Abstract
The aim of the present study was to investigate the expression of activin A in esophageal carcinoma and its association with tumor differentiation and metastasis. A total of 57 esophageal carcinoma patients and 36 controls were included in the current study. The mRNA and protein expression levels of activin A in esophageal tumors or normal esophageal tissues were determined by reverse transcription-quantitative polymerase chain reaction and immunohistochemical analysis. In addition, the association of activin A expression with esophageal carcinoma differentiation, metastasis and recurrence postoperatively was analyzed. The mRNA and protein expression levels of activin A in esophageal carcinoma were significantly higher compared with those in normal esophageal tissues (P<0.05). The expression of activin A was higher in poorly-/moderately-differentiated esophageal tumor tissues compared with that of well-differentiated or control tissues (P<0.05). Furthermore, the expression of activin A in poorly-differentiated esophageal tumor tissues was higher compared with that of moderately-differentiated tissues (P<0.05). A positive correlation was also observed between differentiation degree and activin A expression. The expression of activin A was higher in patients with lymph node metastasis compared with those without metastasis (P<0.05). The cumulative survival rate of patients with a high expression of activin A at 1, 2 and 3 years postoperatively was significantly decreased compared with that of patients with a lower expression of activin A (P<0.05); by contrast, the cumulative recurrence rate was significantly higher in patients with a lower activin A expression (P<0.05). In conclusion, abnormal expression of activin A was detected in esophageal tumor tissues, which was correlated with the tumor differentiation, metastasis, survival and recurrence. In conclusion, activin A may be used as an auxiliary index in the diagnosis and prognosis of clinical esophageal carcinoma.
Keywords: activin A, esophageal carcinoma, tumor aggressiveness, cell differentiation
Introduction
Esophageal cancer is one of the most common malignancies, its incidence rate ranks eighth among cancers worldwide (1). The incidence rates vary widely between countries, with approximately half of all the diagnosed cases occurring in China (2). In addition, esophageal cancer is ~3 times more common in men than in women (3). The majority of patients are diagnosed with advanced stage disease, since the early symptoms are rarely detected. Systemic metastasis is the major cause of mortality in patients with esophageal cancer (4,5). Therefore, developing novel methods for the early diagnosis of the disease is pivotal for the treatment of esophageal carcinoma patients; however, there is a lack of molecular targets for the diagnosis of esophageal cancer in clinical practice.
Activin A, which is a member of the transforming growth factor β superfamily, was initially isolated from the porcine pituitary follicle and has the ability to induce differentiation of embryonic stem cells (6). There are three types of receptors for activin A: type I, II and III. Activin A binds to the type II receptor with the aid of the type Ⅲ receptor, while it can cause the phosphorylation of type I receptor and then form a ternary complex with the phosphorylated type I receptor (7). Smad2 and Smad3 in the cytosol can be phosphorylated by this complex and subsequently transferred into the nucleus, regulating the expression of certain genes (8–11). Activin A plays an important role in cell proliferation, differentiation, apoptosis and adhesion (12–14). Recent studies have demonstrated that activin A expression is significantly increased in various types of cancer and is correlated with cancer progression and metastasis (15–17). However, to the best of our knowledge no studies have previously investigated the expression of activin A in esophageal cancer tissues. In particular, the association of activin A with esophageal carcinoma differentiation and metastasis, as well as the survival and recurrence rates in esophageal carcinoma patients, remain to be elucidated.
In present study, the expression of activin A in esophageal cancer tissues was detected and its association with tumor differentiation, metastasis, postoperative survival and recurrence was analyzed, which may provide evidence for the development of novel clinical diagnosis methods for esophageal cancer.
Patients and methods
Patients
A total of 57 esophageal cancer tissue specimens were included in the study, which had been resected from patients receiving surgery at Xinxiang Central Hospital (Xinxiang, China), between June 2010 and June 2013. All the samples were pathologically diagnosed as esophageal carcinomas and termed the observation group. The 57 patients included 36 males and 21 females, with an age range of 44–67 years and mean age of 50.4±10.4 years. In total, 17 tumor tissue specimens were well-differentiated, 25 were moderately-differentiated and 15 were poorly-differentiated. Lymph node metastasis was observed in 37 patients, while the other 20 patients presented no metastasis. All these patients were followed-up for 3 years after surgery. In addition, 36 esophageal tissue samples from individuals who underwent physical examination but did not present any pathological changes were selected as the control group. The 36 control patients included 25 males and 11 females, with an age range of 42–69 years and mean ages of 51.7±11.1 years. The two groups demonstrated no statistically significant differences in their age, gender and other indicators (P>0.05). This study was conducted in accordance with the Declaration of Helsinki (The Seventh Revision, 2013) and with approval from the Ethics Committee of Xinxiang Central Hospital (Xinxiang, China). Written informed consent was obtained from all participants.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The esophageal tumor tissues or normal esophageal tissues were homogenized in 1 ml TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA), and then 200 µl chloroform was added and mixed. The mixture was naturally stratified on ice and then centrifuge at 15,000 × g for 15 min, and 500 µl of the supernatant was transferred and mixed with an equal volume of isopropanol. Next, the mixture was placed on ice for 15 min, followed by collection of total RNA by centrifugation at 15,000 × g for 10 min. The mixture was then washed twice with 75% cooling ethanol, and the precipitation was redissolved in diethylpyrocarbonate-treated sterilized water. Total RNA was quantified and reverse-transcribed into cDNA using an M-MLV Reverse Transcription kit. (Takara Biotechnology Co., Ltd., Dalian, China), and then used for qPCR.
Primers for qPCR were designed according to the mRNA sequence from GenBank (accession no. AY578797.1) using Primer Premier software, version 5.0 (Premier Biosoft, Palo Alto, CA, USA). The primers used were as follows: activin A forward, 5′-TTCTCGCTGTACTGCTGCAGA-3′, and reverse, 5′-CTTCCTGCATGTCTTCAAGAGATG-3′; β-actin (internal control) forward, 5′-GCGGGAAATCGTGCGTGAC-3′, and reverse, 5′-CGTCATACTCCTGCTTGCTG-3′. The reaction mixture was prepared with SYBR Green Master Mix (Takara Biotechnology Co., Ltd.), 10 µmmol/l of each primer and 20–50 µg cDNA, with a final volume of 20 µl. qPCR was performed under the following cycling parameters: 30 sec at 95°C, followed by 40 cycles of 3 sec at 95°C and 30 sec at 60°C. The specificity of the product was determined by melting curve analysis. Data acquired were analyzed using the 2−ΔΔCt method (18).
Immunohistochemical assays
The tissue samples were embedded in paraffin and cut into 5-µm sections, then mounted on polylysine-treated glass slides and dried for 1 h at 50°C. Next, the slides were deparaffinized in xylene, rehydrated by graded ethanol, washed three times with distilled water and then heated for 8 min in sodium citrate solution. Subsequent to washing three times in phosphate-buffered saline (PBS), the tissues were quenched in 3% H2O2 in methanol and then washed three times in PBS for 5 min each time. The tissues were blocked with PBS-Tween 20 containing 10% goat serum for 30 min at 37°C and then incubated with mouse anti-human activin A antibody (1:100; sc-35644; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C overnight. After washing three times in PBS to remove excess antibodies, the slides were incubated with diluted goat anti-mouse antibody (1:100; sc-38462; Santa Cruz Biotechnology, Inc.) for 30 min at 37°C. Subsequent to washing for a further three times, 50 µl of 3,3′-diaminobenzidine was used as the chromogen. Finally, the slides were rinsed with water, dehydrated in ethanol gradient and xylene, and then mounted using neutral resin (Dingguo Inc., Beijing, China).
Observation indexes and evaluation criteria
The mRNA and protein expression levels of activin A in the two groups (esophageal carcinoma or normal tissues) were observed. The expression levels of activin A in esophageal carcinoma patients with different degrees of differentiation and metastasis status were analyzed, as well as their association with the survival and tumor recurrence of patients. Tissue samples with >50% activin A-positive cells in the immunohistochemical assay were defined as activin A-positive patients, while samples with <50% positive cells were defined as activin A-negative patients.
Statistical analysis
The data are expressed as the mean ± standard deviation and analyzed using the SPSS version 13.0 software (SPSS, Inc., Chicago, IL, USA). Measurement data was performed using Student's t-test. Comparisons of cumulative recurrence rate or cumulative survival rate were performed using the Kaplan-Meier method and log-rank test, respectively. P<0.05 was considered to indicate a statistically significant difference.
Results
mRNA expression levels of activin A in esophageal carcinoma tissues
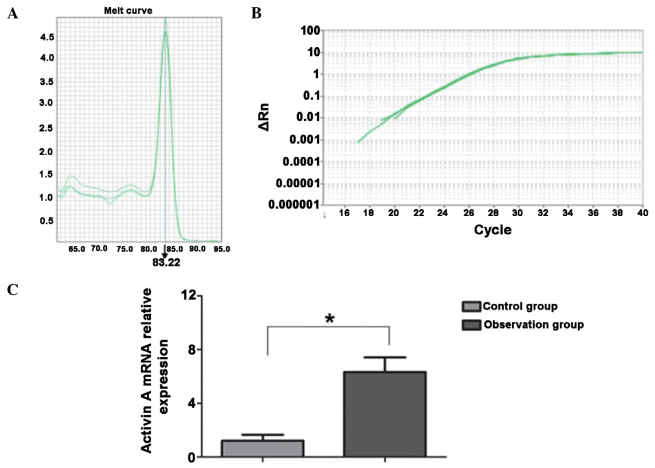
The mRNA level of activin A was detected using the RT-qPCR method. As shown in Fig. 1, the primers used for activin A were specific to the activin A gene and the melting curve was a simplex. In esophageal carcinoma tissues, the mRNA expression of activin A was significantly increased when compared with that in the normal esophageal tissues, and the difference was statistically significant (P<0.05).
Figure 1.
Comparison of mRNA expression levels of activin A in esophageal cancer and normal esophageal tissues. (A) Melting curve of activin A. (B) Amplification curve of activin A. (C) Analysis of expression levels of activin A in the observation (cancer) and control (normal tissues) groups. *P<0.05
Protein expression of activin A in esophageal carcinoma tissues
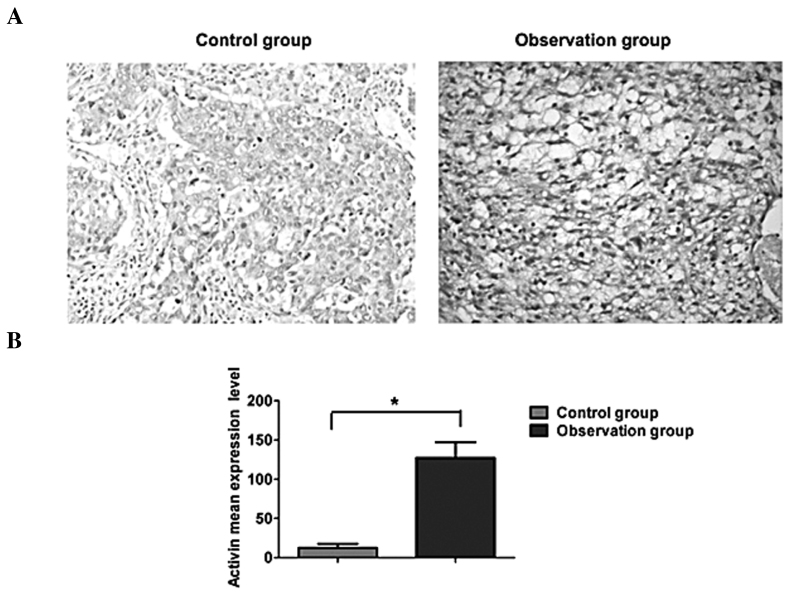
As shown in Fig. 2, activin A was expressed mainly in the cytoplasm, which appeared as brown staining in the immunohistochemical assay. A low expression of activin A was observed in the normal esophageal tissues, while the expression was evidently increased in esophageal carcinoma tissues. Quantitative analysis revealed that activin A expression in esophageal cancer was significantly higher compared with that in normal esophageal tissues, and the difference was statistically significant (P<0.05).
Figure 2.
Comparison of protein expression levels of activin A in esophageal cancer and normal esophageal tissues. (A) Immunohistochemical analysis and (B) quantitative analysis, revealing increased activin A expression in the observation group (esophageal cancer tissues), compared with the control group (normal esophageal squamous tissues).
Expression levels of activin A in esophageal carcinoma tissues with various differentiation degrees
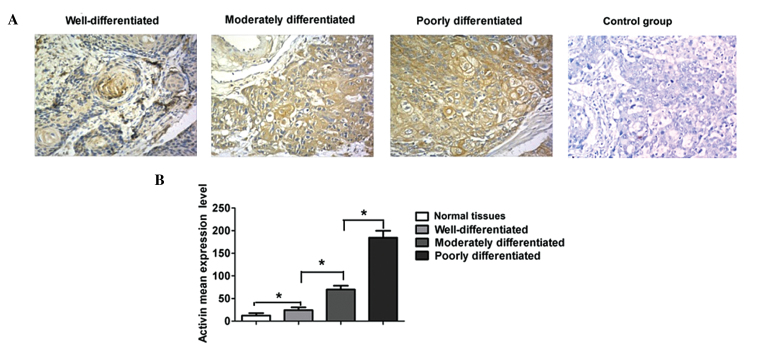
As shown in Fig. 3, the expression of activin A was relatively low in well-differentiated esophageal carcinoma tissues, higher in moderately-differentiated esophageal carcinoma tissues and the highest in poorly-differentiated tumors. Quantitative analysis demonstrated that the expression of activin A was significantly higher in poorly- and moderately-differentiated tumor tissues compared with that in well-differentiated tumors and normal esophageal tissues (P<0.05). By contrast, activin A expression in poorly-differentiated carcinoma tissues was significantly higher compared with that in moderately-differentiated tumor tissues (P<0.05); in addition, the expression in well-differentiated tumors was significantly higher compared with the control normal tissues (P<0.05).
Figure 3.
Comparison of activin A expression levels in cancer tissues with various degrees of differentiation. (A) Immunohistochemical analysis and (B) quantitative analysis, revealing the expression of activin A in well-, moderately- and poorly-differentiated cancer tissues.
Correlation between activin A and metastasis in esophageal cancer
Among the 57 patients included in the current study, 37 subjects presented lymph node metastasis and the remaining 20 patients presented no metastasis. Patients were divided into two groups according to the presence or absence of lymph node metastasis. The two groups demonstrated no statistically significant differences in the gender and age index, and were considered to be comparable (P>0.05). The results revealed that the expression of activin A was markedly higher in metastatic esophageal carcinomas compared with tumors without metastasis, and the difference was statistically significant (P<0.05; Fig. 4).
Figure 4.
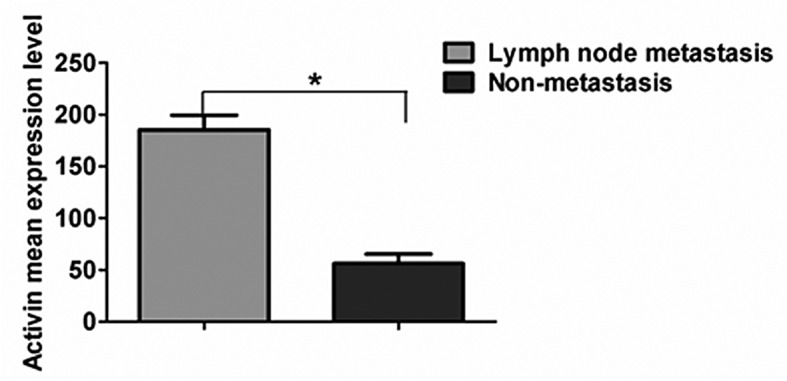
Comparison of activin A expression levels in patients with lymph node metastasis or no metastasis.
Correlation of activin A with survival and recurrence rates
The 57 patients were divided into two groups according to the evaluation standard for activin A expression in the immunohistochemical assay, which included 38 activin A-positive subjects or 19 activin A-negative subjects. Subsequently, the recurrence and survival rates of each group were analyzed. As shown in Fig. 5, the cumulative survival rates at 1, 2 and 3 years postoperatively in the positive group were 71.05, 50.00 and 21.05%, respectively, while in the negative group the rates were 84.21, 63.16 and 36.84%, respectively. Patients with a positive expression of activin A had a significantly shorter survival time compared with that of patients with a negative expression of activin A (P<0.05). Furthermore, the cumulative recurrence rates at 1, 2 and 3 years postoperatively in the positive group were found to be 23.68, 42.11 and 71.00%, respectively, while in the negative group the rates were 5.26, 21.05 and 47.37%, respectively. The positive group presented an evidently higher cumulative recurrence rate compared with the negative group (P<0.05).
Figure 5.
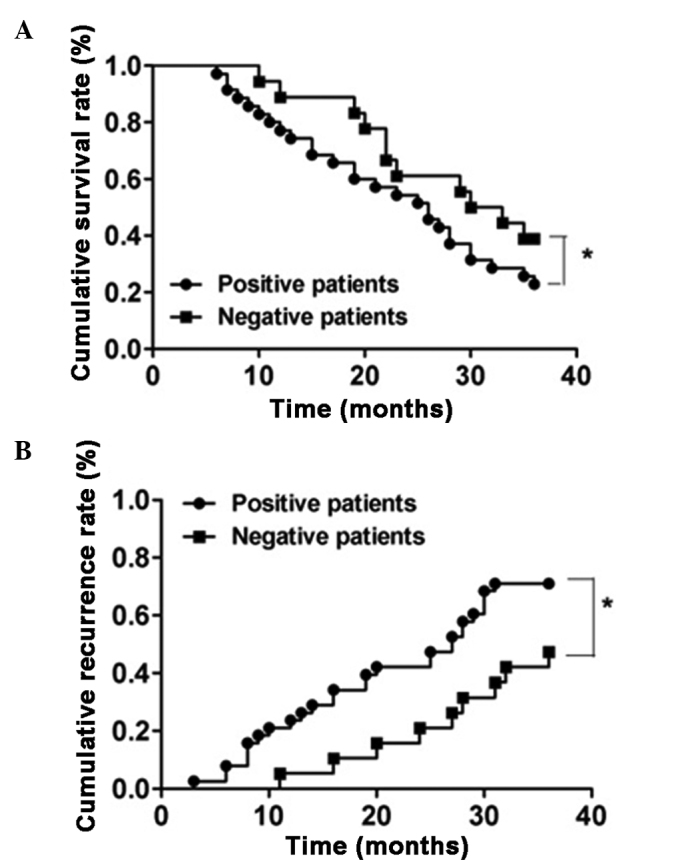
Comparison of postoperative metastasis in activin A-positive and -negative patients. (A) Cumulative survival rate and (B) cumulative recurrence rate in activin A-positive and -negative patients. *P<0.05.
Discussion
Esophageal carcinoma is one of the most common cancer types. A previous study has reported that abnormal expression of activin A was detected in the serum of patients with esophageal carcinoma (19). Therefore, investigating the correlation of the activin A expression with the tumorigenicity and metastasis of human esophageal cancer is essential. However, only a limited number of studies have been conducted on the expression of activin A in esophageal tumor tissues. The present study detected the mRNA and protein expression levels of activin A in esophageal tumor and normal esophageal tissues. The results revealed that the mRNA and protein expression levels of activin A were significantly higher in esophageal cancer compared with those in normal esophageal tissues, which is consistent with the findings of previous studies (20,21).
In addition, the expression of activin A in esophageal tumor tissues with various degrees of differentiation was investigated. The immunohistochemical assay demonstrated that the protein expression of activin A was the highest in poorly-differentiated tumor tissues and the lowest in well-differentiated esophageal tumor tissues, which revealed an association between activin A and the differentiation degree of esophageal carcinoma. Thus, a high expression of activin A can be considered to represent a high degree of malignancy, while a low expression of activin A may indicate a benign tumor. Furthermore, analysis of the lymph node metastasis demonstrated that the expression of activin A was higher in esophageal tumors with presence of lymph node metastasis compared with absence of metastasis. This result indicates that activin A may be involved in the metastasis of esophageal cancer, which is consistent with the aforementioned association with the differentiation degree of esophageal carcinoma. Malignant tumors are generally more likely to metastasize, while benign tumors demonstrate few metastases. The results of the present study revealed that activin A plays an important role in tumor metastasis and grade malignancy, which has also been observed in numerous other types of cancer (22–24).
The primary measure for the treatment of esophageal carcinoma is surgery; however, recurrence is observed postoperatively in certain patients and affects the survival of these patients (25). The present study confirmed that patients with high expression of activin A preserved a lower cumulative survival rate and a higher cumulative recurrence rate compared with patients with low expression of activin A. These results suggest that activin A is closely associated with the recurrence and survival of esophageal cancer patients, which may be due to its correlation with the metastasis and malignancy of esophageal tumors.
In conclusion, the present study identified that activin A was abnormally expressed in esophageal carcinoma tissues and correlated with the tumor differentiation, metastasis, recurrence and survival of esophageal carcinoma patients. Therefore, activin A may be a potential molecular marker for the diagnosis and postoperative prognosis of esophageal cancer.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Jiang L, Zhao X, Meng X, Yu J. Involved field irradiation for the treatment of esophageal cancer: Is it better than elective nodal irradiation. Cancer Lett. 2015;357:69–74. doi: 10.1016/j.canlet.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 3.McK Manson J, Beasley W. A personal perspective on controversies in the surgical management of oesophageal cancer. Ann R Coll Surg Engl. 2014;96:575–578. doi: 10.1308/003588414X13946184901605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba Y, Watanabe M, Yoshida N, Baba H. Neoadjuvant treatment for esophageal squamous cell carcinoma. World J Gastrointest Oncol. 2014;6:121–128. doi: 10.4251/wjgo.v6.i5.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6:112–120. doi: 10.4251/wjgo.v6.i5.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ling N, Ying SY, Ueno N, Shimasaki S, Esch F, Hotta M, Guillemin R. A homodimer of the beta-subunits of inhibin A stimulates the secretion of pituitary follicle stimulating hormone. Biochem Biophys Res Commun. 1986;138:1129–1137. doi: 10.1016/S0006-291X(86)80400-4. [DOI] [PubMed] [Google Scholar]
- 7.Lin HS, Gong JN, Su R, et al. miR-199a-5p inhibits monocyte/macrophage differentiation by targeting the activin A type 1B receptor gene and finally reducing C/EBPα expression. J Leukoc Biol. 2014;96:1023–1035. doi: 10.1189/jlb.1A0514-240R. [DOI] [PubMed] [Google Scholar]
- 8.Kariyawasam HH, Semitekolou M, Robinson DS, Xanthou G. Activin-A: a novel critical regulator of allergic asthma. Clin Exp Allergy. 2011;41:1505–1514. doi: 10.1111/j.1365-2222.2011.03784.x. [DOI] [PubMed] [Google Scholar]
- 9.Maeshima A, Miya M, Mishima K, Yamashita S, Kojima I, Nojima Y. Activin A: autocrine regulator of kidney development and repair. Endocr J. 2008;55:1–9. doi: 10.1507/endocrj.KR-113. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Oakley J, McGee EA. Stage-specific expression of Smad2 and Smad3 during folliculogenesis. Biol Reprod. 2002;66:1571–1578. doi: 10.1095/biolreprod66.6.1571. [DOI] [PubMed] [Google Scholar]
- 11.Zalzman M, Anker-Kitai L, Efrat S. Differentiation of human liver-derived, insulin-producing cells toward the beta-cell phenotype. Diabetes. 2005;54:2568–2575. doi: 10.2337/diabetes.54.9.2568. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira MC, Witz CA, Hammes LS, Kirma N, Petraglia F, Schenken RS, Reis FM. Activin A increases invasiveness of endometrial cells in an in vitro model of human peritoneum. Mol Hum Reprod. 2008;14:301–307. doi: 10.1093/molehr/gan016. [DOI] [PubMed] [Google Scholar]
- 13.Schwall RH, Robbins K, Jardieu P, Chang L, Lai C, Terrell TG. Activin induces cell death in hepatocytes in vivo and in vitro. Hepatology. 1993;18:347–356. doi: 10.1002/hep.1840180219. [DOI] [PubMed] [Google Scholar]
- 14.Thissen JP, Loumaye A. Role of activin A and myostatin in cancer cachexia. Ann Endocrinol (Paris) 2013;74:79–81. doi: 10.1016/j.ando.2013.03.004. (In French) [DOI] [PubMed] [Google Scholar]
- 15.Hoda MA, Münzker J, Ghanim B, Schelch K, Klikovits T, Laszlo V, Sahin E, Bedeir A, Lackner A, Dome B, et al. Suppression of activin A signals inhibits growth of malignant pleural mesothelioma cells. Br J Cancer. 2012;107:1978–1986. doi: 10.1038/bjc.2012.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofland J, van Weerden WM, Steenbergen J, Dits NF, Jenster G, de Jong FH. Activin A stimulates AKR1C3 expression and growth in human prostate cancer. Endocrinology. 2012;153:5726–5734. doi: 10.1210/en.2011-2065. [DOI] [PubMed] [Google Scholar]
- 17.Kim YI, Kim BH, Khang I, Cho BN, Lee HK. Cell growth regulation through apoptosis by activin in human gastric cancer SNU-16 cell lines. Oncol Rep. 2009;21:491–497. [PubMed] [Google Scholar]
- 18.Wang G, Wang L, Sun S, Wu J, Wang Q. Quantitative measurement of serum microRNA-21 expression in relation to breast cancer metastasis in Chinese females. Ann Lab Med. 2015;35:226–232. doi: 10.3343/alm.2015.35.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold E, Risbridger G. Activins and activin antagonists in the prostate and prostate cancer. Mol Cell Endocrinol. 2012;359:107–112. doi: 10.1016/j.mce.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Bao YL, Yang MT, Wu Y, Yu CL, Huang YX, Sun Y, Zheng LH, Li YX. Activin A induces SLC5A8 expression through the Smad3 signaling pathway in human colon cancer RKO cells. Int J Biochem Cell Biol. 2010;42:1964–1972. doi: 10.1016/j.biocel.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 21.de Kretser DM, O'Hehir RE, Hardy CL, Hedger MP. The roles of activin A and its binding protein, follistatin, in inflammation and tissue repair. Mol Cell Endocrinol. 2012;359:101–106. doi: 10.1016/j.mce.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Yoshinaga K, Yamashita K, Mimori K, Tanaka F, Inoue H, Mori M. Activin a causes cancer cell aggressiveness in esophageal squamous cell carcinoma cells. Ann Surg Oncol. 2008;15:96–103. doi: 10.1245/s10434-007-9631-1. [DOI] [PubMed] [Google Scholar]
- 23.Yoshinaga K, Mimori K, Yamashita K, Utsunomiya T, Inoue H, Mori M. Clinical significance of the expression of activin A in esophageal carcinoma. Int J Oncol. 2003;22:75–80. [PubMed] [Google Scholar]
- 24.Liu SG, Li HC, Zhao BS, Cao F. Expression of activin A in tissue and serum of patients with esophageal squamous cell carcinoma and its clinical significance. Zhonghua Zhong Liu Za Zhi. 2013;35:843–847. (In Chinese) [PubMed] [Google Scholar]
- 25.Zhang C, Wang QT, Liu H, et al. Advancement and prospects of tumor gene therapy. Chin J Cancer. 2011;30:182–188. doi: 10.5732/cjc.010.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]