Abstract
The aim of the present study was to investigate the mechanism by which hydrogen sulfide (H2S) inhalation protects against oxidative stress in rats with cotton smoke inhalation-induced lung injury. A total of 24 male Sprague-Dawley rats were separated randomly into four groups, which included the control, H2S, smoke and smoke + H2S groups. A rat model of cotton smoke inhalation-induced lung injury was established following inhalation of 30% oxygen for 6 h. In addition, H2S (80 ppm) was inhaled by the rats in the H2S and smoke + H2S groups for 6 h following smoke or sham-smoke inhalation. Enzyme-linked immunosorbent assays were performed to measure various indices in the rat lung homogenate, while the levels of nuclear factor (NF)-κBp65 in the lung tissue of the rats were determined and semiquantitatively analyzed using immunohistochemistry. In addition, quantitative fluorescence polymerase chain reaction was employed to detect the mRNA expression of inducible nitric oxide synthase (iNOS) in the rat lung tissue. The concentrations of malondialdehyde (MDA), nitric oxide (NO), inducible iNOS and NF-κBp65, as well as the sum-integrated optical density of NF-κBp65 and the relative mRNA expression of iNOS, in the rat lung tissue from the smoke + H2S group were significantly lower when compared with the smoke group. The concentrations of MDA, NO, iNOS and NF-κBp65 in the H2S group were comparable to that of the control group. Therefore, inhalation of 80 ppm H2S may reduce iNOS mRNA transcription and the production of iNOS and NO in rats by inhibiting NF-κBp65 activation, subsequently decreasing oxidative stress and cotton smoke inhalation-induced lung injury.
Keywords: acute lung injury, smoke inhalation injury, oxidative stress, hydrogen sulfide
Introduction
Smoke inhalation injury (SII) mainly affects the airways and lung parenchyma, causing severe toxic pneumonitis or pulmonary edema. These conditions may rapidly develop into acute lung injury (ALI) and acute respiratory distress syndrome, which increase the morbidity and mortality rates in patients (1). Oxidative stress is an important SII mechanism, as high-temperature smoke contains a high concentration of strong oxidants. The resultant inflammatory response, if uncontrolled, causes abundant inflammatory cell accumulation in the lungs, producing excessive reactive oxygen species (ROS) and inducing oxidative stress injury.
Previous studies have shown that hydrogen sulfide (H2S) exerts antioxidative (2) and antifibrotic effects, and plays important roles in vasodilation (3–6) and the regulation of the inflammatory response (7), endocrine and reproductive systems (8). Inhalation of 80 ppm H2S for 6 h has been demonstrated to suppress the systemic inflammatory response and enhance survival rates in mouse models of endotoxin-induced ALI (9,10). In addition, inhalation of H2S has been shown to reduce lung damage in mouse models of hyperventilation-induced ALI by inhibiting pulmonary inflammation and alveolar epithelial cell apoptosis (11). In a previous study, increased oxidative stress was observed in rats with cotton smoke inhalation-induced lung injury (12). The aim of the present study was to observe the effect of 80 ppm H2S inhalation for 6 h on oxidative stress in rats with SII.
Materials and methods
Animals and grouping
A total of 24 healthy, clean, adult male Sprague-Dawley rats (weight, 150–250 g) were provided by the Laboratory Animal Center of the Academy of Military Medical Sciences [SCXK-(Military)-2012-0004] and fed in the Laboratory Animal Center of the Navy General Hospital [SCXK-(Military)-2012-0012]. The rats were randomly divided into four groups, including the control, H2S, smoke and smoke + H2S groups (n=6 in each), in accordance with the regulations for the administration of affairs concerning experimental animals (13). This study was conducted in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (14). Furthermore, the animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the Second Military Medical University (Beijing, China).
Establishment of the SII model and H2S inhalation
A rat model of SII was established by placing two randomly selected rats from the smoke and smoke + H2S groups into a smoke chamber. The smoke was generated by sealing 2 g cotton (Sunny Cotton Company, Xinjiang, China) in a soldering machine (Fudi HT-B, Hongtai Hardware and Electric Equipment Company, Guangzhou, China) at 300°C, which was subsequently channeled into the chamber containing the rats. The rats were left to inhale the smoke for 2 min or until red/purple spots developed on the plantar skin, with symptoms of restlessness, tachypnea, mouth breathing, Kussmaul breathing and stridor (15–17). The smoke chamber was opened to allow the rats to breathe fresh air for 7 min before the chamber was resealed. This procedure was repeated three to five times, until the rats remained unconscious after 7 min of air inhalation. The rats in the control and H2S groups underwent the same procedure, but without smoke inhalation. Following the smoke or sham smoke inhalation, rats in the H2S and smoke + H2S groups inhaled 80 ppm H2S + 30% oxygen for 6 h, while rats in the control and smoke groups inhaled 30% oxygen for 6 h. The rats had free access to food and water.
Enzyme-linked immunosorbent assay (ELISA)
Rats were euthanized with an intraperitoneal injection of pentobarbital sodium. A double-antibody sandwich avidin-biotin-peroxidase complex-ELISA (Jiamay Biotech Co. Ltd., Beijing, China) was performed to determine the levels of nitric oxide (NO), inducible nitric oxide synthase (iNOS) and nuclear factor (NF)-κBp65 in the lower right lung homogenate. In addition, the concentration of malondialdehyde (MDA) was measured using colorimetry.
Immunohistochemistry (IHC) of NF-κBp65
The right middle lobes of the rat lungs were fixed in 4% paraformaldehyde for 72 h, embedded in paraffin, sectioned at 3 µm and preheated at 60–65°C for 4 h. This was followed by deparaffinization, rehydration, washing in phosphate-buffered saline, high temperature antigen retrieval, endogenous peroxidase blocking in 3% H2O2 and normal goat serum blocking. The slides were incubated with 50 µl anti-NF-κBp65 primary antibody (ab16502; Abcam, Cambridge, UK) at 1:200 dilution overnight (4°C), and a secondary horseradish peroxidase-conjugated anti-mouse/rabbit IgG antibody (KIT-5020; Maixin-Bio, Fuzhou, China) for 20 min at room temperature. The slides were subsequently stained with diaminobenzidine and counterstained with hematoxylin. For the negative control, the primary antibody was replaced with serum. Positive expression was observed as yellow or brown staining. Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA) was used for semiquantitative analysis by randomly selecting five high-power fields (magnification, x1,000) from each slide. Image-Pro Plus 6.0 software was used to calculate the sum-integrated optical density (IOD) of the mean density, and the IOD of the positive staining in each field, as well as the mean value of these parameters.
Quantitative fluorescence-polymerase chain reaction (qF-PCR)
Forward and reverse primers for iNOS were synthesized by Jiamay Biotech Co. Ltd. as follows: 5-ACACCGATTCCACTCAACTA-3 and 5-ACCACCTGTTAGTTCAAGCC-3′, respectively. The amplified products were 159 bp in length and β-actin was used as an internal reference gene (CW0918; CWbio Co., Ltd., Beijing, China). Total RNA was extracted using an Ultrapure RNA Kit (CW0581; CWbio Co., Ltd.) and analyzed (5 µl) with 1% agarose gel electrophoresis. The total RNA was reverse transcribed with a HiFi-MMLV-cDNA First-Strand cDNA Synthesis kit (CW0744; CWbio Co., Ltd.) and amplified using an UltraSYBR Mixture with ROX (CW0956; CWbio Co., Ltd.) under the following conditions: 95°C for 10 min, 40 cycles of 95°C for 15 sec and 60°C for 60 sec. qF-PCR was performed with a LightCycler® 480 II PCR system (Roche Diagnostics, Basel, Switzerland), and the 2−ΔΔCt method was employed to analyze the relative changes in gene expression.
Statistical analysis
Data were analyzed with SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA). Measurement data are presented as the mean ± standard deviation, and comparisons were performed using one-way analysis of variance. Comparisons between groups were performed using Fishers least significant difference test. P<0.05 was considered to indicate a statistically significant difference.
Results
ELISA
Significantly higher concentrations of MDA, NO, iNOS and NF-κBp65 were observed in the rat lung homogenate from the smoke group, as compared with those in the control or smoke + H2S groups (P<0.001). Furthermore, the H2S group exhibited a higher concentration of iNOS than the control group (Table I). The levels of all the measured indicators were lower in the H2S group when compared with those in the smoke group.
Table I.
Concentrations of indicators in the rat lung tissue.
| Group | MDA (nmol/ml) | NO (µM/ml) | iNOS (pg/ml) | NF-κBp65 (pg/ml) |
|---|---|---|---|---|
| Control | 161.24±15.68a | 85.25±10.07a | 320.11±30.91a | 7636.77±535.48a |
| H2S | 188.29±20.44a | 78.75±6.61a | 394.11±34.95a,b | 9543.63±755.25a |
| Smoke | 332.00±52.23b | 179.00±16.04b | 603.44±50.67b | 13803.19±2196.37b |
| Smoke + H2S | 240.38±24.26a,b | 93.09±5.33a | 406.33±52.45a,b | 8123.51±2095.33a,b |
| F-value | 34.120 | 123.124 | 46.967 | 18.729 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 |
Results are expressed as the mean ± standard deviation (n=6).
P<0.05, vs. smoke group
P<0.05, vs. control group (LSD test). MDA, malondialdehyde; NO, nitric oxide; iNOS, inducible nitric oxide synthase; NF-κBp65, nuclear factor-κBp65; LSD, Fishers least significant difference.
Rat body weight and relative mRNA expression of iNOS
The mean rat body weight was 186.68±28.79 g and was comparable between the groups; thus, body weight was determined to have no effect on the results (P>0.05). The relative mRNA expression of iNOS was significantly higher in the smoke, smoke + H2S and H2S groups when compared with the control group (P<0.01); however, the levels were markedly lower in the smoke + H2S and H2S groups when compared with the smoke group (P<0.001; Table II).
Table II.
Results of immunohistochemistry in rat lung tissue.
| Group | iNOS mRNA | p65 Density (mean) | p65 IOD (sum) | Weight (g) |
|---|---|---|---|---|
| Control | 0.07±0.03a | 0.244±0.016a | 9275.25±1219.39a | 182.13±14.29 |
| H2S | 0.26±0.05a,b | 0.218±0.005b | 22536.16±3107.68a,b | 200.43±23.23 |
| Smoke | 2.20±0.21b | 0.219±0.009b | 32782.06±4826.13b | 195.42±47.38 |
| Smoke + H2S | 1.04±0.24a,b | 0.218±0.010b | 25668.15±2420.81a,b | 168.75±9.80 |
| F-value | 221.670 | 8.814 | 57.700 | 1.576 |
| P-value | <0.001 | 0.001 | <0.001 | 0.226 |
Results are expressed as the mean ± standard deviation (n=6).
P<0.05, vs. smoke group
P<0.05, vs. control group (LSD test). iNOS, inducible nitric oxide synthase; IOD, integrated optical density; LSD, Fishers least significant difference.
IHC of NF-κBp65
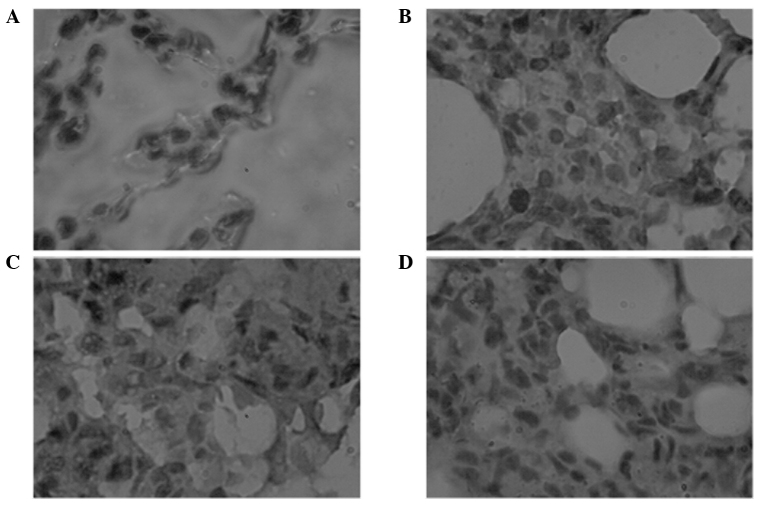
IHC results showing NF-κBp65 expression in the rat lungs are presented in Fig. 1. The sum-IOD of NF-κBp65 expression was higher in the smoke group when compared with the control group (P<0.001). However, in the smoke + H2S and H2S groups, the sum-IOD was lower when compared with the smoke group (P<0.01), but higher when compared with the control group (P<0.001). The mean density values of NF-κBp65 expression in the rat lungs were comparable in the smoke, smoke + H2S and H2S groups, which were all lower than the value observed in the control group (P<0.01; Table II).
Figure 1.
Histopathological observations of NF-κBp65 expression in the lung tissues of the (A) air, (B) H2S, (C) smoke and (D) smoke + H2S groups (6 h) using immunohistochemistry (hematoxylin and eosin; magnification, x1,000). NF, nuclear factor; H2S, hydrogen sulfide.
Discussion
The complex composition of smoke determines the complex SII pathogenesis, in which oxidative stress is important (18). Smoke inhalation can stimulate lung macrophages, neutrophils, vascular endothelial and smooth muscle cells to release abundant cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, −6 and −8, which activate NF-κB. Following NF-κB decomposition in the cytoplasm, active fragments of NF-κBp65 are translocated to the nucleus to promote downstream iNOS gene transcription, enhancing the synthesis of iNOS. Arginine is subsequently metabolized, generating large amounts of NO that react with the superoxide free radical to synthesize peroxynitrite, leading to lipid peroxidation in the cell membrane (19). Simultaneously, NO, nitrous oxide, disulfur monoxide and other oxidizing particles in smoke are strong oxidants and stimulate granulocytes to release large quantities of oxyradicals. In addition, oxygen therapy following hypoxia increases the production of oxyradicals, which results in lipid peroxidation, membrane destruction and the activation of inflammatory mediator synthesis. Subsequently, energy metabolism is affected, causing protein denaturation and dysfunction. These factors enhance alveolar-capillary permeability, leading to exudation of the blood component into the alveolar space and pulmonary edema. Moreover, SII-induced ROS causes excessive NO synthesis, which results in vascular leakage, failure of hypoxic pulmonary vasoconstriction and an increase in the production of cytotoxic reactive nitrogen species (RNS), which further aggravate pulmonary injury (20–22). A previous study confirmed that cotton smoke inhalation for 6 h in rats induced typical lung injury (12). The present study showed the concentration and sum-IODs of NF-κBp65, and the relative mRNA expression and concentration of iNOS and NO were increased in the rat lung tissue after 6 h of smoke inhalation. In addition, there was an increase in MDA, a peroxide produced in the reaction of free radicals and polyunsaturated fatty acids in the cell membranes, indicating an intensified oxidative stress response, lipid peroxidation and tissue injury.
Early treatment for SII following smoke inhalation is crucial. With regard to the pathogenesis of SII, interrupting oxidative stress pathways and decreasing downstream products by inhibiting NF-κB activation may theoretically ameliorate lung injury. A previous study demonstrated that continuous intravenous infusion of arginine vasopressin at a low dose can suppress the excessive generation of NO by iNOS, significantly reducing lung injury induced by burning and smoke inhalation (23).
H2S is a harmful gas, commonly associated with the smell of rotten eggs, and has recently emerged as the third gaseous signaling molecule in addition to NO and CO (24,25). Animal experiments have confirmed that intravenous infusion of NaHS or H2S inhalation has antioxidative, anti-inflammatory and antiapoptotic effects in animal models of various types of lung injury (2,9,10,26). However, the effects of H2S inhalation on SII have not yet been investigated. In macrophages activated in vitro by lipopolysaccharide, H2S can inhibit the activation of the NF-κB signaling pathway, reduce NO production and exert an antioxidative effect (27). Therefore, the aim of the present study was to investigate the effects of H2S inhalation on SII.
The present study revealed that inhalation of 80 ppm H2S for 6 h immediately after smoke inhalation markedly reduced rat lung injury. This reduction in injury was characterized by decreases in the concentration and sum-IOD of NF-κBp65, relative mRNA expression of iNOS and concentrations of iNOS, NO and MDA in the rat lung tissue, indicating that H2S inhalation may reduce iNOS mRNA transcription, and iNOS and NO production, by inhibiting NF-κBp65 activation. Subsequently, oxidative stress and cotton smoke inhalation-induced lung injury were reduced. The mean density of NF-κBp65 in the rat lungs may not be used as an indicator, since it represents the depth of positive IHC staining intensity, but not the total quantity of positive staining.
In the H2S group, the concentrations of MDA, NO and NF-κBp65 were comparable to those in the control group. However, the iNOS concentration, relative mRNA expression of iNOS and the sum-IOD of NF-κBp65 were higher in the H2S group compared with the control group, suggesting that inhalation of 80 ppm H2S for 6 h caused no damage to the rats, but may activate NF-κBp65 signaling pathways to increase iNOS synthesis, which correlated with the negative feedback.
Recently, research into the effects of H2S in vivo has been increasing. Different cell types or stimulations may lead to opposite results in terms of the effects of H2S on NF-κB signaling pathways (28). For example, H2S amplifies the IL-1β-activated NF-κB signaling pathway, increases iNOS expression and NO production in rat vascular smooth muscle cells (29). In addition, H2S activates the NF-κB signaling pathway to increase the production of proinflammatory cytokines in human monocytes pretreated with interferon-γ (30). However, H2S inhibits the activation of the NF-κB signaling pathway to reduce iNOS expression in lipopolysaccharide-activated macrophages (27) or microgliacytes (31). NF-κB is a nuclear transcription factor that maintains cell sensitivity to oxidative stress, and TNF-α, IL-1β and NO can activate the signaling cascade of the NF-κB pathway. This pathway is influenced by numerous factors, including the status of potential sites sensitive to oxidative stress, ROS and RNS, selectivity of signaling pathways and cell types, which may affect the process of oxidative stress reactions (32). Inhalation of strong oxidant H2S disturbs the redox balance in vivo and enhances reduction reactions, which activates the NF-κB signaling pathway, increases iNOS synthesis and produces NO against the reducing property of H2S to complete the negative feedback loop, without evident injury in rats. However, smoke inhalation increases the oxidizing reactions, which activates the NF-κB signaling pathway, elevates iNOS expression and NO synthesis, causing an increase in oxidative stress that cannot be regulated by the antioxidant system (33). Therefore, H2S inhalation provides a negative feedback system, inhibiting the activation of the NF-κB signaling pathway and reducing iNOS expression and NO synthesis, which subsequently decreases lung injury in rats. H2S regulates NF-κB activity via the extracellular signal-regulated kinase signaling pathway (29,30), p38-mitogen-activated protein kinase signaling pathway (31), heme oxygenase-1 and heat shock protein 70 (27). A previous study investigating the mechanisms of H2S (34) revealed that H2S-mediated sulfhydration (the binding of H2S to active-site cysteine residues of target proteins to induce protein sulfhydration and post-translational modification) may be central to the H2S mechanism.
The present study expands the application of H2S inhalation. Moreover, strong reductant H2S in airways can directly neutralize oxidants, including the superoxide anion, hydrogen peroxide, superoxide nitrogen and hypochlorous acid (35,36), to protect the cell membrane against free radical-induced injury. H2S inhalation is a convenient treatment for SII; however, further investigation is required. In the present study, H2S inhalation was applied for only 6 h and the long-term effects require further study. H2S can activate ATP-sensitive potassium channels to dilate blood vessels (6), regulate Fas/Fas ligand death receptor pathways to reduce apoptosis, and inhibit neurogenic inflammation and the co-interaction of the three gaseous signaling molecules (37); these mechanisms may play roles in the treatment of SII. However, further confirmation is required with regard to the mechanisms underlying the effects of H2S in SII.
In conclusion, inhalation of 80 ppm H2S reduces iNOS mRNA transcription and iNOS and NO production by inhibiting NF-κBp65 activation, which subsequently decreases oxidative stress and cotton smoke inhalation-induced lung injury.
Acknowledgements
This study was funded by the Research Project of the ‘Twelfth Five-year Plan’ for Medical Science Development of PLA (no. CWS11J180).
References
- 1.Ballard-Croft C, Sumpter LR, Broaddus R, Alexander J, Wang D, Zwischenberger JB. Ovine smoke/burn ARDS model: a new ventilator-controlled smoke delivery system. J Surg Res. 2010;164:e155–e162. doi: 10.1016/j.jss.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 2.Esechie A, Kiss L, Olah G, Horváth EM, Hawkins H, Szabo C, Traber DL. Protective effect of hydrogen sulfide in a murine model of acute lung injury induced by combined burn and smoke inhalation. Clin Sci (Lond) 2008;115:91–97. doi: 10.1042/CS20080021. [DOI] [PubMed] [Google Scholar]
- 3.Papapetropoulos A, Pyriochou A, Altaany Z, et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci USA. 2009;106:21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szabó C, Papapetropoulos A. Hydrogen sulphide and angiogenesis: mechanisms and applications. Br J Pharmacol. 2011;164:853–865. doi: 10.1111/j.1476-5381.2010.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang G, Wu L, Jiang B, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muzaffar S, Jeremy JY, Sparatore A, Del Soldato P, Angelini GD, Shukla N. H2S-donating sildenafil (ACS6) inhibits superoxide formation and gp91phox expression in arterial endothelial cells: role of protein kinases A and G. Br J Pharmacol. 2008;155:984–994. doi: 10.1038/bjp.2008.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li PC, Chen WC, Chang LC, Lin SC. Substance P acts via the neurokinin receptor 1 to elicit bronchoconstriction, oxidative stress, and upregulated ICAM-1 expression after oil smoke exposure. Am J Physiol Lung Cell Mol Physiol. 2008;294:L912–L920. doi: 10.1152/ajplung.00443.2007. [DOI] [PubMed] [Google Scholar]
- 8.Zhu XY, Gu H, Ni X. Hydrogen sulfide in the endocrine and reproductive systems. Expert Rev Clin Pharmacol. 2011;4:75–82. doi: 10.1586/ecp.10.125. [DOI] [PubMed] [Google Scholar]
- 9.Tokuda K, Kida K, Marutani E, et al. Inhaled hydrogen sulfide prevents endotoxin-induced systemic inflammation and improves survival by altering sulfide metabolism in mice. Antioxid Redox Signal. 2012;17:11–21. doi: 10.1089/ars.2011.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faller S, Zimmermann KK, Strosing KM, et al. Inhaled hydrogen sulfide protects against lipopolysaccharide-induced acute lung injury in mice. Med Gas Res. 2012;2:26. doi: 10.1186/2045-9912-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faller S, Ryter SW, Choi AM, Loop T, Schmidt R, Hoetzel A. Inhaled hydrogen sulfide protects against ventilator-induced lung injury. Anesthesiology. 2010;113:104–115. doi: 10.1097/ALN.0b013e3181de7107. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Han Z, Duan Y, et al. The influence of inhalation H2S lung in rats. Jie Fang Jun Yi Xue Yuan Xue Bao. 2014;35:1241–1244. [Google Scholar]
- 13.McPherson C. Regulation of animal care and research? NIH's opinion. J Animal Sci. 1980;51:492–496. doi: 10.2527/jas1980.512492x. [DOI] [PubMed] [Google Scholar]
- 14.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals, corp-author. Guide for the Care and Use of Laboratory Animals. 8th. National Academies Press (US); Washington (DC): 2011. [Google Scholar]
- 15.Lee HM, Greeley GH, Herndon DN, Sinha M, Luxon BA, Englander EW. A rat model of smoke inhalation injury: influence of combustion smoke on gene expression in the brain. Toxicol Appl Pharmacol. 2005;208:255–265. doi: 10.1016/j.taap.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Huang PS, Tang GJ, Chen CH, Kou YR. Whole-body moderate hypothermia confers protection from wood smoke-induced acute lung injury in rats: the therapeutic window. Crit Care Med. 2006;34:1160–1167. doi: 10.1097/01.CCM.0000207342.50559.0F. [DOI] [PubMed] [Google Scholar]
- 17.Zou YY, Lu J, Poon DJ, Kaur C, Cao Q, Teo AL, Ling EA. Combustion smoke exposure induces up-regulated expression of vascular endothelial growth factor, aquaporin 4, nitric oxide synthases and vascular permeability in the retina of adult rats. Neuroscience. 2009;160:698–709. doi: 10.1016/j.neuroscience.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Rehberg S, Maybauer MO, Enkhbaatar P, Maybauer DM, Yamamoto Y, Traber DL. Pathophysiology, management and treatment of smoke inhalation injury. Expert Rev Respir Med. 2009;3:283–297. doi: 10.1586/ers.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enkhbaatar P, Traber DL. Pathophysiology of acute lung injury in combined burn and smoke inhalation injury. Clin Sci (Lond) 2004;107:137–143. doi: 10.1042/CS20040135. [DOI] [PubMed] [Google Scholar]
- 20.Cox RA, Jacob S, Oliveras G, et al. Pulmonary expression of nitric oxide synthase isoforms in sheep with smoke inhalation and burn injury. Exp Lung Res. 2009;35:104–118. doi: 10.1080/01902140802446832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westphal M, Enkhbaatar P, Schmalstieg FC, et al. Neuronal nitric oxide synthase inhibition attenuates cardiopulmonary dysfunctions after combined burn and smoke inhalation injury in sheep. Crit Care Med. 2008;36:1196–1204. doi: 10.1097/CCM.0b013e31816a1a0c. [DOI] [PubMed] [Google Scholar]
- 22.Rehberg S, Maybauer MO, Maybauer DM, Traber LD, Enkhbaatar P, Traber DL. The role of nitric oxide and reactive nitrogen species in experimental ARDS. Front Biosci (Schol Ed) 2010;2:18–29. doi: 10.2741/s43. [DOI] [PubMed] [Google Scholar]
- 23.Westphal M, Rehberg S, Maybauer MO, et al. Cardiopulmonary effects of low-dose arginine vasopressin in ovine acute lung injury. Crit Care Med. 2011;39:357–363. doi: 10.1097/CCM.0b013e3181feb802. [DOI] [PubMed] [Google Scholar]
- 24.Calvert JW. The summer of hydrogen sulfide: highlights from two international conferences. Med Gas Res. 2013;3:5. doi: 10.1186/2045-9912-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gadalla MM, Snyder SH. Hydrogen sulfide as a gasotransmitter. J Neurochem. 2010;113:14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu WL, Liu ZW, Li TS, Wang C, Zhao B. Hydrogen sulfide donor regulates alveolar epithelial cell apoptosis in rats with acute lung injury. Chin Med J (Engl) 2013;126:494–499. [PubMed] [Google Scholar]
- 27.Oh GS, Pae HO, Lee BS, et al. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic Biol Med. 2006;41:106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 28.Wagner F, Asfar P, Calzia E, Radermacher P, Szabó C. Bench-to-bedside review: Hydrogen sulfide - the third gaseous transmitter: applications for critical care. Crit Care. 2009;13:213. doi: 10.1186/cc7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong SO, Pae HO, Oh GS, et al. Hydrogen sulfide potentiates interleukin-1beta-induced nitric oxide production via enhancement of extracellular signal-regulated kinase activation in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;345:938–944. doi: 10.1016/j.bbrc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Zhi L, Ang AD, Zhang H, Moore PK, Bhatia M. Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK-NF-kappaB pathway. J Leukoc Biol. 2007;81:1322–1332. doi: 10.1189/jlb.1006599. [DOI] [PubMed] [Google Scholar]
- 31.Hu LF, Wong PT, Moore PK, Bian JS. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem. 2007;100:1121–1128. doi: 10.1111/j.1471-4159.2006.04283.x. [DOI] [PubMed] [Google Scholar]
- 32.Janssen-Heininger YM, Poynter ME, Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic Biol Med. 2000;28:1317–1327. doi: 10.1016/S0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 33.LaLonde C, Nayak U, Hennigan J, Demling R. Plasma catalase and glutathione levels are decreased in response to inhalation injury. J Burn Care Rehabil. 1997;18:515–519. doi: 10.1097/00004630-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Paul BD, Snyder SH. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 35.Whiteman M, Armstrong JS, Chu SH, et al. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite scavenger? J Neurochem. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 36.Whiteman M, Cheung NS, Zhu YZ, et al. Hydrogen sulphide: a novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem Biophys Res Commun. 2005;326:794–798. doi: 10.1016/j.bbrc.2004.11.110. [DOI] [PubMed] [Google Scholar]
- 37.Arai M, Yoshioka S, Nishimura R, Okuda K. FAS/FASL-mediated cell death in the bovine endometrium. Anim Reprod Sci. 2014;151:97–104. doi: 10.1016/j.anireprosci.2014.10.004. [DOI] [PubMed] [Google Scholar]