Abstract
Mesenchymal stem cells (MSCs) are primarily isolated by their adherence to plastic and their in vitro growth characteristics. Expansion of these cells from an adherent culture is the only method to obtain a sufficient number of cells for use in clinical practice and research. However, little is known with regard to the effect of adherence to plastic on the phenotype of the cells. In the present study, bone marrow CD45−CD31−CD44− stem cell antigen (Sca)-1+ MSCs were sorted by flow cytometry and expanded in adherent cultures. The expression levels of the adhesion molecule, Sca-1, in the adherent cultures were compared with those from nonadherent cultures at different time points. The flow cytometry results indicated that the expression levels of Sca-1 decreased in the MSCs in the nonadherent cultures grown in ultra-low-adherent plates. Furthermore, the result was confirmed by quantitative polymerase chain reaction at the same time points. Therefore, the results demonstrated that the loss of plastic adherence downregulated the expression of Sca-1. The observations may provide novel insights into the molecular mechanisms underlying plastic adherent culture.
Keywords: adherent culture, mesenchymal stem cells, nonadherent culture, phenotype, stem cell antigen-1
Introduction
Mesenchymal stem cells (MSCs) are considered to be one of the most promising therapeutic cell sources for regenerative medicine, primarily due to their multipotency and immunosuppressive functions. These cells can be isolated from multiple types of tissue, including bone marrow (BM), skin, adipose and umbilical cord tissue (1–3), and are able to differentiate into osteoblasts, adipocytes, chondrocytes and myocardial cells in vitro (4,5). Previous studies have confirmed that MSCs are also able to secrete bioactive factors that alter the milieu of dysfunctional tissues (6,7). These observations provide strong evidence of the potential therapeutic role of MSCs in the treatment of various types of diseases.
However, the therapeutic use of MSCs has been limited due to a number of factors, including difficulties in obtaining sufficient numbers of cells and the unsuccessful engraftment of the cells following transplantation. Current methods include the in vitro expansion of MSCs in plastic adherent culture (8), and the subsequent use of these cells for transplantation into patients. However, in vitro expansion in a standard adherent culture can markedly alter the cell phenotype, which may lead to lung entrapment of the cells and little or no engraftment of the cells in the target organs (8–10). At present, seldom studies have been conducted with the aim to investigate the factors that cause a variation in the MSC phenotype in vitro, and one of the important reasons for this is the lack of suitable research methods.
In 2011, Vunjak-Novakovic and Scadden classified the cellular and acellular components of the stem cell niche (11). The authors demonstrated the important role of the environment in determining the characteristics of MSCs. In addition, the in vitro conditions of the adherent culture may play a vital role in determining the stem cell niche; thus, may affect MSCs. A previous study revealed that CD44− BM cells contained almost all clonogenic cells with a multilineage differentiation potential (12). However, in vitro culture of CD44− BM cells resulted in their conversion to a CD44+ phenotype. With regard to these observations, it was hypothesized that plastic adherence in culture may affect the cell phenotype of MSCs that undergo amplification in vitro.
Stem cell antigen-1 (Sca-1) is enriched on freshly isolated BM MSCs (13–15), and Sca-1+ MSCs are known to have an important function in improving cardiac function in myocardial infarction (16). To determine whether adherence to plastic during culture affects the expression of Sca-1 in MSCs, a novel method of seeding MSCs in ultra-low-attachment culture plates was applied to analyze the effects of plastic adherence on Sca-1 expression. The Sca-1 cell surface marker on MSCs was analyzed using flow cytometry. In addition, the mRNA expression of Sca-1 was assessed by quantitative polymerase chain reaction (qPCR) to confirm the differences in Sca-1 expression between the cells grown in adherent and nonadherent culture conditions.
Materials and methods
Isolation of mononuclear cells from BM in mice
FVB/N mice (age, 9–15 weeks) were obtained from the Karolinksa Institute (Stockholm, Sweden) and 6 mice were sacrificed for this study. Mice were sacrificed by cervical dislocation. Animal experimental protocols were performed with approval from the Local Ethics Committee at Karolinska Institute. Mononuclear cells were isolated from the BM of the mice using a previously described method (12). Briefly, femurs, tibias and iliac crests were crushed in Dulbecco's phosphate-buffered saline (DPBS; Gibco Life Technologies, Paisley, Scotland) and 10% fetal bovine serum (FBS; #10500064; Gibco Life Technologies, Divinopolis, Brazil). Cells from the bone samples were obtained following treatment of the bone fragments with 0.1% collagenase II (Worthington Biochemical Corporation, Lakewood, NJ, USA) and 0.05% trypsin-EDTA (#25300062; Gibco Life Technologies, Grand Island, NY, USA) for 30–45 min at 37°C. Bone and BM cells were pooled and centrifuged at 300 × g (Sorvall ST 16R; Thermo Fisher Scientific, Osterode, Germany) for 5–10 min at room temperature, after which the cells were resuspended in DPBS plus 10% FBS for BM MSC isolation. This research was performed at the Center for Hematology and Regenerative Medicine (HERM) of Karolinska Institute.
Multicolor fluorescence-activated cell sorting (FACS) isolation of mouse MSCs
In a previous study, CD44− MSCs were determined to have properties of MSCs (12). Therefore, BM CD45−CD31−CD44−Sca-1+ MSCs were selected for the study. Briefly, the hematopoietic cells in the BM mononuclear cell preparations were initially depleted by incubating the cells with a purified rat anti-mouse CD45 primary antibodies against CD45 (#140451; eBioscience Inc., San Diego, CA, USA), TER119 (#116202), GR1 (#108402; 1:50), B220 (#103202; 1:100), CD4 (#100506), CD8 (#100802) and MAC1 (#101202; 1:200; BioLegend Inc., San Diego, CA, USA). Cells were subsequently incubated with sheep anti-rat Dynabeads (Dynal Biotech, Inc., New York, NY, USA). The remaining hematopoietic cells were visualized using a goat anti-rat tricolor antibody and fluorescence-conjugated anti-CD45 (#553082; BD Biosciences, Franklin Lakes, NJ, USA), anti-TER119 (#116210) and anti-CD19 (#115510; BioLegend Inc.) antibodies to remove any hematopoietic cells. Dead cells were excluded by propidium iodide staining. CD44−Sca-1+ stromal cells were gated based on the Fluorescence Minus One controls for CD44 Sca-1 expression using a FACSAria III flow cytometer (BD Biosciences), as shown in Fig. 1. These experimental procedures were performed at the Center for HERM of Karolinska Institute.
Figure 1.
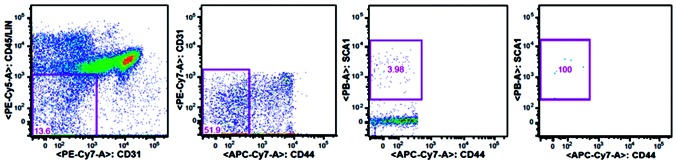
Fluorescence-activated cell sorting (FACS) isolation of mouse bone marrow mesenchymal stem cells (BM MSCs). FACS profile showing the gating strategy for the sorting of BM MSCs (CD45−LIN−CD31−CD44−Sca-1+). The CD31−CD45−LIN− cells were first gated within the live cells (PI−), after which the CD44−Sca-1+ cells were gated. Sca, stem cell antigen; LIN, lineage markers; PI, propidium iodide.
Expansion of MSCs and nonadherence in vitro culture conditions
Sorted MSCs were plated in 20 ml complete Dulbecco's modified Eagle's medium (#10569010; Gibco Life Technologies, Grand Island, NY, USA), containing 10% FBS, 10 mM N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (#15630106; Gibco Life Technologies, Paisley, Scotland), 100 U/ml penicillin and 100 mg/ml streptomycin (15140122; Gibco Life Technologies, Grand Island, NY, USA), in a T75 tissue culture flask (#430641; Corning, Inc., Corning, NY, USA). A hypoxic environment is known to greatly improve the genetic stability and expression of chemokine receptors during in vitro expansion (17). Thus, to increase the accuracy of the results, the cells were incubated in a incubator (FORMA3131; Thermo Fisher Scientific) with 1% O2 and 5% CO2. Complete medium was changed every 5–7 days. When the culture cells exhibited 90% fusion, the cells were suspended by incubation in 0.05% trypsin-EDTA for 5 min at 37°C, and the MSCs were reseeded at a density of 2,500 cells/cm2 in a T75 tissue culture flask.
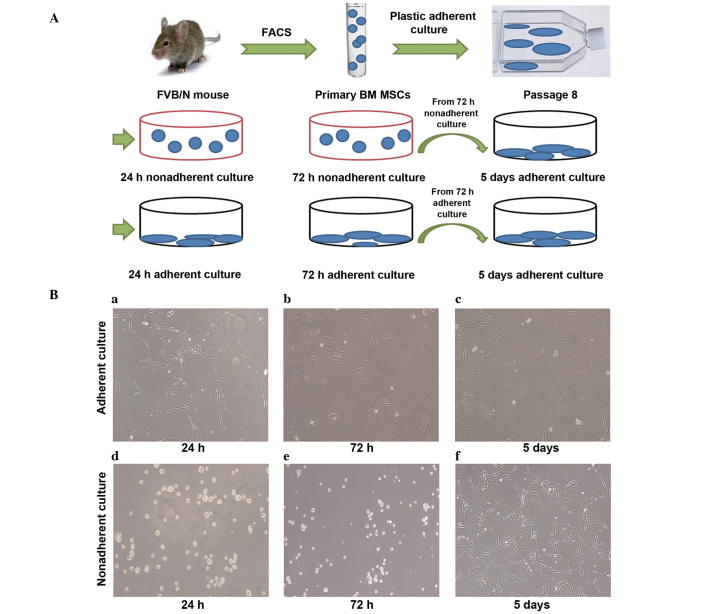
For this plastic-expansion approach, the cells were passaged eight times. Cells in passage 9–11 were transferred into the nonadherent cultures and grown for 24 or 72 h. The cells were plated at a density of 2,500 cells/cm2 in ultra-low-attachment tissue culture plates (#3471; Corning, Inc.). At the same time, an equal number of cells were transferred into adherent culture plates (#353046; BD Biosciences) and grown for 24 or 72 h as a control. In addition, nonadherent culture cells and adherent culture cells from the 72-h cultures were reseeded (2,500 cells/cm2) in the adherent culture plates and grown for 5 days under adherent culture conditions (Fig. 2A).
Figure 2.
Experimental procedure and morphology of nonadherent and adherent cultured cells. (A) MSCs were sorted by FACS and expanded to eight passages by culturing in plastic. Subsequently, the MSCs were seeded into ultra-low-attachment culture plates and resuspended in the medium (nonadherent culture). As a control, the same number of MSCs were seeded in tissue culture plates and attached to the bottom of the plate (adherent culture). Nonadherent and adherent cultured cells obtained after 72 h were reseeded into tissue culture plates and incubated for 5 days (5 days adherent culture). FACS and quantitative polymerase chain reaction were performed at 24 h, 72 h and 5 days. (B) Morphology of the MSCs. MSCs were plated in the tissue culture plates and cultured for (a) 24 h, (b) 72 h and (c) 5 days. All the MSCs in images a-c were attached to the bottom of the plastic plate. MSCs were plated in ultra-low-attachment tissue culture plates and cultured for (d) 24 h and (e) 72 h. All the MSCs from stages d and e were suspended in the medium. (f) MSCs cultured for 72 h in the nonadherent culture were reseeded into tissue culture plates and cultured for 5 days. The cells were attached to the bottom of the plastic plate. Scale bar, 100 µm. FACS, fluorescence-activated cell sorting; BM MSCs, bone marrow mesenchymal stem cells.
FACS analysis of the Sca-1 MSC surface marker
The control adherent cultured cells at 24 h, 72 h and 5 days were detached with 0.05% trypsin-EDTA and collected in a 50-ml centrifuge tube. The nonadherent cultured cells were also collected in a 50-ml centrifuge tube simultaneously. The cells were centrifuged at 300 × g for 5 min and resuspended in phosphate-buffered saline (PBS; Gibco Life Technologies, Taicang, China) for analysis on a BD FACSCalibur (BD Biosciences). Adherent and nonadherent cultured cells were stained with a rat anti-mouse Sca-1 antibody (#557405; BD Biosciences) to analyze the expression of this cell surface marker. An isotype control (PE-R3–34 immunoglobulin; #554685; BD Biosciences) was added at the indicated concentration (0.25 µg), and BD FACSComp software (BD Biosciences) was used for data analysis.
qPCR
Adherent and nonadherent cultured cells grown for 24 h, 72 h and 5 days were collected in 1.5-ml Eppendorf tubes. The total RNA was extracted from the adherent and nonadherent culture cells using a High Pure RNA isolation kit (#11828665001; Roche Diagnostics, Laval, QC, Canada), according to the manufacturer's instructions. The cDNA was synthesized using SuperScript III and Oligo primers (#E6300S; New England Biolabs, Inc., Ipswich, MA, USA), according to the manufacturer's instructions. The primers used for Sca-1 were as follows: Forward, 5′-AGGAGGCAGCAGTTATTGTGG-3′, and reverse, 5′-CGTTGACCTTAGTACCCAGGA-3′. β-actin (ACTB) was used as the reference gene, with the following primers: Forward, 5′-GGCTGTATTCCCCTCCATCG-3′, and reverse, 5′-CCAGTTGGTAACAATGCCATGT-3′ (designed by Gibco Life Technologies, Shanghai, China). qPCR analysis of the Sca-1 gene was performed using the Light-Cycler 480 platform (Roche Diagnostics, Basel, Switzerland) with a SYBR Green I PCR kit (#4887352001; Roche Diagnostics, Laval, QC, Canada). The mixture contained 10 ml SYBR Green I Master, 6 ml RNase-free H2O, 1 ml PCR forward primer (10 mM), 1 ml PCR reverse primer (10 mM) and 2 ml cDNA in a final reaction volume of 20 ml. Sca-1 mRNA expression levels were normalized against the Ct of the ACTB RNA (ΔΔCt). The Ct method was applied to determine the fold changes using the Light-Cycler 480 software. The experiment was repeated three times, and each experiment was performed in triplicate.
Statistical analysis
GraphPad Prism software, version 5 (GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analysis. The Student's t-test was applied to identify the statistical significance of the differences between the culture condition groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Morphology of the adherent and nonadherent cultured cells at different time points
MSCs seeded in tissue culture plates (2,500 cells/well) at 24 h, 72 h and 5 days were attached to the bottom of the plastic tissue culture plates and were shown to exhibit flat morphology (Fig. 2Ba–c). However, MSCs seeded in the ultra-low-attachment culture plates at 24 and 72 h were suspended and scattered throughout the medium, exhibiting a rounded morphology (Fig. 2Bd–e). When nonadherent culture cells obtained after 72 h were reseeded in the tissue culture plates and incubated for 5 days in the adherent culture conditions, the cells exhibited a similar morphology to the adherent culture cells (Fig. 2Bf).
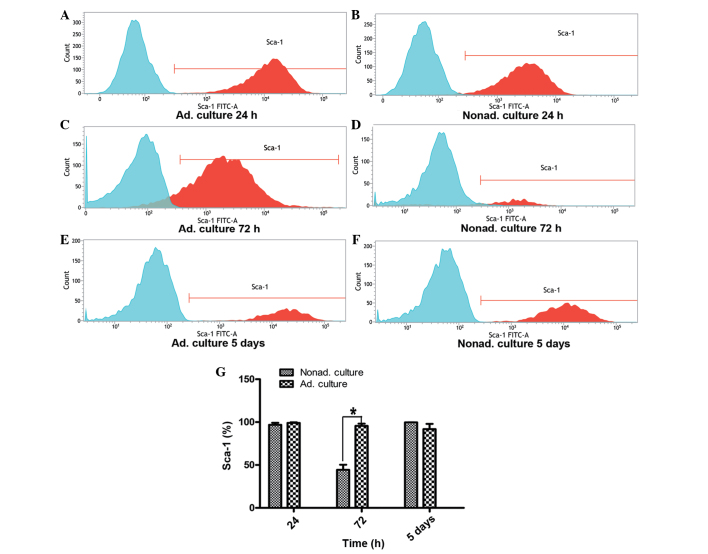
FACS analysis of the Sca-1 MSC surface marker
Sca-1 expression on the MSCs between passages 9 and 11 was analyzed by FACS. At 24 h, the expression of Sca-1 on the nonadherent cultured cells was similar to that of the control group of adherent cultured cells (P>0.05). However, Sca-1 expression at 72 h differed significantly between the adherent and nonadherent cultured cells. At 72 h, the expression level of Sca-1 in the nonadherent cells was approximately one half of that observed in the adherent cultured cells (P<0.05). However, when the nonadherent cultured cells grown for 72 h were reseeded in the adherent conditions and cultured for 5 days, Sca-1 expression recovered to the level exhibited by the adherent cultured cells (P>0.05; Fig. 3).
Figure 3.
Flow cytometry analysis of the expression of the Sca-1 surface marker. (A, C and E) Graphs represent Sca-1 expression on the adherent cultured cells at 24 h, 72 h and 5 days, respectively. (B, D and F) Graphs represent Sca-1 expression on the nonadherent cultured cells at 24 h, 72 h and 5 days, respectively. Expression of the Sca-1 surface marker was analyzed by fluorescence-activated cell sorting, and the expression levels were compared between the adherent and nonadherent cultured cells. Three independent experiments were performed with the cells between passages 9 and 11, which all showed similar Sca-1 expression. (G) Statistical histogram shows the differences in Sca-1 expression levels at 24 h (P>0.05), 72 h (*P<0.05) and 5 days (P>0.05) between the nonadherent and adherent cultured cells. FITC, fluorescein isothiocyanate; Ad, adherent; Nonad; nonadherent; Sca, stem cell antigen.
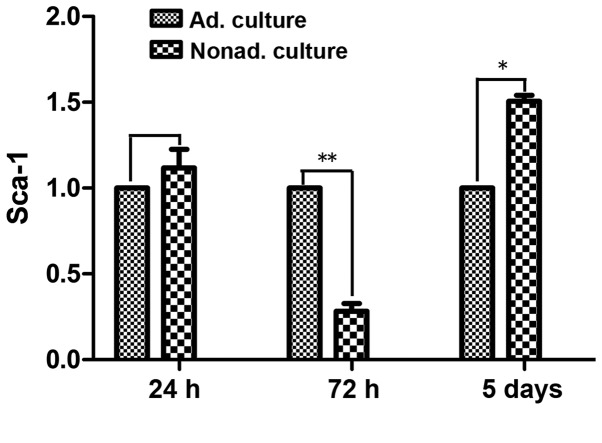
qPCR
qPCR was used to compare the mRNA expression levels of Sca-1 following culture for 24 h, 72 h and 5 days between the adherent and nonadherent cultured cells. At 24 h, Sca-1 mRNA expression levels did not differ significantly between the nonadherent and adherent cultured cells (P>0.05). However, at 72 h, the mRNA expression levels of Sca-1 were ~3 times lower in the nonadherent cultured cells, as compared with the control adherent cultured cells (P<0.01). After culture for 5 days in the adherent conditions, Sca-1 mRNA expression levels were upregulated, and were 1.5 times higher in the nonadherent cultured cells compared with the control adherent cultured cells (P<0.05; Fig. 4).
Figure 4.
Quantitative polymerase chain reaction (qPCR) analysis. Expression of the Sca-1 gene was determined by qPCR. Adherent and nonadherent cultured cells between passages 9 and 11 were used in the experiment. The data are the average of three independent experiments. (A) Sca-1 expression in the nonadherent cultured cells at 24 h, as compared with the adherent cultured cells at 24 h (P>0.05). (B) Sca-1 expression in the nonadherent cultured cells at 72 h compared with the adherent cultured cells at 72 h (**P<0.01). (C) Sca-1 expression in the nonadherent cultured cells at 5 days, as compared with the adherent cultured cells at 5 days (*P<0.05). These experiments demonstrated that the mRNA expression levels of Sca-1 in the mesenchymal stem cells were downregulated after 72 h in the nonadherent culture; however, Sca-1 mRNA expression recovered following culture for 5 days in the adherent culture conditions. Sca, stem cell antigen; Ad, adherent; Nonad, nonadherent.
Discussion
Sca-1 is widely recognized as a marker that can be used to enrich stem cells in a number of tissues (18–20). In addition, Sca-1 expression has been shown to be enriched on isolated mouse BM MSCs with a regenerative and self-renewal capacity (15,19,21,22). In the present study, a novel method of seeding MSCs in ultra-low-attachment culture plates was used, and Sca-1 expression in MSCs was shown to change following culture in nonadherent conditions.
As previous research has shown, CD44− MSCs exhibit similar properties to MSCs (12). Thus, to analyze Sca-1 expression, the CD45−CD31−CD44−Sca-1+ MSCs were sorted. In addition, a previous study reported that MSCs enriched by low-density culture undergo senescence and lose their stem cell properties (23). However, low-density hypoxic culture is a method for efficiently expanding MSCs without losing their stem cell properties or increasing tumorigenicity (17,24). Therefore, to ensure the presence of MSCs with stable stem-cell properties, low-density hypoxic conditions (1% O2) were utilized in the present study.
The expression of Sca-1 on MSCs was analyzed using FACS analysis, and the expression levels were compared between the nonadherent and adherent cultured cells at three time points. At 24 h, there was no statistically significant difference in Sca-1 expression between the nonadherent and adherent cultured cells. However, a notable change in the expression of the Sca-1 cell surface marker was observed at 72 h and 5 days. At 72 h, the nonadherent cultured cells had significantly lower expression levels of Sca-1 compared with the adherent cultured cells. This observation directly confirmed that the nonadherent culture conditions downregulated Sca-1 expression on the MSCs. Notably, the expression of the Sca-1 cell surface marker was shown to recover to the level of the adherent cultured cells when the nonadherent cultured cells, obtained after 72 h, were reseeded in the tissue culture plates and incubated for 5 days in the adherent conditions. These results indicate that the adherent culture conditions increased the expression of Sca-1 following the downregulation by the nonadherent culture. Therefore, these data suggest that nonadherent culture treatment can downregulate the expression of Sca-1 on MSCs. Accordingly, the results from the qPCR analysis confirmed these observations. The pattern of Sca-1 mRNA expression was similar to that of the cell surface expression of the protein on the MSCs. Therefore, the results indicate that the cell surface protein expression and the mRNA expression of Sca-1 can be changed by a variation in culture conditions between nonadherent and adherent culture. Consequently, the cell surface protein and mRNA expression levels of Sca-1 in the MSCs were affected by the nonadherent culture method. In addition, plastic adherence in culture may affect the cell phenotype and gene expression in MSCs that undergo amplification in vitro.
A previous study indicated that the upregulated expression of Sca-1 is associated with a more destructive tumor phenotype (25). Furthermore, in mouse models, Sca-1 has been shown to be associated with greater tumorigenic potential (26). Despite the lack of clear evidence for the malignant transformation of MSCs during in vitro culture, the susceptibility of these cells to functional transformation should not be ignored. Consequently, clinicians and scientists are concerned with regard to the biological safety of MSC transplantation. Adherence to plastic conditions is a critical factor for MSC proliferation in vitro; however, the molecular processes that drive proliferation are complex, and the underlying mechanisms remain unclear. Therefore, the nonadherent culture technique used in the present study may provide a novel method to study the mechanisms underlying plastic adherent culture.
In conclusion, Sca-1 expression in the MSCs were affected by the nonadherent culture method. Further study is required to clarify the association between phenotype variation and biological characteristics in MSCs that undergo amplification in vitro.
Acknowledgements
The authors thank Hong Qian from the Center for HERM at Karolinska Institute for the valuable advice and contribution to the cell sorting (FACS) isolation undertaken in the present study. This study was supported by grants from the International Program of Project 985, Sun Yat-sen University and the ‘One Hundred Talented Scholars’ of Sun Yat-sen University (no. F002009011).
References
- 1.Mundra V, Gerling IC, Mahato RI. Mesenchymal stem cell-based therapy. Mol Pharm. 2013;10:77–89. doi: 10.1021/mp3005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang EJ, Byun JH, Choi YJ, et al. In vitro and in vivo osteogenesis of porcine skin-derived mesenchymal stem cell-like cells with a demineralized bone and fibrin glue scaffold. Tissue Eng Part A. 2010;16:815–827. doi: 10.1089/ten.tea.2009.0439. [DOI] [PubMed] [Google Scholar]
- 3.Tong CK, Vellasamy S, Tan BC, et al. Generation of mesenchymal stem cell from human umbilical cord tissue using a combination enzymatic and mechanical disassociation method. Cell Biol Int. 2011;35:221–226. doi: 10.1042/CBI20100326. [DOI] [PubMed] [Google Scholar]
- 4.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 5.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schinköthe T, Bloch W, Schmidt A. In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev. 2008;17:199–206. doi: 10.1089/scd.2007.0175. [DOI] [PubMed] [Google Scholar]
- 7.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabani V, Shahsavani M, Gharavi M, Piryaei A, Azhdari Z, Baharvand H. Mesenchymal stem cell infusion therapy in a carbon tetrachloride-induced liver fibrosis model affects matrix metalloproteinase expression. Cell Biol Int. 2010;34:601–605. doi: 10.1042/CBI20090386. [DOI] [PubMed] [Google Scholar]
- 9.Qian H, Badaloni A, Chiara F, et al. Molecular characterization of prospectively isolated multipotent mesenchymal progenitors provides new insight into the cellular identity of mesenchymal stem cells in mouse bone marrow. Mol Cell Biol. 2013;33:661–677. doi: 10.1128/MCB.01287-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Vunjak-Novakovic G, Scadden DT. Biomimetic platforms for human stem cell research. Cell Stem Cell. 2011;8:252–261. doi: 10.1016/j.stem.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian H, Le Blanc K, Sigvardsson M. Primary mesenchymal stem and progenitor cells from bone marrow lack expression of CD44 protein. J Biol Chem. 2012;287:25795–25807. doi: 10.1074/jbc.M112.339622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morikawa S, Mabuchi Y, Kubota Y, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura Y, Arai F, Iwasaki H, et al. Isolation and characterization of endosteal niche cell populations that regulate hematopoietic stem cells. Blood. 2010;116:1422–1432. doi: 10.1182/blood-2009-08-239194. [DOI] [PubMed] [Google Scholar]
- 15.Forte G, Franzese O, Pagliari S, et al. Interfacing Sca-1(pos) mesenchymal stem cells with biocompatible scaffolds with different chemical composition and geometry. J Biomed Biotechnol. 2009;2009:910610. doi: 10.1155/2009/910610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughey CC, Ma L, James FD, et al. Mesenchymal stem cell transplantation for the infarcted heart: therapeutic potential for insulin resistance beyond the heart. Cardiovasc Diabetol. 2013;12:128. doi: 10.1186/1475-2840-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haque N, Rahman MT, Abu Kasim NH, Alabsi AM. Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. Scientific World Journal. 2013;2013:632972. doi: 10.1155/2013/632972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forte G, Carotenuto F, Pagliari F, et al. Criticality of the biological and physical stimuli array inducing resident cardiac stem cell determination. Stem Cells. 2008;26:2093–2103. doi: 10.1634/stemcells.2008-0061. [DOI] [PubMed] [Google Scholar]
- 19.Nadri S, Soleimani M, Hosseni RH, Massumi M, Atashi A, Izadpanah R. An efficient method for isolation of murine bone marrow mesenchymal stem cells. Int J Dev Biol. 2007;51:723–729. doi: 10.1387/ijdb.072352ns. [DOI] [PubMed] [Google Scholar]
- 20.Gong X, Sun Z, Cui D, et al. Isolation and characterization of lung resident mesenchymal stem cells capable of differentiating into alveolar epithelial type II cells. Cell Biol Int. 2014;38:405–411. doi: 10.1002/cbin.10240. [DOI] [PubMed] [Google Scholar]
- 21.Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25:1339–1347. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 22.Lee CH, Park JH, Lee JH, et al. Replacement of mouse embryonic fibroblasts with bone marrow stromal cells for use in establishing and maintaining embryonic stem cells in mice. Cell Biol Int. 2012;36:537–543. doi: 10.1042/CBI20110395. [DOI] [PubMed] [Google Scholar]
- 23.Shibata KR, Aoyama T, Shima Y, et al. Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem Cells. 2007;25:2371–2382. doi: 10.1634/stemcells.2007-0225. [DOI] [PubMed] [Google Scholar]
- 24.Tsai CC, Chen YJ, Yew TL, et al. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011;117:459–469. doi: 10.1182/blood-2010-05-287508. [DOI] [PubMed] [Google Scholar]
- 25.Witz IP. Differential expression of genes by tumor cells of a low or a high malignancy phenotype: the case of murine and human Ly-6 proteins. J Cell Biochem Suppl. 2000;34:61–66. doi: 10.1002/(SICI)1097-4644(2000)77:34+<61::AID-JCB11>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 26.Yuan H, Upadhyay G, Yin Y, Kopelovich L, Glazer RI. Stem cell antigen-1 deficiency enhances the chemopreventive effect of peroxisome proliferator-activated receptorγ activation. Cancer Prev Res (Phila) 2012;5:51–60. doi: 10.1158/1940-6207.CAPR-11-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]