Abstract
Upper tract urothelial carcinoma (UTUC) is a relatively rare and highly aggressive tumor. However, the prognosis of UTUC is rarely predicted accurately due to the lack of reliable biomarkers. C-reactive protein (CRP) has been found to be correlated with several types of cancer. In this study, we performed a systematic review and meta-analysis to determine the association between CRP levels and prognosis in UTUC. A computerized search was conducted through PubMed, Embase, Web of Science, the Cochrane Library and CBM databases to identify clinical studies that have evaluated the association between preoperative CRP levels and prognosis of UTUC. The prognostic outcomes included recurrence-free survival (RFS), cancer-specific survival (CSS) and overall survival (OS). We extracted and synthesized corresponding hazard ratios (HRs) and confidence intervals (CIs) using Review Manager 5.3 software. We identified 7 retrospective cohort studies including a total of 1,919 patients and analyzed these studies using univariate and multivariate models. Our meta-analysis results revealed that RFS and CSS were significantly different between patients with elevated CRP levels and those with low CRP levels (P<0.0001 and P<0.00001, respectively); however, that was not the case for OS (P=0.22) in the multivariate or the univariate model. The pooled HR of RFS was 2.90 (95% CI: 1.87–4.51, P<0.00001) in the univariate analysis and 1.57 (95% CI: 1.26–1.97, P<0.0001) in the multivariate analysis. The pooled HRs of CSS were 2.78 (95% CI: 1.75–4.43, P<0.0001) and 1.64 (95% CI: 1.32–2.03, P<0.00001) in the univariate and multivariate analysis, respectively. However, the pooled HRs of OS were not significant in the univariate [1.24 (95% CI: 0.72–2.15, P=0.43)] or the multivariate analysis [1.24 (95% CI: 0.88–1.75, P=0.22)]. In conclusion, our meta-analysis results suggested that CRP level may be a prognostic predictor in UTUC.
Keywords: upper tract urothelial carcinoma, C-reactive protein, prognosis, survival, meta-analysis
Introduction
Upper tract urothelial carcinoma (UTUC) is a rare and highly aggressive urinary tumor, with a high recurrence rate (mainly intravesical) and a poor prognosis. A bladder recurrence rate of 44% was previously reported (1). Radical nephroureterectomy with bladder cuff excision is recommend as the standard treatment by the National Comprehensive Cancer Network and European Association of Urology guidelines (2,3). However, the high rate of recurrence and metastasis of UTUC pose a significant challenge for clinical physicians. Neoadjuvant and adjuvant chemotherapy have been proven to effectively decrease recurrence and improve survival (4,5). Therefore, prognosis assessment is critical for designing a therapy plan and increasing survival rate in patients with UTUC. However, the number of studies investigating predictors of UTUC is limited, due to its low incidence rate.
As a systematic inflammation-based biomarker, an elevated C-reactive protein (CRP) level has been proven to be a risk factor in several types of cancer (6). A previous meta-analysis demonstrated a significant correlation between CRP level and renal cell carcinoma (7). Therefore, the aim of this study was to perform a systematic review and determine the prognostic value of CRP in UTUC.
Materials and methods
Study selection process
A systematic literature search through PubMed, Embase, Web of Science, the Cochrane Library and CBM databases was conducted to identify the relevant clinical studies up to November, 2014. The search terms were as follows: (CRP OR C-reactive protein) AND (ureteral neoplasms OR ureter cancer OR ureter carcinoma OR renal pelvis cancer OR renal pelvic carcinoma OR upper tract urothelial carcinoma OR UTUC). The reference lists of the retrieved articles were searched for any additional relevant studies. The initial selection was performed based on the title and abstract by two independent investigators (Y. Luo and D.L. She). Thereafter, the full text was reviewed according to the eligibility criteria. To be eligible for this analysis, studies were required to evaluate the association between preoperative CRP and UTUC prognosis, including recurrence-free survival (RFS), cancer-specific survival (CSS) or overall survival (OS). Studies with duplicated or overlapping patient data and studies without sufficient available data were excluded.
Quality assessment and data extraction
The quality of the studies was independently assessed by the Newcastle-Ottawa scale (NOS) (8). Data were extracted by two independent investigators (Y. Luo and D.L. She) and cross-checked. Disagreements were resolved through discussion. The extracted data included the name of the first author, year of publication, source of patients, cut-off value of CRP, prognostic outcomes delineated, sample size, geographical region and follow-up time. The data were extracted from the original articles. When required data were missing, the original research data were requested directly from the corresponding authors via e-mail. When the original data were obtained, the cut-off value was set at 0.5 mg/dl; alternatively, the provided survival or mortality curves were used to calculate the hazard ratio (HR) and 95% confidence interval (CI), as described by Tierney et al (9).
Statistical analysis
Review Manager 5.3 software (The Cochrane Collaboration, Copenhagen, Denmark) was used for the analysis. As the number of included studies was <10, quantitative publication bias detection with Egger's regression intercept test or meta-regression analysis was not performed (10–12). HR was the time-to-event effect estimate for survival analysis and the generic inverse variance method was used. First, Cochran's Q test and Higgins I2 statistic were used to estimate heterogeneity (11). when P≥0.1 and I2≤50%, there was no significant heterogeneity and the fixed-effects model was used. In any other case, the random-effects model was used to calculate pooled HR. Univariate and multivariate analyses were performed and two-tailed P-values of <0.05 were considered to indicate statistically significant differences. All the results are presented in the forest plots.
Results
Eligible studies and quality assessment
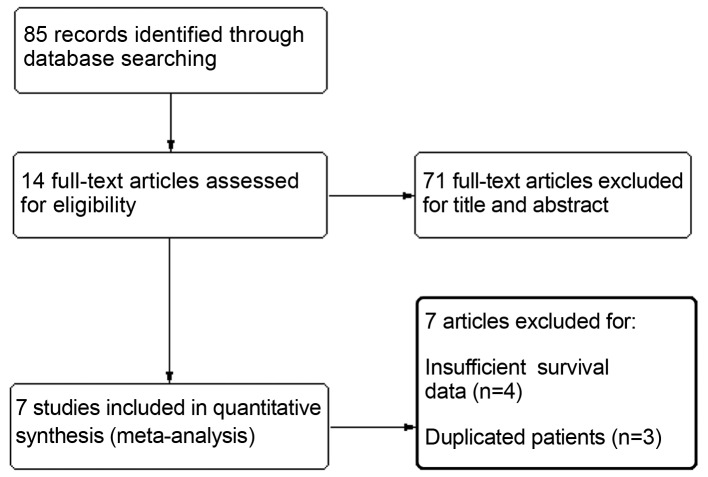
A total of 85 citations were selected by the initial search strategy. After reading the title and abstract, 71 articles were excluded for unrelated information. The remaining 14 articles were read and relative information was extracted. A further 3 studies were excluded due to duplicated references or overlapping patients and 4 studies due to insufficient survival data. Finally, 7 retrospective cohort studies including a total of 1,919 patients were entered in the analysis (13–19). The flow chart of the study selection process is presented in Fig. 1 and the basic characteristics of the included studies are summarized in Table I. The NOS scores were used to identify included studies of high methodological quality. A total of 5 articles reported RFS, 6 reported CSS and only 2 reported OS.
Figure. 1.
Flow diagram of the study selection process.
Table I.
Characteristics of included studies.
| Author (year) | Country | Duration | Disease | No. of pts. | CRP cut.off (mg/dl) | Outcomes | Median follow.up, months (IQR/range) | NOS scorea | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|
| Saito et al (2007) | Japan | 1990–2005 | Non-metastatic | 130 | 0.5 | RFS-CSS | 47 | 6 | (16) |
| UTUC | (3–190) | ||||||||
| Azuma et al (2013) | Japan | 1994–2008 | UTUC | 137 | 0.5 | RFS-CSS | 60.9 | 5 | (14) |
| (1.9–187.3) | |||||||||
| Sakano et al (2013) | Japan | 1995–2009 | Non-metastatic | 536 | 0.13 | CSS | 40.9 | 6 | (17) |
| UTUC | (3–200) | ||||||||
| Stein et al (2013) | Germany | 1981–2011 | UTUC | 115 | 0.5 | CSS | 15.1 | 7 | (18) |
| (7.2–37.7) | |||||||||
| Aziz et al (2014) | Germany | 1990–2012 | Non-metastatic | 265 | 0.9 | RFS-CSS-OS | 23 | 7 | (13) |
| UTUC | (10–48) | ||||||||
| Cho et al (2014) | Korea | 2004–2012 | Non-metastatic | 172 | 0.5 | RFS-OS | 33 | 5 | (15) |
| UTUC | (1–191) | ||||||||
| Tanaka et al (2014) | Japan | 1993–2010 | Non.metastatic, locally confined or advanced | 564 | 0.5 | RFS-CSS | 32 | 7 | (19) |
| UTUC | (15–62) |
A score of >5 stars reflected a relatively high quality. CRP, C-reactive protein; NOS, Newcastle-Ottawa scale; UTUC, upper tract urothelial carcinoma; RFS, recurrence-free survival; CSS, cancer-specific survival; OS, overall survival; IQR, interquartile range.
Meta-analysis
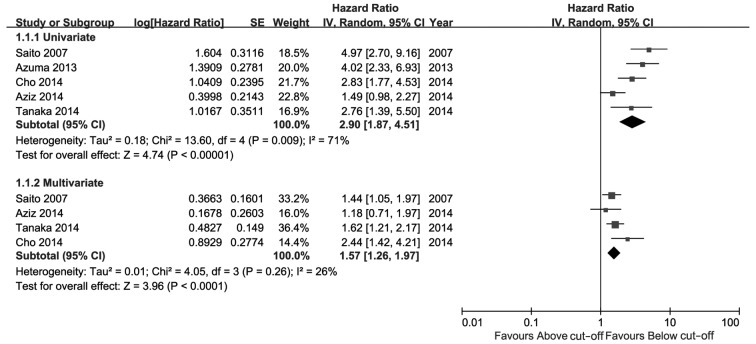
The meta-analysis results are presented in Figs. 2–4. As shown in Fig. 2, high CRP level was a significant risk factor for UTUC recurrence in the univariate as well as the multivariate analysis. The comprehensive HR for RFS was 2.90 (95% CI: 1.87–4.51, P<0.00001) in the univariate and 1.57 (95% CI: 1.26–1.97, P<0.0001) in the multivariate analysis. As shown in Fig. 3, high CRP level was a predictive risk factor for CSS of UTUC in the univariate as well as the multivariate analysis, with pooled HRs of 2.78 (95% CI: 1.75–4.43, P<0.0001) and 1.64 (95% CI: 1.32–2.03, P<0.00001), respectively. Patients with a high preoperative CRP level exhibited poorer CSS compared with patients with a low CRP level. Only 2 studies reported OS (Fig. 4); the pooled HRs of univariate and multivariate analysis were not significant [1.24 (95% CI: 0.72–2.15, P=0.43) and 1.24 (95% CI: 0.88–1.75, P=0.22), respectively].
Figure. 2.
Hazard ratio of C-reactive protein levels for recurrence-free survival. CI, confidence interval; SE, standard error.
Figure. 4.
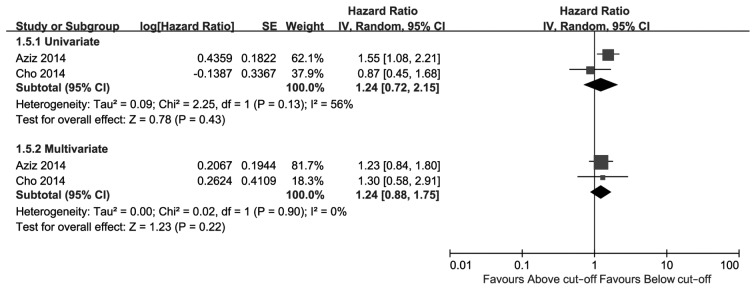
Hazard ratio of C-reactive protein levels for overall survival. CI, confidence interval; SE, standard error.
Figure 3.
Hazard ratio of C-reactive protein levels for cancer-specific survival. CI, confidence interval; SE, standard error.
Of note, 2 of the included studies utilized inconsistent cut-off values for CRP level, which caused significant heterogeneity as verified by a sensitivity analysis (data not shown). However, the statistical significance remained the same even following sensitivity analysis. A more significant effect may have been observed if the cut-off value in these two studies was set at 0.5 mg/dl (compared with 0.13 or 0.9 mg/dl; Table I).
Discussion
CRP is a non-specific biomarker of systemic inflammation. Several studies have demonstrated an association between CRP levels and tumor progression (6,7,20–26), suggesting that a high CRP level predicts poor prognosis. This association may be interpreted by the theory that the tumor itself produces inflammatory cytokines, which promote tumor growth and metastasis (19,27). If this theory is tenable, radical or cytoreductive tumor resection may significantly decrease the levels CRP and those of other inflammatory markers following surgery. Should this hypothesis be validated, CRP may prove to be a valuable response monitoring marker of UTUC or other tumors. However, no relative studies were retrieved.
Several systematic reviews have verified CRP as a tumor risk factor. A previous meta-analysis including 24 studies demonstrated a significant association of elevated CRP levels with tumor stage and grade in renal cell carcinoma, as well as with OS, CSS and progression-free survival. The CRP level was directly correlated with stage and grade and inversely correlated with outcomes (7). Similar associations were reported in lung (20), colorectal (22), gastroesophageal (23,25) and prostate cancer (21), hepatocellular carcinoma (26) and osteosarcoma (24). Our results are consistent with those findings and amplify the evidence. In our meta-analysis, an elevated CRP level indicated poor prognostic outcomes, but exerted no significant effect on OS in terms of the current evidence.
CRP was not the unique predictor of prognosis; other prognostic risk factors were found to be significantly associated with UTUC prognosis. Lughezzani et al (28) summarized the prognostic factors of UTUC, including clinical performance factors, pathological and biomarkers. Clinicopathological parameters were the primary prognostic risk factors of UTUC (19). In fact, certain pre- or intraoperative manipulations may lead to metastasis. It was previously demonstrated that preoperative diagnostic ureteroscopy may increase intravesical seeding by irrigation and instrument adhesion and, thus, lead to bladder cancer recurrence (29,30).
Although our results revealed a significant association between CRP and UTUC patient survival, there were several limitations to our study. In the forest plots, 2 studies did not report the multivariate HRs; the stepwise regression model was used in these studies, the results of which are not displayed if they are not significant risk factors. Although the respective corresponding authors were contacted for the original data, there have been no replies. Thus, this may result in inevitable reporting bias. Additionally, there was a small-study effect due to our limited study sample.
In conclusion, our meta-analysis demonstrated that CRP is a predictive serum biomarker for UTUC survival and a high preoperative CRP level is significantly associated with poor prognosis. However, considering the potential reporting bias risk and small sample, further studies with more detailed information are required to reach a definitive conclusion.
Acknowledgements
The authors are grateful to Dr Eu Chang Hwang, Department of Urology, Chonnam National University Hwasun Hospital (Chonnam National University Medical School, Republic of Korea), for the original clinical data cited in this study (15).
References
- 1.Raman JD, Sosa RE, Vaughan ED, Jr, Scherr DS. Pathologic features of bladder tumors after nephroureterectomy or segmental ureterectomy for upper urinary tract transitional cell carcinoma. Urology. 2007;69:251–254. doi: 10.1016/j.urology.2006.09.065. [DOI] [PubMed] [Google Scholar]
- 2.Clark PE, Agarwal N, Biagioli MC, et al. National Comprehensive Cancer Network (NCCN): Bladder cancer. J Natl Compr Canc Netw. 2013;11:446–475. doi: 10.6004/jnccn.2013.0059. [DOI] [PubMed] [Google Scholar]
- 3.Rouprêt M, Babjuk M, Compérat E, et al. European Association of Urology: European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol. 2013;63:1059–1071. doi: 10.1016/j.eururo.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 4.Leow JJ, Martin-Doyle W, Fay AP, Choueiri TK, Chang SL, Bellmunt J. A systematic review and meta-analysis of adjuvant and neoadjuvant chemotherapy for upper tract urothelial carcinoma. Eur Urol. 2014;66:529–541. doi: 10.1016/j.eururo.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Porten S, Siefker-Radtke AO, Xiao L, Margulis V, Kamat AM, Wood CG, Jonasch E, Dinney CP, Matin SF. Neoadjuvant chemotherapy improves survival of patients with upper tract urothelial carcinoma. Cancer. 2014;120:1794–1799. doi: 10.1002/cncr.28655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo YZ, Pan L, Du CJ, Ren DQ, Xie XM. Association between C-reactive protein and risk of cancer: A meta-analysis of prospective cohort studies. Asian Pac J Cancer Prev. 2013;14:243–248. doi: 10.7314/APJCP.2013.14.1.243. [DOI] [PubMed] [Google Scholar]
- 7.Hu Q, Gou Y, Sun C, et al. The prognostic value of C reactive protein in renal cell carcinoma: A systematic review and meta-analysis. Urol Oncol. 2014;32:50.e1–e8. doi: 10.1016/j.urolonc.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Wells GA, Shea B, O'Connell D, et al. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Dec 20;2014 ];The Newcastle Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta analyses. Accessed. [Google Scholar]
- 9.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JPT, Green S, editors. http://handbook.cochrane.org/ [Dec 20;2014 ];Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. 2011 Mar; updated. Accessed. [Google Scholar]
- 12.Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: A large survey. CMAJ. 2007;176:1091–1096. doi: 10.1503/cmaj.060410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aziz A, Rink M, Gakis G, et al. Preoperative C-reactive protein in the serum: A prognostic biomarker for upper urinary tract urothelial carcinoma treated with radical nephroureterectomy. Urol Int. 2014;93:352–360. doi: 10.1159/000362248. [DOI] [PubMed] [Google Scholar]
- 14.Azuma T, Matayoshi Y, Odani K, Sato Y, Sato Y, Nagase Y, Oshi M. Preoperative neutrophil-lymphocyte ratio as an independent prognostic marker for patients with upper urinary tract urothelial carcinoma. Clin Genitourin Cancer. 2013;11:337–341. doi: 10.1016/j.clgc.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Cho YH, Seo YH, Chung SJ, et al. Predictors of intravesical recurrence after radical nephroureterectomy for upper urinary tract urothelial carcinoma: An inflammation-based prognostic score. Korean J Urol. 2014;55:453–459. doi: 10.4111/kju.2014.55.7.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito K, Kawakami S, Ohtsuka Y, Fujii Y, Masuda H, Kumagai J, Kobayashi T, Kageyama Y, Kihara K. The impact of preoperative serum C-reactive protein on the prognosis of patients with upper urinary tract urothelial carcinoma treated surgically. BJU Int. 2007;100:269–273. doi: 10.1111/j.1464-410X.2007.06934.x. [DOI] [PubMed] [Google Scholar]
- 17.Sakano S, Matsuyama H, Kamiryo Y, et al. Yamaguchi Uro-Oncology Group: Risk group stratification based on preoperative factors to predict survival after nephroureterectomy in patients with upper urinary tract urothelial carcinoma. Ann Surg Oncol. 2013;20:4389–4396. doi: 10.1245/s10434-013-3259-0. [DOI] [PubMed] [Google Scholar]
- 18.Stein B, Schrader AJ, Wegener G, Seidel C, Kuczyk MA, Steffens S. Preoperative serum C-reactive protein: A prognostic marker in patients with upper urinary tract urothelial carcinoma. BMC Cancer. 2013;13:101. doi: 10.1186/1471-2407-13-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka N, Kikuchi E, Shirotake S, et al. The predictive value of C-reactive protein for prognosis in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy: A multi-institutional study. Eur Urol. 2014;65:227–234. doi: 10.1016/j.eururo.2012.11.050. [DOI] [PubMed] [Google Scholar]
- 20.Jin Y, Sun Y, Shi X, Zhao J, Shi L, Yu X. Prognostic value of circulating C-reactive protein levels in patients with non-small cell lung cancer: A systematic review with meta-analysis. J Cancer Res Ther. 2014;10:C160–C166. doi: 10.4103/0973-1482.145854. (Suppl) [DOI] [PubMed] [Google Scholar]
- 21.Liu ZQ, Chu L, Fang JM, Zhang X, Zhao HX, Chen YJ, Xu Q. Prognostic role of C-reactive protein in prostate cancer: A systematic review and meta-analysis. Asian J Androl. 2014;16:467–471. doi: 10.4103/1008-682X.123686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh PP, Zeng ISL, Srinivasa S, Lemanu DP, Connolly AB, Hill AG. Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg. 2014;101:339–346. doi: 10.1002/bjs.9354. [DOI] [PubMed] [Google Scholar]
- 23.Warschkow R, Tarantino I, Ukegjini K, Beutner U, Müller SA, Schmied BM, Steffen T. Diagnostic study and meta-analysis of C-reactive protein as a predictor of postoperative inflammatory complications after gastroesophageal cancer surgery. Langenbecks Arch Surg. 2012;397:727–736. doi: 10.1007/s00423-012-0944-6. [DOI] [PubMed] [Google Scholar]
- 24.Yi JH, Wang D, Li ZY, Hu J, Niu XF, Liu XL. C-reactive protein as a prognostic factor for human osteosarcoma: A meta-analysis and literature review. PLoS One. 2014;9:e94632. doi: 10.1371/journal.pone.0094632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Q, Yu XF, Zhang SD, Wang HH, Wang HY, Teng LS. Prognostic role of C-reactive protein in gastric cancer: A meta-analysis. Asian Pac J Cancer Prev. 2013;14:5735–5740. doi: 10.7314/APJCP.2013.14.10.5735. [DOI] [PubMed] [Google Scholar]
- 26.Zheng Z, Zhou L, Gao S, Yang Z, Yao J, Zheng S. Prognostic role of C-reactive protein in hepatocellular carcinoma: A systematic review and meta-analysis. Int J Med Sci. 2013;10:653–664. doi: 10.7150/ijms.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lughezzani G, Burger M, Margulis V, Matin SF, Novara G, Roupret M, Shariat SF, Wood CG, Zigeuner R. Prognostic factors in upper urinary tract urothelial carcinomas: A comprehensive review of the current literature. Eur Urol. 2012;62:100–114. doi: 10.1016/j.eururo.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 29.Ishikawa S, Abe T, Shinohara N, et al. Impact of diagnostic ureteroscopy on intravesical recurrence and survival in patients with urothelial carcinoma of the upper urinary tract. J Urol. 2010;184:883–887. doi: 10.1016/j.juro.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 30.Luo HL, Kang CH, Chen YT, Chuang YC, Lee WC, Cheng YT, Chiang PH. Diagnostic ureteroscopy independently correlates with intravesical recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma. Ann Surg Oncol. 2013;20:3121–3126. doi: 10.1245/s10434-013-3000-z. [DOI] [PubMed] [Google Scholar]