ABSTRACT
The contractile vacuole complex (CVC) of Trypanosoma cruzi, the etiologic agent of Chagas disease, collects and expels excess water as a mechanism of regulatory volume decrease after hyposmotic stress; it also has a role in cell shrinking after hyperosmotic stress. Here, we report that, in addition to its role in osmoregulation, the CVC of T. cruzi has a role in the biogenesis of acidocalcisomes. Expression of dominant-negative mutants of the CVC-located small GTPase Rab32 (TcCLB.506289.80) results in lower numbers of less-electron-dense acidocalcisomes, lower content of polyphosphate, lower capacity for acidocalcisome acidification and Ca2+ uptake that is driven by the vacuolar proton pyrophosphatase and the Ca2+-ATPase, respectively, as well as less-infective parasites, revealing the role of this organelle in parasite infectivity. By using fluorescence, electron microscopy and electron tomography analyses, we provide further evidence of the active contact of acidocalcisomes with the CVC, indicating an active exchange of proteins between the two organelles.
KEY WORDS: Acidocalcisome, Calcium, Contractile vacuole, Polyphosphate, Vacuolar pyrophosphatase
Summary: In addition to its role in osmoregulation, the contractile vacuole of Trypanosoma cruzi, the agent of Chagas disease, has a role in the biogenesis of acidocalcisomes.
INTRODUCTION
Trypanosoma cruzi (Clark, 1959), the etiologic agent of Chagas disease, together with Leishmania spp. (Figarella et al., 2007) and a number of monogenetic trypanosomes (Baqui et al., 2000; Linder and Staehelin, 1979), possess a contractile vacuole complex (CVC) involved in osmoregulation. In T. cruzi, the CVC has been shown to be important for regulatory volume decrease (RVD) after hyposmotic stress (Rohloff et al., 2004) and for shrinking of the cells when subjected to hyperosmotic stress (Li et al., 2011). In addition, we have recently reported a role for the CVC in trafficking glycosylphosphatidylinositol (GPI)-anchored proteins to the plasma membrane (Niyogi et al., 2014). Previous studies in T. cruzi (Hasne et al., 2010) and Dictyostelium discoideum (Heuser et al., 1993; Moniakis et al., 1999; Sesaki et al., 1997; Sriskanthadevan et al., 2009) suggest that some soluble (Sesaki et al., 1997; Sriskanthadevan et al., 2009) and membrane (Hasne et al., 2010; Heuser et al., 1993; Moniakis et al., 1999) proteins can also be transported through the CVC to the plasma membrane. The presence of Rab11, a small GTPase that localizes in recycling endosomes in most cells – including Trypanosoma brucei (Jeffries et al., 2001) – and in the CVC of T. cruzi (Ulrich et al., 2011) and D. discoideum (Harris et al., 2001), suggests that the CVC could be an evolutionary precursor to the recycling endosomal system in other eukaryotes (Docampo et al., 2013; Harris et al., 2001).
In a previous proteomic and bioinformatic study of the CVC of T. cruzi, we identified a number of proteins that have roles in trafficking, among them SNARE 2.1 and SNARE 2.2, VAMP1 (an ortholog of mammalian VAMP7), AP180 and the small GTPases Rab11 and Rab32 (TcCLB.506289.80) (Ulrich et al., 2011). Rab proteins mediate tethering of incoming vesicles to the correct target organelle through cycling between a GDP-bound inactive and a GTP-active form (Zerial and McBride, 2001). They have also been implicated in vesicle budding and in the interaction with cytoskeletal elements (Zerial and McBride, 2001). Different Rab GTPases are localized to different organelles, and this represents an important determinant of the identity of each organelle (Bright et al., 2010; Munro, 2002; Pfeffer, 2001; Seabra and Wasmeier, 2004; Turkewitz and Bright, 2011). Rab32 and its close homolog Rab38 are predominantly expressed in cells that produce lysosome-related organelles (LROs), such as melanocytes and platelets (Bultema et al., 2012), and it has been suggested that these Rabs could be the specificity factors that work in concert with the ubiquitous trafficking machinery to direct transport toward LROs (Bultema et al., 2012). It has been proposed that LROs arise through the delivery of specific cargoes from the early endosomal network, comprising sorting and recycling endosomes (Delevoye et al., 2009; Raposo and Marks, 2007).
T. cruzi possesses organelles that have similarities to LROs of mammalian cells, known as acidocalcisomes (Docampo et al., 2005, 1995; Docampo and Moreno, 2011). Like LROs of human platelets (Ruiz et al., 2004; Smith et al., 2006) and mast cells (Moreno-Sanchez et al., 2012), acidocalcisomes have rounded morphology, are acidic, and are rich in Ca2+, pyrophosphate (PPi) and polyphosphate (polyP). In addition, adaptor protein complex-3 (AP-3), the system known to be involved in the transport of membrane proteins to LROs of mammalian cells (Theos et al., 2005), is also involved in the biogenesis of acidocalcisomes (Besteiro et al., 2008; Huang et al., 2011). Interestingly, electron microscopy analyses have previously provided evidence of the fusion of acidocalcisomes to the CVC of T. cruzi (Montalvetti et al., 2004) and D. discoideum (Marchesini et al., 2002). Also, under hyposmotic stress, acidocalcisomes fuse to the CVC, which results in translocation of an aquaporin [T. cruzi (Tc)AQP1] (Rohloff et al., 2004). In this work, we demonstrate that the expression of dominant-interfering TcRab32 mutants alters osmoregulation, acidocalcisome morphology and content, as well as parasite infectivity. The results suggest that the CVC and TcRab32 participate in the trafficking of proteins involved in acidocalcisome biogenesis, and reaffirm the role of the CVC as a trafficking hub.
RESULTS
The localization in the CVC of TcRab32, a Rab usually associated with LROs (Bultema and Di Pietro, 2013), suggests that the CVC, in addition to its role in osmoregulation, could be involved in the biogenesis of acidocalcisomes. We first confirmed the localization of TcRab32 in the CVC using specific antibodies and then investigated whether expression of dominant-negative TcRab32 affected osmoregulation and the biogenesis of acidocalcisomes. We analyzed the interaction between the organelles, as well as the enzymatic activities (vacuolar H+-pyrophosphatase and Ca2+-ATPase), composition and number of acidocalcisomes, and finally the relevance of this interaction for the infectivity of the parasites.
Localization of TcRab32 at different T. cruzi stages
We have reported previously that N-terminal tagging of TcRab32 with green fluorescent protein (GFP) results in fluorescent labeling of the CVC of epimastigotes and additional punctate staining (Ulrich et al., 2011). We confirmed this localization by using indirect immunofluorescence analysis with specific affinity-purified antibody against TcRab32, which was raised in mice against the recombinant protein (supplementary material Fig. S1A). Supplementary material Fig. S2A,B shows that TcRab32 localized to the CVC of wild-type epimastigotes, trypomastigotes and amastigotes, as evidenced by the circular staining close to the flagellar pocket; additional punctate staining was also observed, especially in epimastigotes and trypomastigotes. Western blot analysis of parasite lysates, using the same antibody, revealed a band of ∼26 kDa, corresponding to the native protein (supplementary material Fig. S2C). A double band was detected in amastigote lysates, indicative of some cross-reaction with another protein or post-translational modification that occurs at this life-cycle stage. Control experiments using pre-immune serum were negative. The CVC localization of GFP–TcRab32 was also confirmed by using immunogold electron microscopy and antibodies against GFP (supplementary material Fig. S2D,E), which was negative when wild-type cells were used.
In vitro prenylation studies of TcRab32
TcRab32 possesses the sequence CSC at the carboxyl terminus (supplementary material Fig. S1B), and it is known that Rab prenylation at cysteine residues of the carboxyl end retains Rabs at membranes (Jean and Kiger, 2012). To examine whether TcRab32 is geranylgeranylated, we performed in vitro prenylation experiments (Fig. 1A) using recombinant TcRab32 as substrate in the presence of a cytosolic epimastigote extract as the source of prenyltransferases. When tritiated geranylgeranyl pyrophosphate ([3H]GGPP) was used as the isoprenoid donor, His-tagged TcRab32 was efficiently geranylgeranylated as shown by the labeled band of 42 kDa that was detected, corresponding to the His-tagged protein. The intensity of the prenylated band was strongest at 30 min, the optimum incubation time. Conversely, when tritiated farnesyl pyrophosphate ([3H]FPP) was used as the donor, we were unable to detect prenylation of recombinant TcRab32 (data not shown). Therefore, TcRab32 is specifically geranylgeranylated. Previous studies of recombinant T. cruzi protein geranylgeranyl transferase I (GGTI) using a panel of mammalian and yeast protein substrates report that two mammalian Rab-family GTPases containing the C-terminal CXC sequence do not serve as substrates for this enzyme, as expected (Yokoyama et al., 2008; Nepomuceno-Silva et al., 2001). Accordingly, Prenylation Prediction Suite (PrePS) predicts that geranylgeranyl transferase II (GGTII) is the enzyme involved in the prenylation of this protein.
Fig. 1.
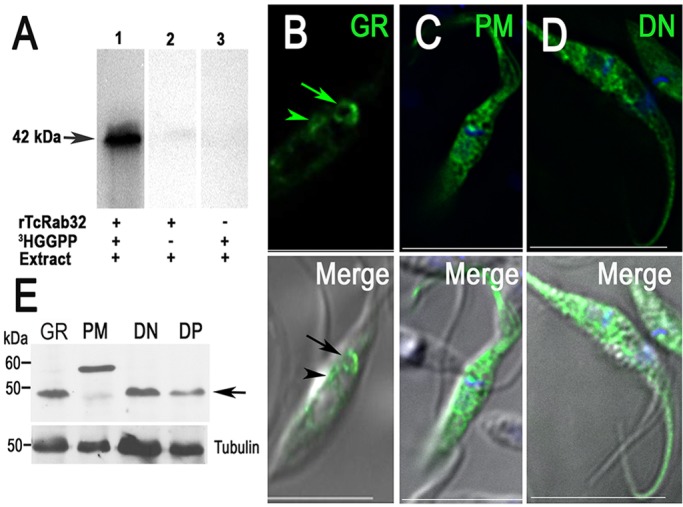
TcRab32 is geranylgeranylated in vitro. (A) Radiolabeled proteins were analyzed using SDS-PAGE on a 15% gel followed by autoradiography. Lane 1, in the presence of all reactants – rTcRab32, epimastigote extract and [3H]GGPP. Lanes 2 and 3 are negative controls. The enzyme assay was performed for 30 min. A radioactive band of 42 kDa was observed, corresponding to the His-tagged protein (arrow). (B) GFP–TcRab32-expressing epimastigotes (GR) show preferential localization in the contractile vacuole (arrow) and perinuclear region (arrowhead). (C) GFP–TcRab32 prenylation-motif mutants have a cytosolic localization. (D) Mutant dominant-negative GFP–TcRab32, which mimics the GDP-bound state of the protein, has a cytosolic localization. Scale bars: 10 µm. (E) Western blot analysis of lysates from epimastigotes expressing GFP–TcRab32 (GR) or expressing the prenylation mutant (PM) or dominant negative (DN) TcRab32 using an antibody against TcRab32. Tubulin (antibody dilution 1:10,000) was used as a loading control. Arrow shows the molecular mass of GR and dominant-negative TcRab32, as well as the endogenous Rab32 in the PM lane. The prenylation mutant has a higher molecular mass.
Localization of TcRab32 mutants
To examine the role of the prenylation motif in the targeting of TcRab32 to cell membranes, we generated mutants in which alanine replaced the cysteine residues at the prenylation motif, and we studied the effect of this mutation on the localization of the protein. The geranyl-geranyl tails of Rabs tether the proteins to cell membranes and help to restrict free diffusion through the cytoplasm (Rak et al., 2003). In transfected T. cruzi epimastigotes, GFP–TcRab32 mainly localized to the CVC (Fig. 1B), as reported previously (Ulrich et al., 2011), although it also showed some localization to the perinuclear endoplasmic reticulum (ER), which is probably a product of its overexpression, whereas the prenylation mutant GFP–TcRab32C241A/C243A exhibited cytosolic localization (Fig. 1C). We also engineered an expression plasmid encoding a TcRab32 mutant that mimics the GDP-bound form (dominant-negative, TcRab32T24N) (Fig. 1D). In transfected T. cruzi epimastigotes, the dominant-negative GFP–TcRab32 exhibited cytosolic localization, which included localization to the cytoplasm along the flagellum. Taken together, these results indicate that TcRab32 localizes to the membrane of the CVC in a GTP- and geranylgeranyl-dependent manner. The morphology of the CVC remained unaffected when the mutant proteins were expressed. We confirmed tagging of the mutant proteins using western blot analyses (Fig. 1E). The anomalous migration of the GFP–TcRab32 prenylation-motif mutants could be due to lack of prenylation or other post-translational modifications of this protein in the absence of prenylation (Beranger et al., 1994).
Colocalization of GFP–TcRab32 with VP1 under osmotic stress
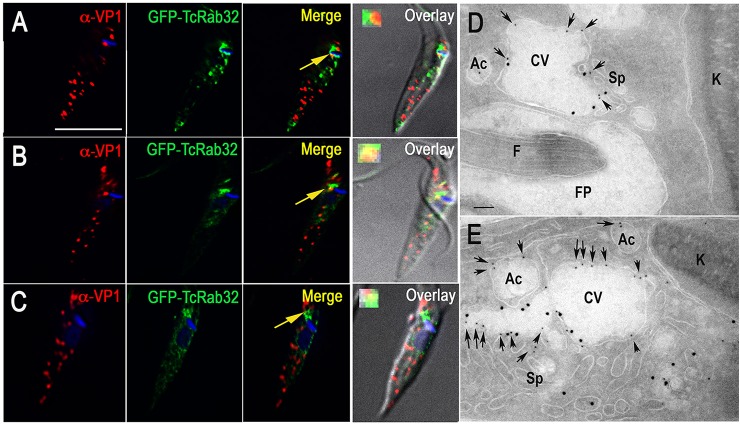
It has been reported that mammalian (Tamura et al., 2009) and Xenopus (Park et al., 2007) Rab32 partially localizes to melanosomes, which are LROs. We therefore investigated whether TcRab32 partially co-localizes with the acidocalcisome marker vacuolar proton pyrophosphatase (V-H+-PPase, VP1). We did not observe any substantial overlap between the labeling of VP1 and GFP–TcRab32 under isosmotic conditions (Fig. 2A). The observed additional punctate staining of GFP–TcRab32 could correspond to endosomes. However, under hyposmotic (Fig. 2B) or hyperosmotic (Fig. 2C) conditions, we observed that staining of VP1 overlapped with that of GFP at the CVC region, in agreement with the previously reported fusion of these organelles under osmotic stress (Montalvetti et al., 2004; Rohloff et al., 2004). The lack of colocalization between GFP–TcRab32 and the acidocalcisome marker under isosmotic conditions could be attributed to the dynamic nature of the interaction between these two organelles. These results were confirmed by analyses using cryo-immunogold electron microscopy. After hyposmotic stress, it was possible to detect colocalization of VP1 staining and GFP–TcRab32 in the contractile vacuole bladder and in the tubules of the spongiome (Fig. 2D,E).
Fig. 2.
Colocalization of GFP–TcRab32 with staining of VP1 under osmotic stress. (A) There is no colocalization between VP1 (red) and GFP–TcRab32 (green), as detected with antibodies against T. brucei VP1 and GFP under isosmotic conditions. (B,C) Overlap between signals for VP1 (red) and GFP–TcRab32 (green) in the CVC occurs under hyposmotic (B) or hyperosmotic (C) stress conditions (arrows, and enlarged insets in the merged images). (D,E) Cryo-immunogold electron microscopy analysis of epimastigotes subjected to hyposmotic conditions (150 mosm/l) and incubated with rabbit anti-GFP antibodies (1:25) and mouse anti-VP1 antibodies (1:25, 12-nm gold particles, arrows) at 4°C overnight, and then treated with secondary antibodies (goat anti-mouse conjugated to 12-nm colloidal gold, arrows) and goat anti-rabbit 18-nm gold for 1 h at room temperature. The images show colocalization in the contractile vacuole (CV) bladder and the spongiome (Sp). Ac, acidocalcisome; FP, flagellar pocket; F, flagellum; K, kinetoplast. Scale bars: 10 µm (A, applies to A–C); 2 µm (D,E).
We have previously reported that GFP-tagged VAMP1 (the ortholog of mammalian VAMP7, here on referred to as TcVAMP7) localizes to the contractile vacuole bladder of epimastigotes that have been submitted to osmotic stress (Ulrich et al., 2011). Immunogold electron microscopy analyses of GFP localization also revealed labeling of the spongiome, flagellar pocket, small vesicles and plasma membrane (supplementary material Fig. S3A,B). No labeling was observed when wild-type cells were used. Correct tagging of the protein was demonstrated using western blot analysis (supplementary material Fig. S3C). We now report that when tagged with GFP at its C-terminus, TcVAMP7–GFP localizes predominantly to the acidocalcisomes, as revealed by its colocalization with VP1 (Fig. 3A) and by using cryo-immunogold electron microscopy (Fig. 3B). TcVAMP7–GFP also weakly labeled the CVC, as shown by using immunofluorescence analyses (Fig. 3D). When the cells were submitted to hyposmotic stress, an increase in TcVAMP7–GFP labeling of the CVC was observed (Fig. 3C,E,F), and once regulatory volume decrease (RVD) had been completed, acidocalcisome labeling predominated again (Fig. 3G), suggesting transient fusion of both organelles. Live-cell microscopy analyses of epimastigotes that expressed TcVAMP7–GFP and were labeled with BODIPY–ceramide (Fig. 3H,I; supplementary material Movies 1 and 2) shows how, after hyposmotic stress, acidocalcisomes that had been labeled with TcVAMP7–GFP make contact with the CVC, reinforcing the hypothesis that these two organelles interact and exchange proteins.
Fig. 3.
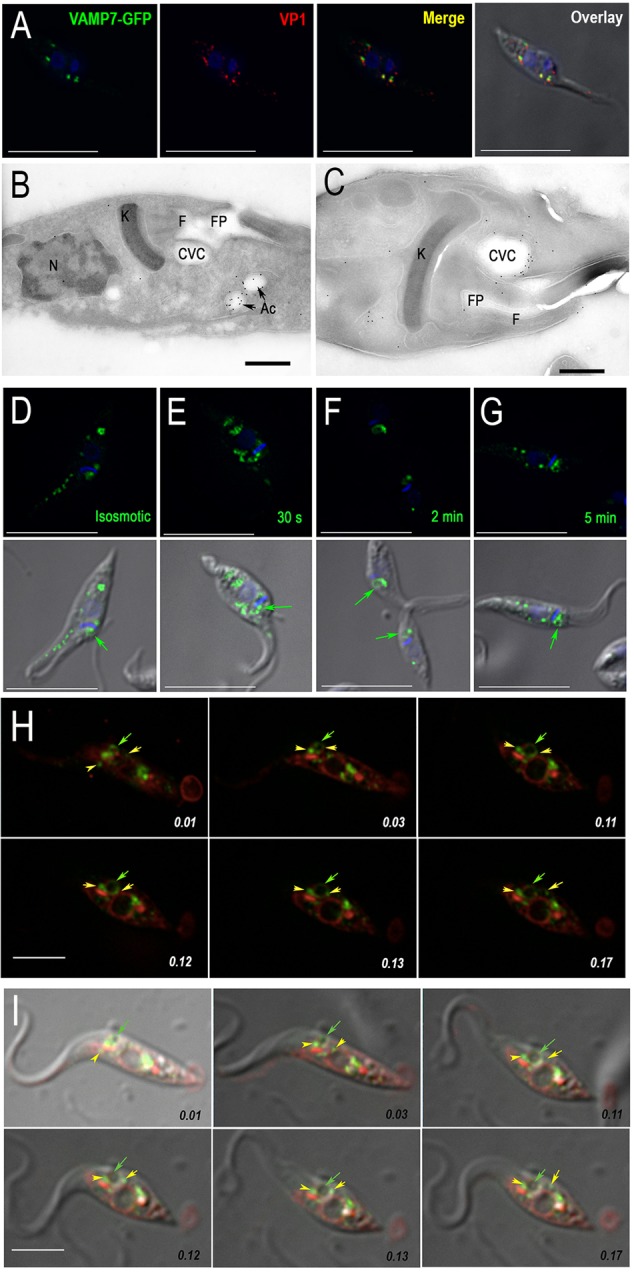
Translocation of TcVAMP7–GFP to the CVC under hyposmotic conditions. (A) Colocalization of VAMP7–GFP (green) with VP1 (red) using a polyclonal antibody against GFP and a monoclonal antibody against TcVP1, respectively. (B,C) Cryo-immunogold electron microscopy analyses using anti-GFP antibodies show predominant labeling of acidocalcisomes (Ac) under isosmotic conditions (B) and of the CVC under hyposmotic conditions (C). FP, flagellar pocket; F, flagellum; K, kinetoplast; N, nucleus. (D–G) Immunofluorescence analysis of cells imaged under isosmotic conditions (D) after 30 s (E), 2 min (F) and 5 min (G) of hyposmotic shock (150 mosm/l) using anti-GFP antibodies (green). DAPI staining labels DNA (blue). (H) Labeling of TcVAMP7–GFP-overexpressing parasites (green) with BODIPY–ceramide (red). The time indicated on each frame corresponds to 10, 30, 110, 120, 130 and 170 s (top row, left to right then bottom row, left to right). Note the dilation of the contractile vacuole bladder. (I) Overlay of differential interference contrast with the green and red channels at the same time points as those in H. In H and I, yellow arrows show acidocalcisomes, and green arrows show the localization of the CVC. Scale bars: 10 µm (A,D–G); 2 µm (B,C); 5 µm (H,I).
Additional evidence of contact of acidocalcisomes with the CVC complex under osmotic stress
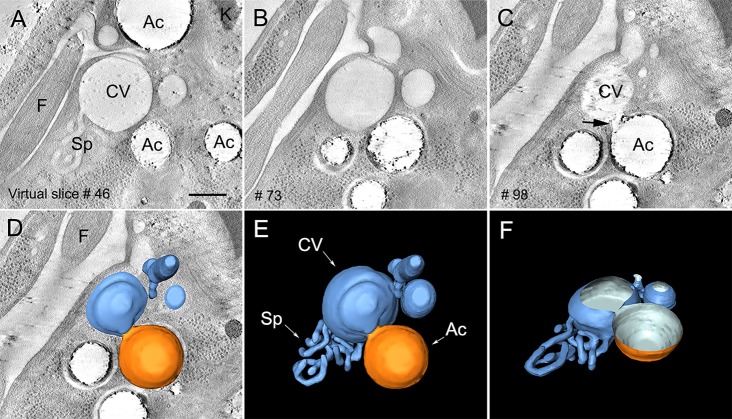
By using electron tomography to examine the CVC under hyposmotic stress, we observed an organized network of interconnected tubules (spongiome) that was attached to the central vacuole or bladder (Fig. 4A), confirming previous results (Girard-Dias et al., 2012). Reconstruction of the whole volume of the organelle by using serial electron tomography showed that the CVC was surrounded by acidocalcisomes, which were identified by their remaining electron-dense content that was observed along the depth of each organelle (Fig. 4A–C; supplementary material Movie 3). Inspection of the virtual sections through the tomogram provided further evidence for the close apposition of acidocalcisomes with the bladder of the CVC under hyposmotic conditions (Fig. 4C,D–F; supplementary material Movie 3). Taken together with the results presented thus far, these results are suggestive of fusion events (Fig. 3H,I and Fig. 4; supplementary material Movies 1–3).
Fig. 4.
Close apposition of an acidocalcisome with the CVC, indicating a fusion event. (A–C) Virtual section (1-nm thickness) sequence of a tomogram showing the anterior region of the parasite. The CVC is represented by the central vacuole of bladder (CV) and the spongiome (Sp). Acidocalcisomes (Ac) in the neighboring region are observed in close contact with the CVC. In the left lower corner, the section number is shown. In C, it is possible to observe a close apposition between acidocalcisome and CVC membranes, which are suggestive of a fusion event (arrow) between the two organelles. (D–F) 3D models of the CVC (blue) and its close contact with an acidocalcisome (orange). (F) Tilted view of the 3D model at 45° around the x axis. Spongiome (Sp) and flagellum (‘F’) are shown. Scale bar: 200 nm.
Response of dominant-negative Rab32 mutants to hyposmotic and hyperosmotic stress
Fusion of acidocalcisomes to the CVC is important for the response of the parasites to osmotic stress (Rohloff et al., 2004). We therefore investigated whether epimastigotes that expressed dominant-negative GFP–TcRab32 were deficient in their response to hyposmotic and hyperosmotic stresses. Wild-type epimastigotes and epimastigotes expressing GFP–TcRab32 or the dominant-negative mutant were subjected to hyposmotic stress, and their RVD was measured using the light-scattering technique, as described previously (Niyogi et al., 2014). Dominant-negative mutants were less able to recover their volume after hyposmotic stress than wild-type cells, whereas recovery was faster in GFP–TcRab32-expressing cells (Fig. 5A), probably as a result of overexpression of this protein. When subjected to hyperosmotic stress, epimastigotes expressing dominant-negative TcRab32 shrank less, whereas cells expressing GFP–TcRab32 shrank more than control cells (Fig. 5B), and in all cases, they did not recover their volume during the time of the experiment, as has been reported before (Li et al., 2011).
Fig. 5.
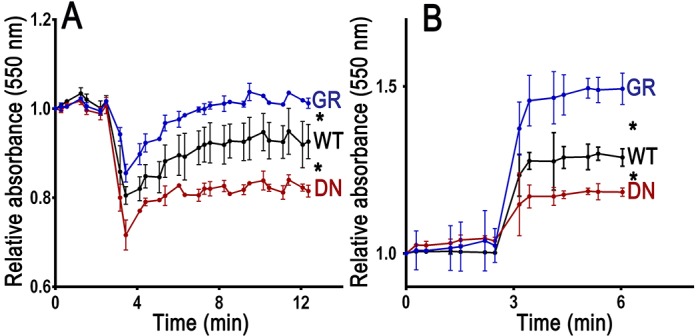
Effect of TcRab32 mutation on the parasite response to hyposmotic and hyperosmotic stress conditions. Epimastigotes were pre-incubated in isosmotic buffer for 3 min and then subjected to hyposmotic (final osmolarity=150 mosm/l) (A) or hyperosmotic (final osmolarity=650 mosm/l) (B) stress. Monitoring absorbance at 550 nm by light scattering was used to follow the relative changes in cell volume. As compared with wild-type cells (WT), cells expressing dominant-negative GFP–TcRab32 (DN) failed to fully recover their volume after hyposmotic stress and shrank less after hyperosmotic stress, whereas cells expressing GFP–TcRab32 (GR) recovered their volume faster after hyposmotic stress and shrank more after hyperosmotic stress. Values are means±s.d. of three independent experiments. Asterisks indicate statistically significant differences, P<0.05 (Bonferroni's multiple comparison ‘a posteriori’ test of one-way ANOVA), at all time points after the induction of osmotic stress (after 4 min).
Deficient acidocalcisome biogenesis and function in cells expressing dominant-negative TcRab32
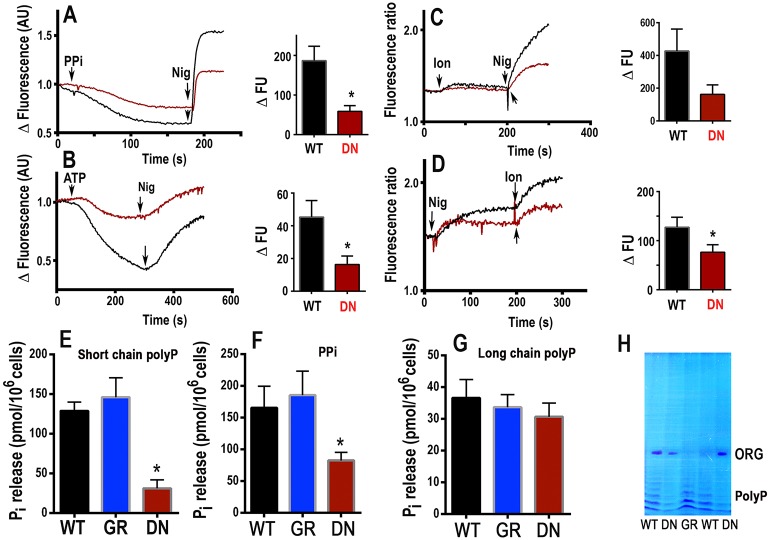
VP1 is a marker of acidocalcisomes in T. cruzi, and its activity was investigated in digitonin-permeabilized epimastigotes by measuring the uptake of Acridine Orange, which was induced by the addition of PPi. The decrease in fluorescence, after a delay that was due to plasma membrane permeabilization, indicated increasing vacuolar acidity (Fig. 6A). The vacuolar pH was neutralized, and Acridine Orange was released by the K+/H+ exchanger nigericin. The initial rate of PPi-induced acidification was greatly decreased in epimastigotes expressing dominant-negative GFP–TcRab32 (Fig. 6A).
Fig. 6.
Deficient PPi-dependent H+ uptake, ATP-dependent Ca2+ uptake, acidic Ca2+ stores, short-chain polyP and PPi levels in epimastigotes expressing dominant-negative GFP–TcRab32 in comparison with wild-type and GFP–TcRab32-expressing epimastigotes. (A) Wild type (black) or epimastigotes expressing dominant-negative GFP–TcRab32 (red, DN) (5×107/ml) were added to the standard buffer containing 125 mM sucrose, 65 mM KCl, 10 mM Hepes, pH 7.2, 2.5 mM potassium phosphate, 1 mM MgCl2, to which 3 µM Acridine Orange and 40 µM digitonin were added. PPi (0.1 mM) and nigericin (1 µM, Nig) were added where indicated. (B) Epimastigotes, as above, were added to the standard buffer (in A), to which 1 µM Calcium Green-5N and 40 µM digitonin were added. ATP (1 mM) and nigericin (1 µM) were added where indicated. The bar graphs (A,B) show quantification of the initial rate of H+ (A) or Ca2+ (B) uptake after adding PPi (A) or ATP (B) post-permeabilization. Three independent experiments were used for quantification, and results are expressed as means±s.e.m. (C,D) Amastigotes (5×107/ml) loaded with Fura-2 were incubated in buffer A in the presence of 100 µM EGTA, and, where indicated, 1 µM ionomycin (Ion) or 1 µM nigericin was added. Bar graphs in C,D show quantification of the amount of Ca2+ released by the combination ionomycin-nigericin as means±s.e.m. of three independent experiments. Asterisks in A,B,D indicate that differences were significant (P<0.05). In panel C, the difference was marginal (P<0.07). Extracts from epimastigotes expressing dominant-negative GFP–TcRab32 (DN) showed ∼80% reduction in short-chain polyP levels (E) and ∼50% reduction in PPi levels (F) with no significant changes in long-chain polyP levels (G) in comparison with wild-type (WT) or GFP–TcRab32-expressing epimastigotes (GR). Values are means±s.e.m. of three independent experiments. *Differences are statistically significant as compared with respective controls, P<0.05 (0.041, DN versus GR, and 0.031, DN versus WT for E; 0.028, DN versus GR, and 0.003, DN versus WT for F) (Student's t-test). (H) Extracts of short-chain polyP produced by epimastigotes expressing GFP–TcRab32 (GR) or dominant-negative GFP–TcRab32 (DN). Two samples were resolved by using urea-PAGE and visualized using Toluidine Blue. ORG represents migration of Orange G dye. Bands corresponding to short-chain polyP of different lengths are shown as a series of bands in the lower part of the gel; these bands had lower intensities in lanes corresponding to the dominant-negative mutant (two samples) in comparison with those of cells transfected with GFP–TcRab32 (GR) and of control wild-type cells (WT) (two samples).
Acidocalcisomes are capable of taking up Ca2+ using a plasma-membrane-type Ca2+-ATPase (PMCA) (Lu et al., 1998). Ca2+ uptake by digitonin-permeabilized epimastigotes was measured using Calcium Green-5N (Fig. 6B). The initial rate of Ca2+ uptake was greatly decreased in cells that expressed dominant-negative GFP–TcRab32 (Fig. 6B). Ca2+ was released by the addition of nigericin (Fig. 6B). Ca2+ uptake under these conditions measures not only Ca2+ uptake by acidocalcisomes but also by the sarcoplasmic-endoplasmic reticulum-type Ca2+-ATPase (SERCA). We therefore evaluated whether cells expressing dominant-negative GFP–TcRab32 were deficient in acidic Ca2+ content. In previous work with T. cruzi epimastigotes, we noticed that epimastigotes were deficient in esterases that were able to hydrolyze Fura-2/AM, which is commonly used to detect Ca2+ pools in live cells, but this was not the case with infective stages (Docampo et al., 1993; Moreno et al., 1992). We therefore loaded amastigotes that had been obtained by differentiation of wild-type epimastigotes and epimastigotes expressing dominant-negative GFP–TcRab32, as described in Materials and Methods, and examined their acidic Ca2+ content using ionophores, as reported previously (Moreno et al., 1992). Addition of ionomycin to Fura-2-loaded amastigotes in Ca2+-free medium (with the addition of 100 µM EGTA) resulted in Ca2+ release from neutral or alkaline compartments because ionomycin binds to essentially no Ca2+ below pH 7.0 and it cannot carry Ca2+ out of acidic compartments. However, further addition of nigericin, which alkalinize acidic compartments, resulted in a greater release of Ca2+ (Fig. 6C). If the order of addition was reversed, nigericin caused a transient increase in Ca2+, which was greatly increased after ionomycin addition (Fig. 6D). The amount of Ca2+ released by the combination of ionomycin and nigericin was considerably lower in cells expressing dominant-negative GFP–TcRab32 (Fig. 6C,D), suggesting that the acidocalcisomes of these mutant cells contain less Ca2+.
Most PPi and polyP in trypanosomes accumulate in acidocalcisomes (Docampo et al., 2005; Docampo and Moreno, 2011). It is unknown whether PPi is taken up from the cytosol or synthesized inside acidocalcisomes, whereas synthesis of polyP is through the activity of polyP kinases, such as that formed by the vacuolar transporter chaperone (VTC) complex (Lander et al., 2013; Ulrich et al., 2014). This is a complex of at least two subunits in trypanosomatids, VTC1 and VTC4, both of which localize to the membrane of acidocalcisomes, and VTC4 is the catalytic subunit (Lander et al., 2013; Ulrich et al., 2014). We hypothesized that if TcRab32 is important for the biogenesis of acidocalcisomes, these organelles will have a reduced ability to synthesize these compounds, and this is what we found. Expression of the dominant-negative form of TcRab32 led to a significant reduction in the levels of short-chain polyP (∼80%) (Fig. 6E) and PPi (∼50%) (Fig. 6F). There was, however, no significant change in the content of long chain polyP (>300 up to 700–800 phosphate units) (Fig. 6G), suggesting that only the activity of the VTC complex, which is mainly involved in the synthesis of short-chain polyP (Lander et al., 2013; Ulrich et al., 2014), is affected in these mutants. The results were further verified by visualization of short-chain polyP that had been extracted from the above cell lines, resolved by urea-PAGE and stained with Toluidine Blue (Fig. 6H).
Changes in acidocalcisome electron-density and the number of cells expressing dominant-negative GFP–TcRab32
In previous work (Mendoza et al., 2002; Urbina et al., 1999), electron microscopy techniques have been used to demonstrate that treatment of fixed trypanosomes with yeast pyrophosphatase results in loss of the electron-density of acidocalcisomes, as observed in whole unstained cells, suggesting that PPi (complexed with cations) is the main electron-dense material in these organelles (Urbina et al., 1999). In agreement with the considerable decrease in PPi and short chain polyP content in epimastigotes expressing dominant-negative GFP-TcRab32, we detected an increase in the presence of empty and dilated vacuoles in intact unstained epimastigotes expressing the mutant protein by using direct transmission electron microscopy (compare Fig. 7A,B). There was also a substantial reduction in the number of acidocalcisomes per cell (Fig. 7B,G). Of the cells expressing the dominant-negative protein, 84% had empty dilated vacuoles (Fig. 7H) with an average of ∼10 empty vacuoles per dominant-negative cell. Similar results were obtained with trypomastigotes (compare Fig. 7C,D) and amastigotes (compare Fig. 7E,F) expressing dominant-negative GFP–TcRab32.
Fig. 7.
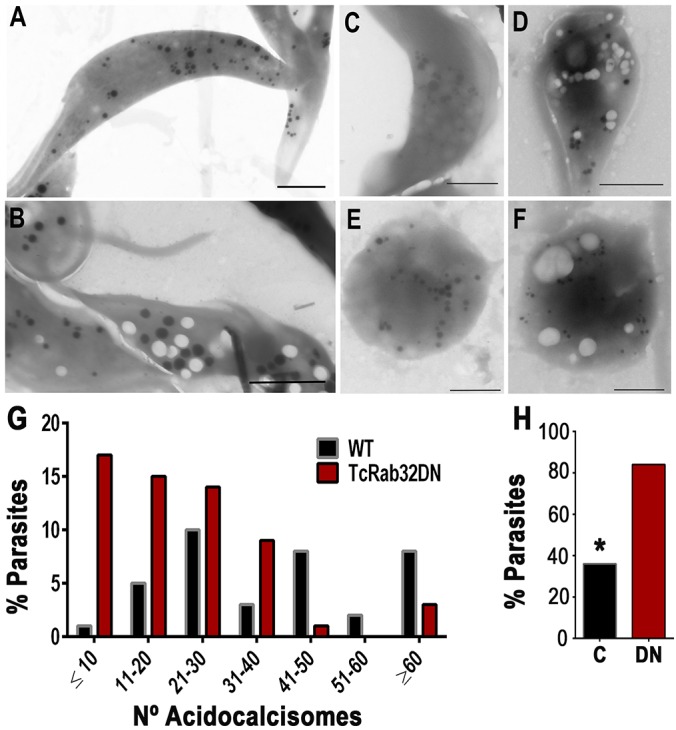
Reduction in electron-dense acidocalcisomes and a considerable increase in empty vacuoles in cells expressing dominant-negative TcRab32 in comparison with wild-type cells. (A,B) Direct transmission electron microscopy image from whole unstained and unfixed epimastigotes expressing dominant-negative GFP–TcRab32 (DN) show the presence of numerous empty vacuoles (B) in comparison with wild-type epimastigotes (A). (C–F) A similar increase in the number of empty vacuoles was observed in trypomastigotes (C,D) and amastigotes (E,F) expressing dominant-negative GFP–TcRab32 (D,F) as compared with that of wild-type cells (C,E). (G) The number of acidocalcisomes per epimastigote was counted in 70 random cells from two independent experiments, and the numeric distribution of acidocalcisomes showed that the majority of epimastigotes expressing dominant-negative TcRab32 (TcRab32DN) had <10 or between 11 and 20 electron-dense acidocalcisomes. The results in wild-type (WT) cells are similar to those reported previously for trypanosomatids. (H) An increase in the percentage of epimastigotes showing empty vacuoles. In order to quantify the phenotype of empty vacuoles, by using transmission electron microscopy, we examined 50 random wild-type parasites, ‘C’, and parasites expressing the dominant-negative protein (DN), and counted the number of parasites with empty vacuoles for each. In comparison with wild type, we found that there was a significant increase in the percentage of parasites with empty vacuoles when they expressed dominant-negative TcRab32. *Differences between control and the dominant-negative protein are statistically significant, P<0.05. Scale bars: 2 µm (A,B); 1 µm (C–F).
Cells expressing dominant-negative GFP–TcRab32 have reduced growth and infectivity
The growth rate of the epimastigotes expressing dominant-negative GFP–TcRab32 was reduced as compared to that of control epimastigotes expressing GFP alone (Fig. 8A).
Fig. 8.
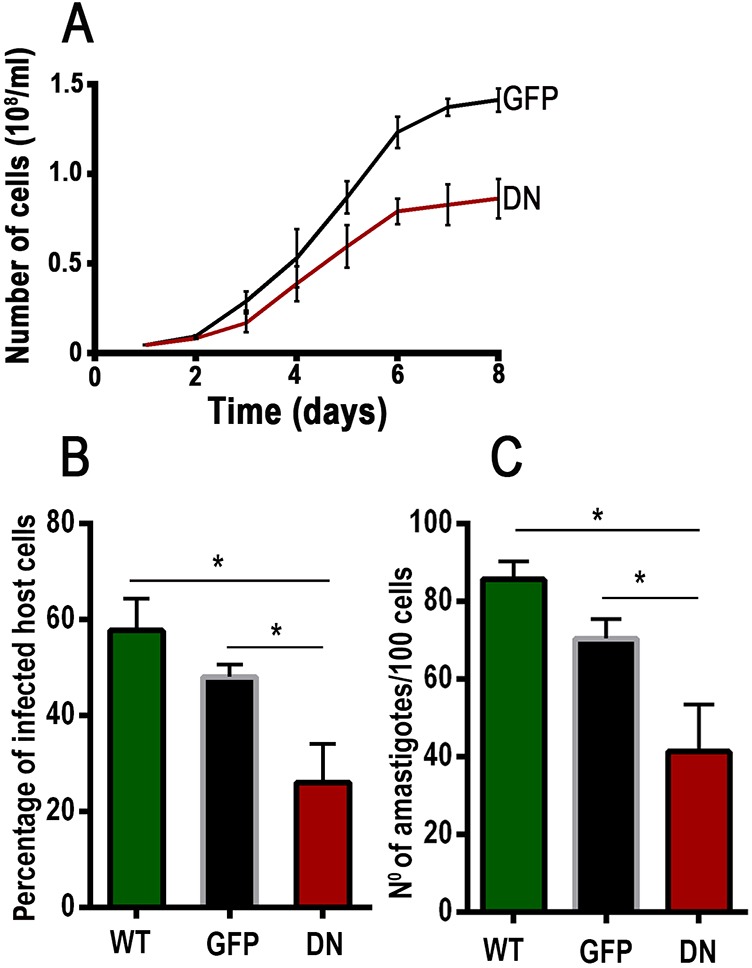
Reduced infectivity of GFP–TcRab32 mutant trypomastigotes. (A) Growth rate of epimastigotes expressing dominant-negative (DN) GFP–TcRab32 in comparison to GFP-expressing epimastigotes (GFP). Values are means±s.d. from three independent experiments. (B,C) The effect of expression of dominant-negative GFP–TcRab32 (DN) on the invasion by trypomastigotes of host cells in vitro in comparison with wild-type trypomastigotes (WT) or GFP-expressing trypomastigotes (GFP). Values are mean±s.d. (n=3). *Differences are statistically significant, P<0.05 (one way ANOVA with Bonferroni post-hoc test).
To study the infectivity of cells expressing dominant-negative GFP–TcRab32, we fully differentiated them into cell-derived trypomastigotes, as described in Materials and Methods. Invasion of culture cells with parasites expressing dominant-negative GFP–TcRab32 was substantially reduced as compared with that of controls that had been infected with parasites transfected with GFP alone or with wild-type parasites (Fig. 8B,C). Cytosolic localization of cells expressing dominant-negative GFP–TcRab32 was maintained when epimastigotes were differentiated into trypomastigotes and intracellular amastigotes (supplementary material Fig. S4). These results suggest a role for TcRab32 in infectivity.
DISCUSSION
We show here that expression of a dominant-negative form of the GTPase TcRab32 results in alterations in the morphology and content of acidocalcisomes, and in a deficient response to osmotic stress, growth in vitro and invasion of host cells. The results suggest that the CVC, where TcRab32 is located, is involved in the biogenesis and function of acidocalcisomes.
We have reported previously that GFP-tagged TcRab32 localizes to the CVC of T. cruzi epimastigotes (Ulrich et al., 2014). We now confirm those observations using antibodies against the protein and find it distributed in the CVC at different stages of the parasite life cycle, with additional punctate staining in epimastigotes and trypomastigotes. Dominant-negative GFP–TcRab32 or the TcRab32 protein lacking the prenylation motif, however, have a cytosolic localization, indicating that localization to the CVC is geranylgeranyl- and GTP-dependent. Dominant-negative TcRab32 mutants might act by blocking or reducing the function of endogenous TcRab32 by competing or sequestering Rab32 effector proteins.
TcRab32, like other Rab32 proteins, contains amino acid sequences that are shared with only a small number of other Rab sequences (Hirota and Tanaka, 2009). For example, threonine in the WDTAGQE sequence (GTP binding site), which is conserved in almost all Rab proteins, is replaced by isoleucine (WDIAGQE). A similar replacement is found in Rab38 and Rab29/Rab7L1 of mammalian cells, in RabE from D. discoideum (Hirota and Tanaka, 2009) and in Tetrahymena thermophila Rab32 (Bright et al., 2010), but there are no orthologs of any of the other Rabs in T. cruzi (Berriman et al., 2005). TcRab32 also possesses three amino acids – Gly-75, Gln-76 and Val-80 – that are only conserved in the switch II region of Rab32 and Rab38 alone and not in any of the other 58 Rabs of mammalian cells (Tamura et al., 2011). Val-80 is required for binding of mammalian Rab32 to its effector VPS9-ankyrin-repeat protein (Varp)/Ankrd27, and this interaction is important for trafficking of tyrosinase-related protein 1 to melanosomes (Tamura et al., 2011). By contrast, TcRab32 [as well as T. thermophila Rab32 (Bright et al., 2010)] has a phenylalanine residue at amino acid position 194 instead the alanine residue in mammalian Rab32 (supplementary material Fig. S1B). This alanine is an anchoring determinant for regulatory subunit IIα (RIIα subunit) of protein kinase A and is responsible for mammalian Rab32 interaction with mitochondria (Alto et al., 2002). In agreement with those studies, we found that TcRab32 does not associate with mitochondria in colocalization experiments using Mitotracker (data not shown). Interestingly, other authors have also been unable to confirm the association of human Rab32 with the mitochondria of COS cells (Hirota and Tanaka, 2009), and T. thermophila Rab32 associates with the phagolysosomal system (Bright et al., 2010).
Rab proteins participate in membrane trafficking events that involve membrane fusion, fission and motility. Although our data do not distinguish between these events, the localization of TcRab32 in the CVC, as well as the deficient morphology and content of acidocalcisomes upon expression of its dominant-negative form, suggests that the CVC acts as an equivalent to the early endosomes of mammalian cells where TcRab32 functions as a tether to facilitate cargo loading into fused vesicles (Bultema and Di Pietro, 2013). The fusion of CVC with acidocalcisomes would facilitate the exchange of membrane proteins between the organelles, such as translocation of TcAQP1 from acidocalcisomes to the CVC (Rohloff et al., 2004), or of membrane enzymes and transporters involved in acidification, as well as Ca2+ transport from the CVC to the acidocalcisomes. In support of this hypothesis, in previous studies we have presented the results of electron microscopy analyses showing the localization of VP1 (Montalvetti et al., 2004) and a Ca2+-ATPase (Lu et al., 1998) in large vacuoles of T. cruzi, which were comparable in the size and the location of the CVC. The fact that acidocalcisomes exhibited deficiencies in the transport of H+ and Ca2+ in epimastigotes expressing dominant-negative GFP–TcRab32 is compatible with deficiencies in the transfer of these proteins to the organelles. Our electron tomography results provide direct evidence of contact of acidocalcisomes with the CVC under osmotic stress. Transient colocalization of VP1 with GFP–TcRab32 under hyposmotic and hyperosmotic stress, and the transfer of TcVAMP7–GFP from acidocalcisomes to the CVC under hyposmotic stress support those results. CVC-located TcRab32 is likely to function as a tether in order to attract acidocalcisomes, facilitating fusion that is mediated by the CVC SNAREs (Ulrich et al., 2011) and the acidocalcisome TcVAMP-7, leading to an exchange of proteins. This model is consistent with that in the mammalian system, where Rab32 effector proteins interact with VAMP7 (a vesicle SNARE protein that is involved in vesicle fusion; Tamura et al., 2011, 2009) and the δ-subunit of the AP-3 complex (Martinez-Arca et al., 2003) (a complex known to be involved in the biogenesis of acidocalcisomes; Besteiro et al., 2008; Huang et al., 2011). Other proteins could be involved in these interactions. For example, myosin heavy chain (myosin VI and myosin VII), which is an actin-based motor, has been detected in proteomic analyses of the CVC of T. cruzi (Ulrich et al., 2011). In humans, myosin VC is an effector of Rab32, and is involved in melanosome biogenesis and in the trafficking of integral membrane proteins to the melanosome (Bultema et al., 2014).
Our results are also in agreement with a role for acidocalcisomes in osmoregulation. Fusion of acidocalcisomes with the contractile vacuole, and the concomitant hydrolysis of polyP has been postulated to lead to an increase in phosphate and cations in the bladder, resulting in water accumulation (Docampo et al., 2013; Rohloff and Docampo, 2008). These changes are likely to be accompanied by the transfer of a phosphate transporter and cation exchangers (Ulrich et al., 2011) from the acidocalcisome membranes, together with TcAQP1 (Rohloff et al., 2004), providing the means for water accumulation (through TcAQP1), as well as for the return of cations (cation exchangers) and phosphate (Pi transporter) to the cytosol after water elimination (Docampo et al., 2013). Our results are consistent with several possible interactions, including unloading of the acidocalcisome content into the CVC, moving proteins from acidocalcisomes to the CVC and remodeling of acidocalcisomes through the transfer of proteins from the CVC to acidocalcisomes.
In conclusion, we propose that the CVC is a trafficking hub that is not only involved in the transfer of GPI-anchored proteins to the plasma membrane (Niyogi et al., 2014) but is also a specialized endosomal system that can be used to deliver membrane proteins that are important for the biogenesis of acidocalcisomes.
MATERIALS AND METHODS
Cell culture
Epimastigotes from T. cruzi were cultured in liver infusion tryptose (LIT) medium containing 10% fetal calf serum at 28°C. T. cruzi epimastigotes that had been transfected with GFP–TcRab32, dominant-negative GFP–TcRab32 or GFP-TcRab32C241A/C243A, as well as GFP–TcVAMP7 and TcVAMP7–GFP, were maintained in the presence of 250 µg/ml geneticin (G418). Human foreskin fibroblasts (HFF) were grown in low glucose Dulbecco’s modified Eagle's medium (DMEM) supplemented with 10% Cosmic Calf Serum (HyClone) and 0.1% l-glutamine. Vero cells were grown in RPMI supplemented with 10% fetal bovine serum. L6E9 myoblasts were grown in high glucose DMEM supplemented with 10% fetal bovine serum. Host cells were maintained at 37°C with 5% CO2. Tissue-culture-derived trypomastigotes were obtained from Vero cells that had been infected with metacyclic trypomastigotes from stationary cultures of parasites that expressed TcGFP, GFP–TcRab32 and dominant-negative GFP–TcRab32. T. cruzi amastigote and trypomastigote forms were collected from the culture medium of infected myoblasts using a modification of the method of Schmatz and Murray (Schmatz and Murray, 1982), as described previously (Moreno et al., 1994). We determined the growth of epimastigotes by measuring the change in optical density at 600 nm in a Gilford spectrophotometer with a starting culture of 4.5×106 epimastigotes.
Chemicals and reagents
Fetal bovine serum, Dulbecco's PBS and Hank's solution, 4′,6-diamidino-2-phenylindole (DAPI), DMEM and RPMI media, paraformaldehyde, bovine serum albumin and protease inhibitors were purchased from Sigma (St Louis, MO). Restriction enzymes were purchased from New England BioLabs (Ipswich, MA). pCR2.1-TOPO cloning kit, 1 kb plus DNA ladder, rabbit antibodies against GFP and Gene Tailor Site-Directed Mutagenesis System were from Invitrogen (Life Technologies, Grand Island, NY). Hybond-N nylon membranes were obtained from PerkinElmer (Waltham, MA). Pierce ECL western blotting substrate and BCA protein assay reagent was from Pierce (Thermo Fisher Scientific, Rockford, IL). All other reagents were analytical grade. The oligonucleotides were ordered from Sigma or Integrated DNA Technologies (Coralville, IA). Vector pET32 Ek/LIC, Benzonase® Nuclease and anti-histidine-tag antibodies were from Novagen (EMD Millipore, Billerica, MA). [1-3H(N)]-farnesyl pyrophosphate, triammonium salt (23.0 Ci/mmol), [1-3H(N)]-geranylgeranyl pyrophosphate, triammonium salt (22.4 Ci/mmol) and EN3HANCE were from Perkin Elmer.
In vitro infection assay
HFF or irradiated myoblasts (6×105 cells per well) were equally distributed in a 12-well plate on a sterile coverslip in their respective growth medium (as mentioned above) and were incubated for 24 h at 37°C under a 5% CO2 atmosphere. The following day, the cells were washed once with Dulbecco's Hanks' solution, and 6×106 wild-type trypomastigotes or trypomastigotes expressing GFP, GFP–TcRab32 or dominant-negative GFP–TcRab32 were added to each well (10 trypomastigotes per myoblast or HFF), and they were incubated for 4 h at 37°C under a 5% CO2 atmosphere. To decrease the chances of contamination of cell-derived-trypomastigotes with extracellular amastigotes, collections of parasites were centrifuged and incubated at 37°C for 2 h to allow trypomastigotes to swim to the surface. The supernatant was collected and used for subsequent invasion assays. Next, the parasites were removed from the plate, and the infected cells were washed extensively with Dulbecco's Hank's solution and fixed for immunofluorescence assays. For attachment and internalization assays, recently internalized parasites, and parasites that were caught in the process of invasion, were included and manually counted in at least 200 DAPI-stained cells in three independent experiments. The percentage of infected cells and the average number of parasites per infected cell were determined.
Immunofluorescence and western blot analyses
For immunofluorescence microscopy, parasites were fixed in PBS, pH 7.4, with 4% paraformaldehyde, adhered to polylysine coverslips and permeabilized for 3 min with PBS, pH 7.4, containing 0.3% Triton X-100. Permeabilized cells were quenched for 30 min at room temperature with 50 mM NH4Cl and blocked overnight with 3% BSA in PBS, pH 8.0. Samples were incubated with both primary and secondary antibodies for 1 h at room temperature. Coverslips were mounted by using mounting medium containing DAPI at 5 µg/ml for staining DNA-containing organelles. For imaging of intracellular parasites, mammalian cells were seeded onto sterile coverslips in 12-well culture plates and allowed to grow for 24 h. To semi-synchronize the infection, we added the parasites at a ratio of 10:1 (parasite:host cell) for 4 h, washed the cells to eliminate extracellular parasites and then fixed them in cold methanol for 30 min. The dilutions used for primary antibodies were as follows: mouse anti-Rab32 (1:200); rabbit polyclonal anti-GFP (1:500); polyclonal rabbit against T. brucei VP1 (1:250) (Lemercier et al., 2002) and monoclonal rabbit against T. cruzi VP1 (1:250). Differential interference contrast (DIC) and direct fluorescence images were obtained by using an Olympus IX-71 inverted fluorescence microscope with a Photometrix CoolSnapHQ charge-coupled device (CCD) camera driven by Delta Vision softWoRx3.5.1 (Applied Precision, Issaquah, WA). Images were deconvolved for ten cycles using the same software and applying the ‘noise filter’ at ‘medium’ mode. This is automatic deconvolution software and was applied to all channels; brightness and contrast were the same in all channels. The figures were composed by using Adobe Photoshop 13.0.5×64 (Adobe System, Inc., San Jose, CA).
BODIPY-ceramide labeling and live cell-imaging
Epimastigotes overexpressing TcVAMP7–GFP were collected by centrifugation in the exponential phase of growth and resuspended at a density of 1×107 parasites/ml in LIT medium containing 5 µM of BODIPY-TR C5 ceramide (Invitrogen). After 1 h of incubation at 28°C, the cells were collected by centrifugation at 1600g and washed twice in PBS, pH 7.4. Labeled epimastigotes were then placed onto glass-bottomed petri dishes that had been previously treated with polylysine. Cells were allowed to settle in the dish for 2 min, and loose parasites were washed away with isosmotic buffer (Jimenez and Docampo, 2012). Live-cell imaging was started in isosmotic conditions at 1 frame/s, and after 30 s hyposmotic buffer was added to the cells to reach a final osmolarity of 117 mosm/l. Cell swelling and recovery was captured for 5 min. Imaging was performed using an Olympus IX-71 inverted fluorescence microscope, as described above.
Generation of dominant-negative and prenylation-motif TcRab32 mutants, and transfection
Dominant-negative (GFP–TcRab32T24N) and prenylation-motif mutant (GFP–TcRab32C241A/C243A) forms of TcRab32 were constructed using site-directed mutagenesis by the use of the Gene Tailor Site-Directed Mutagenesis System. This method involved methylating the TOPO blunt-end vector containing the coding sequence for TcRab32 with DNA methylase at 37°C for 1 h, followed by amplification of the plasmid in a mutagenesis reaction with two overlapping primers, forward, TcRab32T24N Forw: 5′-GTGAGGGAGGCACGGGGAAAAACTG-3′ and reverse, TcRab32T24N Rev: 5′-TTTCCCCGTGCCTCCCTCACCAATGA-3′ (for dominant-negative mutants); and forward, TcRab32 C241A/C243A Forw: 5′-AAGAAAAGTCGGGCGCCTCCGCTTAA-3′ and reverse, TcRab32 C241A/C243A Rev 5′-GGAGCAGCCCGACTTTTCTTCCCGTC-3′ (for prenylation mutants) of which the forward primer had the target mutation, resulting in the mutation of amino acid threonine to asparagine (dominant negative), or cysteine to alanine (prenylation-motif mutant). Mutations were confirmed by sequencing (Yale DNA Analysis Facility, Yale University, New Haven, Connecticut). After transformation, the resulting plasmids in TOPO were digested with restriction enzymes BamHI and HindIII. The circular pTEX-N-GFP vector was linearized by the corresponding restriction enzymes. Finally, TcRab32T24N, and TcRab32C241A/C243A inserts were ligated to pTEX-N-GFP followed by transformation. The plasmids pTEX-GFPTcRab32T24N/C241A/C243A were sequenced to confirm that the correct reading frames were used. T. cruzi Y strain epimastigotes were transfected in cytomix (120 mM KCl, 0.15 mM CaCl2, 10 mM K2HPO4, 2 mM EDTA, 5 mM MgCl2, pH 7.6) containing 50 μg of the plasmid construct in a 4-mm cuvette. The cuvette was cooled on ice for 10 min and pulsed three times (1.5 kV, 25 μF) with a Gene Pulser Xcell™ (Bio-Rad), and expression of GFP-fusion proteins was verified by western blot analyses. Stable cell lines were established under drug selection with G418 at 250 μg/ml. Enrichment of GFP-fluorescent parasites was performed with a high-speed cell sorter when needed (MoFlo Legacy; Beckman-Coulter, Hialeah, FL).
Generation of TcVAMP7-overexpressing parasites
TcVAMP7 tagged at the N-terminal with GFP constructs were generated as previously described (Ulrich et al., 2011). For tagging of the C-terminal with GFP, the TcVAMP7 open reading frame (ORF) was amplified from genomic DNA from CL-strain parasites with primers forward 5′-GAATTCATGGCCATTATATCATCTTTTGTT-3′ and reverse 5′-AAGCTTTTTTTTGCACTTTTTAAAATC-3′. The ORF, flanked by EcoRI and HindIII restriction sites, was cloned into TOPO blunt vector, sequenced and subcloned into pTREX-GFP vector. Transfection of epimastigotes with linearized pTREX-VAMP7-GFP was performed as described above. Parasites were selected with G418 at 250 μg/ml to obtain a stable population overexpressing TcVAMP7–GFP.
Cell volume measurements
T. cruzi epimastigotes (wild type and those expressing GFP–TcRab32 or dominant-negative GFP–TcRab32) at log phase of growth (3 days) were collected at 1600 g for 10 min (at a density of 1×108/ml), and volume measurement experiments after stress were performed exactly as described previously (Li et al., 2011).
Recombinant protein expression, purification and antibody generation
The DNA sequence corresponding to the entire ORF of TcRab32 was PCR-amplified from T. cruzi Y strain gDNA (forward primer: 5′-GACGACGACAAGATGTCATACTCGAA-3′, reverse primer: 5′-GAGGAGAAGCCCGGTTTAACAGGAGCAGCCCGAC-3′) and inserted into vector pET32 Ek/LIC using ligation-independent cloning for heterologous expression in bacteria. The sequence of several recombinant clones was verified, and they were transformed by heat shock into E. coli BL21 Codon Plus (DE3)-RIPL chemically competent cells. Expression of recombinant protein was obtained by induction in 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG) in Luria Bertani (LB) broth overnight at 37°C. His-tagged recombinant protein was purified under denaturing conditions with His-Bind cartridges (Novagen). Recombinant TcRab32 was used as the immunogen for production of polyclonal antibody in mice. This antibody was generated at the Monoclonal Antibody Facility of the College of Veterinary Medicine, University of Georgia (Athens, GA).
In vitro prenylation
In vitro prenylation reactions were performed as described previously (Cuevas et al., 2005) with minor modifications. A total of 2 µCi of [3H]-FPP or [3H]-GGPP was used as the isoprenoid donor. The assay reaction was carried out at 30°C for 30 min, 1 h and 3 h. The optimum reaction time for this assay was 30 min. Products were resolved by using 10% SDS-PAGE. The gel was incubated in EN3HANCE, dried, and exposed to film at −80°C for 2 weeks.
H+ and Ca2+ transport
Ca2+ uptake by digitonin-permeabilized epimastigotes was measured using the fluorescence indicator Calcium Green-5N, as described previously (Huang et al., 2013). Acidification of digitonin-permeabilized cells was followed by measuring the changes in the fluorescence of Acridine Orange in a fluorometer, as described previously (Docampo et al., 1995). Differences in Ca2+ and H+ uptake were evaluated by measuring the rates of Ca2+ or Acridine Orange uptake, respectively, during the first 20 s after uptake initiation (linear rate), and rates are expressed as changes in arbitrary fluorescence units.
Fura-2 measurements
Fura-2 determinations were performed, essentially, as described previously (Docampo et al., 1995). Excitation was at 340 nm and 380 nm, and emission was at 510 nm. The Fura-2 fluorescence response to intracellular Ca2+ concentration was calibrated from the ratio of 340/380 nm fluorescence values after subtraction of 340/380 nm fluorescence of the cells at 340 and 380 nm, as described previously (Grynkiewicz et al., 1985).
Quantification of PPi, and short-chain and long-chain polyP
Cells (2×108) in log phase were harvested and washed twice with buffer A (116 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, 50 mM Hepes, pH 7.2, and 5.5 mM glucose). The PPi and short-chain polyP were extracted using 0.5 M perchloric acid (HClO4) (Ruiz et al., 2001), and the long chain polyP was extracted using glass milk (Molecular Probes) as described previously (Ault-Riche et al., 1998). PPi levels were determined by the amount of Pi released upon treatment with an excess of Saccharomyces cerevisiae inorganic pyrophosphatase (catalog no. I-1891, Sigma). The free Pi (released) amount was determined by using a standard curve. Briefly, the enzymatic reaction was performed on 96-well plates with 50 mM Tris-HCl (pH 7.4), 6 mM MgCl2, inorganic pyrophosphatase and extracted PPi samples at a final volume of 100 µl. After incubation at 30°C for 10 min, the reaction was immediately stopped by the addition of an equal amount of the fresh mixture of three parts of 0.045% malachite green with one part of 4.2% ammonium molybdate (Sigma), which was filtered before use. The absorbance at 660 nm was read using a SpectraMax M2e plate reader (Molecular Devices, Sunnyvale, CA).
Short-chain and long-chain polyP levels were determined by the amount of Pi released upon treatment with an excess of the purified recombinant exopolyphosphatase of S. cerevisiae (rScPPX1) freshly purified in our laboratory (Ruiz et al., 2001)
Short-chain polyP that had been extracted from 5×108 cells was mixed with 6× dye (0.01% Orange G, 30% glycerol, 10 mM Tris-HCl, pH 7.4, 1 mM EDTA) and resolved by using 20% urea-PAGE. Samples were run at 600 V, 6 mA overnight at 4°C until the Orange G had run through two-thirds of the gel. Gels were stained with 0.1% Toluidine Blue.
Transmission electron microscopy
For imaging whole epimastigote forms, cells were washed with filtered buffer A twice, and directly applied to Formvar-coated copper grids, allowed to adhere for 10 min, carefully blotted dry and then observed using a JEM-1210 electron microscope operating at 80 kV. Whole unfixed wild-type epimastigotes and epimastigotes expressing dominant-negative TcRab32 were randomly selected, and the number of acidocalcisome per cell was counted in 70 cells from two different preparations.
T. cruzi epimastigotes expressing GFP–TcRab32, GFP–TcVAMP7 or TcVAMP7–GFP were washed twice in 0.1 M sodium cacodylate buffer, pH 7.4, and fixed for 1 h on ice with 0.1% glutaraldehyde, 4% paraformaldehyde and 0.1 M sodium cacodylate buffer, pH 7.4. Samples were processed for cryo-immunoelectron microscopy at the Molecular Microbiology Imaging Facility, Washington University School of Medicine. GFP-fusion protein localization was detected with a polyclonal antibody against GFP (Invitrogen) and anti-rabbit IgG conjugated to gold as a secondary antibody.
Electron tomography 3D reconstruction
Cells were prepared as previously described (Girard-Dias et al., 2012). Briefly, a pellet of cells was sandwiched between 3×0.5 mm aluminium carries (Bal-Tec, Liechtenstein) or inserted into 2-mm pieces of 200-µm Ø cellulose capillaries and frozen using a Bal-Tec HPM 010 high-pressure freezing machine (Bal-Tec, Liechtenstein). Freeze substitution was performed at −80°C for 72 h in a medium comprising acetone with 2% osmium tetroxide, 0.1% glutaraldehyde and 1% of water, using a Leica EMP apparatus. After substitution, samples were embedded in Epon. For electron tomography analyses, 200-nm sections or ribbons of serial sections were collected in 200 mesh copper grids or onto Formvar-coated slot copper grids and stained. Finally, the sections were coated with 5 nm of carbon and observed under a FEI G2 transmission electron microscope (Tecnai G2, FEI Company, Eindhoven) that was equipped with a 4 k×4 k CCD camera (Eagle, FEI Company, Eindhoven). Tilt series from −65° to +65° with an angular increment of 1° were acquired. Alignments were applied using fiducial markers, and weighted back projections were performed using IMOD software package (University of Colorado). For segmentation and data display, AMIRA (Visage Imaging) and IMOD were used.
Supplementary Material
Acknowledgements
We thank Melina Galizzi (University of Georgia, GA) for help in the preparation of T. cruzi infective stages; Melissa Storey and Melina Galizzi for help in the preparation of the antibody against Rab32; Mary Ard (University of Georgia, GA) for help with transmission electron microscopy; and Wandy L. Beatty (Washington University, St Louis, MO) for the cryo-immunoelectron microscopy.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
S.N., V.J., W.d.S., K.M. and R.D. designed experiments. S.N., V.J., W.G.-D. and K.M. performed experiments; S.N. and R.D. wrote the manuscript. V.J. and K.M. reviewed the manuscript.
Funding
This work was supported by the US National Institutes of Health (NIH) [grant AI107663 to R.D.]. V.J. was supported by the NIH [grant AI101167]. W.G.-D., W.d.S. and K.M. were supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Universal grants 480184/2012-7, 449256/2014-6, INCT em Biologia Estrutural e Bioimagem); Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ-Programa Núcleos Emergentes grant E-26/111.185/2011); Coordenação de Aperfeiçoamento do Pessoal de Nível Superior (CAPES); and Financiadora de Estudos e Projetos (FINEP). Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.169466/-/DC1
References
- Alto N. M., Soderling J. and Scott J. D. (2002). Rab32 is an A-kinase anchoring protein and participates in mitochondrial dynamics. J. Cell Biol. 158, 659-668. 10.1083/jcb.200204081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault-Riche D., Fraley C. D., Tzeng C. M. and Kornberg A. (1998). Novel assay reveals multiple pathways regulating stress-induced accumulations of inorganic polyphosphate in Escherichia coli. J. Bacteriol. 180, 1841-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baqui M. M. A., De Moraes N., Milder R. V. and Pudles J. (2000). A giant phosphoprotein localized at the spongiome region of Crithidia luciliae thermophila. J. Eukaryot. Microbiol. 47, 532-537. 10.1111/j.1550-7408.2000.tb00086.x [DOI] [PubMed] [Google Scholar]
- Beranger F., Paterson H., Powers S., de Gunzburg J. and Hancock J. F. (1994). The effector domain of Rab6, plus a highly hydrophobic C terminus, is required for Golgi apparatus localization. Mol. Cell. Biol. 14, 744-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berriman M., Ghedin E., Hertz-Fowler C., Blandin G., Renauld H., Bartholomeu D. C., Lennard N. J., Caler E., Hamlin N. E., Haas B. et al. (2005). The genome of the African trypanosome Trypanosoma brucei. Science 309, 416-422. 10.1126/science.1112642 [DOI] [PubMed] [Google Scholar]
- Besteiro S., Tonn D., Tetley L., Coombs G. H. and Mottram J. C. (2008). The AP3 adaptor is involved in the transport of membrane proteins to acidocalcisomes of Leishmania. J. Cell Sci. 121, 561-570. 10.1242/jcs.022574 [DOI] [PubMed] [Google Scholar]
- Bright L. J., Kambesis N., Nelson S. B., Jeong B. and Turkewitz A. P. (2010). Comprehensive analysis reveals dynamic and evolutionary plasticity of Rab GTPases and membrane traffic in Tetrahymena thermophila. PLoS Genet. 6, e1001155 10.1371/journal.pgen.1001155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultema J. J. and Di Pietro S. M. (2013). Cell type-specific Rab32 and Rab38 cooperate with the ubiquitous lysosome biogenesis machinery to synthesize specialized lysosome-related organelles. Small GTPases 4, 16-21. 10.4161/sgtp.22349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultema J. J., Ambrosio A. L., Burek C. L. and Di Pietro S. M. (2012). BLOC-2, AP-3, and AP-1 proteins function in concert with Rab38 and Rab32 proteins to mediate protein trafficking to lysosome-related organelles. J. Biol. Chem. 287, 19550-19563. 10.1074/jbc.M112.351908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultema J. J., Boyle J. A., Malenke P. B., Martin F. E., Dell'Angelica E. C., Cheney R. E. and Di Pietro S. M. (2014). Myosin Vc interacts with Rab32 and Rab38 proteins and works in the biogenesis and secretion of melanosomes. J. Biol. Chem. 289, 33513-33528. 10.1074/jbc.M114.578948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark T. B. (1959). Comparative morphology of four genera of trypanosomatidae. J. Protozool. 6, 227-232. 10.1111/j.1550-7408.1959.tb04362.x [DOI] [Google Scholar]
- Cuevas I. C., Rohloff P., Sanchez D. O. and Docampo R. (2005). Characterization of farnesylated protein tyrosine phosphatase TcPRL-1 from Trypanosoma cruzi . Eukaryot. Cell 4, 1550-1561. 10.1128/EC.4.9.1550-1561.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevoye C., Hurbain I., Tenza D., Sibarita J.-B., Uzan-Gafsou S., Ohno H., Geerts W. J. C., Verkleij A. J., Salamero J., Marks M. S. et al. (2009). AP-1 and KIF13A coordinate endosomal sorting and positioning during melanosome biogenesis. J. Cell Biol. 187, 247-264. 10.1083/jcb.200907122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R. and Moreno S. N. J. (2011). Acidocalcisomes. Cell Calcium 50, 113-119. 10.1016/j.ceca.2011.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R., Moreno S. N. J. and Vercesi A. E. (1993). Effect of thapsigargin on calcium homeostasis in Trypanosoma cruzi trypomastigotes and epimastigotes. Mol. Biochem. Parasitol. 59, 305-313. 10.1016/0166-6851(93)90228-P [DOI] [PubMed] [Google Scholar]
- Docampo R., Scott D. A., Vercesi A. E. and Moreno S. N. (1995). Intracellular Ca2+ storage in acidocalcisomes of Trypanosoma cruzi. Biochem. J. 310, 1005-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R., de Souza W., Miranda K., Rohloff P. and Moreno S. N. (2005). Acidocalcisomes - conserved from bacteria to man. Nat. Rev. Microbiol. 3, 251-261. 10.1038/nrmicro1097 [DOI] [PubMed] [Google Scholar]
- Docampo R., Jimenez V., Lander N., Li Z.-H. and Niyogi S. (2013). New insights into roles of acidocalcisomes and contractile vacuole complex in osmoregulation in protists. Int. Rev. Cell Mol. Biol. 305, 69-113. 10.1016/B978-0-12-407695-2.00002-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figarella K., Uzcategui N. L., Zhou Y., LeFurgey A., Ouellette M., Bhattacharjee H. and Mukhopadhyay R. (2007). Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: possible role in volume regulation and osmotaxis. Mol. Microbiol. 65, 1006-1017. 10.1111/j.1365-2958.2007.05845.x [DOI] [PubMed] [Google Scholar]
- Girard-Dias W., Alcântara C. L., Cunha-e-Silva N., de Souza W. and Miranda K. (2012). On the ultrastructural organization of Trypanosoma cruzi using cryopreparation methods and electron tomography. Histochem. Cell Biol. 138, 821-831. 10.1007/s00418-012-1002-8 [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M. and Tsien R. Y. (1985). A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440-3450. [PubMed] [Google Scholar]
- Harris E., Yoshida K., Cardelli J. and Bush J. (2001). Rab11-like GTPase associates with and regulates the structure and function of the contractile vacuole system in dictyostelium. J. Cell Sci. 114, 3035-3045. [DOI] [PubMed] [Google Scholar]
- Hasne M.-P., Coppens I., Soysa R. and Ullman B. (2010). A high-affinity putrescine-cadaverine transporter from Trypanosoma cruzi. Mol. Microbiol. 76, 78-91. 10.1111/j.1365-2958.2010.07081.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J., Zhu Q. and Clarke M. (1993). Proton pumps populate the contractile vacuoles of Dictyostelium amoebae. J. Cell Biol. 121, 1311-1327. 10.1083/jcb.121.6.1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y. and Tanaka Y. (2009). A small GTPase, human Rab32, is required for the formation of autophagic vacuoles under basal conditions. Cell. Mol. Life Sci. 66, 2913-2932. 10.1007/s00018-009-0080-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G., Fang J., Sant'Anna C., Li Z.-H., Wellems D. L., Rohloff P. and Docampo R. (2011). Adaptor protein-3 (AP-3) complex mediates the biogenesis of acidocalcisomes and is essential for growth and virulence of Trypanosoma brucei. J. Biol. Chem. 286, 36619-36630. 10.1074/jbc.M111.284661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G., Vercesi A. E. and Docampo R. (2013). Essential regulation of cell bioenergetics in Trypanosoma brucei by the mitochondrial calcium uniporter. Nat. Commun. 4, 2865 10.1038/ncomms3865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean S. and Kiger A. A. (2012). Coordination between RAB GTPase and phosphoinositide regulation and functions. Nat. Rev. Mol. Cell Biol. 13, 463-470. 10.1038/nrm3379 [DOI] [PubMed] [Google Scholar]
- Jeffries T. R., Morgan G. W. and Field M. C. (2001). A developmentally regulated Rab11 homologue in Trypanosoma brucei is involved in recycling processes. J. Cell Sci. 114, 2617-2626. [DOI] [PubMed] [Google Scholar]
- Jimenez V. and Docampo R. (2012). Molecular and electrophysiological characterization of a novel cation channel of Trypanosoma cruzi. PLoS Pathog. 8, e1002750 10.1371/journal.ppat.1002750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander N., Ulrich P. N. and Docampo R. (2013). Trypanosoma brucei vacuolar transporter chaperone 4 (TbVtc4) is an acidocalcisome polyphosphate kinase required for in vivo infection. J. Biol. Chem. 288, 34205-34216. 10.1074/jbc.M113.518993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemercier G., Dutoya S., Luo S., Ruiz F. A., Rodrigues C. O., Baltz T., Docampo R. and Bakalara N. (2002). A vacuolar-type H+-pyrophosphatase governs maintenance of functional acidocalcisomes and growth of the insect and mammalian forms of Trypanosoma brucei. J. Biol. Chem. 277, 37369-37376. 10.1074/jbc.M204744200 [DOI] [PubMed] [Google Scholar]
- Li Z.-H., Alvarez V. E., De Gaudenzi J. G., Sant'Anna C., Frasch A. C. C., Cazzulo J. J. and Docampo R. (2011). Hyperosmotic stress induces aquaporin-dependent cell shrinkage, polyphosphate synthesis, amino acid accumulation, and global gene expression changes in Trypanosoma cruzi. J. Biol. Chem. 286, 43959-43971. 10.1074/jbc.M111.311530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder J. C. and Staehelin L. A. (1979). A novel model for fluid secretion by the trypanosomatid contractile vacuole apparatus. J. Cell Biol. 83, 371-382. 10.1083/jcb.83.2.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. G., Zhong L., de Souza W., Benchimol M., Moreno S. N. and Docampo R. (1998). Ca2+ content and expression of an acidocalcisomal calcium pump are elevated in intracellular forms of Trypanosoma cruzi. Mol. Cell. Biol. 18, 2309-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini N., Ruiz F. A., Vieira M. and Docampo R. (2002). Acidocalcisomes are functionally linked to the contractile vacuole of Dictyostelium discoideum. J. Biol. Chem. 277, 8146-8153. 10.1074/jbc.M111130200 [DOI] [PubMed] [Google Scholar]
- Martinez-Arca S., Rudge R., Vacca M., Raposo G., Camonis J., Proux-Gillardeaux V., Daviet L., Formstecher E., Hamburger A., Filippini F. et al. (2003). A dual mechanism controlling the localization and function of exocytic v-SNAREs. Proc. Natl. Acad. Sci. USA 100, 9011-9016. 10.1073/pnas.1431910100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza M., Mijares A., Rojas H., Rodrı´guez J. P., Urbina J. A. and DiPolo R. (2002). Physiological and morphological evidences for the presence acidocalcisomes in Trypanosoma evansi: single cell fluorescence and 31P NMR studies. Mol. Biochem. Parasitol. 125, 23-33. 10.1016/S0166-6851(02)00166-4 [DOI] [PubMed] [Google Scholar]
- Moniakis J., Coukell M. B. and Janiec A. (1999). Involvement of the Ca2+-ATPase PAT1 and the contractile vacuole in calcium regulation in Dictyostelium discoideum. J. Cell Sci. 112, 405-414. [DOI] [PubMed] [Google Scholar]
- Montalvetti A., Rohloff P. and Docampo R. (2004). A functional aquaporin co-localizes with the vacuolar proton pyrophosphatase to acidocalcisomes and the contractile vacuole complex of Trypanosoma cruzi. J. Biol. Chem. 279, 38673-38682. 10.1074/jbc.M406304200 [DOI] [PubMed] [Google Scholar]
- Moreno S. N. J., Vercesi A. E., Pignataro O. P. and Docampo R. (1992). Calcium homeostasis in Trypanosoma cruzi amastigotes: presence of inositol phosphates and lack of an inositol 1,4,5-trisphosphate-sensitive calcium pool. Mol. Biochem. Parasitol. 52, 251-261. 10.1016/0166-6851(92)90057-Q [DOI] [PubMed] [Google Scholar]
- Moreno S. N., Silva J., Vercesi A. E. and Docampo R. (1994). Cytosolic-free calcium elevation in Trypanosoma cruzi is required for cell invasion. J. Exp. Med. 180, 1535-1540. 10.1084/jem.180.4.1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Sanchez D., Hernandez-Ruiz L., Ruiz F. A. and Docampo R. (2012). Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes. J. Biol. Chem. 287, 28435-28444. 10.1074/jbc.M112.385823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. (2002). Organelle identity and the targeting of peripheral membrane proteins. Curr. Opin. Cell Biol. 14, 506-514. 10.1016/S0955-0674(02)00350-2 [DOI] [PubMed] [Google Scholar]
- Nepomuceno-Silva J. L., Yokoyama K., de Mello L. D. B., Mendonca S. M., Paixao J. C., Baron R., Faye J.-C., Buckner F. S., Van Voorhis W. C., Gelb M. H. et al. (2001). TcRho1, a farnesylated Rho family homologue from Trypanosoma cruzi: cloning, trans-splicing, and prenylation studies. J. Biol. Chem. 276, 29711-29718. 10.1074/jbc.M102920200 [DOI] [PubMed] [Google Scholar]
- Niyogi S., Mucci J., Campetella O. and Docampo R. (2014). Rab11 regulates trafficking of trans-sialidase to the plasma membrane through the contractile vacuole complex of Trypanosoma cruzi. PLoS Pathog. 10, e1004224 10.1371/journal.ppat.1004224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M., Serpinskaya A. S., Papalopulu N. and Gelfand V. I. (2007). Rab32 regulates melanosome transport in Xenopus melanophores by protein kinase a recruitment. Curr. Biol. 17, 2030-2034. 10.1016/j.cub.2007.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R. (2001). Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11, 487-491. 10.1016/S0962-8924(01)02147-X [DOI] [PubMed] [Google Scholar]
- Rak A., Pylypenko O., Durek T., Watzke A., Kushnir S., Brunsveld L., Waldmann H., Goody R. S. and Alexandrov K. (2003). Structure of Rab GDP-dissociation inhibitor in complex with prenylated YPT1 GTPase. Science 302, 646-650. 10.1126/science.1087761 [DOI] [PubMed] [Google Scholar]
- Raposo G. and Marks M. S. (2007). Melanosomes--dark organelles enlighten endosomal membrane transport. Nat. Rev. Mol. Cell Biol. 8, 786-797. 10.1038/nrm2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohloff P. and Docampo R. (2008). A contractile vacuole complex is involved in osmoregulation in Trypanosoma cruzi. Exp. Parasitol. 118, 17-24. 10.1016/j.exppara.2007.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohloff P., Montalvetti A. and Docampo R. (2004). Acidocalcisomes and the contractile vacuole complex are involved in osmoregulation in Trypanosoma cruzi . J Biol. Chem. 279, 52270-52281. 10.1074/jbc.M410372200 [DOI] [PubMed] [Google Scholar]
- Ruiz F. A., Rodrigues C. O. and Docampo R. (2001). Rapid changes in polyphosphate content within acidocalcisomes in response to cell growth, differentiation, and environmental stress in Trypanosoma cruzi. J. Biol. Chem. 276, 26114-26121. 10.1074/jbc.M102402200 [DOI] [PubMed] [Google Scholar]
- Ruiz F. A., Lea C. R., Oldfield E. and Docampo R. (2004). Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J. Biol. Chem. 279, 44250-44257. 10.1074/jbc.M406261200 [DOI] [PubMed] [Google Scholar]
- Schmatz D. M. and Murray P. K. (1982). Cultivation of Trypanosoma cruzi in irradiated muscle cells: improved synchronization and enhanced trypomastigote production. Parasitology 85, 115-125. 10.1017/S0031182000054202 [DOI] [PubMed] [Google Scholar]
- Seabra M. C. and Wasmeier C. (2004). Controlling the location and activation of Rab GTPases. Curr. Opin. Cell Biol. 16, 451-457. 10.1016/j.ceb.2004.06.014 [DOI] [PubMed] [Google Scholar]
- Sesaki H., Wong E. F. S. and Siu C.-H. (1997). The cell adhesion molecule DdCAD-1 in Dictyostelium is targeted to the cell surface by a nonclassical transport pathway involving contractile vacuoles. J. Cell Biol. 138, 939-951. 10.1083/jcb.138.4.939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. A., Mutch N. J., Baskar D., Rohloff P., Docampo R. and Morrissey J. H. (2006). Polyphosphate modulates blood coagulation and fibrinolysis. Proc. Natl. Acad. Sci. USA 103, 903-908. 10.1073/pnas.0507195103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriskanthadevan S., Lee T., Lin Z., Yang D. and Siu C.-H. (2009). Cell adhesion molecule DdCAD-1 is imported into contractile vacuoles by membrane invagination in a Ca2+- and conformation-dependent manner. J. Biol. Chem. 284, 36377-36386. 10.1074/jbc.M109.057257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Ohbayashi N., Maruta Y., Kanno E., Itoh T. and Fukuda M. (2009). Varp is a novel Rab32/38-binding protein that regulates Tyrp1 trafficking in melanocytes. Mol. Biol. Cell 20, 2900-2908. 10.1091/mbc.E08-12-1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Ohbayashi N., Ishibashi K. and Fukuda M. (2011). Structure-function analysis of VPS9-ankyrin-repeat protein (Varp) in the trafficking of tyrosinase-related protein 1 in melanocytes. J. Biol. Chem. 286, 7507-7521. 10.1074/jbc.M110.191205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theos A. C., Tenza D., Martina J. A., Hurbain I., Peden A. A., Sviderskaya E. V., Stewart A., Robinson M. S., Bennett D. C., Cutler D. F. et al. (2005). Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol. Biol. Cell 16, 5356-5372. 10.1091/mbc.E05-07-0626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkewitz A. P. and Bright L. J. (2011). A Rab-based view of membrane traffic in the ciliate Tetrahymena thermophila. Small GTPases 2, 222-226. 10.4161/sgtp.2.4.16706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich P. N., Jimenez V., Park M., Martins V. P., Atwood J. III, Moles K., Collins D., Rohloff P., Tarleton R., Moreno S. N. J. et al. (2011). Identification of contractile vacuole proteins in Trypanosoma cruzi. PLoS ONE 6, e18013 10.1371/journal.pone.0018013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich P. N., Lander N., Kurup S. P., Reiss L., Brewer J., Soares Medeiros L. C., Miranda K. and Docampo R. (2014). The acidocalcisome vacuolar transporter chaperone 4 catalyzes the synthesis of polyphosphate in insect-stages of Trypanosoma brucei and T. cruzi. J. Eukaryot. Microbiol. 61, 155-165. 10.1111/jeu.12093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbina J. A., Moreno B., Vierkotter S., Oldfield E., Payares G., Sanoja C., Bailey B. N., Yan W., Scott D. A., Moreno S. N. J. et al. (1999). Trypanosoma cruzi contains major pyrophosphate stores, and its growth in vitro and in vivo is blocked by pyrophosphate analogs. J. Biol. Chem. 274, 33609-33615. 10.1074/jbc.274.47.33609 [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Gillespie J. R., Van Voorhis W. C., Buckner F. S. and Gelb M. H. (2008). Protein geranylgeranyltransferase-I of Trypanosoma cruzi. Mol. Biochem. Parasitol. 157, 32-43. 10.1016/j.molbiopara.2007.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M. and McBride H. (2001). Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2, 107-117. 10.1038/35052055 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.