Abstract
Sorafenib demonstrated a survival benefit in the treatment of advanced hepatocellular carcinoma (HCC) in phase III trials. However, almost all the patients included in those trials exhibited well-preserved liver function (Child-Pugh A). The aim of this study was to describe our experience with sorafenib in Child-Pugh B HCC patients. A database of patients with advanced HCC treated with sorafenib was retrospectively evaluated. The median overall survival of Child-Pugh B patients (n=20) was 2.53 months [95% confidence interval (CI): 0.33–5.92 months] and of Child-Pugh A patients (n=100) 9.71 months (95% CI: 6.22–13.04). Child-Pugh B patients had a significantly poorer survival compared to Child-Pugh A patients (P=0.002). The toxicities were similar between the two groups. Metastasis, vascular invasion and α-fetoprotein level >1,030 ng/ml were not associated with survival among Child-Pugh B patients (P=0.281, 0.189 and 0.996, respectively). Although the survival outcomes were worse in Child-Pugh B patients treated with sorafenib, the toxicity profile was manageable. Therefore, there remains the question of whether to treat this subgroup of patients and more data are required to define the role of sorafenib in the context of liver dysfunction.
Keywords: hepatocellular carcinoma, liver cirrhosis, sorafenib, liver disease
Introduction
The natural history of hepatocellular carcinoma (HCC), the fifth most common malignancy worldwide, is clearly associated with liver function and the stage of the underlying liver disease (1). It is estimated that ~80% of all HCC patients also have cirrhosis and a number of them experience lethal complications from cirrhosis prior to cancer progression (2). According to previously published data, the median survival of patients with untreated HCC is ~2.5 times lower in patients with liver dysfunction compared with that in patients with well-preserved liver function (3).
HCC is diagnosed at an advanced stage in 50% of the cases and the systemic therapy options in this setting are limited (4). Sorafenib is an oral multitarget tyrosine kinase inhibitor directed against vascular endothelial growth factor receptor-1, −2 and −3, platelet-derived growth factor receptor-β, c-KIT, RET, FLT-3 and RAF. This agent proved to offer a survival benefit based on the results of two placebo-controlled randomized trials (5,6). In the SHARP trial, sorafenib 400 mg twice daily significantly prolonged overall survival (OS) from 7.9 to 10.7 months (5). The Asia-Pacific trial, in the Eastern population, also demonstrated an improvement in median OS in favor of sorafenib (6.5 vs. 4.2 months) (6). However, those trials conducted a rigorous patient selection. In the SHARP and Asia-Pacific trials, 95 and 97.3% of the patients, respectively, exhibited well-preserved liver function (Child-Pugh A) (5,6).
Considering the strict patient selection in the aforementioned trials, the safety and efficacy of sorafenib in patients with Child-Pugh B cirrhosis have not yet been clearly determined.
In a phase II study evaluating sorafenib for HCC, 28% of the patients had Child-Pugh B cirrhosis. The median OS for Child-Pugh A patients was 41 weeks and for Child-Pugh B patients 14 weeks (7). Several studies also demonstrated that Child-Pugh B patients fare worse compared with Child-Pugh A patients and also present with worsening of cirrhosis more frequently during treatment (8–10).
Sorafenib is widely used in clinical practice in Child-Pugh B patients, as a large number of patients with advanced HCC also exhibit liver dysfunction. However, there is lack of data to appropriately define the management of HCC in this subgroup in clinical practice. In the present study, we aimed to retrospectively describe our single-center experience with sorafenib in Child-Pugh B patients with advanced HCC.
Patients and methods
Patients and methods
A database of 120 patients treated at the Cancer Institute of the State of Sao Paulo, University of Sao Paulo (Sao Paulo, Brazil), was retrospectively evaluated. We identified 20 (16.7%) Child-Pugh B and 100 (83.3%) Child-Pugh A patients. The study protocol was approved by the local Ethics Committee.
The patients met the diagnostic criteria for HCC based on radiological or histological findings, according to the American Association for the Study of Liver Diseases (11). All the patients received sorafenib as first-line systemic treatment between July, 2009 and November, 2013 and were followed up at our institution in the division of Clinical Oncology.
Data collection included age, gender, Eastern Cooperative Oncology Group (ECOG) performance status, preexisting hepatopathy, Child-Pugh score, extrahepatic spread, vascular invasion, laboratory findings, prior treatments for HCC, sorafenib treatment duration, dose reductions, toxicity and OS. The last update of the outcome data was on January 28th, 2014.
Treatment and methods
Sorafenib was administered orally, usually at a starting dose of 400 mg twice daily. Adverse events were managed by dose reductions. Treatment was continued until evidence of disease progression, unacceptable toxicity or death, according to the decision of the treating physician. Regular physical examination, laboratory assessment and imaging studies (computed tomography, ultrasonography or magnetic resonance imaging) were performed during follow-up with varying intervals, depending on the decision of the treating physician and the particularities of each case.
Statistical analysis
Continuous variables are presented as median and range and categorical variables as percentages. The data were evaluated using SPSS software, version 11.0 (SPSS. Inc., Chicago, IL, USA).
The OS, calculated from the initiation of sorafenib treatment until the date of death or the last follow-up, was estimated using the Kaplan-Meier method. Comparisons of OS between the Child-Pugh A and B groups were performed using the log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 120 patients were treated at the Cancer Institute of State of Sao Paulo between July, 2009 and November, 2013. Of the 120 patients, 100 exhibited well-preserved liver function (Child-Pugh A), whereas 20 patients were classified as Child-Pugh B.
The median age of the Child-Pugh B patients was 56 years (range, 28–73 years). The majority of the patients were male (70%) and had an ECOG performance status of 0 or 1 (80%). Extrahepatic spread was observed in 65% and vascular invasion in 40% of the cases. Hepatitis C, B and alcohol-related liver disease were preexisting in 65, 10 and 10% of the patients, respectively. The detailed characteristics of the Child-Pugh A and B patients are presented in Table I.
Table I.
Baseline demographic and clinicopathological characteristics of the patients (n=120).
| Characteristics | Child-Pugh B, no. (%) (n=20) | Child-Pugh A, no. (%) n=100 (100%) |
|---|---|---|
| Age, years | ||
| Median (range) | 56 (28–73) | 61 (19–80) |
| Gender | ||
| Male | 14 (70.0) | 73 (73.0) |
| Female | 6 (30.0) | 27 (27.0) |
| ECOG PS | ||
| 0 or 1 | 16 (80.0) | 87 (87.0) |
| 2 or 3 | 4 (20.0) | 13 (13.0) |
| Coexisting liver disease | ||
| Hepatitis B | 2 (10.0) | 12 (12.0) |
| Hepatitis C | 13 (65.0) | 27 (27.0) |
| Alcohol-related | 2 (10.0) | 13 (13.0) |
| Other/unknown | 3 (15.0) | 48 (48.0) |
| Extrahepatic spreada | 13 (65.0) | 51 (51.0) |
| Lung | 6 (30.0) | 20 (20.0) |
| Lymph nodes | 5 (25.0) | 10 (10.0) |
| Bone | 4 (20.0) | 18 (18.0) |
| Macroscopic vascular invasion | 8 (40.0) | 43 (43.0) |
| BCLC stage | ||
| B | 4 (20.0) | 18 (18.0) |
| C | 16 (80.0) | 82 (82.0) |
| Previous locoregional treatment | 7 (35.0) | 38 (38.0) |
| Child-Pugh class | ||
| A5 | 68 (68.0) | |
| A6 | 32 (32.0) | |
| B7 | 19 (95.0) | |
| B8 | 1 (05.0) | |
| Ascites | 13 (65.0) | 17 (17.0) |
| Biochemical analysis | ||
| Albumin, g/dl | ||
| Median (range) | 3.15 (2.2–4.0) | 3.90 (2.8–5.10) |
| TBIL, mg/dl | ||
| Median (range) | 1.9 (0.28–26.38) | 0.88 (0.24–2.42) |
| AFP, ng/ml | ||
| Median (range) | 1,030 (3.0–60,500) | 568.3 (0.9–60,500) |
| ALP, U/l | ||
| Median (range) | 181 (9.8–3,200) | 158 (55–805) |
Some patients had more than one site of metastasis. ECOG PS, Eastern Cooperative Oncology Group performance status; BCLC, Barcelona Clinic Liver Cancer; TBIL, total bilirubin; AFP, α-fetoprotein; ALP, alkaline phosphatase.
Treatment safety and tolerability
The median duration of sorafenib treatment was 60 days (range, 22–308 days) for Child-Pugh B and 117 days (range, 12.9–1,002 days) for Child-Pugh A patients. A total of 17 patients (85%) in Child-Pugh B group and 88 patients (88%) in Child-Pugh A group were started on 800 mg sorafenib daily. During treatment, 3 (15%) Child-Pugh B and 26 (26%) Child-Pugh A patients required dose reductions for management of adverse events. Discontinuation due to adverse events was required in 3 (15%) and 15 (15%) of Child-Pugh B and A patients, respectively.
Among the most common sorafenib-related adverse events in Child-Pugh B patients were fatigue (30%), hand-foot syndrome (30%), diarrhea (15%) and mucositis (10%). Grade 3 or 4 toxicities were observed in 3 Child-Pugh B patients (15%), namely diarrhea (5%), pneumonitis (5%) and thrombocytopenia (5%). Table II shows detailed side effects related to sorafenib observed.
Table II.
Sorafenib-related adverse events.
| Adverse events | Child-Pugh B, no. (%) (n=20) | Child-Pugh A, no. (%) (n=100) |
|---|---|---|
| Grade 1 or 2 | 13 (65.0) | 64 (64.0) |
| Fatigue | 6 (30.0) | 45 (45.0) |
| Diarrhea | 3 (15.0) | 39 (39.0) |
| Hand-foot syndrome | 4 (20.0) | 35 (35.0) |
| Nausea | 3 (15.0) | 18 (18.0) |
| Vomiting | 3 (15.0) | 14 (14.0) |
| Mucositis | 2 (10.0) | 7 (7.0) |
| Thrombocytopenia | 1 (5.0) | 9 (9.0) |
| Neutropenia | 0 (0.0) | 4 (4.0) |
| Anemia | 3 (15.0) | 3 (3.0) |
| Grade 3 or 4 | 3 (15.0) | 20 (20.0) |
| Fatigue | 0 (0.0) | 9 (9.0) |
| Diarrhea | 1 (5.0) | 9 (9.0) |
| Pneumonitis | 1 (5.0) | 0 (0.0) |
| Thrombocytopenia | 1 (5.0) | 0 (0.0) |
OS, prognostic factors and tumor response
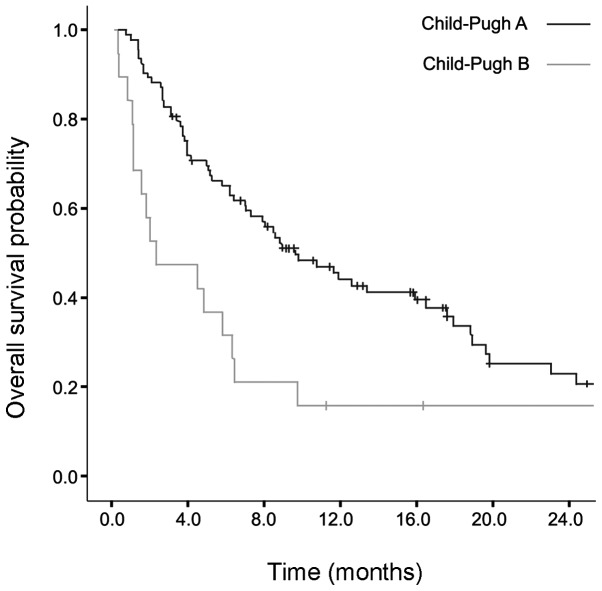
The median follow-up for Child-Pugh B patients was 97 days (range, 24–423 days). The median survival of Child-Pugh B patients was 2.53 months [95% confidence interval (CI): 0.33–5.92] and that of Child-Pugh A patients 9.71 months (95% CI: 6.22–13.04). The median OS was significantly higher in Child-Pugh A patients (P=0.002) (Fig. 1).
Figure 1.
Overall survival probability in months according to the Child-Pugh classification status.
Considering Child-Pugh B patients, 15 (75%) were followed-up with sequential imaging examinations and, therefore, a response analysis could be performed. The disease remained stable in 10 (66.7%) patients, 4 (26.7%) developed progressive disease and 1 (6.7%) exhibited partial response (data not shown).
The presence of metastasis, vascular invasion and α-fetoprotein levels >1,030 ng/ml were not associated with poor survival among Child-Pugh B patients (univariate P=0.281, 0.189 and 0.996, respectively; data not shown).
Discussion
In this retrospective study, we observed that Child-Pugh B patients had a worse OS compared with Child-Pugh A patients, although the toxicity profile was similar between the two groups and considered to be manageable.
In pivotal trials, strict inclusion criteria focused on Child-Pugh A patients, although 2.7–5% of the cases were classed as Child-Pugh B (5,6). Therefore, the Food and Drug Administration and the European Medicines Agency do not contraindicate sorafenib for Child-Pugh B patients. In clinical practice, however, a number of studies reported poor outcomes and questioned whether sorafenib is beneficial for such patients (8–10,12).
Hollebecque et al (9) prospectively evaluated patients with advanced HCC treated with sorafenib and observed a higher OS among Child-Pugh A patients (11.1 months) compared with Child-Pugh B patients (4.5 months). Pressiani et al (10) reported an OS of 10 vs. 3.8 months for Child-Pugh A vs. B patients, respectively, with similar adverse events in the two groups. Recently, the GIDEON study, a global, non-interventional study, was conducted to evaluate the safety of sorafenib for HCC treatment under real-life practice conditions, particularly in Child-Pugh B patients. A shorter median OS was also observed in this group (4.8 vs. 10.3 months in Chilh-Pugh B vs. A patients, respectively); however, the safety profile favored the use of this agent in Child-Pugh B patients (13).
The possible explanations for the poor prognosis of Child-Pugh B patients treated with sorafenib include i) reduced efficacy of sorafenib under conditions of liver dysfunction, ii) unfavorable toxicity profile and iii) progression of the natural history of cirrhosis, leading to cirrhosis-related death.
Sorafenib is primarily metabolized in the liver and its metabolism is mediated via cytochrome P450 3A4 and uridine diphosphate glucuronosyltransferase 1A19. The differences in sorafenib pharmacokinetics between Child-Pugh A and B patients, considering the maximal concentration and the geometric means of area under curve at steady state, were not clinically significant, which makes the hypothesis of lower efficacy in liver dysfunction unlikely (12,14).
However, a shorter progression-free survival was previously reported in Child-Pugh B patients (7,10). This finding may be an argument in favor of liver dysfunction affecting the efficacy of sorafenib. However, the assessment of disease progression in patients with HCC treated with targeted therapy is a complex issue, since measuring tumor dimensions does not take into consideration aspects such as tumor necrosis and vascular modification.
The hypothesis of worse toxicity profile of sorafenib in Child-Pugh B patients was previously evaluated, but the reported data are controversial. Several studies support the viability of sorafenib treatment in this group (9,12,15,16). In addition, an increase in the incidence of cirrhosis-related adverse events was not reported in the Asia-Pacific trial, in which only 2.7% of the patients discontinued treatment due to upper gastrointestinal hemorrhage and ascites (6). Another study, however, observed that sorafenib was more frequently associated with diarrhea, skin reactions and grade 3–4 liver toxicity in Child-Pugh B and C compared with Child-Pugh A patients (17).
In the present study, we observed similar rates of grade 1/2 or 3/4 adverse events in the two groups, which supports the manageable toxicity profile of sorafenib in Child-Pugh B patients.
A reasonable explanation for the less favorable survival observed in Child-Pugh B patients is the emergence of cirrhosis-related complications as a consequence of the natural progression of cirrhosis, rather than due to the occurrence of adverse events or reduced efficacy of sorafenib in Child-Pugh B patients. There was a lack of data regarding the immediate cause of death in our cohort to conclude this hypothesis, which is a limitation of our study; other limitations included its retrospective design and that almost all the Child-Pugh B patients scored 7, what may overestimate the OS and underestimate toxicity.
In conclusion, we demonstrated that OS was worse in Child-Pugh B patients with advanced HCC treated with sorafenib. The question of whether to treat this patient subgroup remains open to discussion and more data are required to appropriately define the safety and efficacy of sorafenib in the context of liver dysfunction. A randomized placebo-controlled trial including only Child-Pugh B patients should be conducted. Based on the natural history of liver cirrhosis and the poor prognosis of Child-Pugh B patients with advanced HCC, careful patient selection is crucial in clinical practice.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso MC, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: Rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Fuster J, Bruix J. Barcelona-Clínic Liver Cancer Group: The Barcelona approach: Diagnosis, staging and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10:S115–S120. doi: 10.1002/lt.20034. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, et al. SHARP Investigators Study Group: Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 8.Abou Alfa GK, Amadori D, Santoro A, et al. Is sorafenib (S) safe and effective in patients (pts) with hepatocellular carcinoma (HCC) and Child-Pugh B (CPB) cirrhosis? J Clin Oncol. 2008 May 20;26:4518. Suppl. [Google Scholar]
- 9.Hollebecque A, Cattan S, Romano O, et al. Safety and efficacy of sorafenib in hepatocellular carcinoma: The impact of the Child-Pugh score. Aliment Pharmacol Ther. 2011;34:1193–1201. doi: 10.1111/j.1365-2036.2011.04860.x. [DOI] [PubMed] [Google Scholar]
- 10.Pressiani T, Boni C, Rimassa L, et al. Sorafenib in patients with Child-Pugh class A and B advanced hepatocellular carcinoma: A prospective feasibility analysis. Ann Oncol. 2013;24:406–411. doi: 10.1093/annonc/mds343. [DOI] [PubMed] [Google Scholar]
- 11.Bruix J, Sherman M. American Association for the Study of Liver Diseases: Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JE, Ryoo BY, Ryu MH, Chang HM, Suh DJ, Lee HC, Lim YS, Kim KM, Kang YK. Sorafenib for hepatocellular carcinoma according to Child-Pugh class of liver function. Cancer Chemother Pharmacol. 2011;68:1285–1290. doi: 10.1007/s00280-011-1616-x. [DOI] [PubMed] [Google Scholar]
- 13.Lencioni R, Kudo M, Ye SL, et al. First interim analysis of the GIDEON (global investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafenib) non-interventional study. Int J Clin Pract. 2012;66:675–683. doi: 10.1111/j.1742-1241.2012.02940.x. [DOI] [PubMed] [Google Scholar]
- 14.Keating GM, Santoro A. Sorafenib: A review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69:223–240. doi: 10.2165/00003495-200969020-00006. [DOI] [PubMed] [Google Scholar]
- 15.Pinter M, Sieghart W, Graziadei I, Vogel W, Maieron A, Königsberg R, Weissmann A, Kornek G, Plank C, Peck-Radosavljevic M. Sorafenib in unresectable hepatocellular carcinoma from mild to advanced stage liver cirrhosis. Oncologist. 2009;14:70–76. doi: 10.1634/theoncologist.2008-0191. [DOI] [PubMed] [Google Scholar]
- 16.Iavarone M, Cabibbo G, Piscaglia F, Zavaglia C, Grieco A, Villa E, Cammà C, Colombo M. SOFIA (sorafenib Italian assessment) study group: Field-practice study of sorafenib therapy for hepatocellular carcinoma: A prospective multicenter study in Italy. Hepatology. 2011;54:2055–2063. doi: 10.1002/hep.24644. [DOI] [PubMed] [Google Scholar]
- 17.Wörns MA, Weinmann A, Pfingst K, et al. Safety and efficacy of sorafenib in patients with advanced hepatocellular carcinoma in consideration of concomitant stage of liver cirrhosis. J Clin Gastroenterol. 2009;43:489–495. doi: 10.1097/MCG.0b013e31818ddfc6. [DOI] [PubMed] [Google Scholar]