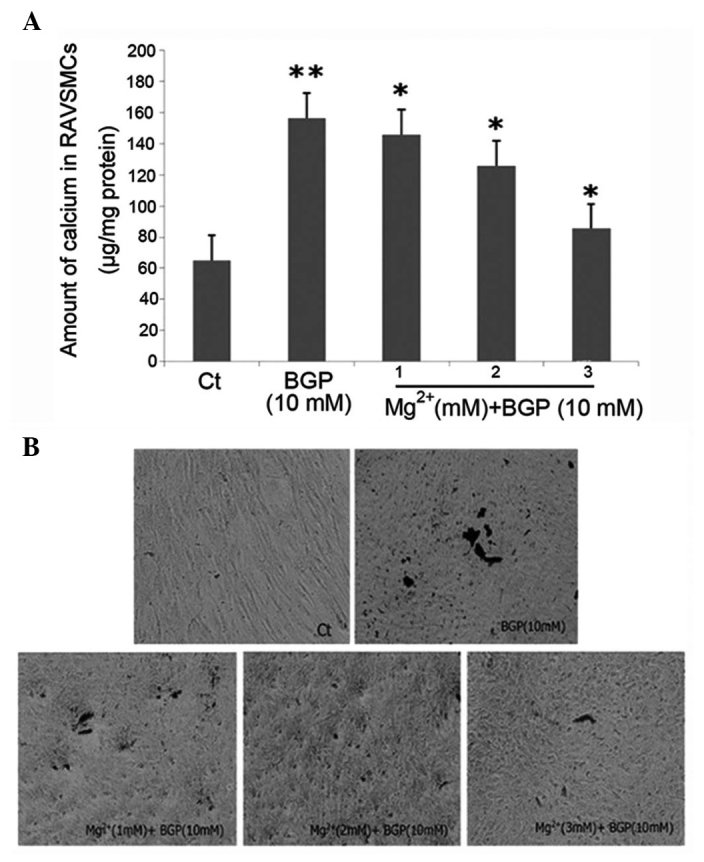
(A) Effect of magnesium on β-glycerophosphate (BGP)-induced deposition of calcium in rat aortic vascular smooth muscle cells (RAVSMCs) following intervention with 1, 2 and 3 mM Mg2+. *P<0.05 vs. BGP group; **P<0.05 vs. Ct group. In parallel, (B) alizarin red staining was performed with cells treated for 7 days under the same conditions as mentioned above.
Mg2+ inhibits BGP-induced calcification in RAVSMCs
To investigate the effects of different Mg2+ concentrations on VC, the calcium content was measured of the cells incubated with the indicated conditions. AR staining was also performed on day 7 to further confirm the occurrence of calcium/phosphate (Ca/P) deposition. In the presence of 10 mM BGP disodium, Mg2+ at a total concentration of 1, 2 and 3 mM in the medium was effective to significantly inhibit the calcium content on day 7. After 7 days of intervention, Mg2+ decreased the calcium content significantly in a dose-dependent manner (Fig. 1A). The effect of Mg2+ was evident at 1 mM, and became clearer as the concentration increased and reached the maximum at 3 mM. In addition to the biochemical analysis, Ca/P deposits were also identified by AR staining (Fig. 1B). The size and amount of the granular calcified formations appear to decrease with the addition of Mg2+ in a dose-dependent manner.
Effects of Mg2+ on Cbfα1 expression and activity of ALP in RAVSMCs
To further validate the effects of Mg2+ on the differentiation of RAVSMCs, the gene expression associated with osteoblast differentiation was examined on day 7 after intervention of Mg2+ by RT-PCR. RT-PCR showed that the mRNA expression of Cbfα1 was downregulated following intervention with 1, 2 and 3 mM Mg2+ (P<0.05) (Fig. 2). After 7 days of intervention, the ALP activity of the BGP group was significantly higher than those of the control group (P<0.05). Mg2+ decreased the activity of ALP significantly in a dose-dependent manner (Fig. 3). The effect of Mg2+ was evident at 1 mM, became clearer as the concentration increased and reached the maximum at 3 mM. These data demonstrated that Mg2+ plays an important role in preventing BGP-induced calcification in RAVSMCs
Figure 2.
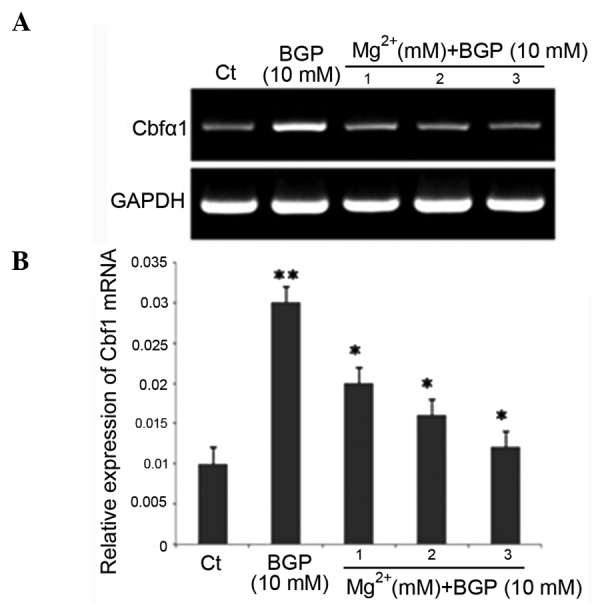
Messenger RNA (mRNA) expression of core binding factor α-1 (Cbfα1) in β-glycerophosphate (BGP)-induced rat aortic vascular smooth muscle cells following intervention with 1, 2 and 3 mM Mg2+. (A) Representative mRNA levels of Cbfα1; (B) quantification of the relative mRNA expression levels of Cbfα1. *P<0.05 vs. BGP group; **P<0.05 vs. Ct group.
Figure 3.
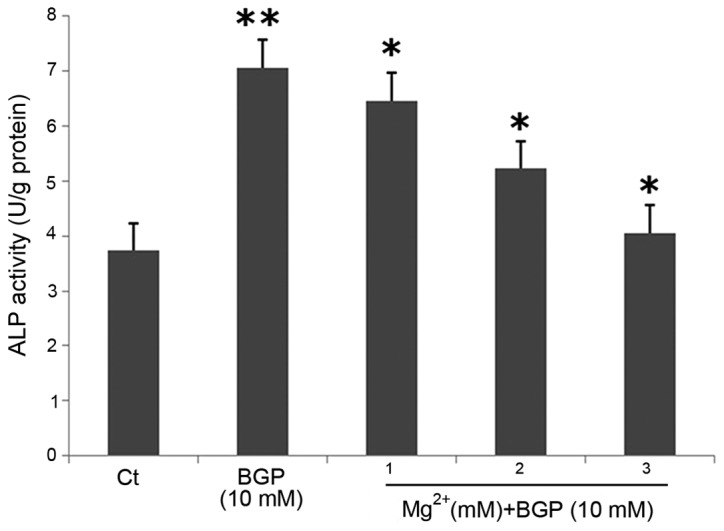
Effect of magnesium on the alkaline phosphatase (ALP) acitvity of β-glycerophosphate (BGP)-induced rat aortic vascular smooth muscle cells following intervention with 1, 2 and 3 mM Mg2+. *P<0.05 vs. BGP group; **P<0.05 vs. Ct group.
Discussion
Results obtained from previous clinical studies have indicated that lower magnesium levels are associated with increased VC in uremic and non-uremic conditions, and higher serum magnesium results in a decreased risk of peripheral VC in non-diabetic haemodialysis patients (13,23–26). Furthermore, magnesium supplementation improved the media thickness in patients on hemodialysis (12). Notably, the dietary magnesium level influenced the ectopic mineralization in an animal model, which may have broader implications (13,27). However, the detailed mechanisms by which magnesium acts on VC were not clear and the relevant studies were few and limited. The aim of the present study was to provide in vitro evidence for the preventive effect of magnesium on calcification in a cell culture model of RAVSMCs.
Magnesium is the second most abundant intracellular divalent cation and functions as an allosteric modulator of several enzymes, or bridges structurally distinct molecules (28). Previous clinical studies showed that a low magnesium status has an important role in the pathogenesis of cardiovascular disease (29), hypertension (30) and thrombosis (6) in subjects without kidney disease. In the present experimental model, BGP-induced calcification was strongly inhibited by increasing magnesium concentrations in the media of RAVSMCs in a dose-dependent manner and was stable over a period of ≤7 days. Increasing magnesium concentrations decreased calcium deposition, providing evidence that magnesium is able to impair hydroxyapatite crystal growth in in vitro experiments, which is in agreement with the previous studies. It has been suggested that the effect of magnesium on VC may be mediated via this mechanism (31–33). The present study also found that magnesium was not only able to prevent RAVSMC calcification but also inhibit progression of already established calcification. Even partly reverted calcification was observed with longer incubation time, which renewed the attraction in the therapeutic applications of magnesium on VC. Although phosphate binders are administered to patients of CKD in order to lower serum phosphorus concentrations, as one aspect of VC development, VC progressed overtime. Therefore, magnesium administration or magnesium-containing phosphate binder supplementation in CKD patients may be beneficial as it may partially revert or at least inhibit the progression of VC.
VC is an active, cell-mediated multistep process that includes apoptosis, and the osteochondrogenic differentiation of VSMCs is similar to bone formation, with the role of deposition of calcium-phosphate in terms of matrix mineralization (7,8,34). The bone-related factors, particularly the specific osteogenic transcription factor Cbfα1, were shown to be upregulated in VSMCs undergoing BGP-induced transdifferentiation into osteoblast-like cells. Factors such as ALP, which may contribute to the mineralization process, were also upregulated by elevated phosphate concentrations in in vitro models (35). In the present study, high phosphorus induced the enhanced expression of Ca2+ content, Cbfα1 and ALP, while these decreased when administrated with higher magnesium levels, clearly showing that the protective effect of magnesium on RAVSMCs calcification by not only decreasing the deposition of calcium in the cells but also affecting the osteogenic transdifferentiation. However, it appears unlikely, that the inhibitory effect of magnesium on calcification will also account in the bone, as it has been shown that the physiological magnesium concentrations present in bone are not able to inhibit crystal growth (32). The present study suggests that magnesium inhibits the pathological transdifferentiation process from VSMCs into osteoblast-like cells, which does not occur in the physiological process of calcification in the bone. However, detailed clinical investigations regarding the effect of magnesium on bone metabolism, specifically in renal failure patients, are rare and (24) contradictory (36,37). Well designed in vitro and clinical studies to further evaluate the influence of magnesium on bone are required.
In conclusion, higher magnesium levels prevented calcification and further progression of already established calcification in RAVSMCs. Increased magnesium concentrations not only reduced the deposition of calcium, but also inhibited osteogenic transdifferentiation, which are consistent with experimental animal studies and with clinical studies linking elevated magnesium levels to beneficial effects on VC (11,26,38). However, the detailed mechanism by which magnesium may influence this process is unclear. Thus, it appears to be that magnesium influences the molecular processes associated with VC, providing an important insight into the clinical application of magnesium. More clinical research is required to observe the serum magnesium levels in patients with renal failure and to confirm a possible protective effect of magnesium against VC. There is a large gap concerning controlled interventional studies providing unchallengeable results to improve the understanding of its role.
Acknowledgements
The present study was supported by the project of the Hebei Natural Science Fund (grant no. H2012206157) and the project of the Hebei Major Medical Science (grant no. GL2011-51).
References
- 1.Nugent RA, Fathima SF, Feigl AB, Chyung D. The burden of chronic kidney disease on developing nations: A 21st century challenge in global health. Nephron Clin Pract. 2011;118:c269–c277. doi: 10.1159/000321382. [DOI] [PubMed] [Google Scholar]
- 2.Eknoyan G, Lameire N, Barsoum R, Eckardt KU, Levin A, Levin N, Locatelli F, MacLeod A, Vanholder R, Walker R, et al. The burden of kidney disease: Improving global outcomes. Kidney Int. 2004;66:1310–1314. doi: 10.1111/j.1523-1755.2004.00894.x. [DOI] [PubMed] [Google Scholar]
- 3.Yano Y, Fujimoto S, Asahi K, Watanabe T. Prevalence of chronic kidney disease in China. Lancet. 2012;380:213–216. doi: 10.1016/S0140-6736(12)61208-2. [DOI] [PubMed] [Google Scholar]
- 4.Liu ZH. Nephrology in China. Nat Rev Nephrol. 2013;9:523–528. doi: 10.1038/nrneph.2013.146. [DOI] [PubMed] [Google Scholar]
- 5.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 6.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 7.Cozzolino M, Missaglia E, Ortiz A, Bellasi A, Adragao T, Apostolous T, Vescovo G, Gallieni M. Vascular calcification in chronic kidney disease. Recenti Prog Med. 2010;101:442–452. (In Italian) [PubMed] [Google Scholar]
- 8.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–567. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 9.Spiegel DM. Magnesium in chronic kidney disease: Unanswered questions. Blood Purif. 2011;31:172–176. doi: 10.1159/000321837. [DOI] [PubMed] [Google Scholar]
- 10.M de Francisco AL, Rodriguez M. Magnesium - its role in CKD. Nefrologia. 2013;33:389–399. doi: 10.3265/Nefrologia.pre2013.Feb.11840. [DOI] [PubMed] [Google Scholar]
- 11.Ishimura E, Okuno S, Kitatani K, Tsuchida T, Yamakawa T, Shioi A, Inaba M, Nishizawa Y. Significant association between the presence of peripheral vascular calcification and lower serum magnesium in hemodialysis patients. Clin Nephrol. 2007;68:222–227. doi: 10.5414/CNP68222. [DOI] [PubMed] [Google Scholar]
- 12.Turgut F, Kanbay M, Metin MR, Uz E, Akcay A, Covic A. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int Urol Nephrol. 2008;40:1075–1082. doi: 10.1007/s11255-008-9410-3. [DOI] [PubMed] [Google Scholar]
- 13.van den Broek FA, Beynen AC. The influence of dietary phosphorus and magnesium concentrations on the calcium content of heart and kidneys of DBA/2 and NMRI mice. Lab Anim. 1998;32:483–491. doi: 10.1258/002367798780599758. [DOI] [PubMed] [Google Scholar]
- 14.Planells E, Llopis J, Perán F, Aranda P. Changes in tissue calcium and phosphorus content and plasma concentrations of parathyroid hormone and calcitonin after long-term magnesium deficiency in rats. J Am Coll Nutr. 1995;14:292–298. doi: 10.1080/07315724.1995.10718510. [DOI] [PubMed] [Google Scholar]
- 15.Nakatani S, Mano H, Ryanghyok IM, Shimizu J, Wada M. Excess magnesium inhibits excess calcium-induced matrix-mineralization and production of matrix gla protein (MGP) by ATDC5 cells. Biochem Biophys Res Commun. 2006;348:1157–1162. doi: 10.1016/j.bbrc.2006.07.180. [DOI] [PubMed] [Google Scholar]
- 16.Kircelli F, Peter ME, Sevinc Ok E, Celenk FG, Yilmaz M, Steppan S, Asci G, Ok E, Passlick-Deetjen J. Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrol Dial Transplant. 2012;27:514–521. doi: 10.1093/ndt/gfr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong IK, Oh H, Park SJ, Kang JH, Kim S, Lee MS, Kim MJ, Hwang YC, Ahn KJ, Chung HY, et al. Inhibition of NF-κB prevents high glucose-induced proliferation and plasminogen activator inhibitor-1 expression in vascular smooth muscle cells. Exp Mol Med. 2011;43:684–692. doi: 10.3858/emm.2011.43.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhai K, Hubert F, Nicolas V, Ji G, Fischmeister R, Leblais V. β-Adrenergic cAMP signals are predominantly regulated by phosphodiesterase type 4 in cultured adult rat aortic smooth muscle cells. PLoS One. 2012;7:e47826. doi: 10.1371/journal.pone.0047826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang HY, Huang RP, Han P, Xue DB, Li HB, Liu B, Shan P, Wang QS, Li KS, Li HL. The effects of artemisinin on the proliferation and apoptosis of vascular smooth muscle cells of rats. Cell Biochem Funct. 2014;32:201–208. doi: 10.1002/cbf.2995. [DOI] [PubMed] [Google Scholar]
- 20.Skalli O, Pelte MF, Peclet MC, Gabbiani G, Gugliotta P, Bussolati G, Ravazzola M, Orci L. Alpha-smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. J Histochem Cytochem. 1989;37:315–321. doi: 10.1177/37.3.2918221. [DOI] [PubMed] [Google Scholar]
- 21.Chen NX, O'Neill KD, Duan D, Moe SM. Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells. Kidney Int. 2002;62:1724–1731. doi: 10.1046/j.1523-1755.2002.00625.x. [DOI] [PubMed] [Google Scholar]
- 22.Lowry OH, Roberts NR, Wu ML, Hixon WS, Crawford EJ. The quantitative histochemistry of brain. II. Enzyme measurements. J Biol Chem. 1954;207:19–37. [PubMed] [Google Scholar]
- 23.Shioi A, Nishizawa Y, Jono S, Koyama H, Hosoi M, Morii H. Beta-glycerophosphate accelerates calcification in cultured bovine vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1995;15:2003–2009. doi: 10.1161/01.ATV.15.11.2003. [DOI] [PubMed] [Google Scholar]
- 24.Meema HE, Oreopoulos DG, Rapoport A. Serum magnesium level and arterial calcification in end-stage renal disease. Kidney Int. 1987;32:388–394. doi: 10.1038/ki.1987.222. [DOI] [PubMed] [Google Scholar]
- 25.Tzanakis I, Pras A, Kounali D, Mamali V, Kartsonakis V, Mayopoulou-Symvoulidou D, Kallivretakis N. Mitral annular calcifications in haemodialysis patients: A possible protective role of magnesium. Nephrol Dial Transplant. 1997;12:2036–2037. doi: 10.1093/ndt/12.9.2036. [DOI] [PubMed] [Google Scholar]
- 26.Tzanakis I, Virvidakis K, Tsomi A, Mantakas E, Girousis N, Karefyllakis N, Papadaki A, Kallivretakis N, Mountokalakis T. Intra- and extracellular magnesium levels and atheromatosis in haemodialysis patients. Magnes Res. 2004;17:102–108. [PubMed] [Google Scholar]
- 27.LaRusso J, Li Q, Jiang Q, Uitto J. Elevated dietary magnesium prevents connective tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6(–/–)) J Invest Dermatol. 2009;129:1388–1394. doi: 10.1038/jid.2008.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf FI, Cittadini A. Magnesium in cell proliferation and differentiation. Front Biosci. 1999;4:D607–D617. doi: 10.2741/Wolf. [DOI] [PubMed] [Google Scholar]
- 29.Liao F, Folsom AR, Brancati FL. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 1998;136:480–490. doi: 10.1016/S0002-8703(98)70224-8. [DOI] [PubMed] [Google Scholar]
- 30.Peacock JM, Folsom AR, Arnett DK, Eckfeldt JH, Szklo M. Relationship of serum and dietary magnesium to incident hypertension: The Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol. 1999;9:159–165. doi: 10.1016/S1047-2797(98)00040-4. [DOI] [PubMed] [Google Scholar]
- 31.Ennever J, Vogel JJ. Magnesium inhibition of apatite nucleation by proteolipid. J Dent Res. 1981;60:838–841. doi: 10.1177/00220345810600041301. [DOI] [PubMed] [Google Scholar]
- 32.Blumenthal NC, Posner AS. Hydroxyapatite: Mechanism of formation and properties. Calcif Tissue Res. 1973;13:235–243. doi: 10.1007/BF02015413. [DOI] [PubMed] [Google Scholar]
- 33.Boskey AL, Posner AS. Effect of magnesium on lipid-induced calcification: An in vitro model for bone mineralization. Calcif Tissue Int. 1980;32:139–143. doi: 10.1007/BF02408533. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 35.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 36.Gonella M, Ballanti P, Della Rocca C, Calabrese G, Pratesi G, Vagelli G, Mazzotta A, Bonucci E. Improved bone morphology by normalizing serum magnesium in chronically hemodialyzed patients. Miner Electrolyte Metab. 1988;14:240–245. [PubMed] [Google Scholar]
- 37.Morinière P, Vinatier I, Westeel PF, Cohemsolal M, Belbrik S, Abdulmassih Z, Hocine C, Marie A, Leflon P, Roche D, et al. Magnesium hydroxide as a complementary aluminium-free phosphate binder to moderate doses of oral calcium in uraemic patients on chronic haemodialysis: Lack of deleterious effect on bone mineralisation. Nephrol Dial Transplant. 1988;3:651–656. doi: 10.1093/oxfordjournals.ndt.a091722. [DOI] [PubMed] [Google Scholar]
- 38.Adamopoulos C, Pitt B, Sui X, Love TE, Zannad F, Ahmed A. Low serum magnesium and cardiovascular mortality in chronic heart failure: A propensity-matched study. Int J Cardiol. 2009;136:270–277. doi: 10.1016/j.ijcard.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


