Abstract
Postmenopausal osteoporosis (PMOP) is a systemic bone metabolism disease, characterized by progressive bone loss following menopause and a subsequent increase in fracture risk. Estrogen deficiency as a result of menopause is known to increase bone resorption and accelerate bone loss. Furthermore, postmenopausal women may exhibit iron accumulation, in addition to estrogen deficiency. Elevated iron levels are a risk factor for PMOP in postmenopausal women, and reducing the iron overload has been demonstrated to benefit bone cell metabolism in vitro and improve the bone in vivo by normalizing osteoclastic bone resorption and formation. The identification of hepcidin was a key development in the field of iron metabolism in the previous decade. We hypothesize that hepcidin may aid in the prevention and treatment of PMOP due to its capacity to control body iron stores and its intrinsic effects on osteoblast function. The aim of the current review was to highlight the role of iron accumulation in the pathogenesis of PMOP and to evaluate the possible use of hepcidin as a potential therapy for this condition.
Keywords: iron, osteoporosis, hepcidin, postmenopause
1. Introduction
Due to the progressive aging of populations worldwide, osteoporosis is a growing public health concern, with increasing prevalence among aging individuals, particularly postmenopausal women. Although osteoporosis has been recognized as a disease entity for almost a century, therapeutic approaches are limited, since the pathogenesis of postmenopausal osteoporosis (PMOP) is complex and not yet fully elucidated. Thus, recent progress towards understanding the role of iron accumulation in PMOP is crucial, since it may expose the underlying mechanisms and aid the treatment of this bone disease.
Iron is one of the most abundant transition metals in the human body, and serves a key function in numerous biological processes, including oxygen transport, DNA synthesis and energy production (1). However, excessive iron is deleterious to organ function (2). If the iron concentration in the circulation exceeds the binding capacity of transferrin, an iron-binding blood plasma glycoprotein, then free iron or non-transferrin-bound iron becomes abnormally enriched in various organs, including the liver, heart, brain and pancreas (3). As a consequence, organs are subject to potentially irreversible damage. Previously, Weinberg (4,5) hypothesized that iron overload is a risk factor for osteoporosis. In women, the levels of iron in the form of ferritin (an iron storage protein) have been observed to increase markedly following menopause (6). Furthermore, previous studies have indicated that increasing iron concentrations contribute towards the development of PMOP by enhancing bone resorption and suppressing bone formation, a mode of action which is independent from that of estrogen (7,8). A reduction in iron levels, using either hepcidin (a negative regulator of iron absorption) or an iron chelator, targets the underlying cause and may provide a viable therapeutic option for mitigating the iron accumulation associated with PMOP. The aim of the present review was to investigate the role of iron accumulation in the development of PMOP and to evaluate the use of iron mitigation as a potential therapy for this clinical condition.
2. Iron accumulation in postmenopausal women
Iron overload is defined as the presence of high serum ferritin concentrations of ≥300 µg/l in men and ≥200 µg/l in women (9). In recent years, an increasing number of studies have investigated the associations among ferritin, estrogen and PMOP, in order to determine the reason for the enhanced risk of developing osteoporosis in women compared with men. By compiling studies on the levels of ferritin and sex hormones in various populations, it was concluded that as women age, their serum levels of estrogen decrease, while serum ferritin levels increase (10). These results demonstrated a negative correlation between ferritin and estrogen levels during the menopausal transition period (Fig. 1A) (6). With regard to the changes in ferritin and testosterone levels in men, a synchronized pattern was observed as the men age, in which ferritin levels decreased gradually following ʻandropauseʼ (Fig. 1B) (11). However, serum ferritin levels in women and men did not reach levels defined as iron overload. Collectively, these results indicated that iron accumulation was a common process in aging women, but not iron overload, which may account for the observed differences between genders in the incidence of osteoporosis. Our retrospective study indicated that women aged >70 years with a hip fracture possessed higher serum ferritin levels and significantly reduced bone mineral density (BMD) in the lumbar spine and hip, as compared with a control group (12). In order to eliminate the possibility that osteoporosis itself, but not iron accumulation, exerted an effect on bone metabolism, a team of scientists in Seoul conducted a three-year longitudinal health promotion center-based study on 1,729 subjects, which included 789 middle-aged men and 940 postmenopausal women (13). Subjects with illnesses known to affect ferritin levels or bone metabolism, such as inflammatory diseases, chronic liver diseases or a history of transfusion, were excluded from the study. The results revealed a linear association between vertebral fracture prevalence and serum ferritin levels in women; however, this correlation was not observed in the male subjects (14), which partially supported the previous observations (12). In addition, previous studies have demonstrated that in healthy individuals, increased serum ferritin levels were associated with an accelerated rate of bone loss, which was most marked in women aged >45 years (13,14). Notably, levels of serum ferritin were markedly increased in the women aged >45 years, as is shown in Fig. 1A. Assuming these results were not a coincidence, 45 years of age, typically during the perimenopausal period, appears to be a critical time point at which the routine examination of biological markers of iron levels may be advisable, in order to monitor the development of iron accumulation.
Figure 1.

Alterations in the levels of ferritin, E2 and testosterone in women and men over time. (A) Serum levels of E2 were converted to a percentage of the normal value in the serum of 25-year-old women (500 pg/ml). (B) Serum levels of testosterone were converted to a percentage of the normal value in the serum of 20-year-old men (4.4 ng/ml). Levels of ferritin are expressed as ng/ml serum. E2, 17β-estradiol; T, testosterone.
3. Involvement of estrogen in iron homeostasis
On the basis of the aforementioned clinical results, an investigation into the interaction between estrogen and iron levels was conducted. Menstruation is a key process in women of a reproductive age, which is characterized by periodic fluctuations in estrogen and the discharge of blood. For menstruating women, the excretion of endogenous iron occurs primarily through blood loss, resulting in reduced levels of ferritin and an increased prevalence of iron deficiency (15,16). Following menopause, iron is no longer lost through menstruation, and the metal ion increasingly accumulates in the body. However, the interaction between estrogen and iron is not exclusively a result of the effects of estrogen on menstrual blood flow. Through investigating the effect of estrogen on hepcidin, a negative regulator of iron absorption, estrogen was observed to transcriptionally suppress the expression of hepcidin by binding to the estrogen response element in the hepcidin promoter (17,18). Notably, this process provides a compensatory mechanism through which estrogen prevents the rapid reduction in body iron in menstruating women, in addition to mitigating the accumulation of iron in postmenopausal women.
4. Iron overload and abnormal bone metabolism
A number of experimental models of iron overload have been established in vivo in order to confirm the adverse effect of iron on bone metabolism. Tsay et al (7) generated a group of iron-overloaded mice via injection with iron dextran for two months. The results indicated that the iron-overloaded mice exhibited alterations in bone microarchitecture, including the trabecular number, thickness and bone volume fraction, in addition to an increase in bone resorption, as compared with the control group. Similarly, postmenopausal rats fed an iron lactate diet for 4 weeks exhibited a significant increase in urinary deoxypyridinoline, indicating an increase in bone resorption activity (19). In an additional study, pigs were administered 300 mg iron dextran per day intramuscularly for 36 days, after which the pigs appeared to have accumulated large iron deposits in the osteoblasts and bone matrix. Furthermore, the bone mineralization and formation in the pigs were shown to have significantly decreased (8).
The mechanisms underlying the impact of iron on bone metabolism are yet to be fully elucidated. However, in vitro data indicated that iron-induced bone damage was predominantly attributable to the function of iron in catalyzing the formation of reactive oxygen species (ROS) via the Fenton reaction (20). Wnt signaling is essential for bone formation through the stimulation of osteoblastogenesis (21). However, ROS are able to antagonize Wnt signaling in osteoblast precursors by diverting β-catenin from T cell factor to Forkhead Box O-mediated transcription, thereby attenuating bone formation (22). Furthermore, a previous study indicated that ferric ion promotes the differentiation of osteoclasts and increases bone resorption via the generation of ROS (23). In summary, the risk of osteoporosis is increased through the suppression of bone formation and enhancing bone resorption.
5. Reducing iron overload for the prevention of bone loss
A previous study reported that iron overload was associated with osteoporosis in ovariectomized (OVX) rats (24). When the OVX rats were fed orally with a bone-targeted chelator (1-N-Docosyl-triethylenetetramine pentaacetic acid), bone loss was alleviated significantly in the chelator-treated OVX rats when compared with the untreated-OVX controls (24,25). Desferrioxamine (DFO), an iron chelator isolated from Streptomyces pilosus, is currently used in clinical practice for the treatment of iron overload in patients with thalassemia, hemochromatosis and sickle cell anemia (26–28). Experimental results have indicated that DFO is able to inhibit osteoclastic differentiation, which has been associated with reduced mitochondrial biogenesis and the production of ROS (29). Furthermore, OVX rats treated with DFO have been shown to exhibit reduced bone resorption and an improved three-dimensional bone structure (29). In addition, our unpublished preliminary data indicated that OVX rats intraperitoneally treated with DFO for three months presented with significantly increased BMD values, accompanied with reduced serum ferritin levels. On the basis of the knowledge that menopause results in iron accumulation, which may independently increase the risk of osteoporosis, the manipulation of iron levels using an iron chelator is hypothesized to be a viable therapeutic approach for the treatment of PMOP.
6. Hepcidin treatment: A potential approach for the reduction of iron overload
Iron homeostasis is closely regulated at the point of iron absorption and storage. Hepcidin, a peptide hormone produced by the liver, is the master regulator of iron homeostasis (30). Hepcidin functions by inhibiting the efflux of cellular iron into the circulation through the transmembrane protein receptor, ferroportin. To date, ferroportin is the only known cellular iron exporter in vertebrates, and is known to be highly expressed in cells involved with iron handling, such as duodenal enterocytes, which absorb iron from the diet, and splenic macrophages, which recycle iron from senescent erythrocytes (31). Hepcidin binds to ferroportin on the surface of duodenal enterocytes and splenic macrophages, and induces the internalization and lysosomal degradation of ferroportin, thereby reducing the body's iron stores and iron deposition in the bone (Fig. 2) (32). Therefore, if the hepcidin-induced downregulation of ferroportin is inadequate or ineffective, ferroportin activity is upregulated and iron overload may occur (33–35).
Figure 2.
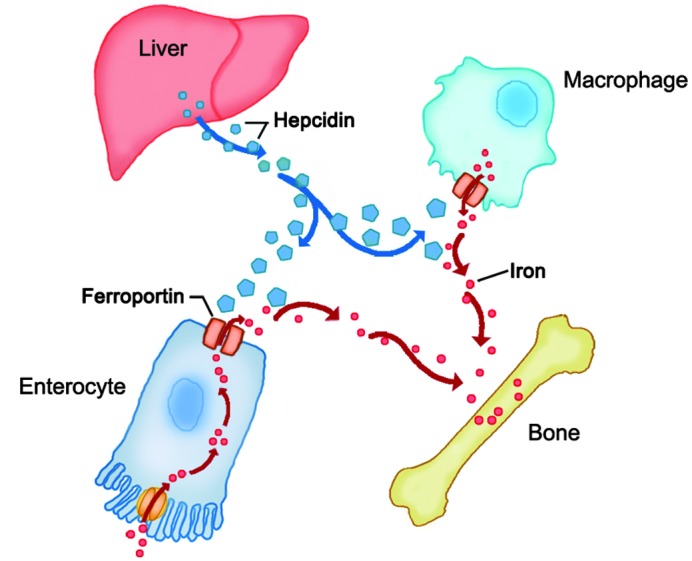
Hepcidin-ferroportin interaction controls the entry of iron into bone tissues. The rate of iron entry into the bone tissues depends primarily on the serum levels of hepcidin. When serum hepcidin levels are reduced, the ferroportin activity is not blocked effectively. Thus, the release of iron from enterocytes and macrophages increases, resulting in elevated serum iron levels and increased iron deposition in bone tissues.
The Hfe gene encodes a membrane protein that is implicated in the stimulation of hepcidin expression (37). In a previous study using Hfe−/− mice, the trabeculae surface was found to be markedly labeled with Prussian blue (used for detecting ferric iron), indicating a considerable quantity of iron deposition in the skeletal tissues. In addition, the Hfe−/− mice manifested an osteoporotic phenotype characterized by low bone mass and impaired bone microarchitecture, in addition to an increased number of osteoclasts along the trabeculae surfaces (38). The results suggested that hepcidin deficiency increases the bone iron content and reduces the quantity of bone tissue. Furthermore, constitutive activation of hepcidin expression or treatment with synthetic hepcidin has been demonstrated to prevent iron overload and the corresponding complications in Hfe−/− mice (39,40). In mice with β-thalassemia, increasing hepcidin expression was shown to induce a reduction in iron content and an improvement in anemia (41). Collectively, these results indicate that hepcidin may possess therapeutic potential for iron-overload diseases (42–44).
In a rat model of osteoporosis, liver hepcidin gene expression was observed to reduce over time, which further suggested that the development of osteoporosis was associated with reduced levels of hepcidin (45). Furthermore, increased mineralization and reduced rates of apoptosis were observed in human osteoblasts treated with hepcidin (46). In addition, a previous study observed that hepcidin was able to increase the intracellular calcium concentration in cultured osteoblasts, an effect that was more evident in cells growing in a high iron concentration environment (47). By reducing the calcium influx from extracellular spaces using nimodipine (a specific L-type Ca2+ channel blocker) or EDTA (an extracellular calcium chelator), hepcidin-mediated calcium inflow was found to occur predominantly via L-type Ca2+ channels (48). Furthermore, the intracellular calcium induced by hepcidin was sourced primarily from the endoplasmic reticulum, which is triggered by calcium influx (47). Thus, increased levels of intracellular calcium may be associated with the anti-osteoporosis effect of hepcidin. Furthermore, considering that postmenopausal women exhibit enhanced iron accumulation, we hypothesize that hepcidin may provide a viable therapeutic option for the prevention and treatment of PMOP by reducing the iron content in the body and enhancing osteoblast mineralization. In recent years, a patent was filed in the USA detailing the treatment of osteoporosis with hepcidin in perimenopausal and postmenopausal women (49). However, further studies are required to validate this hypothesis.
Notably, there is a potential receptor-based mechanism through which hepcidin may interact with osteoblasts. Previous studies have indicated that the mechanism underlying hepcidin-mediated internalization of ferroportin may result from the activation of the Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway (50,51). Furthermore, activation of the JAK2/STAT3 pathway has been reported to promote osteoblast differentiation (52,53), while inhibition of the JAK2/STAT3 pathway using the JAK2 inhibitor, AG490, has been shown to reduce human osteoblast differentiation and mineralization (54). Recently, our research group recognized that ferroportin can be detected in human hFOB 1.19 cultured cells, which indicates that osteoblasts are a potential target of hepcidin activity (55). Based on these collective results, a possible mechanism through which hepcidin stimulates osteoblast differentiation was proposed (Fig. 3).
Figure 3.

Mechanism through which hepcidin stimulates osteoblast differentiation. The binding of hepcidin to ferroportin activates the Jak2 protein kinase, which subsequently results in osteoblast differentiation mediated by the Stat3 transcription factor. Jak2, Janus kinase 2; Stat3, signal transducer and activator of transcription 3.
7. Future prospects
In the previous decade, research into iron metabolism and bone metabolism has progressed rapidly; the results of which have improved the understanding of the pathogenesis underlying PMOP (44). The maintenance of iron homeostasis in postmenopausal women has been recognized as crucial, and indicates the therapeutic potential of the manipulation of iron levels for treating PMOP. An artificial, biologically active form of hepcidin, known as ʻminihepcidinʼ, has been developed by Preza et al (56).
The following are the key recommendations for clinical research and practice, based on the present review. Firstly, well-designed prospective studies are required to investigate whether DFO or other iron chelators are able to mitigate bone loss in patients with iron overload conditions, such as thalassemia, hemachromatosis and sickle cell anemia. Secondly, the symptoms of iron overload are insensitive and nonspecific, which differs from the activity of other biologically important metal ions, such as potassium. Therefore, routine examination of the biochemical markers of iron stores may be advisable in order to predict the future patient risk of PMOP. As aforementioned, the optimum age for initiating iron store examination in an aging population is ~45 years. Thirdly, the epidemiological profile of iron deficiency remains among the most prevalent micronutrient deficiencies worldwide, increasing the risk of diminished bone metabolism in animals and humans (57,58). Therefore, the maintenance of normal iron levels is essential in clinical practice for healthy bone homeostasis.
Acknowledgements
The authors thank Dr Yi-Lin Yan and Dr Han Wang for critically reading the manuscript. The study was supported by grants from the National Natural Science Foundation of China (nos. 81273090 and 81302438), the Special Program for Clinic of Jiangsu Province (no. BL2014044) and the Natural Science Foundation of Soochow University (no. SDY2013A33).
References
- 1.MacKenzie EL, Iwasaki K, Tsuji Y. Intracellular iron transport and storage: From molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:997–1030. doi: 10.1089/ars.2007.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olivieri NF, Liu PP, Sher GD, et al. Brief report: Combined liver and heart transplantation for end-stage iron-induced organ failure in an adult with homozygous beta-thalassemia. N Engl J Med. 1994;330:1125–1127. doi: 10.1056/NEJM199404213301605. [DOI] [PubMed] [Google Scholar]
- 3.Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J. Body iron metabolism and pathophysiology of iron overload. Int J Hematol. 2008;88:7–15. doi: 10.1007/s12185-008-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinberg ED. Iron loading: A risk factor for osteoporosis. Biometals. 2006;19:633–635. doi: 10.1007/s10534-006-9000-8. [DOI] [PubMed] [Google Scholar]
- 5.Weinberg ED. Role of iron in osteoporosis. Pediatr Endocrinol Rev. 2008;6:81–85. (Suppl 1) [PubMed] [Google Scholar]
- 6.Jian J, Pelle E, Huang X. Iron and menopause: Does increased iron affect the health of postmenopausal women? Antioxid Redox Signal. 2009;11:2939–2943. doi: 10.1089/ars.2009.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsay J, Yang Z, Ross FP, et al. Bone loss caused by iron overload in a murine model: Importance of oxidative stress. Blood. 2010;116:2582–2589. doi: 10.1182/blood-2009-12-260083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vernejoul MC, Pointillart A, Golenzer CC, et al. Effects of iron overload on bone remodeling in pigs. Am J Pathol. 1984;116:377–384. [PMC free article] [PubMed] [Google Scholar]
- 9.Adams PC, Chakrabarti S. Genotypic/phenotypic correlations in genetic hemochromatosis: Evolution of diagnostic criteria. Gastroenterology. 1998;114:319–323. doi: 10.1016/S0016-5085(98)70483-4. [DOI] [PubMed] [Google Scholar]
- 10.Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM. Association of age, sex, and race with body iron stores in adults: Analysis of NHANES III data. Am Heart J. 2000;140:98–104. doi: 10.1067/mhj.2000.106646. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Xu Y, Partridge NC. Dancing with sex hormones, could iron contribute to the gender difference in osteoporosis? Bone. 2013;55:458–460. doi: 10.1016/j.bone.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu YJ, Sirois P, Li K. Iron overload plays a unique role in osteoporosis. http://www.bloodjournal.org/content/116/14/2582.e-letters#iron-overload-plays-a-unique-role-in-osteoporosis. [May 6;2015 ];Blood (E-letter) Accessed. [Google Scholar]
- 13.Kim BJ, Ahn SH, Bae SJ, et al. Iron overload accelerates bone loss in healthy postmenopausal women and middle-aged men: A 3-year retrospective longitudinal study. J Bone Miner Res. 2012;27:2279–2290. doi: 10.1002/jbmr.1692. [DOI] [PubMed] [Google Scholar]
- 14.Kim BJ, Lee SH, Koh JM, Kim GS. The association between higher serum ferritin level and lower bone mineral density is prominent in women ≥45 years of age (KNHANES 2008–2010) Osteoporos Int. 2013;24:2627–2637. doi: 10.1007/s00198-013-2363-0. [DOI] [PubMed] [Google Scholar]
- 15.Clark SF. Iron deficiency anemia. Nutr Clin Pract. 2008;23:128–141. doi: 10.1177/0884533608314536. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370:511–520. doi: 10.1016/S0140-6736(07)61235-5. [DOI] [PubMed] [Google Scholar]
- 17.Yang Q, Jian J, Katz S, Abramson SB, Huang X. 17beta-Estradiol inhibits iron hormone hepcidin through an estrogen responsive element half-site. Endocrinology. 2012;153:3170–3178. doi: 10.1210/en.2011-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou Y, Zhang S, Wang L, et al. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene. 2012;511:398–403. doi: 10.1016/j.gene.2012.09.060. [DOI] [PubMed] [Google Scholar]
- 19.Isomura H, Fujie K, Shibata K, et al. Bone metabolism and oxidative stress in postmenopausal rats with iron overload. Toxicology. 2004;197:93–100. doi: 10.1016/j.tox.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Kalinowski DS, Richardson DR. The evolution of iron chelators for the treatment of iron overload disease and cancer. Pharmacol Rev. 2005;57:547–583. doi: 10.1124/pr.57.4.2. [DOI] [PubMed] [Google Scholar]
- 21.Bennett CN, Longo KA, Wright WS, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almeida M, Han L, Martin-Millan M, O'Brien CA, Manolagas SC. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem. 2007;282:27298–27305. doi: 10.1074/jbc.M702811200. [DOI] [PubMed] [Google Scholar]
- 23.Jia P, Xu YJ, Zhang ZL, et al. Ferric ion could facilitate osteoclast differentiation and bone resorption through the production of reactive oxygen species. J Orthop Res. 2012;30:1843–1852. doi: 10.1002/jor.22133. [DOI] [PubMed] [Google Scholar]
- 24.Liu G, Men P, Kenner GH, Miller SC. Age-associated iron accumulation in bone: implications for postmenopausal osteoporosis and a new target for prevention and treatment by chelation. Biometals. 2006;19:245–251. doi: 10.1007/s10534-005-6666-2. [DOI] [PubMed] [Google Scholar]
- 25.Liu G, Men P, Kenner GH, Miller SC. Therapeutic effects of an oral chelator targeting skeletal tissue damage in experimental postmenopausal osteoporosis in rats. Hemoglobin. 2008;32:181–190. doi: 10.1080/03630260701726707. [DOI] [PubMed] [Google Scholar]
- 26.Maggio A, Filosa A, Vitrano A, et al. Iron chelation therapy in thalassemia major: A systematic review with meta-analyses of 1520 patients included on randomized clinical trials. Blood Cells Mol Dis. 2011;47:166–175. doi: 10.1016/j.bcmd.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Fabio G, Minonzio F, Delbini P, Bianchi A, Cappellini MD. Reversal of cardiac complications by deferiprone and deferoxamine combination therapy in a patient affected by a severe type of juvenile hemochromatosis (JH) Blood. 2007;109:362–364. doi: 10.1182/blood-2006-04-016949. [DOI] [PubMed] [Google Scholar]
- 28.Kalpatthi R, Peters B, Kane I, et al. Safety and efficacy of high dose intravenous desferrioxamine for reduction of iron overload in sickle cell disease. Pediatr Blood Cancer. 2010;55:1338–1342. doi: 10.1002/pbc.22660. [DOI] [PubMed] [Google Scholar]
- 29.Ishii KA, Fumoto T, Iwai K, et al. Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med. 2009;15:259–266. doi: 10.1038/nm.1910. [DOI] [PubMed] [Google Scholar]
- 30.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–788. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 31.Donovan A, Brownlie A, Zhou Y, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 32.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 33.Lesbordes-Brion JC, Viatte L, Bennoun M, et al. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood. 2006;108:1402–1405. doi: 10.1182/blood-2006-02-003376. [DOI] [PubMed] [Google Scholar]
- 34.Nicolas G, Bennoun M, Devaux I, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci USA. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadziahmetovic M, Song Y, Ponnuru P, et al. Age-dependent retinal iron accumulation and degeneration in hepcidin knockout mice. Invest Ophthalmol Vis Sci. 2011;52:109–118. doi: 10.1167/iovs.10-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roetto A, Papanikolaou G, Politou M, et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2003;33:21–22. doi: 10.1038/ng1053. [DOI] [PubMed] [Google Scholar]
- 37.Ahmad KA, Ahmann JR, Migas MC, et al. Decreased liver hepcidin expression in the Hfe knockout mouse. Blood Cells Mol Dis. 2002;29:361–366. doi: 10.1006/bcmd.2002.0575. [DOI] [PubMed] [Google Scholar]
- 38.Guggenbuhl P, Fergelot P, Doyard M, et al. Bone status in a mouse model of genetic hemochromatosis. Osteoporos Int. 2011;22:2313–2319. doi: 10.1007/s00198-010-1456-2. [DOI] [PubMed] [Google Scholar]
- 39.Nicolas G, Viatte L, Lou DQ, et al. Constitutive hepcidin expression prevents iron overload in a mouse model of hemochromatosis. Nat Genet. 2003;34:97–101. doi: 10.1038/ng1150. [DOI] [PubMed] [Google Scholar]
- 40.Moran-Jimenez MJ, Mendez M, Santiago B, et al. Hepcidin treatment in Hfe-/- mice diminishes plasma iron without affecting erythropoiesis. Eur J Clin Invest. 2010;40:511–517. doi: 10.1111/j.1365-2362.2010.02291.x. [DOI] [PubMed] [Google Scholar]
- 41.Gardenghi S, Ramos P, Marongiu MF, et al. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in beta-thalassemic mice. J Clin Invest. 2010;120:4466–4477. doi: 10.1172/JCI41717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrews NC. Closing the iron gate. N Engl J Med. 2012;366:376–377. doi: 10.1056/NEJMcibr1112780. [DOI] [PubMed] [Google Scholar]
- 43.Ganz T, Nemeth E. The hepcidin-ferroportin system as a therapeutic target in anemias and iron overload disorders. Hematology Am Soc Hematol Educ Program. 2011;2011:538–542. doi: 10.1182/asheducation-2011.1.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li GF, Pan YZ, Sirois P, Li K, Xu YJ. Iron homeostasis in osteoporosis and its clinical implications. Osteoporos Int. 2012;23:2403–2408. doi: 10.1007/s00198-012-1982-1. [DOI] [PubMed] [Google Scholar]
- 45.Ma Y, Xu YJ, Wang AD, Yu C, Wang B, Zhang P, Zhang ZD. A preliminary report of expression of hepcidin gene in SD rats osteoporosis model. Su Zhou Da Xue Zue Bao. 2006;26:367–369. (In Chinese) [Google Scholar]
- 46.Zhang P, Xu YJ, Zhao DY, et al. Increased intracellular iron and mineralization of cultured hFOB 1.19 cells following hepcidin activation through ferroportin-1. Saudi Med J. 2010;31:1303–1308. [PubMed] [Google Scholar]
- 47.Li GF, Xu YJ, He YF, et al. Effect of hepcidin on intracellular calcium in human osteoblasts. Mol Cell Biochem. 2012;366:169–174. doi: 10.1007/s11010-012-1294-y. [DOI] [PubMed] [Google Scholar]
- 48.Xu Y, Li G, Du B, et al. Hepcidin increases intracellular Ca2+ of osteoblast hFOB1.19 through L-type Ca2+ channels. Regul Pept. 2011;172:58–61. doi: 10.1016/j.regpep.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Xi Huang., inventor. Treatment of osteoporosis in peri- and post-menopausal women with hepcidin. US Patent 0,204,122. 2010 2010 Feb 11 12;Aug 11 12; Filed. issued.
- 50.De Domenico I, Lo E, Ward DM, Kaplan J. Hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Jak2. Proc Natl Acad Sci USA. 2009;106:3800–3805. doi: 10.1073/pnas.0900453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Domenico I, Zhang TY, Koening CL, et al. Hepcidin mediates transcriptional changes that modulate acute cytokine-induced inflammatory responses in mice. J Clin Invest. 2010;120:2395–2405. doi: 10.1172/JCI42011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellido T, Borba VZ, Roberson P, Manolagas SC. Activation of the Janus kinase/STAT (signal transducer and activator of transcription) signal transduction pathway by interleukin-6-type cytokines promotes osteoblast differentiation. Endocrinology. 1997;138:3666–3676. doi: 10.1210/endo.138.9.5364. [DOI] [PubMed] [Google Scholar]
- 53.Nishimura R, Moriyama K, Yasukawa K, Mundy GR, Yoneda T. Combination of interleukin-6 and soluble interleukin-6 receptors induces differentiation and activation of JAK-STAT and MAP kinase pathways in MG-63 human osteoblastic cells. J Bone Miner Res. 1998;13:777–785. doi: 10.1359/jbmr.1998.13.5.777. [DOI] [PubMed] [Google Scholar]
- 54.Barhanpurkar AP, Gupta N, Srivastava RK, et al. IL-3 promotes osteoblast differentiation and bone formation in human mesenchymal stem cells. Biochem Biophys Res Commun. 2012;418:669–675. doi: 10.1016/j.bbrc.2012.01.074. [DOI] [PubMed] [Google Scholar]
- 55.Xu Y, Zhang W, Zhang P, et al. Downregulation of ferroportin 1 expression in hFOB1.19 osteoblasts by hepcidin. Inflammation. 2012;35:1058–1061. doi: 10.1007/s10753-011-9411-8. [DOI] [PubMed] [Google Scholar]
- 56.Preza GC, Ruchala P, Pinon R, et al. Minihepcidins are rationally designed small peptides that mimic hepcidin activity in mice and may be useful for the treatment of iron overload. J Clin Invest. 2011;121:4880–4888. doi: 10.1172/JCI57693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katsumata S, Tsuboi R, Uehara M, Suzuki K. Dietary iron deficiency decreases serum osteocalcin concentration and bone mineral density in Rats. Biosci Biotechnol Biochem. 2006;70:2547–2550. doi: 10.1271/bbb.60221. [DOI] [PubMed] [Google Scholar]
- 58.Maurer J, Harris MM, Stanford VA, et al. Dietary iron positively influences bone mineral density in postmenopausal women on hormone replacement therapy. J Nutr. 2005;135:863–869. doi: 10.1093/jn/135.4.863. [DOI] [PubMed] [Google Scholar]


