Abstract
The aim of the present study was to assess the association between the efficacy and adverse events (AEs) of transcatheter arterial chemoembolization (TACE) combined with sorafenib in patients with unresectable hepatocellular carcinoma (HCC). Between July 2008 and May 2011, 50 patients with unresectable HCC were enrolled and assigned to receive TACE combined with sorafenib in the present study. The primary outcomes were considered as time to disease progression (TTP) and sorafenib-related AEs. In the present study, 34 of 50 patients had disease progression with a median TTP (mTTP) of 210 days. The most common AEs included hand-foot skin reaction (HFSR), fatigue, diarrhea and hypertension. The mTTP of patients with HFSR extended 140 days compared to that of the patients without HFSR. Kaplan-Meier analysis was used for mTTP between the two groups of patients. This difference was statistically significant when analyzed by the univariate COX proportional hazards regression model. In conclusion, TACE in combination with sorafenib had an acceptable safety profile in the treatment of unresectable HCC. Additionally, it also revealed that HFSR served as a good prognostic predictor in using combination therapy. Therefore, discontinuation of sorafenib treatment should be prevented to avoid disease progression.
Keywords: hepatocellular carcinoma, transcatheter arterial chemoembolization, sorafenib, efficacy, adverse events
Introduction
Hepatocellular carcinoma (HCC) is emerging as a common malignancy in China. However, the majority of patients presenting with HCC were diagnosed at intermediate-advanced stages, and thus could not be treated by surgical resection (1). Currently, transcatheter arterial chemoembolization (TACE) is the preferred treatment for the palliation of unresectable HCC (2–4). Additionally, according to the Barcelona Clinic Liver Cancer (BCLC) staging system, TACE is the standard treatment for BCLC stage B HCC. In addition to incomplete embolization and collateral circulation, another study has shown that TACE can cause enhanced expression of vascular endothelial growth factor (VEGF), which subsequently stimulates tumor angiogenesis, resulting in the progression and metastasis of residual tumor, or even formation of new tumor lesions (5). Therefore, HCC patients treated with TACE alone could not survive for longer durations.
Recently, sorafenib, which is a molecular-targeting agent, has been shown to inhibit tumor cell proliferation and tumor angiogenesis. Sorafenib has been successfully used in the treatment of patients with Child-Pugh class C HCC. This is a key reason for why sorafenib is approved as the standard treatment option for advanced HCC, and is based on the BCLC staging system.
Currently, the majority of attention has been directed on the use of sorafenib in combination with TACE for treating unresectable HCC. The major purpose of the present study was to prospectively analyze the efficacy and safety of TACE in combination with sorafenib in the treatment of unresectable HCC. The association between sorafenib-related adverse events (AEs) and antitumor efficacy of combinatorial therapy was assessed in the study.
Patients and methods
Patients and selection criteria
Patients meeting the following inclusion criteria were included in the study: i) Patients who were diagnosed with HCC via imaging, α-fetoprotein testing or pathological examination (HCC as defined by the expert consensus on the diagnosis of primary liver cancer), and were further categorized as having BCLC stage B or C HCC (according to the BCLC staging system); ii) patients with at least one target lesion, which could be measured [according to modified Response Evaluation Criteria in Solid Tumors Group (mRECIST) guidelines]; iii) patients with unresectable HCC, and those with an estimated life expectancy of ≥3 months; and iv) patients with Eastern Cooperative Oncology Group performance status score (ECOG PS) of ≤2. Patients were excluded if they received sorafenib for <3 months during the study, with no involvement of disease progression or mortality. Additionally, patients who discontinued sorafenib treatment due to AEs or for other reasons for >1 month were also excluded from the analysis.
TACE protocol
A catheter was inserted into the celiac, hepatic and superior mesenteric arteries via femoral arterial puncture using the Seldinger technique (6). Subsequently, angiography was performed to identify the tumor burden (feeding artery, numbers of nodules, blood supply and presence of vascular invasions and arteriovenous fistula). Having safely positioned the catheter within the feeding artery, a mixture of iodized oil and 2–3 types of the selected chemotherapeutic agents was injected into the target artery. When required, the embolic particles were used as supplemental embolic agents. The type and dose of chemotherapeutic agents, including epirubicin (50–100 mg), pirarubicin (30–50 mg), hydroxycamptothecine (10–30 mg) and fluorouracil (500–1,000 mg), were determined by the number and size of the lesions, and liver and kidney function of the patient. In addition, the dose of the embolic agents was adjusted according to the number and size of the patient's tumor lesions, and the blood supply. All the patients received protection of liver function and symptomatic treatment following surgery. The examination of imaging was repeated every 4–6 months, and subsequently the interval between each TACE cycle was dependent on the imaging characteristics.
Sorafenib treatment
All the patients were assigned to receive continuous oral treatment with 400 mg of sorafenib twice daily before and after 1 week of TACE. The high-fat diet was prohibited pre- and post-treatment with sorafenib. Dose reduction (400 mg once daily or 200 mg twice daily) or temporary interruption of sorafenib therapy for ≤1 month was permitted if intolerance to the treatment occurred. After the adverse symptoms were alleviated, the patients continued to receive sorafenib at the regular or reduced dose until evidence of disease occurred, there was evidence of disease progression or the patient succumbed. The total duration of sorafenib treatment was continued for ≥3 months.
Outcomes and assessment
The entire follow-up was completed in September 2011, and concluded at the fatality of the patient during follow-up. Patients were followed every 4–6 weeks following treatment. The primary outcomes of the study included time to disease progression (TTP) and the secondary outcome was sorafenib-related AEs and differences in overall survival (OS). The TTP and/or mortality were recorded at each follow-up visit. Additionally, enhanced computed tomography and/or magnetic resonance imaging were performed every 4–6 weeks following administration of sorafenib to evaluate the therapeutic outcomes. Tumor response rates were evaluated as complete remission (CR), partial remission (PR), stable disease (SD) and progressive disease (PD), according to the mRECIST criteria (7).
Adverse events
Sorafenib-related AEs following TACE were observed and recorded using the Common Terminology Criteria for Adverse Events version 3.0.
Statistical analysis
All the statistical analyses were performed using the statistical software package SPSS version 14.0 (SPSS Inc., Chicago, IL, USA). All the measurement data are expressed as median, maximum and minimum values. The enumeration and ranked data are expressed as numbers of patients and percentage. The Kaplan-Meier method was adopted to calculate the survival and plot the survival curves, and the differences in terms of median TTP (mTTP) according to the independent influencing factors, including the presence and severity of AEs, were evaluated by the log-rank test. In addition, the hazard ratio (HR) was analyzed using the COX proportional hazards regression model, with a 95% confidence interval (CI). P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical characteristics
Between July 2008 and May 2011, 50 patients who fulfilled the inclusion and exclusion criteria were cumulatively enrolled, including 44 males and 6 females, with a median age of 56 years (range, 31–81 years). According to the Child-Pugh grade, there were 44 patients with Child-Pugh class A and 6 with Child-Pugh class B. Additionally, 26 patients were diagnosed at BCLC stage B and the remaining 24 were diagnosed at BCLC stage C, in accordance with the BCLC staging system. The baseline characteristics are shown in Table I. The median number of TACE cycles administered was 3 (range, 1–12 cycles). After a course of sorafenib treatment (median duration, 42 days; range 29–184 days), the median size of the tumor was 5.7 cm (range, 1.8–16.5 cm).
Table I.
Baseline characteristics of patients with HCC.
| Variable | Patients |
|---|---|
| Age, median years (range) | 56 (31–81) |
| Gender, n | |
| Male | 44 |
| Female | 6 |
| Child-Pugh score, n | |
| A | 44 |
| B | 6 |
| BCLC stage, n | |
| B | 26 |
| C | 24 |
| ECOG PS, n | |
| 0 | 23 |
| 1 | 27 |
| Number of lesions, n | |
| 1 | 22 |
| ≥2 | 28 |
| Hepatitis virus, n | |
| HbsAg positive | 42 |
| Anti-HCV positive | 2 |
| HbsAg and anti-HCV, n | |
| Positive | 2 |
| No | 4 |
| AFP, n | |
| <400 ng/ml | 24 |
| ≥400 ng/ml | 26 |
| AST/ALT, n | |
| <2 | 8 |
| ≥2 | 42 |
| Vascular invasion, n | |
| Yes | 23 |
| No | 27 |
| Extrahepatic metastasis | |
| Pathological confirmation, n | |
| Yes | 7 |
| No | 43 |
| Yes | 7 |
| No | 43 |
| Previous therapy | |
| TACE, n (median cycle; cycle range) | 43 (3; 1–12) |
| TACE combined with sorafenib, n | 7 |
| Surgical resection, n | 6 |
| Radiofrequency therapy, n | 4 |
| Radiotherapy, n | 2 |
HCC, hepatocellular carcinoma; BCLC, Barcelona Clinic Liver Cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; AFP, α-fetoprotein; AST/ALT, aspartate aminotransferase/alanine aminotransferase (AST/ALT); TACE, transcatheter arterial chemoembolization.
Treatment outcomes
The median follow-up time was 310 days for the enrolled patients. Fourteen fatalities were reported when the study closed during February 2011. The cumulative survival rates at 1 and 2 years were 86 and 72%, respectively. The time of survival ranged from 109 to 1,231 days, and the median OS (mOS) had not been reached at the time of analysis. According to the mRECIST criteria, 4 patients achieved a PR, 12 achieved SD and 34 had PD, but there were no observed cases of CR.
Adverse events
All the sorafenib-related AEs are listed in Table II. Of the 50 patients enrolled in the study, 4 (8%) did not experience adverse AEs. The most common AEs included hand-foot skin reaction (HFSR), fatigue, diarrhea, hypertension, myalgia, erythra and oral ulcers, which were observed in 29 (58%), 27 (54%), 20 (40%), 19 (38%), 13 (26%), 10 (20%) and 7 (14%) cases, respectively. Grade 3 AEs were found in 32 patients without any grade 4 AEs during the course of the study. All the symptoms were alleviated with symptomatic treatment, dose reduction or temporary interruption of sorafenib. Nine patients required the sorafenib dose to be reduced by 50% due to AEs. Of these, 5 continued sorafenib treatment with a full dose and 4 remained with sorafenib reduced until their symptoms were alleviated. Additionally, dose interruption resulting from AEs was observed in 4 patients. Among these patients, 1 continued to receive full-dose sorafenib and 3 continued at the 50% dose reduction. During the same period, 23 patients were withdrawn from the study, of whom 10 discontinued the treatment for >1 month as a result of AEs, including grade 3 HFSR in 4 patients, grade 3 fatigue in 3, grade 3 diarrhea in 2, grade 3 hypertension in 1, grade 4 hypertension in 1 and grade 4 oral ulcers in 1 patient. The remaining 13 patients were excluded as the duration of sorafenib treatment was <3 months.
Table II.
Adverse events induced by sorafenib after TACE.
| Grade, n | ||||
|---|---|---|---|---|
| Adverse event | 1 | 2 | 3 | 4 |
| HFSR (n=29) | 8 | 8 | 13 | 0 |
| Lack of force (n=27) | 8 | 13 | 6 | 0 |
| Diarrhea (n=20) | 11 | 4 | 5 | 0 |
| Hypertension (n=19) | 11 | 4 | 4 | 0 |
| Muscle pain (n=13) | 9 | 2 | 2 | 0 |
| Erythra (n=10) | 5 | 3 | 2 | 0 |
| Oral ulcers (n=7) | 6 | 1 | 0 | 0 |
TACE, transcatheter arterial chemoembolization; HFSR, hand-foot skin reaction.
Correlation between treatment duration and mTTP
During the study, 34 enrolled patients had disease progression, with an mTTP of 210 days (range, 33–714 days; 95% CI, 159–261 days). The mTTP for the 10 patients who discontinued sorafenib treatment for >1 month was 102 days (range, 28–202 days; 95% CI, 60–144). The Kaplan-Meier analysis demonstrated a significant difference in the mTTP between the two groups of patients (χ2=14.692, P<0.001). Additionally, this difference in mTTP was statistically significant as analyzed by the univariate COX proportional hazards regression model, with a HR of 0.247 (95% CI, 0.114–0.535; P<0.001, Fig. 1).
Figure 1.
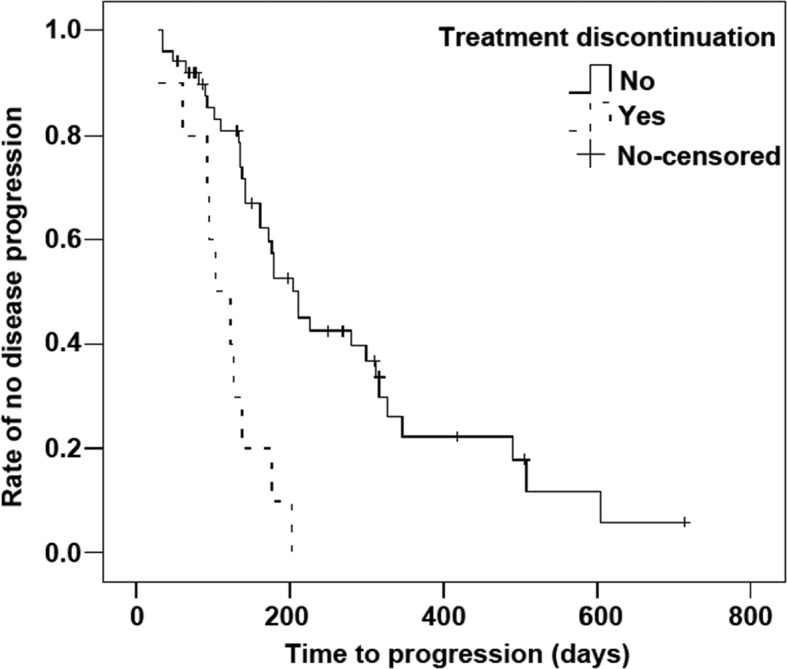
Kaplan-Meier plot for TTP in the 50 patients enrolled and 10 patients excluded from the study due to treatment discontinuation. The median TTP was 210 days for the 50 patients enrolled versus 102 days for the patients excluded from the study due to treatment discontinuation, with a significant difference (χ2=14.692, P<0.001). TTP, time to disease progression.
Correlation between the presence and severity of adverse events and mTTP
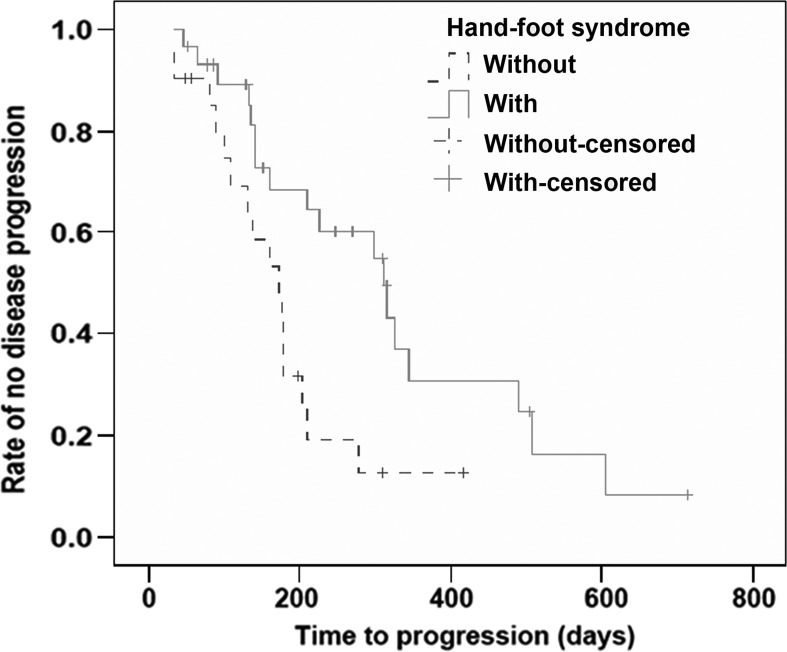
Compared to the patients who developed mild or no AEs, the mTTP was significantly longer in those that experienced moderate or severe AEs (279 vs. 141 days; χ2=5.079, P=0.024). By univariate analysis, the calculated HR for mTTP in patients with moderate or severe AEs was 0.435, with a significant difference when compared to those with mild or no AEs (95% CI, 0.206–0.918, P=0.029). Patients with HFSR showed significantly longer mTTP than those without HFSR (312 vs. 172 days; χ2=6.643, P=0.010). The HR for mTTP in patients with HFSR was 0.394, which was significantly different from those without HFSR (95% CI, 0.189–0.820, P=0.013). Additionally, there was no association between the presence of fatigue, diarrhea or hypertension and mTTP (Figs. 2 and 3, Tables III and IV).
Figure 2.
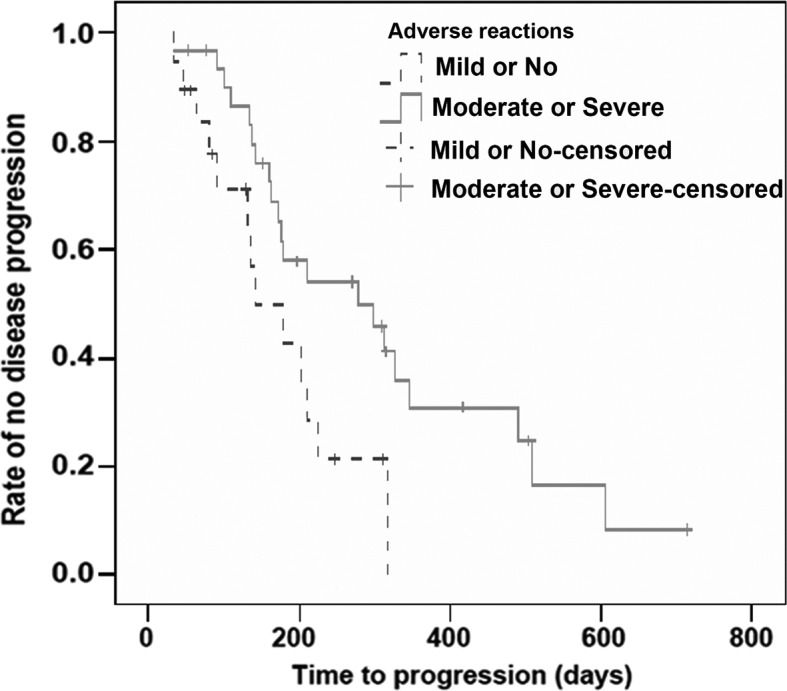
Kaplan-Meier plot for TTP in the 50 patients enrolled based on the severity of adverse reactions. The mTTP was 279 days for the patients with moderate or severe adverse events versus 141 days for the patients with mild or no adverse events, with a significant difference (χ2=5.079, P=0.024). TTP, time to disease progression.
Figure 3.
Kaplan-Meier plot for TTP in the 50 patients enrolled based on the presence and absence of HFSR. The mTTP was 312 days for the patients with HFSR versus 172 days for the patients without HFSR, with a significant difference (χ2=6.643, P=0.010). TTP, time to disease progression; HFSR, hand-foot skin reaction.
Table III.
Kaplan-Meier analysis of mTTP based on the presence and severity of adverse events.
| Variable | Patients, n | mTTP, days | χ2 | P-value |
|---|---|---|---|---|
| Severity of adverse events | ||||
| No or mild | 19 | 141 | 5.079 | 0.024 |
| Moderate or severe | 31 | 279 | ||
| HFSR | ||||
| Without | 21 | 172 | 6.643 | 0.010 |
| With | 29 | 312 | ||
| Fatigue | ||||
| Without | 23 | 210 | 1.600 | 0.206 |
| With | 27 | 203 | ||
| Diarrhea | ||||
| Without | 30 | 178 | 1.649 | 0.193 |
| With | 20 | 298 | ||
| Hypertension | ||||
| Without | 31 | 203 | 0.041 | 0.804 |
| With | 19 | 210 | ||
mTTP was calculated by Kaplan-Meier method and the differences in mTTP were evaluated using log-rank test (P<0.05 was considered statistically significant). mTTP, median time to progression; HFSR, hand-foot skin reaction.
Table IV.
COX proportional hazards regression model of mTTP based on the presence and severity of adverse events.
| Variable | Hazard ratio | 95% CI | P-value |
|---|---|---|---|
| Severity of adverse reactions (moderate or severe:no or mild) | 0.435 | 0.206–0.918 | 0.029 |
| HFSR (with:without) | 0.394 | 0.189–0.820 | 0.013 |
| Fatigue (with:without) | 0.642 | 0.320–1.286 | 0.211 |
| Diarrhea (with:without) | 0.628 | 0.309–1.276 | 0.199 |
| Hypertension (with:without) | 0.931 | 0.466–1.862 | 0.841 |
mTTP, median time to progression; HFSR, hand-foot skin reaction. The hazard ratio was calculated by COX proportional hazards regression model (P<0.05 was considered statistically significant).
Discussion
Currently, patients with primary HCC at an early stage are treated predominantly by surgical resection. However, in China, HCC is more frequently complicated by hepatitis B and post-hepatitic cirrhosis, with an incidence as high as 85.0–95.0%. Therefore, surgical resection is an option for <20% of patients. Furthermore, patients with HCC who underwent resection often exhibited higher incidences of postoperative recurrence, and the reported 1- and 5-year recurrence rates were 35.0–50.0% and 61.8%, respectively (8).
TACE is currently accepted as a palliative treatment for unresectable HCC, and shown to significantly prolong survival of the patients in several clinical trials (2–4). According to the BCLC staging system, TACE is considered standard therapy in patients with intermediate-stage HCC. However, it has been shown that TACE alone rarely produced complete embolization of tumor-feeding arteries and complete necrosis of lesions, and only a few patients (16.1%) with HCC showed complete tumor necrosis following TACE (9).
In addition, the presence of collateral circulation was associated with poor outcomes of TACE. Previous studies showed that TACE increases VEGF levels via induction of hypoxia and nutritional deficiency of tumor cells and liver tissues. This stimulates tumor angiogenesis, thereby contributing to tumor invasion and metastasis, disease progression and formation of new lesions (5,10,11). In recent years, elevated VEGF levels induced by TACE have been extensively demonstrated. It was concluded that TACE with an anti-VEGF therapy, and enhanced long-term efficacy in HCC patients.
Sorafenib (Nexavar®) is an oral multi-kinase inhibitor that exerts its inhibitory effects on tumor cell proliferation and apoptosis by targeting the serine-threonine kinase Raf in the Raf/mitogen-activated protein kinase/extracellular signal-related kinase-signaling pathway. Additionally, sorafenib exhibits antiangiogenic properties, which can inhibit the receptor tyrosine kinase activity of VEGFR-2, VEGFR-3 and platelet-derived growth factor receptor-B (12,13). A Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol trial involving patients from Europe with advanced-stage HCC demonstrated a potential therapeutic efficacy of sorafenib. In this study, sorafenib treatment resulted in an mOS of 10.7 months and an mTTP of 5.5 months, which were increased by 44.0 and 73.0%, in comparison with those treated with placebo, respectively (14). In a trial in the Asia-Pacific region, compared to the placebo group, a large increase in the mOS (73.0%, 6.5 vs. 4.2 months) and mTTP (74.0%, 2.8 vs. 1.4 months) was observed in HCC patients treated with sorafenib, respectively (15). Sorafenib is offered as a first-line treatment for patients with advanced HCC in the BCLC staging system based on the data as provided above in the results.
In recent years, numerous studies have explored combination therapy of TACE with sorafenib in treating intermediate-stage HCC to investigate whether or not this combination inhibits tumor angiogenesis, reduces tumor recurrence and metastasis, and improves the therapeutic effects of HCC (16). Additionally, sorafenib-related AEs (Table V) have been extensively studied in the clinic (16–23). Similar to previously published studies (16–18,20,21,23), HFSR, fatigue, diarrhea and hypertension were the most common AEs in the present study, observed in 29 (58%), 27 (54%), 20 (40%) and 19 (38%) patients, respectively. The toxicity profile in the present study has been previously reported. All the cases of AEs occurred frequently following sorafenib treatment, and persisted for 1 week to 1 month, in varying degrees. However, the symptoms were generally mild and could be managed with symptomatic treatment. In addition, severe AEs associated with sorafenib could be alleviated following treatment with other drugs (including ointments or lubricants containing urea and corticosteroids, such as antifungal drugs or antibiotics) for HFSR, anti-diarrheal drugs such as loperamide for diarrhea, adjustment of antihypertensive drugs for hypertension, and stimulants for fatigue. Temporary dose reduction was provided if intolerance to sorafenib occurred. Sorafenib treatment could then be restarted at the original level until alleviation of symptoms. When the patients were intolerant to reduced doses, temporary discontinuation of sorafenib for ≤1 month was permitted, following which their treatment was promptly continued.
Table V.
Results from the literature on sorafenib-related adverse events.
| First author (year) | Patients, n | Treatment regimen | Prognosis | Common adverse events | Conclusions | (Refs.) |
|---|---|---|---|---|---|---|
| Yan (2010) | 13 | TACE combined with sorafenib | mOS of 36.6 months | Diarrhea, HFSR, hypertension and fatigue | Adverse events were controllable | (15) |
| Li (2010) | 36 | TACE combined with sorafenib | mOS 12.41 months, mTTP 8.62 months | HFSR, diarrhea, fatigue and loss of appetite | Adverse events were tolerable | (16) |
| Cabrera (2011) | 47 | Sorafenib combined with TACE | mOS, 18.5 months | Fatigue, hand-foot skin reaction and diarrhea | Grade 4 adverse events occurred at a low incidence | (17) |
| Pawlik (2011) | 35 | TACE with doxorubicin-eluting beads (DEB) and continuous administration of sorafenib | Fatigue, loss of appetite, alterations in liver enzymes and skin toxicities | Adverse events was manageable with dose adjustment of sorafenib | (18) | |
| Song (2011) | 40 | Sorafenib | mOS for patients with AEs, 21.5 months; mOS for patients without AEs, 7.6 months | HFSR, hypertension, diarrhea and fatigue | Presence of adverse events predicted improved survival | (19) |
| Vincenzi (2010) | 65 | Sorafenib | mTTP for patients with skin toxicities during the first month of sorafenib treatment, 8.1 months; mTTP for patients without skin toxicities during the first month of sorafenib treatment, 4.0 months | Skin toxicities (rash and HFSR) | Early skin toxicities were considered a predictive factor for tumor control in HCC patients treated with sorafenib | (20) |
| Otsuka (2012) | 94 | Sorafenib | mOS for patients with skin toxicities, 16.8 months; mOS for patients without skin toxicities, 5.9 months | Skin toxicities (palmar-plantar erythrodysesthesia syndrome, rash, pruritus and alopecia) and hypertension | Skin toxicities were correlated with good clinical outcomes, but hypertension had no significant correlation with survival | (21) |
mTTP, median time to disease progression; mOS, median overall survival; HFSR, hand-foot skin reaction.
During the same time period, 23 patients were withdrawn from the study. Of these, 10 patients discontinued sorafenib treatment for >1 month due to AEs or the economic burdens of the patients. The remaining 13 patients were excluded as they received sorafenib treatment for a period of time <3 months. Given that the discontinuation of sorafenib and shorter treatment duration may influence the efficacy assessment of combination therapy, the study therefore decided to exclude 23 patients. In these excluded patients, the most frequently reported AEs following TACE were nausea, vomiting, leucopenia, pyrexia and abdominal pain. As compared to the patients treated with TACE alone, increased frequency and severity of the symptoms described above were not observed in patients receiving TACE combined with sorafenib.
In patients who experienced moderate or severe AEs, their mTTP was significantly longer as compared to that of the patients who had mild or no AEs (279 vs. 141 days). This indicated that the severity of AEs was positively correlated with antitumor efficacy. These results are consistent with those of Song et al (21) who identified that for patients with HCC treated with sorafenib, the occurrence of sorafenib-related AEs resulted in longer mOS. Additionally, the patients with HFSR showed significantly longer mTTP than those without HFSR (312 vs. 172 days). This suggested that the incidence of HFSR could serve as a prognostic predictor in this combination therapy, which is consistent with those reported in previous studies (22,23). Additionally, the presence or absence of fatigue, diarrhea and hypertension was not significantly different in mTTP, which indicated that the above AEs were not linked to the prognosis of HCC patients. Otsuka et al (23) also detected no association between the presence of hypertension that was induced by sorafenib and survival.
In addition, of the 50 patients enrolled in the present study, 34 had disease progression with an mTTP of 210 days. Ten patients who discontinued sorafenib treatment for >1 month also demonstrated disease progression, and the mTTP was reported to be 102 days, which was significantly shorter compared to that of the enrolled patients who experienced disease progression. Additionally, the study also showed that disease would eventually progress following a long-term discontinuation of sorafenib treatment. Furthermore, a previous study had demonstrated the rebound of tumor growth following cessation of sorafenib therapy in patients with HCC (24). We conclude that proper management of sorafenib-related AEs following TACE plays an important role in contributing the prognostic improvement of patients with HCC.
The present study revealed that HFSR was more prevalent in patients with HCC treated with sorafenib, which predicted better efficacy. Additionally, the occurrence of moderate and/or severe sorafenib-related AEs were associated with improved efficacy. Patients also experienced disease progression after sorafenib discontinuation for a long time. Therefore, appropriate management of sorafenib-related AEs is necessary, and permanent cessation of sorafenib treatment should be avoided to prevent disease progression. The majority of AEs were clinically manageable in the study. In conclusion, the combination of sorafenib and TACE provides a new regimen for the treatment of unresectable HCC, with improved efficacy following correct management of sorafenib-related AEs.
References
- 1.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48:20–37. doi: 10.1016/j.jhep.2008.01.022. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 2.Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6–25. doi: 10.1007/s00270-006-0062-3. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 5.Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914–921. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 6.Dufour JF, Hoppe H, Heim MH, et al. Continuous administration of sorafenib in combination with transarterial chemoembolization in patients with hepatocellular carcinoma: results of a phase I study. Oncologist. 2010;15:1198–1204. doi: 10.1634/theoncologist.2010-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Zhijun W, Maoqiang W. Combination of TACE with molecularly targeted agent in management of advanced HCC. Int J Med Radiol. 2009;32:374–377. [Google Scholar]
- 9.Enhua X. Pathological changes of molecular biology in treatment of liver cancer with TACE. CJIR. 2008;2:132–135. [Google Scholar]
- 10.Shim JH, Park JW, Kim JH, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99:2037–2044. doi: 10.1111/j.1349-7006.2008.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Marschall Z, Cramer T, Hocker M, Finkenzeller G, Wiedenmann B, Rosewicz S. Dual mechanism of vascular endothelial growth factor upregulation by hypoxia in human hepatocellular carcinoma. Gut. 2001;48:87–96. doi: 10.1136/gut.48.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilhelm SM, Carter C, Tang L, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 13.Chang YS, Adnane J, Trail PA, et al. Sorafenib (BAY 43–9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. 2007;59:561–574. doi: 10.1007/s00280-006-0393-4. [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 15.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 16.Strebel BM, Dufour JF. Combined approach to hepatocellular carcinoma: A new treatment concept for nonresectable disease. Expert Rev Anticancer Ther. 2008;8:1743–1749. doi: 10.1586/14737140.8.11.1743. [DOI] [PubMed] [Google Scholar]
- 17.Dong Y, Dezhong L, Huiying Z, et al. Primary study of TACE combined with sorafenib in hepatocellular carcinoma. Chin Clin Oncol. 2010;15:359–361. [Google Scholar]
- 18.Li Y, Huang JW, Lu LG, Shao PJ, Hu BS, Huang GM, Wei ZG, Zhang L. Clinical analysis of the treatment: Transcatheter arterial chemoembolization combined with sorafenib in advanced hepatocellular carcinoma. Zhonghua Yi Xue Za Zhi. 2010;90:2187–2192. (In Chinese) [PubMed] [Google Scholar]
- 19.Cabrera R, Pannu DS, Caridi J, et al. The combination of sorafenib with transarterial chemoembolisation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;34:205–213. doi: 10.1111/j.1365-2036.2011.04697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 2011;29:3960–3967. doi: 10.1200/JCO.2011.37.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song T, Zhang W, Wu Q, Kong D, Ma W. A single center experience of sorafenib in advanced hepatocellular carcinoma patients: evaluation of prognostic factors. Eur J Gastroenterol Hepatol. 2011;23:1233–1238. doi: 10.1097/MEG.0b013e32834bd2d0. [DOI] [PubMed] [Google Scholar]
- 22.Vincenzi B, Santini D, Russo A, et al. Early skin toxicity as a predictive factor for tumor control in hepatocellular carcinoma patients treated with sorafenib. Oncologist. 2010;15:85–92. doi: 10.1634/theoncologist.2009-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otsuka T, Eguchi Y, Kawazoe S, et al. Skin toxicities and survival in advanced hepatocellular carcinoma patients treated with sorafenib. Hepatol Res. 2012;42:879–886. doi: 10.1111/j.1872-034X.2012.00991.x. [DOI] [PubMed] [Google Scholar]
- 24.van Malenstein H, Dekervel J, Verslype C, Van Cutsem E, Windmolders P, Nevens F, van Pelt J. Long-term exposure to sorafenib of liver cancer cells induces resistance with epithelial-to-mesenchymal transition, increased invasion and risk of rebound growth. Cancer Lett. 2013;329:74–83. doi: 10.1016/j.canlet.2012.10.021. [DOI] [PubMed] [Google Scholar]