Abstract
Dracorhodin perchlorate (DP) has recently been revealed to induce apoptosis in various types of cancer. However, the antitumor potential and molecular mechanisms of DP in human lung cancer have not been previously reported. Therefore, the present study aimed to investigate the effects of DP on cell viability, the cell cycle and apoptosis, using an MTT assay, flow cytometry and western blot studies. DP was identified to induce cellular and DNA morphological changes, and decreased the viability of SK-MES-1 human lung squamous carcinoma cells. DP significantly inhibited the growth of SK-MES-1 cells by inducing apoptosis and G1/G0 cell cycle arrest in a dose-dependent manner via activation of p53 (P<0.05). Furthermore, DP promoted the significant upregulation of B cell lymphoma-2 (Bcl-2)-activated X protein and significant downregulation of Bcl-2 (P<0.05), inducing dissipation of the mitochondrial membrane potential (MMP). In addition, caspase-3 was activated by DP via the cleavage of its substrate, proteolytic cleavage of poly(ADP-ribose) polymerase. DP also induced caspase-independent apoptosis by significantly increasing the protein expression of the apoptosis-inducing factor (P<0.05), which is localized in mitochondria under the physiological conditions and released into the cytoplasm when MMP is dissipated. Furthermore, the present study demonstrated that DP significantly increased the generation of reactive oxygen species (P<0.05). In conclusion, the current study revealed that DP is able to induce cell cycle arrest and apoptosis in SK-MES-1 cells via activation of the mitochondrial pathway, indicating that DP may be a potential leading compound for the development of future lung cancer therapeutic regimes.
Keywords: dracorhodin perchlorate, SK-MES-1 cells, apoptosis
Introduction
Lung cancer, the most common type of malignant carcinoma, is the leading cause of cancer-associated mortality worldwide (1). Despite the availability of various therapeutic strategies, chemotherapy plays a major role in the treatment of lung cancer (2). Thus far, alternative strategies to chemotherapy have not resulted in significant improvement in the survival rate of postoperative patients with lung cancer. Therefore, the development of novel agents with selectivity against critical apoptotic targets may provide a rational approach to the treatment of cancer. Over the last decade, an abundance of pharmacological evidence regarding the anticancer properties of conventional natural Chinese products has been obtained (3,4). A number of these anticancer agents have exerted their therapeutic action by inducing apoptosis in human malignant cells (5–7).
Dracorhodin perchlorate (DP) is a synthetic analogue of the anthocyanin red pigment dracorhodin, a major constituent of traditional Chinese medicine, ‘dragon's blood’ (also known as Daemonorops draco) (8–10). A previous study demonstrated that DP inhibited the activation of the phosphoinositide 3-kinase/Akt and nuclear factor-κB signaling pathways, and upregulated the expression of p53 in gastric cancer cells (11). Furthermore, DP was found to inhibit cell growth and trigger apoptosis in melanoma and breast cancer cells (12,13). In addition, DP induced apoptosis through the caspase pathways and generated reactive oxygen species (ROS) in cervical cancer cells (14). However, the effect of DP on human lung cancer cells has yet to be reported.
Apoptosis is a type of programmed cell death that occurs in multicellular organisms, and mitochondrial dysfunction is considered to be the central executioner of the apoptosis pathway. Mitochondrial membrane permeability is regulated by various proteins, including p53, B cell lymphoma-2 (Bcl-2) and Bcl-2-activated X protein (Bax) (15,16). Depolarization of the mitochondrial membrane potential (MMP) stimulates mitochondria to release apoptosis-inducing factor (AIF) and various other proapoptotic molecules that eventually result in the activation of caspase-3 (17,18). Thus, AIF and caspase-3 are able to trigger chromatin condensation and DNA degradation in order to induce programmed cell death.
The present study aimed to examine the possible inhibitory effect of DP and the mechanism of DP-induced apoptosis in SK-MES-1 lung cancer cells.
Materials and methods
Materials
The human lung squamous carcinoma cell line, SK-MES-1, was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). DP, which was purchased from the Tonglian Pharmaceutical Co., Ltd. (Shanghai, China), was dissolved in dimethyl sulfoxide (DMSO; Shengong Biotechnology Co., Shanghai, China) to make a stock solution. Furthermore, fetal bovine serum (FBS) was purchased from Gibco Life Technologies (Carlsbad, CA, USA). The following reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA): 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Hoechst 33342, Dulbecco's modified Eagle's medium (DMEM) and Rhodamine 123 mitochondrial-specific fluorescent dye. The Cell Cycle Analysis [propidium iodide (PI) + RNase A; catalog number (cat. no.) C1052] and the Reactive Oxygen Species Assay kits were purchased from Beyotime Institute of Biotechnology (Shanghai, China). In addition, the BCA Protein Assay and the Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection kits were purchased from Nanjing KeyGen Biotech. Co. Ltd. (Nanjing, China). Polyclonal goat anti-rabbit antibodies against β-actin, (dilution, 1:2000; cat. no. 4967S) Bax (dilution, 1:1000; cat. no. 2772S), Bcl-2 (dilution, 1:1000; cat. no. 2876S), pro-caspase-3 (dilution, 1:1000; cat. no. 9662P), poly(ADP-ribose) polymerase (PARP; dilution, 1:1000; cat. no. 9542S), AIF (dilution, 1:1000; cat. no. 4642S), p53 (dilution, 1:1000; cat. no. 9282S) and phosphorylated retinoblastoma (pRb; dilution, 1:1000; cat. no. 9306S), as well as horseradish peroxidase (HRP)-conjugated secondary antibodies (anti-rabbit IgG; dilution, 1:2000; cat. no. 7074P2), were purchased from Cell Signaling Technology, Inc. (Shanghai, China). A western blot detection kit was purchased from EMD Milipore (Billerica, MA, USA).
Cell culture and treatments
SK-MES-1 human lung squamous carcinoma cells were cultured in DMEM supplemented with 10% FBS and maintained at 37°C in a 5% CO2 humidified atmosphere. Cells were treated with DP dissolved in DMSO with a final DMSO concentration of 1%. DMSO-treated cells were used as the controls in all the experiments. The DMSO concentration was maintained at a concentration of <0.01% in all the cell cultures and did not exert any detectable effect on the rate of cell growth or death.
Cell growth inhibition assay
The inhibition of cell growth was determined by performing an MTT assay, as previously described (13). Briefly, SK-MES-1 cells were seeded in 96-well plates at a density of 1×104 cells/well, and treated with various concentrations of DP (0, 10, 20, 40, 80 and 160 µM) for 24 h. Following treatment, the MTT reagent was added (100 µl/ml) and the cells were incubated at 37°C for an additional 4 h. Next, 150 µl DMSO was added to dissolve the formazan crystals and absorbance was read in a microplate reader (Varioskan Flash; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at a wavelength of 570 nm. The viable cell number was directly proportional to the production of formazan. The growth assay was repeated three times and the half maximal inhibitory concentration (IC50) values were calculated using GraphPad Prism software (version 5.0; GraphPad Software, Inc., La Jolla, CA, USA). The percentage of inhibition was calculated as follows: Inhibitory ratio (%) = (Acontrol / Asample)/Acontrol × 100%, where Asample and Acontrol are the absorbance values of the treated and control groups, respectively, following incubation.
Flow cytometric cell cycle analysis
A PI cell cycle detection kit (PI + RNase A) and flow cytometry were used for cell cycle analysis. Briefly, SK-MES-1 cells were seeded in a 6-well plate at a density of 6×105 cells/well and treated with DP at concentrations of 0, 40 and 80 µM. Following treatment for 24 h, the cells were harvested and fixed in 500 µl 70% ice-cold ethanol at 4°C for 2 h. Next, the samples were washed with phosphate-buffered saline (PBS), and incubated with RNase A and PI staining solution, according to the manufacturer's instructions. Subsequent to staining, the samples were analyzed by performing flow cytometry (EPICS® XL™; Beckman Coulter, Brea, CA, USA).
Flow cytometric apoptosis analysis using Annexin V-FITC/PI staining
SK-MES-1 cells were seeded in a 6-well plate at a density of 3×105 cells/well and treated with DP at concentrations of 0, 40 and 80 µM. Following treatment for 24 h, the cells were collected and washed with PBS. Subsequently, the apoptotic cell death rate was examined by performing Annexin V-FITC and PI double-staining using the Annexin V-FITC apoptosis detection kit, according to the manufacturer's instructions. After staining with Annexin V-FITC/PI, the samples were analyzed using flow cytometry.
Nuclei fragmentation observed by Hoechst 33342 staining
To visualize apoptotic cell death and nuclear morphology, SK-MES-1 cells were stained with Hoechst 33342. Briefly, SK-MES-1 cells were seeded into a 6-well flat bottom plate at a density of 6×105 cells/well and treated with DP at concentrations of 0, 40 and 80 µM. Following treatment for 24 h, the cells were collected, washed and allowed to dry on slides. Subsequently, the nuclei were stained with Hoechst 33342 for 10 min and the apoptotic cells displaying fragmented or condensed nuclei were observed under a fluorescence microscope (BH2-RFL-T3; Olympus Corporation, Tokyo, Japan).
Western blot analysis
To determine the underlying mechanism of the apoptotic effect of DP, western blot analysis was performed for a number of apoptosis-associated proteins. First, SK-MES-1 cells were seeded in a 10 cm culture dish at a density of 60%, treated with DP at concentrations of 0, 40 and 80 µM. Following treatment for 24 h, the cells were collected and washed with PBS. The cells were centrifuged at 1,000 × g for 5 min; the cell pellets were then resuspended in radioimmunoprecipitation assay lysis buffer and lysed on ice using ultrasound (200 W for 15 sec). Following centrifugation at 12,000 × g for 10 min, the supernatant fluids were collected and the protein contents of the supernatant were determined using a BCA Protein Assay kit, with the protein samples stored at −20°C. Subsequently, the protein lysates were separated by performing electrophoresis on a 10% sodium dodecyl sulphate-polyacrylamide gel and transferred to a polyvinylidene fluoride membrane (GE Healthcare, Piscataway, NJ, USA). The membranes were then soaked in blocking buffer (5% skimmed milk) for 2 h. To probe for all the apoptosis-associated proteins, the membranes were incubated overnight at 4°C with the relevant antibodies, followed by appropriate HRP-conjugated secondary antibodies, and enhanced chemiluminescence detection was performed. Gel-Pro Analyzer software (GelPro32, version 4.0; Media Cybernetics, Inc., Rockville, MD, USA) was used to extract valuable qualitative and quantitative data from the electrophoretic gels to document and store the western blot data.
Flow cytometric determination of the MMP
Flow cytometry was performed to evaluate perturbations in the MMP in SK-MES-1 cells treated with Rhodamine 123. Briefly, SK-MES-1 cells were seeded in a 6-well plate at a density of 3×105 cells/well and treated with DP at concentrations of 0, 40 and 80 µM. Following treatment for 24 h, the cells were collected, washed with PBS and incubated with 10 µM Rhodamine 123 at a temperature of 37°C for 20 min. The stained cells were washed and resuspended in 200 µl PBS prior to determining the MMP level using flow cytometry.
Flow cytometric measurement of intracellular ROS generation
A 2′,7′-dichlorofluorescin-diacetate (DCFH-DA) detection kit was used to measure the ROS levels, according to the manufacturers instructions. Briefly, SK-MES-1 cells were treated with DP at concentrations of 0, 40 and 80 µM. Following treatment for 24 h, the cells were collected, washed in DMEM without FBS and incubated with 10 mM DCFH-DA at a temperature of 37°C for 15 min. The stained cells were washed and resuspended in 200 µl DMEM. Intracellular ROS mediate the oxidation of DCFH to the fluorescent compound DCF; thus, the generation of ROS was analyzed using flow cytometry.
Statistical analysis
All the data are expressed as the mean ± standard deviation of at least three independent experiments. For statistical analysis, comparisons between results from different groups were analyzed using SPSS software for Windows (version 17.0; SPSS, Inc., Chicago, IL, USA). Comparisons were performed using one-way analysis of variance, followed by Dunnett's test. P<0.05 was considered to indicate a statistically significant difference.
Results
DP time- and dose-dependently inhibits SK-MES-1 cell growth
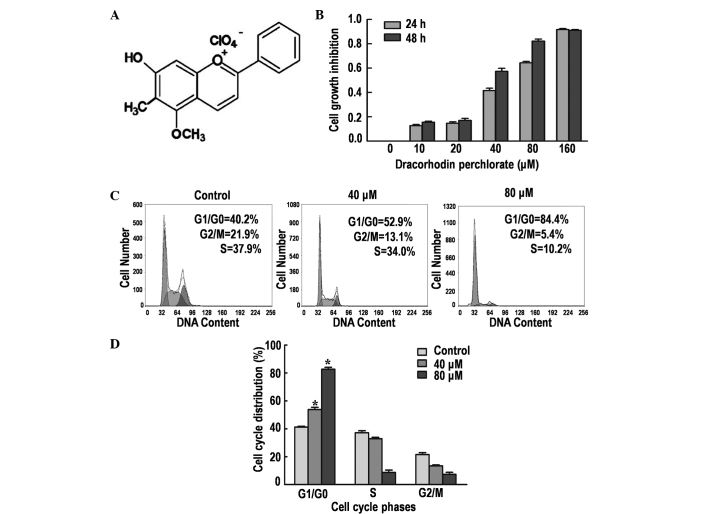
DP (Fig. 1A) is a synthetic analogue of the antimicrobial anthocyanin red pigment dracorhodin, a compound isolated from the exudates of the fruit of Daemonorops draco (9). The antiproliferative effect of DP on SK-MES-1 cells was determined by performing an MTT assay. Treatment with DP for 24 and 48 h reduced the cell viability in a time- and dose-dependent manner (Fig. 1B). The IC50 values were ~50 and ~30 µM following treatment for 24 and 48 h, respectively. Thus, 24-h treatments with 40 and 80 µM DP were selected for the subsequent experiments.
Figure 1.
Effects of dracorhodin perchlorate (DP) treatment on cell viability and the cell cycle in SK-MES-1 cells. (A) Chemical structure of DP. (B) Effect of DP treatment on the growth of SK-MES-1 cells using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Cells were treated with different concentrations of DP (0, 10, 20, 40, 80 and 160 µmol/l) for 24 and 48 h. (C) Analysis of the cell cycle using propidium iodide staining followed by flow cytometric analysis. Cells were treated with 40 and 80 µM DP for 24 h. (D) Cell cycle phase distribution. Data are expressed as the mean ± standard deviation of three independent experiments. *P<0.05 vs. control.
DP induces G1/G0 phase arrest in SK-MES-1 cells
Cell cycle arrest is one of the major causes of cell growth inhibition. Therefore, the induction of cell cycle arrest was analyzed using PI staining and flow cytometry. The results demonstrated that DP treatment caused significant cell cycle arrest at the G1/G0 phase in a dose-dependent manner (P<0.05; Fig. 1C and D). The percentage of cells accumulated in the G1/G0 phase were 40.2, 52.9 and 84.4% following treatment with 0, 40 and 80 µM DP for 24 h, respectively. In addition, a corresponding decrease in G2/M and S phase cells was observed, in part caused by the induction of G1/G0 phase cell cycle arrest.
DP induces apoptosis in SK-MES-1 cells
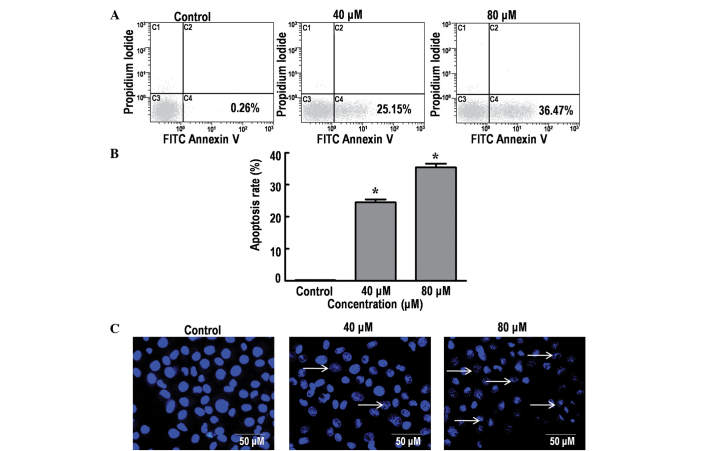
The effect of DP on cell apoptosis was analyzed using Annexin V-FITC/PI and Hoechst 33342 staining. The results indicated a significant increase in the percentage of dead cells in a dose-dependent manner, from 0.26% (control group; 0 µM DP) to 25.15 and 36.47% following treatment with 40 and 80 µM DP for 24 h, respectively (P<0.05; Fig. 2A and B). DNA fragmentation is an important characteristic of apoptosis that can be clearly identified using Hoechst staining (19). Consistent with the aforementioned results, treatment of the SK-MES-1 cells with 40 and 80 µM DP for 24 h resulted in a marked increase in nuclear fragmentation (Fig. 2C). Thus, the current data demonstrated that DP can induce apoptosis in SK-MES-1 cells in a dose-dependent manner.
Figure 2.
Effects of dracorhodin perchlorate (DP) treatment on the apoptosis in SK-MES-1 cells. (A) Analysis of apoptosis using Annexin-V/propidium iodide staining followed by flow cytometry. The percentage of early stage apoptotic cells was determined following treatment with different concentrations of DP for 24 h. (B) Percentage of apoptotic cells in early stage apoptosis. Data are expressed as the mean ± standard deviation of three independent experiments. *P<0.05 vs. control. (C) Nuclear morphological changes of SK-MES-1 cells determined by performing Hoechst 33342 staining followed by fluorescence microscopy. Treatment of cells with 40 and 80 µM DP for 24 h resulted in a significant increase in the number of fragmented nuclei (arrows). FITC, fluorescein isothiocyanate.
Effect of DP on the expression of major cell cycle and mitochondrial apoptosis regulators
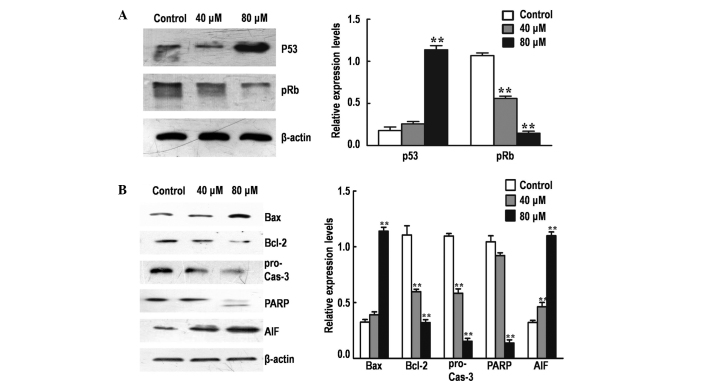
To elucidate the molecular mechanism underlying G1/G0 phase arrest mediated by DP, the protein expression levels of various major cell cycle regulatory proteins (p53 and pRb) were detected by performing western blot analysis. The treatment of SK-MES-1 cells with DP for 24 h resulted in the significant upregulation of p53 and the significant downregulation of pRb in a dose-dependent manner (P<0.05; Fig. 3A).
Figure 3.
Effects of dracorhodin perchlorate (DP) on the expression of major cell cycle and apoptotic regulators determined by western blot analysis. Gel-Pro Analyzer software was employed to extract quantitative data. (A) Protein expression levels of p53 and pRb determined by western blot analysis. (B) Protein expression levels of Bax, Bcl-2, Cas-3, PARP and AIF determined by western blot analysis. Data are expressed as the mean ± standard deviation of three independent experiments. **P<0.05 vs. control. pRb, phosphorylated retinoblastoma; Bcl-2, B-cell lymphoma-2; Bax, Bcl-2-associated X protein; Cas-3, pro-caspase-3; PARP, poly(ADP-ribose) polymerase; AIF, apoptosis-inducing factor.
To investigate the rate of mitochondrial apoptosis in SK-MES-1 cells, western blot analysis was performed to determine the effect of DP treatment on the protein expression levels of various major mitochondrial apoptosis regulatory proteins (Bax, Bcl-2, caspase-3, PARP and AIF; Fig. 3B). The Bax/Bcl-2 expression ratio was significantly increased following treatment with DP (P<0.05), accompanied by activation of procaspase-3 and cleavage of PARP in a dose-dependent manner. In addition, the expression of AIF was significantly increased following treatment (P<0.05), possibly due to the increase in mitochondrial permeability, resulting in the release of AIF from the mitochondria into the cytosol. These results indicate that DP can induce apoptosis in SK-MES-1 cells via the mitochondrial pathway.
DP causes disruption of the MMP in SK-MES-1 cells
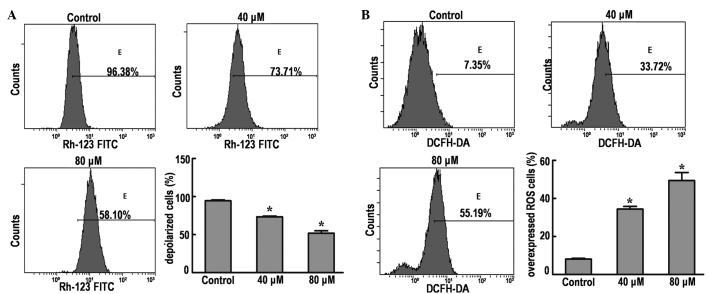
Depolarization of the MMP is a characteristic feature of apoptosis; therefore, the effects of DP on the MMP of SK-MES-1 cells were examined by flow cytometry using Rhodamine 123 staining. The results demonstrated that the MMP was significantly decreased from 96.38% (control group) to 73.71 and 58.10% in cells treated with 40 and 80 µM DP, respectively (P<0.05; Fig. 4A). These results indicate that dissipation of the MMP may be one of mechanisms through which DP induces apoptosis.
Figure 4.
Effects of DP treatment on MMP and ROS expression in SK-MES-1 cells by flow cytometric analysis. (A) Values represent the percentage of Rhodamine-123 fluorescence in SK-MES-1 cells. Cells were treated with 40 and 80 µM DP for 24 h. Representative histograms express the percentage of depolarized cells. (B) Values represent the percentage of DCFH-DA staining in SK-MES-1 cells. Cells were treated with 40 and 80 µM DP for 24 h. Representative histograms express the percentage of ROS overexpressing cells. All the data are expressed as the mean ± standard deviation of three independent experiments. *P<0.05 vs. control. FITC, fluorescein isothiocyanate; DCFH-DA, 2′,7′-dichlorofluorescein-diacetate; ROS, reactive oxygen species.
DP induces increased generation of ROS in SK-MES-1 cells
Intracellular ROS generation in SK-MES-1 cells was evaluated by flow cytometry using DCFH-DA (Fig. 4B). The ROS level in SK-MES-1 cells treated with 40 and 80 µM DP was significantly increased from 7.35% (control group) to 33.72 and 55.19%, respectively.
Discussion
A number of studies have recently identified that DP has a broad spectrum of cytotoxicity towards various human cancer cell lines of different origins (11–13). In the present study, DP was demonstrated to significantly inhibit the growth of SK-MES-1 human lung cancer cells in a dose-dependent manner.
Cell cycle regulation and apoptosis are considered to be major causes of cell growth inhibition (20). The cell cycle is controlled at different checkpoints. These checkpoints ensure that specific processes have been precisely completed at each stage of the cell cycle before allowing progress to the next phase of the cycle. The loss of key checkpoints is a characteristic of cancer cells that results in anomalous proliferation and the promotion of oncogenic transformation (21). The results of the current study indicated that the treatment of lung cancer cells with DP induced G1/G0 phase arrest in a dose-dependent manner.
In addition to cell cycle arrest, DP exhibited a cytotoxic effect in the present study, inducing apoptotic cell death in lung cancer cells. These apoptotic effects are consistent with the results of a number of previous studies, which observed that DP inhibited abnormal proliferation by the induction of apoptosis in various types of cancer cells, including melanoma, gastric cancer and prostate cancer cells (11,12,22).
p53 is an important factor in the regulation of cell cycle progression, checkpoint activation, apoptosis and repair of DNA damage (15,23,24). Once activated, p53 can activate its downstream transcription factor, which can form complexes with cell cycle-dependent protein kinase and inhibit the activity of Rb protein. Rb is critical in the G1/S phase transition, regulating the expression of genes necessary for cell cycle progression (25). The present study determined that treatment with DP significantly upregulated the p53 protein expression levels. This upregulation was accompanied by a significant decrease in the protein expression levels of pRb. These data indicated that DP may disrupt cell cycle progression via the activation of p53 and the inhibition of Rb.
The p53 tumor suppressor is crucial for regulating the expression of various genes that mediate apoptosis. Furthermore, it is well-established that p53 activates apoptosis via the regulation of mitochondrial integrity, resulting in the release of downstream cytokines and, ultimately, the activation of caspases (15,23,24). The Bcl-2 protein family is a well-known family of apoptosis regulating proteins that act via the mitochondrial pathway. This family includes anti-apoptotic proteins, such as Bcl-2, and proapoptotic proteins, such as Bax (17). These proteins function together to regulate mitochondrial membrane permeability and modulate the release of apoptogenic proteins that promote cell death (26–28). In the present study, the expression levels of proteins involved in the mitochondrial pathway were detected by western blot analysis. The current data demonstrated that the expression of Bax significantly increase, while the expression of Bcl-2 significantly decreased, indicating that DP may induce apoptosis via the mitochondrial pathway.
An increase in mitochondrial permeability can result in the release of proapoptotic molecules, leading to the activation of other downstream caspases and, ultimately, the activation of caspase-3 (29–31). Caspases play a key role in the process of apoptosis and frequently catalyze the specific cleavage of numerous pivotal cellular proteins (18). For instance, the cleavage of PARP, which is a DNA repair enzyme, is performed by caspases, in particular caspase-3, and is the hallmark of apoptosis (18,32). The data presented in the current study clearly demonstrated the cleavage of PARP following treatment with DP. These results indicate that the intrinsic mitochondrial-mediated caspase activation pathway is involved in the DP-mediated apoptosis of SK-MES-1 cells.
Dysfunction of the mitochondrial membrane allows the release of AIF from mitochondria into the cytosol. AIF is a mitochondrial intermembrane flavoprotein that can induce apoptosis in a caspase-independent manner. AIF condenses chromatin and fragmented DNA in order to trigger programmed cell death (33,34). Previous studies have indicated that AIF is required for cell death following certain cell stresses (33,34). This has been clarified by the injection of anti-AIF antibodies or knockout of the AIF gene, which alleviated the progression of apoptosis (33,34). The current study demonstrated that treatment with DP significantly increased the expression of AIF, indicating that DP may partially induce the apoptosis of SK-MES-1 cells via a caspase-independent pathway.
Increasing the expression of Bax appears to result in increased mitochondrial membrane permeability and dissipation of the MMP (35). Flow cytometry data obtained in the present study supports this hypothesis, with a significant reduction in MMP observed in the treatment group cells. Thus, the results of the current study indicate that DP treatment may disrupt the integrity of mitochondria by increasing the Bax/Bcl-2 ratio.
A number of studies have indicated that ROS are downstream mediators of p53-dependent apoptosis. However, ROS may transmit a signal for apoptosis as opposed to being a consequence of the cellular changes accompanied by apoptosis (36), as p53 affects the mitochondrial apoptotic pathway and mitochondria are the major target of ROS. In addition, ROS generation may alter the redox status of cells, thus, altering the sensitivity of cells to apoptotic stimuli and ultimately triggering subsequent apoptotic events (37–40). In the current study, DP treatment significantly increased the generation of ROS in SK-MES-1 cells. Our future studies will aim to summarize the association between the current findings, and the cell cycle and apoptosis. Understanding the specific mechanism of ROS generation may provide a novel method for the development of therapeutic agents that are capable of selectively inducing apoptosis in healthy or neoplastic cells.
In conclusion, the current study revealed that treatment of SK-MES-1 cells with DP induced: Mitotic arrest; caspase-dependent apoptosis via an increase in the Bax/Bcl-2 ratio and caspase-3 expression, causing cleavage of PARP; and caspase-independent apoptosis via an increase in AIF expression. Therefore, DP may be a potential compound for the development of future lung cancer therapeutic strategies.
Acknowledgements
The present study was supported by grants from the Science and Technology Services of Jilin Province Scientific and Technological Project (no. 20140521) and the Natural Science Foundation of China (no. 81272472).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Johnson DH. Evolution of cisplatin-based chemotherapy in non-small cell lung cancer: A historical perspective and the eastern cooperative oncology group experience. Chest. 2000;117(4 Suppl 1):133S–137S. doi: 10.1378/chest.117.4_suppl_1.133S. [DOI] [PubMed] [Google Scholar]
- 3.Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100:72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27:2712–2725. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo M, Palumbo R, Mupo A, et al. Flavonoid quercetin sensitizes a CD95-resistant cell line to apoptosis by activating protein kinase Calpha. Oncogene. 2003;22:3330–3342. doi: 10.1038/sj.onc.1206493. [DOI] [PubMed] [Google Scholar]
- 6.Xia MY, Wang MW, Cui Z, et al. Dracorhodin perchlorate induces apoptosis in HL-60 cells. J Asian Nat Prod Res. 2006;8:335–343. doi: 10.1080/10286020500035300. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira CG, Epping M, Kruyt FA, Giaccone G. Apoptosis: Target of cancer therapy. Clin Cancer Res. 2002;8:2024–2034. [PubMed] [Google Scholar]
- 8.Brockmann H, Junge H. Die konstitution des dracorhodins, eines neuen farbstoffes aus dem ‘drachenblut’. Eur J Inorg Chem. 1943;76:751–763. (In German) [Google Scholar]
- 9.Rao GS, Gerhart MA, Lee RT, III, et al. Antimicrobial agents from higher plants. Dragon's blood resin. J Nat. Prod. 1982;45:646–648. doi: 10.1021/np50023a024. [DOI] [PubMed] [Google Scholar]
- 10.Gao WF, Zheng H, Wang YS, et al. Synthesis of dracorhodin. Chin J Pharma. 1989;20:247–250. (In Chinese) [Google Scholar]
- 11.Rasul A, Ding C, Li X, et al. Dracorhodin perchlorate inhibits PI3K/Akt and NF-κB activation, up-regulates the expression of p53, and enhances apoptosis. Apoptosis. 2012;17:1104–1119. doi: 10.1007/s10495-012-0742-1. [DOI] [PubMed] [Google Scholar]
- 12.Xia M, Wang M, Tashiro S, Onodera S, Minami M, Ikejima T. Dracorhodin perchlorate induces A375-S2 cell apoptosis via accumulation of p53 and activation of caspases. Biol Pharm Bull. 2005;28:226–232. doi: 10.1248/bpb.28.226. [DOI] [PubMed] [Google Scholar]
- 13.Yu JH, Zheng GB, Liu CY, Zhang LY, Gao HM, Zhang YH, Dai CY, Huang L, Meng XY, Zhang WY, Yu XF. Dracorhodin perchlorate induced human breast cancer MCF-7 apoptosis through mitochondrial pathways. Int J Med Sci. 2013;10:1149–1156. doi: 10.7150/ijms.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia M, Wang D, Wang M, Tashiro S, Onodera S, Minami M, Ikejima T. Dracorhodin perchlorate induces apoptosis via activation of caspases and generation of reactive oxygen species. J Pharmacol Sci. 2004;95:273–283. doi: 10.1254/jphs.FPJ03102X. [DOI] [PubMed] [Google Scholar]
- 15.Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–9040. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- 16.Adams JM, Cory S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci. 2001;26:61–66. doi: 10.1016/S0968-0004(00)01740-0. [DOI] [PubMed] [Google Scholar]
- 17.Saelens X, Festjens N, Vande Walle L, van Gurp M, van Loo G, Vandenabeele P. Toxic proteins released from mitochondria in cell death. Oncogene. 2004;23:2861–2874. doi: 10.1038/sj.onc.1207523. [DOI] [PubMed] [Google Scholar]
- 18.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 19.Araki M, Iida Y, Taketani S, Watanabe K, Ohta T, Saito T. Characterization of photoreceptor cell differentiation in the rat retinal cell culture. Dev Biol. 1987;124:239–247. doi: 10.1016/0012-1606(87)90475-1. [DOI] [PubMed] [Google Scholar]
- 20.King KL, Cidlowski JA. Cell cycle regulation and apoptosis. Annu Rev Physiol. 1998;60:601–617. doi: 10.1146/annurev.physiol.60.1.601. [DOI] [PubMed] [Google Scholar]
- 21.Pan J, She M, Xu ZX, et al. Farnesyltransferase inhibitors induce DNA damage via reactive oxygen species in human cancer cells. Cancer Res. 2005;65:3671–3681. doi: 10.1158/0008-5472.CAN-04-2744. [DOI] [PubMed] [Google Scholar]
- 22.He Y, Ju W, Hao H, Liu Q, Lv L, Zeng F. Dracorhodin perchlorate suppresses proliferation and induces apoptosis in human prostate cancer cell line PC-3. J Huazhong Univ Sci Technolog Med Sci. 2011;31:215–219. doi: 10.1007/s11596-011-0255-0. [DOI] [PubMed] [Google Scholar]
- 23.Budram-Mahadeo V, Morris PJ, Latchman DS. The Brn-3a transcription factor inhibits the pro-apoptotic effect of p53 and enhances cell cycle arrest by differentially regulating the activity of the p53 target genes encoding Bax and p21(CIP1/Waf1) Oncogene. 2002;21:6123–6131. doi: 10.1038/sj.onc.1205842. [DOI] [PubMed] [Google Scholar]
- 24.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 25.Das SK, Hashimoto T, Shimizu K, et al. Fucoxanthin induces cell cycle arrest at G0/G1 phase in human colon carcinoma cells through up-regulation of p21WAF1/Cip1. Biochim Biophys Acta. 2005;1726:328–335. doi: 10.1016/j.bbagen.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Huang DC, Strasser A. BH3-Only proteins-essential initiators of apoptotic cell death. Cell. 2000;103:839–842. doi: 10.1016/S0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 27.Frenzel A, Grespi F, Chmelewskij W, Villunger A. Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis. 2009;14:584–596. doi: 10.1007/s10495-008-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danial NN. BCL-2 family proteins: critical checkpoints of apoptotic cell death. Clin Cancer Res. 2007;13:7254–7263. doi: 10.1158/1078-0432.CCR-07-1598. [DOI] [PubMed] [Google Scholar]
- 29.Schuler M, Bossy-Wetzel E, Goldstein JC, et al. p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J Biol Chem. 2000;275:7337–7342. doi: 10.1074/jbc.275.10.7337. [DOI] [PubMed] [Google Scholar]
- 30.Toshiyuki M, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 31.Greiner M, Cárdenas S, Parra C, et al. Adrenalectomy regulates apoptotic-associated genes in rat hippocampus. Endocrine. 2001;15:323–333. doi: 10.1385/ENDO:15:3:323. [DOI] [PubMed] [Google Scholar]
- 32.Slee EA, Adrain C, Martin SJ. Executioner caspase −3, −6, and −7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem. 2001;276:7320–7326. doi: 10.1074/jbc.M008363200. [DOI] [PubMed] [Google Scholar]
- 33.Norberg E, Orrenius S, Zhivotovsky B. Mitochondrial regulation of cell death: processing of apoptosis-inducing factor (AIF) Biochem Biophys Res Commun. 2010;396:95–100. doi: 10.1016/j.bbrc.2010.02.163. [DOI] [PubMed] [Google Scholar]
- 34.Candé C, Cohen I, Daugas E, et al. Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie. 2002;84:215–22. doi: 10.1016/S0300-9084(02)01374-3. [DOI] [PubMed] [Google Scholar]
- 35.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 36.Johnson TM, Yu ZX, Ferrans VJ, et al. Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc Natl Acad Sci USA. 1996;93:11848–11852. doi: 10.1073/pnas.93.21.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai J, Jones DP. Mitochondrial redox signaling during apoptosis. J Bioenerg Biomembr. 1999;31:327–334. doi: 10.1023/A:1005423818280. [DOI] [PubMed] [Google Scholar]
- 38.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/A:1009616228304. [DOI] [PubMed] [Google Scholar]
- 39.Akgul C, Moulding DA, Edwards SW. Molecular control of neutrophil apoptosis. FEBS Lett. 2001;487:318–322. doi: 10.1016/S0014-5793(00)02324-3. [DOI] [PubMed] [Google Scholar]
- 40.Cimino F, Esposito F, Ammendola R, Russo T. Gene regulation by reactive oxygen species. Curr Top Cell Regul. 1997;35:123–148. doi: 10.1016/s0070-2137(97)80005-2. [DOI] [PubMed] [Google Scholar]