Abstract
Malignant neoplasia represents the second cause of disease-related mortality and, among all patients diagnosed with cancer, 70% will receive chemotherapy during the course of treatment. As a consequence, an increasing number of researchers have focused their attention on the search for more specific anticancer therapies associated with fewer side effects. Leukopenia is an important adverse effect associated with chemotherapy. Secondary infection is very common among leukopenic patients, directly affecting the continuity of the chemotherapeutic treatment and leading to possible complications in tumor immune defense. Atorvastatin, a type of statin, is a known agent used to control hypercholesterolemia. Trans-caryophyllene, isolated from a resinous oil extracted from the copaiba tree, possesses anti-inflammatory and analgesic properties. The AIM of the present study was to evaluate, through a complete leukocyte count, the systemic immunomodulation potential of pentoxifylline (PTX), atorvastatin and trans-caryophyllene, as well as the possible prophylactic role of these drugs against secondary leukopenia, in an experimental chemotherapy model induced by 5-fluorouracil (5-FU) in wistar rats. A total of 32 male wistar rats were used, 24 of which were submitted to treatment with atorvastatin, PTX and trans-caryophyllene prior to the administration of chemotherapy. The Shapiro-Wilk test was used to verify normality and the Kruskal-Wallis test was used for negative data in the normality test. Among the drugs selected, atorvastatin exhibited the best preventive potential in regards to leukopenia secondary to experimental chemotherapy induced by 5-FU, in comparison to the group receiving saline solution, while PTX amplified such alterations in the leukograms of the animals in this trial.
Keywords: chemotherapy, leukopenia, oral mucositis, atorvastatin, trans-caryophyllene
Introduction
Malignant neoplasia represents the second cause of disease-related mortality worldwide and, among all patients diagnosed with cancer, 70% will receive chemotherapy during the course of treatment (1–3). As a consequence, an increasing number of researchers in several countries have focused their attention on the search for more specific anticancer therapies associated with fewer side effects (3,4).
Among the choices of chemotherapy, methotrexate and 5-fluorouracil (5-FU) are considered as safe drugs, with effective antimitotic activity, although they act on malignant tumor cells as well as normal cells exhibiting unstable mitotic activity, such as several glandular epithelial and lining cells, as well as hematopoietic cells originating in the bone marrow (4).
Leukopenia is a significant adverse effect observed during chemotherapy. Secondary infection is very commom among leukopenic patients, directly affecting the continuity of the chemotherapeutic treatment and leading to possible complications in tumor immune defense (5). Several authors consider leukopenia secondary to chemotherapy as one of the main causes of the anticancer treatment discontinuation, emphasizing the need to monitor the immune competence of the patient through a complete blood count (CBC) during the course of treatment (3,4,6,7).
Chemotherapy is the standard treatment for cancer and leukopenia is the most common side effect, which is directly associated with patient survival (8). Consequently, the oncological experimental research has allowed the establishment of more effective therapeutic protocols, as well as the modulation of biological reactions and its effects on improved clinical results, combined with fewer side effects (9,10).
Among all anticancer drugs, 5-FU has been the primary drug of choice in experimental chemotherapy models, due to its wide clinical application and low cost (1,10,11).
In an attempt to reduce the systemic and local negative effects of chemotherapy, several drugs have been proposed in the literature, the majority of which are immune-modulatory, with the purpose of controlling the development of detrimental side effects throughout the course of treatment (12–14).
Pentoxifylline (PTX) was introduced in the 1990s as a prophylactic drug used for the prevention of thrombosis, as it reduces platelet aggregation and the plasma concentrations of fibrinogen, optimizing the perfusion of the microvasculature (15). Platzer et al (16) and Fujimoto et al (17) also investigated the anti-inflammatory effects of PTX and suggested that PTX inhibits the migration and activation of human lymphocytes and is able to modulate the reactions mediated by T lymphocytes. Of note, PTX is well tolerated at high dosages, with no severe side effects (18).
Atorvastatin, a type of statin, synthetically derived from the fermentation of Aspergillus terreus, is a known agent used for the control of hypercholesterolemia (19–21). In addition to affecting the metabolism of cholesterol, its anti-inflammatory properties have been associated with its ability to modulate the levels of C-reactive protein and nitric oxide by upregulating the expression of nitric oxide synthase (21–23).
Trans-caryophyllene, isolated from a resinous oil extracted from the copaiba tree, has anti-inflammatory and analgesic properties due to its direct vascular action, modulating capillary permeability and blood perfusion (24–27).
The AIM of the present study was to evaluate, through a complete leukocyte count, the systemic immunomodulation potential of PTX, atorvastatin and trans-caryophyllene, as well as the possible prophylactic role of these drugs against secondary leukopenia, in an experimental chemotherapy model induced by 5-FU in wistar rats.
Materials and methods
Animals
A total of 32 male wistar rats were used, with an average age of 7 weeks and average body weight of 220 g. The entire experiment was performed at the Laboratory of Immunopathology and Experimental Pathology, Center of Reproductive Biology, Federal University of Juiz de Fora (MG, Brazil) and was approved by the Animal Research Committee (notion no. 062/2011). The animals were kept in the bioterium in pairs, in cages quilted with shavings, at room temperature and controlled lighting, with ad libitum access to water and feed.
Prophylaxis against leukopenia secondary to experimental chemotherapy with 5-FU
Of the 32 animals used in the experiment, 24 were submitted to treatment with atorvastatin, PTX and trans-caryophyllene prior to the administration of chemotherapy and, subsequently, were randomly divided into the following experimental groups: Group II, animals treated with trans-caryophyllene (n=8); Group III, animals treated with PTX (n=8); and Group IV, animals treated with atorvastatin (n=8).
A control group was also established and comprised animals submitted to experimental chemotherapy priorly treated with saline solution alone (n=8) (Group I).
Trans-caryophyllene was administered to the animals of group II at a dosage of 50 mg/kg/day (24,28) through force-feeding, starting 3 days prior to the administration of 5-FU and for 3 days after the last administration of 5-FU, totalling 9 days of treatment.
PTX was administered to the animals of group III over 15 continuous days prior to the administration of chemotherapy by intraperitoneal injection, dissolved in sterile saline solution to a concentration of 150 mg/ml/day for a total of 18 days of treatment (4,29).
The animals of group IV received 10 mg/kg/day of micro-emulsion of atorvastatin by intraperitoneal injection, initiated 1 week prior to the treatment with 5-FU and during the entire course of treatment, totalling 10 days of medication (19).
The control group (group I) received 0.9% saline solution by peritoneal injections, following the same protocol of the animals on group III.
Experimental chemotherapy treatment
The animals received two intraperitoneal administrations of 5-FU (Eurofarma, São Paulo, SP, Brazil), at the distinct dosages of 100 mg/kg (1st day) and 60 mg/kg (2 days after the first application) (30).
Leukocyte count
The animals (n=32) were euthanized with an overdose of anesthetic (100 mg/kg ketamine and 10 mg/kg xylazine) and their blood was obtained through cardiac puncture. Following collection, the blood was transferred to tubes containing the anticoagulant K2-EDTA.
The global leukocyte count was performed with a Neubauer chamber and the pocH-100iv Automated Hematology Analyzer (Sysmex America, Inc., Lincolnshire, IL, USA) at the Laboratory of Immunopathology and Clinical Immunology (UFJF), at the Center of Reproductive Biology, Federal University of Juiz de Fora, MG, Brazil.
Turk's fluid was used to dilute the blood (standard, 1/20). Following dilution and homogenization, the space between the chamber and the coverslip was carefully filled out to avoid bubble formation.
The leukocyte count was performed under a Bioval binocular microscope, model L2000 A (Bioval, Tocantins, Brazil) at a magnification of x400 at the lateral sets of four quadrants of the Neubauer chamber and the results were expressed in global quantification of leukocytes per MM3 of blood.
Statistical analysis of the data
The Shapiro-Wilk test was used to verify normality and the Kruskal-Wallis test was used for negative data in the normality test. The analysis was performed using GraphPad Prism 5® software (GraphPad Software, Inc., San Diego, CA, USA).
Results
Leukocyte count normality standard
The normality standard of the leukocyte count for wistar rats of average age and weight compatible with the sample, raised and maintained at the bioterium used in this research, corresponds to an average of 10,840 leukocytes/mm3. Of note, the normal value refers to the animal's colony of origin; therefore, not only this global value of the colony must be considered for the analysis of data, but also the reference value of the control group of the experiment (31).
Leukocyte count values
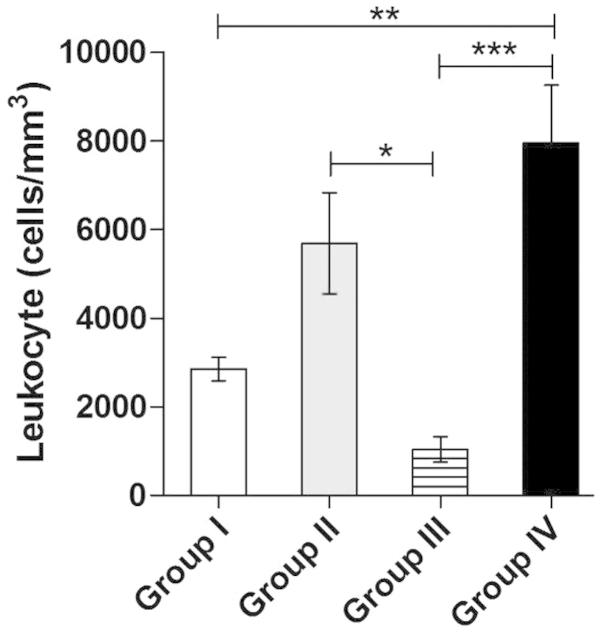
The following values (mean ± standard deviation) in terms of total leukocyte count were observed in the experimental samples: Group I, 2,854±921.8/MM3; group II, 5,688±2,284/MM3; group III, 1,042±694.6/MM3; and group IV, 7,944±3,707/MM3. As regards the normal reference leukocyte count of the colony, the number of leukocytes/mm3 decreased by 73.67% (group I, control), 47.52% (group II, trans-caryophyllene), 90.38% (group III, PTX) and 26.72% (group IV, atorvastatin). The results are presented in Fig. 1.
Figure 1.
The total leukocyte count of group I (control; animals submitted to experimental chemotherapy and treated prophylactically with saline solution) was 2,854±921.8/MM3, of group II (animals submitted to experimental chemotherapy and treated prophylactically with trans-caryophyllene), 5,688±2,284/MM3, of group III (animals submitted to experimental chemotherapy and treated prophylactically with pentoxifylline) 1,042±694.6/MM3 and of group IV (animals submitted to experimental chemotherapy and treated prophylactically with atorvastatin) 7,944±3,707/MM3. The results are expressed as mean ± standard deviation. *P<0.05; **P<0.01; ***P<0.001.
The results demonstrated that the animals of the control group (submitted to experimental chemotherapy and priorly treated with saline solution alone) and of group III (treated prophylactically with PTX) developed more severe leukopenia compared to the other groups; in addition, the group treated with PTX developed more severe leukopenia compared to the control group.
The animals of group II (treated with trans-caryophyllene) did not exhibit a significantly improved leukocyte count compared to that of the control group. However, the animals of group IV (treated prophylactically with atorvastatin) exhibited only a minor decrease in the number of leukocytes, compared to the normal colony parameters (Fig. 1).
Discussion
PTX has been described in the literature as a TNF-α inhibitor in several pathological conditions, in experimental and clinical studies (32). TNF-α was first described as a protein named cachexin, which was associated with weight loss and other complications observed during the establishment of a cachexia state. In the present study, the animals administered 5-FU and preventively treated with PTX exhibited an exacerbation of the leukopenia.
The secondary infection that developed in this group during the experiment was likely a consequence of the leukopenia associated with PTX administration. The blending episodes of leukopenia and secondary infection explain the deterioration in the overall condition of the rats. In addition, PTX-treated rats presented with blending episodes of epistaxis, secondary skin infections and aggravation of systemic symptoms.
The improvement in leukopenia associated with the preventive use of trans-caryophyllene in group II may be explained by its anti-inflammatory properties, mediated by acting directly on blood vessels, modulating capillary permeability. Trans-caryophyllene has been reported to possess a number of pharmacological properties, including antimicrobial and analgesic properties; additionally, it acts on intestinal smooth muscle, blocking the electromechanical and pharmacomechanical excitation-contraction coupling. Due to these activities, trans-caryophyllene is considered as a potential antispasmodic agent in tracheal smooth muscle (33). Therefore, further studies should be conducted to investigate the novel therapeutic possibilities of this molecule. As an analgesic and anti-inflammatory medication, trans-caryophyllene may improve the quality of life of patients undergoing chemotherapy.
Atorvastatin administration significantly prevented the loss of leukocytes compared to the other groups in this experiment at several relevance levels; therefore, this drug demonstrated the best potential regarding the control of leukopenia in animals submitted to experimental chemotherapy.
Similar results were reported by Medeiros et al (34), who conducted a study in an experimental hamster model with the purpose of investigating the occurrence of ulcerated lesions in the buccal mucosa and its asociation with leukocyte count in groups treated with different dosage and treatment duration. The animals were administered 5-FU (on the first and second days, at dosages of 60 and 40 mg/kg, respectively) and treated with atorvastatin at a dosage of 1, 5 and 10 mg/kg, administered 30 min prior to the daily dose of the 5-FU for 5 or 10 days. The results demonstrated that atorvastatin at a dosage of 5 mg/kg amplified the leukopenia (34).
Atorvastatin, initially used as a modulator of hypercholesterolemia, has been recently associated with different functions, such as protection against microvascular damage, antifibrotic and anticalcifying effects; in addition, it exhibits antioxidant properties if administered in high doses for the pretreatment of lesions of the myocardium in heart failure, renal disease and abdominal surgery (35–37). These functions are due to the effect of this drug on populations of mononuclear cells, for example the subpopulations of TCD4+ lymphocytes in reciprocity with or independent from the population of cells of the mononuclear system, such as tissue macrophages, Kupffer cells in the liver, microglial cells in the central nervous system and mucosal dendritic cells (38–40). Furthermore, extremely relevant to the chemotherapy model, atorvastatin was suggested by Su et al (38) as a possible anti-apoptotic drug for renal epithelial cells.
Finally, the results of this study suggest that trans-caryophyllene and atorvastatin may be able to prevent leukopenia. Atorvastatin is a drug commonly used for several human pathological conditions. Therefore, this experimental study may provide a basis for further experimental studies and clinical trials investigating the potential of atorvastatin in preventing leukopenia during chemotherapy in cancer patients.
In conclusion, among the selected drugs, atorvastatin exhibited the best preventive potential in regards to leukopenia secondary to experimental chemotherapy with 5-FU, compared to the group treated with saline solution, while PTX amplified such alterations in the leukograms of the animals in this trial.
Acknowledgements
The authors would like to thank Akinory Nagato, for providing guidance with the statistical methodology.
References
- 1.McCarthy GM, Awde JD, Ghandi H, Vincent M, Kocha WI. Risk factors associated with mucositis in cancer patients receiving 5-fluorouracil. Oral Oncol. 1998;34:484–490. doi: 10.1016/S1368-8375(98)00068-2. [DOI] [PubMed] [Google Scholar]
- 2.Schirmer EM, Ferrari A, Trindade LCT. Oral mucositis evolution after nutritional intervention in cancer patients under palliative care. Rev Dor. 2012;13:141–146. doi: 10.1590/S1806-00132012000200009. [DOI] [Google Scholar]
- 3.Sonis ST. New thoughts on the initiation of mucositis. Oral Dis. 2010;16:597–600. doi: 10.1111/j.1601-0825.2010.01681.x. [DOI] [PubMed] [Google Scholar]
- 4.Lima V, Vidal FD, Rocha FA, Brito GA, Ribeiro RA. Effects of tumor necrosis factor-alpha inhibitors pentoxifylline and thalidomide on alveolar bone loss in short-term experimental periodontal disease in rats. J Periodontol. 2004;75:162–168. doi: 10.1902/jop.2004.75.1.162. [DOI] [PubMed] [Google Scholar]
- 5.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 6.Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am. 2008;52(viii):61–77. doi: 10.1016/j.cden.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonis ST. Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol. 1998;34:39–43. doi: 10.1016/S1368-8375(97)00053-5. [DOI] [PubMed] [Google Scholar]
- 8.Liu W, Zhang CC, Li K. Prognostic value of chemotherapy-induced leukopenia in small-cell lung cancer. Cancer Biol Med. 2013;10:92–98. doi: 10.7497/j.issn.2095-3941.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4:277–284. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 10.Stringer AM. Interaction between host cells and microbes in chemotherapy-induced mucositis. Nutrients. 2013;5:1488–1499. doi: 10.3390/nu5051488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinedo HM, Peters GF. Fluorouracil: biochemistry and pharmacology. J Clin Oncol. 1988;6:1653–1664. doi: 10.1200/JCO.1988.6.10.1653. [DOI] [PubMed] [Google Scholar]
- 12.Alvarino-Martin C, Sarrion-Perez MG. Prevention and treatment of oral mucositis in patients receiving chemotherapy. J Clin Exp Dent. 2014;6:e74–e80. doi: 10.4317/jced.51313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owlia F, Kazemeini SK, Gholami N. Prevention and management of mucositis in patients with cancer: a review article. Iranian J Cancer Prevent. 2012;5:216–220. [PMC free article] [PubMed] [Google Scholar]
- 14.Sonis S, Clark J. Prevention and management of oral mucositis induced by antineoplastic therapy. Oncology (Williston Park) 1991;5:11–18. discussion 18–22. [PubMed] [Google Scholar]
- 15.Sampaio EP, Moraes MO, Nery JA, Santos AR, Matos HC, Sarno EN. Pentoxifylline decreases in vivo and in vitro tumour necrosis factor-alpha (TNF-alpha) production in lepromatous leprosy patients with erythema nodosum leprosum (ENL) Clin Exp Immunol. 1998;111:300–308. doi: 10.1046/j.1365-2249.1998.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platzer C, Meisel C, Vogt K, Platzer M, Volk HD. Up-regulation of monocytic IL-10 by tumor necrosis factor-alpha and cAMP elevating drugs. Int Immunol. 1995;7:517–523. doi: 10.1093/intimm/7.4.517. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto T, Nakamura T, Furuya T, et al. Relationship between the clinical efficacy of pentoxifylline treatment and elevation of serum T helper type 2 cytokine levels in patients with human T-lymphotropic virus type I-associated myelopathy. Intern Med. 1999;38:717–721. doi: 10.2169/internalmedicine.38.717. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Amaro R, Portales-Perez D, Baranda L, et al. Pentoxifylline inhibits adhesion and activation of human T lymphocytes. J Immunol. 1998;161:65–72. [PubMed] [Google Scholar]
- 19.Azevedo AM, Kumakura HS, Alloufa SL, et al. Effect of simvastatin in attenuation of mucositis induced by methotrexate in rats. J Surg Clin Res. 2010;1:22–32. [Google Scholar]
- 20.Lea AP, McTavish D. Atorvastatin. A review of its pharmacology and therapeutic potential in the management of hyperlipidaemias. Drugs. 1997;53:828–847. doi: 10.2165/00003495-199753050-00011. [DOI] [PubMed] [Google Scholar]
- 21.Veillard NR, Mach F. Statins: the new aspirin? Cell Mol Life Sci. 2002;59:1771–1786. doi: 10.1007/PL00012505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponomarev ED, Maresz K, Tan Y, Dittel BN. CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. J Neurosci. 2007;27:10714–10721. doi: 10.1523/JNEUROSCI.1922-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 24.Dias DS, Fontes LB, Crotti AE, et al. Copaiba oil suppresses inflammatory cytokines in splenocytes of C57Bl/6 mice induced with experimental autoimmune encephalomyelitis (EAE) Molecules. 2014;19:12814–12826. doi: 10.3390/molecules190812814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leandro LM, Vargas Fde S, Barbosa PC, Neves JK, da Silva JA, da Veiga-Junior VF. Chemistry and biological activities of terpenoids from copaiba (Copaifera spp.) oleoresins. Molecules. 2012;17:3866–3889. doi: 10.3390/molecules17043866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vilanova C, Ribeiro SM, Machado R, et al. Evaluation of oil-resin activity of Copaifera sp. on gastric emptying in Rattus novergicus. Emirates J Food Agriculture. 2013;25:394–397. doi: 10.9755/ejfa.v25i5.15510. [DOI] [Google Scholar]
- 27.Dias DS, Fontes LBA, Crotti AEM, et al. Copaiba Oil Suppresses Inflammatory Cytokines in Splenocytes of C57Bl/6 Mice Induced with Experimental Autoimmune Encephalomyelitis (EAE) Molecules. 2014;19:12814–12826. doi: 10.3390/molecules190812814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandes ES, Passos GF, Medeiros R, et al. Anti-inflammatory effects of compounds alpha-humulene and (–)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur J Pharmacol. 2007;569:228–236. doi: 10.1016/j.ejphar.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 29.Zargari O. Pentoxifylline: a drug with wide spectrum applications in dermatology. Dermatol Online J. 2008;14:2. [PubMed] [Google Scholar]
- 30.Sonis ST, Tracey C, Shklar G, Jenson J, Florine D. An animal model for mucositis induced by cancer chemotherapy. Oral Surg Oral Med Oral Pathol. 1990;69:437–443. doi: 10.1016/0030-4220(90)90376-4. [DOI] [PubMed] [Google Scholar]
- 31.Lillie LE, Temple NJ, Florence LZ. Reference values for young normal Sprague-Dawley rats: weight gain, hematology and clinical chemistry. Hum Exp Toxicol. 1996;15:612–616. doi: 10.1177/096032719601500802. [DOI] [PubMed] [Google Scholar]
- 32.Correa JO, Aarestrup BJ, Aarestrup FM. Effect of thalidomide and pentoxifylline on experimental autoimmune encephalomyelitis (EAE) Exp Neurol. 2010;226:15–23. doi: 10.1016/j.expneurol.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Pinho-da-Silva L, Mendes-Maia PV, Teofilo TM, et al. trans-Caryophyllene, a natural sesquiterpene, causes tracheal smooth muscle relaxation through blockade of voltage-dependent Ca2+ channels. Molecules. 2012;17:11965–11977. doi: 10.3390/molecules171011965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medeiros CA, Leitao RF, Macedo RN, et al. Effect of atorvastatin on 5-fluorouracil-induced experimental oral mucositis. Cancer Chemother Pharmacol. 2011;67:1085–1100. doi: 10.1007/s00280-010-1409-7. [DOI] [PubMed] [Google Scholar]
- 35.He GX, Tan W. High-dose atorvastatin pretreatment could diminish microvascular impairment in patients undergoing elective percutaneous coronary intervention. J Geriatr Cardiol. 2013;10:355–360. doi: 10.3969/j.issn.1671-5411.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kose E, An T, Kikkawa A, Matsumoto Y, Hayashi H. Effects on serum uric acid by difference of the renal protective effects with atorvastatin and rosuvastatin in chronic kidney disease patients. Biol Pharm Bull. 2014;37:226–231. doi: 10.1248/bpb.b13-00418. [DOI] [PubMed] [Google Scholar]
- 37.Liu D, Cui W, Liu B, et al. Atorvastatin protects vascular smooth muscle cells from TGF-β1-stimulated calcification by inducing autophagy via suppression of the β-catenin pathway. Cell Physiol Biochem. 2014;33:129–141. doi: 10.1159/000356656. [DOI] [PubMed] [Google Scholar]
- 38.Su Q, Li L, Liu Y, Zhou Y, Wang J, Sun Y. Effect of intensive atorvastatin therapy on periprocedural PDCD4 expression in CD4+ T lymphocytes of patients with unstable angina undergoing percutaneous coronary intervention. Cardiology. 2014;127:169–175. doi: 10.1159/000356434. [DOI] [PubMed] [Google Scholar]
- 39.Wang W, Song W, Wang Y, Chen L, Yan X. HMG-CoA reductase inhibitors, simvastatin and atorvastatin, downregulate ABCG1-mediated cholesterol efflux in human macrophages. J Cardiovasc Pharmacol. 2013;62:90–98. doi: 10.1097/FJC.0b013e3182927e7c. [DOI] [PubMed] [Google Scholar]
- 40.Weber MS, Prod'homme T, Youssef S, Dunn SE, Steinman L, Zamvil SS. Neither T-helper type 2 nor Foxp3+ regulatory T cells are necessary for therapeutic benefit of atorvastatin in treatment of central nervous system autoimmunity. J Neuroinflammation. 2014;11:29. doi: 10.1186/1742-2094-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]