Abstract
Long non-coding RNAs (lncRNAs) have previously been reported to be involved in cancer invasion, proliferation and apoptosis. However, the association between the lncRNA, H19, and esophageal cancer (EC) has remained elusive. In the present study, reverse transcription quantitative-polymerase chain reaction revealed that the expression of H19 was significantly increased and associated with tumor depth and metastasis in 133 EC samples. Furthermore, MTT and Transwell assays revealed that overexpression of H19 in vitro promoted the proliferation and invasion of EC cell lines, whereas knockdown of H19 inhibited the proliferation and invasion of EC cell lines. In addition, it was identified that an upregulation of H19 induced epithelial-to-mesenchymal transition, while the opposite effect was observed following the downregulation of H19. In conclusion, H19 has a significant role in the development of EC and may serve as a potential prognostic marker and therapeutic target for EC.
Keywords: epithelial-to-mesenchymal transition, proliferation, H19, invasion, esophageal cancer
Introduction
Esophageal carcinoma (EC) is the eighth most aggressive and malignant type of cancer, with a high incidence that varies according to geographic location and ethnicity (1). Despite progress in the development of diagnostic and therapeutic options, the survival rates for EC patients remain poor. Therefore, the identification of novel genes involved in the tumorigenesis and development of EC is urgently required.
Long non-coding RNAs (lncRNAs) are a class of RNAs that have been reported to be involved in the regulation, invasion, proliferation and apoptosis of multiple tumors (2,3). The association between H19 expression and the progression of various types of cancer has been demonstrated in previous studies. One study found that the overexpression of lncRNA H19 enhanced the carcinogenesis and metastasis of gastric cancer (4). MALAT-1, an abundant lncRNA present in many human cell types, has been suggested to regulate the alternative splicing of a subset of pre-messenger (m)RNAs by modulating serine/arginine splicing factor activity. This factor in turn regulates tissue or cell-type-specific alternative splicing in a phosphorylation-dependent manner (5). However, the role of H19 in EC is yet to be elucidated.
The epithelial-to-mesenchymal transition (EMT) has an important role in the invasion of various types of cancer by transforming adherent and polarized epithelial cells into invasive and motile mesenchymal cells (6,7). A number of transcription factors involved in EMTs, including Twist and Snail, increase the expression level of mesenchymal markers, including fibronectin, collagen and Vimentin, and decrease the expression of epithelial markers, including E-cadherin. The breakdown of tight junctions results in the loss of epithelial markers and the acquisition of mesenchymal markers (8–10).
In the present study, the expression levels of H19 in EC were investigated, in order to elucidate the role of H19 in EC.
Materials and methods
Clinical samples
EC samples with corresponding adjacent esophageal tissues were obtained from 133 patients who had undergone routine surgery at The Fourth Affiliated Hospital of Nantong Medical College (Yancheng, China) between June 2007 and May 2012, and from The First Affiliated Hospital of Nanjing Medical University (Nanjing, China) between March 2010 and June 2013. The tissues were stored at −80°C until the RNA extraction was performed. The institutional committee approved the experiments. The present study was approved by the Ethical Committee of Nantong Medical College, and each patient provided written informed consent.
Cell culture
In total, five EC cell lines (TE-1, TE-10, Eca-1, Eca-109 and KYSE1170) and one normal control cell line (HEEC) were purchased from the Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China) and cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) (Invitrogen Life Technologies, Carlsbad, CA, USA). All cells were maintained in a humidified 37°C incubator with 5% CO2.
Isolation of total RNA and reverse transcription quantitative-polymerase chain reaction (RT-qPCR)
RNA was extracted from the tissue samples using TRIzol reagent (Invitrogen Life Technologies, Shanghai, China). Subsequently, complemetary DNA was synthesized using a reverse transcriptase kit (Takara Bio, Inc., Otsu, Japan) according to the manufacturers' instructions. The relative expression levels of H19 mRNA were determined using a SYBR Green real-time PCR kit (Takara Bio, Inc.) and normalized to GAPDH. RT-PCR was performed using the ABI 7500 Fast Real-Time PCR system (Applied Biosystems Life Technologies, Foster City, CA, USA) and the following gene-specific primers: Forward, 5′-ATCGGTGCCTCAGCGTTCGG-3′ and reverse, 5′-CTGTCCTCGCCGTCACACCG-3′ for H19; forward, 5′-CTGTCCTCGCCGTCACACCG-3′ and reverse, 5′-GGCATGGACTGTGGTCATGAG-3′ for GAPDH. All primers were designed using the National Center for Biotechnology Information Primer-BLAST tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome). PCR was performed under the following conditions: Denaturation at at 50°C for 2 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Protein expression was quantified using the 2−∆CT method, as previously described (11).
Transwell assay
The invasive ability of the cell lines was determined using a polycarbonate membrane, Boyden chamber insert with an 8-µm-pore size in a Transwell apparatus (EMD Millipore, Billerica, MA, USA). The transfected cells were first treated with trypsin/EDTA solution (Invitrogen Life Technologies) and then washed once with a serum-containing RPMI-1640 medium. In total, 1×105 cells in 0.2 ml serum-free RPMI-1640 medium were seeded into the Transwell apparatus. Next, RPMI-1640 supplemented with 600 µl 10% FBS was added to the lower chamber. In addition, an invasion assay was performed following an identical procedure, with the exception that the Transwell chamber filters were coated with 45 µg Matrigel (BD Biosciences, San Jose, CA, USA). Subsequent to a 24-h incubation at 37°C in a 5% CO2 incubator, the cells on the upper surface of the insert were removed using a cotton swab. The cells that had invaded to the lower surface of the insert were fixed in 100% precooling methanol (Lindi, Shanghai, China) for 10 min, stained in 0.5% crystal violet (Beyotime Institute of Biotechnology, Shanghai, China) for 30 min, rinsed in phosphate-buffered saline (Sigma-Aldrich, St. Louis, MO, USA) and analyzed using a microscope (XSP-4C; Changfang, Shanghai, China). Invasive ability values were obtained by counting three fields per membrane and then presented as the average of three independent experiments.
Cell proliferation assay
The various cell lines were seeded into 96-well plates at a density of 2000 cells/well. In total, 20 µl MTT (0.5 mg/ml) was added into each well and incubated at 37°C for 4 h. Next, 200 µl DMSO was added to each well in order to dissolve the precipitate. The optical density was then measured at 490 nm using a microplate reader (Model 550; Bio-Rad Laboratories, Inc., Hercules, CA, USA). Three independent experiments were performed in quintuplicate.
Western blot analysis
The total proteins were extracted from the cultured cells and then quantified using a bicinchoninic acid assay (Beyotime Institute of Biotechnology). Next, the proteins were fractionated by 5% SDS-PAGE (Beyotime Institute of Biotechnology), transferred to a polyvinylidene fluoride membrane (Beyotime Institute of Biotechnology), blocked in 4% dry milk at room temperature for 1 h and then immunostained using primary polyclonal rabbit anti-human E-cadherin (dilution, 1:500; cat. no. ab15148; Abcam, Cambridge, MA, USA), anti-human fibronectin (dilution, 1:1,000; cat. no. ab61214; Abcam), anti-human vimentin (dilution, 1:5,000; cat. no. ab71144; Abcam) and anti-human GAPDH (dilution, 1:5,000; cat. no. ab9385; Abcam) antibodies at 4°C overnight. The membranes were washed four times with PBS/0.1% Tween 20 solution (Sigma-Aldrich) then incubated with horseradish peroxidase-conjugated polyclonal goat anti-rabbit IgG (dilution, 1:2,000; cat. no. ab6721; Abcam) secondary antibodies for 1 h at 37°C. The results were then visualized using a chemiluminescent detection system (Pierce ECL western blotting substrate detection system; Thermo Fisher Scientific, Pittsburgh, PA, USA) and exposed by the Molecular Imager ChemiDoc XRS System (Bio-Rad Laboratories, Inc.). The integrated density of the bands was quantified using Image Lab 4.1 software (Bio-Rad Laboratories, Inc.).
Transfection of small interfering RNAs (siRNAs)
The cells were seeded into six-well plates and transfected with 50 nM H19-targeting siRNA (siRNA/H19; GenePharma, Shanghai, China) using Lipofectamine® 2000 (Invitrogen Life Technologies) according to the manufacturer's instructions. Non-targeting siRNA (siRNA/control) was used as the control. The transfection efficiency was monitored by RT-qPCR. RT-PCR was performed using the ABI 7500 Fast Real-Time PCR system (Applied Biosystems Life Technologies, Foster City, CA, USA) and the following gene-specific primers: Forward, 5′-ATCGGTGCCTCAGCGTTCGG-3′ and reverse, 5′-CTGTCCTCGCCGTCACACCG-3′ for H19; forward, 5′-CTGTCCTCGCCGTCACACCG-3′ and reverse, 5′-GGCATGGACTGTGGTCATGAG-3′ for GAPDH. PCR was performed under the following conditions: Denaturation at 50°C for 2 min, followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min. Protein expression was quantified using the 2−∆CT method, as previously described (11).
Plasmid construction and cell transduction
The H19 sequence was synthesized and subcloned into pCDNA3.1 (Invitrogen Life Technologies) to generate pCDNAH19. Aberrant expression of H19 was achieved by transfection with pCDNAH19. An empty pCDNA vector was used as the control. The Eca-109 cells were cultured on a six-well plate, and transfected with the pCDNA-H19 or empty vector using Lipofectamine 2000 (Invitrogen Life Technologies) according to the manufacturer's instructions. The expression level of H19 was detected by qPCR using the aforementioned primers, and was performed under the following conditions: Denaturation at 50°C for 2 min, followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min. Protein expression was quantified using the 2−∆CT method, as previously described (11).
Statistical analysis
The expression levels of H19 in the tissues were evaluated using χ2 tests. All P-values are two-sided. P<0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed using Stata 11 (StataCorp LP, College Station, TX, USA), and presented with Graph PAD prism version 4.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
H19 expression is increased in human EC tissues and cell lines
The results of the RT-qPCR analysis revealed that the expression of H19 was higher in the 133 EC tissues compared with that of the corresponding adjacent tissues (Fig. 1A). The cases were divided into H19 low- and high-expression groups. The median was used as the cut-off value. The correlation between the expression of H19 and the clinicopathological characteristics of the patients with EC are shown in Table I. A marked correlation was evident between H19 and tumor depth (P=0.007), tumor stage (P=0.001) and metastasis (P=0.000). By contrast, no positive associations with gender, age or histological differentiation were noted. In addition, the expression of H19 was analyzed in the EC cell lines (TE-1, TE-10, Eca-1, Eca-109 and KYSE1170) and in the normal control cell line (HEEC). Compared with the HEEC cells, H19 expression was significantly increased in the EC cell lines (Fig. 1B). These findings suggested that the aberrant expression of H19 may be involved in the development and progression of EC.
Figure 1.
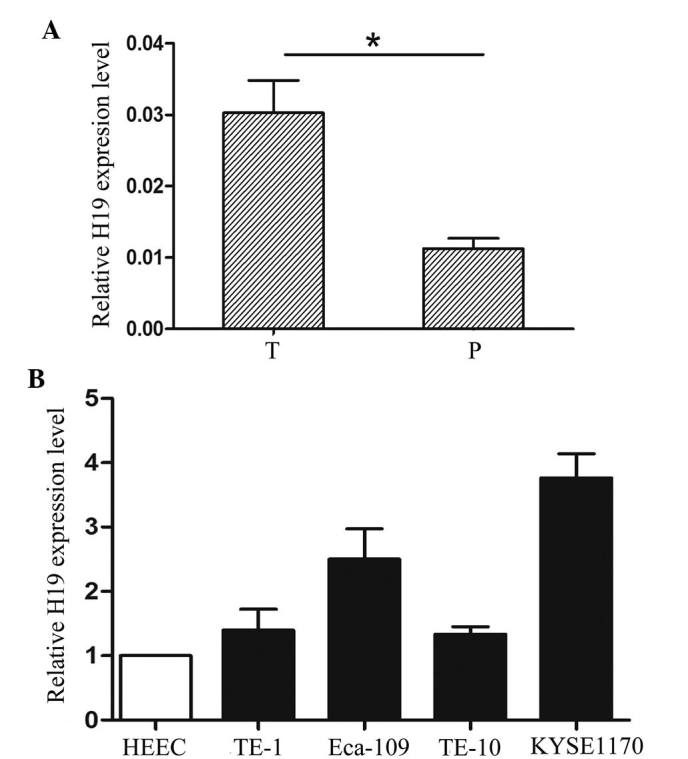
H19 is upregulated in EC patients. (A) Expression levels of H19 in human EC tissues and corresponding adjacent tissues relative to GAPDH were determined by RT-PCR (n=133). (B) Messenger RNA levels of H19 relative to GAPDH were evaluated in five esophageal cancer cell lines (TE-1, TE-10, Eca-1, Eca-109 and KYSE1170) and one normal control cell line (HEEC) using RT-PCR. The results were validated by RT-PCR. The data are presented as the mean ± standard error of the mean. *P<0.05. RT-PCR, real-time polymerase chain reaction; EC, esophageal cancer; T, EC tumor tissue; P, adjacent normal tissue.
Table I.
Expression levels of H19 in esophageal cancer and corresponding adjacent tissues.
| Factors | Patients, n | H19 low expression (≤median), n | H19 high expression (>median), n | P-value |
|---|---|---|---|---|
| Total | 133 | 67 | 66 | |
| Age, years | 0.536 | |||
| <64 | 60 | 32 | 28 | |
| ≥64 | 73 | 35 | 38 | |
| Gender | 0.663 | |||
| Male | 65 | 34 | 31 | |
| Female | 68 | 33 | 35 | |
| Histology | 0.931 | |||
| AC | 66 | 33 | 33 | |
| SCC | 67 | 34 | 33 | |
| Tumor depth | 0.007 | |||
| Tis, T1 | 65 | 25 | 40 | |
| T2, T3, T4 | 68 | 42 | 26 | |
| Stage | 0.001 | |||
| 0, I | 63 | 22 | 41 | |
| II, III, IV | 70 | 45 | 25 | |
| Metastasis | 0.000 | |||
| Yes | 40 | 30 | 10 | |
| No | 93 | 37 | 56 |
AC, adenocarcinoma; SCC, squamous cell carcinoma; Tis, carcinoma in situ; T, tumor stage.
H19 regulates EC cell invasion in Eca-109 cells
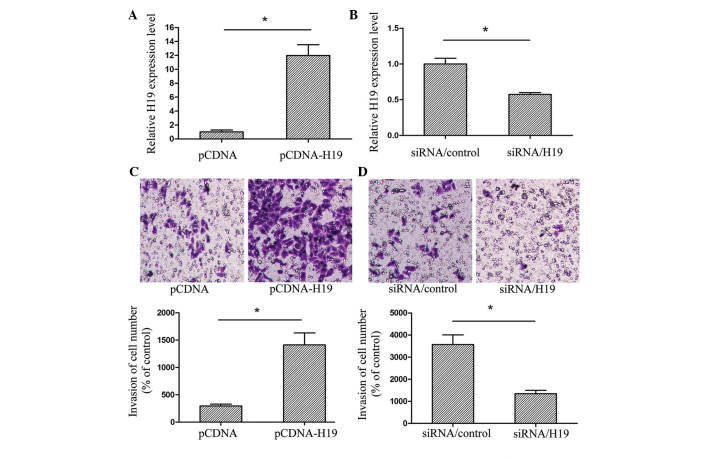
Northern blot analysis has previously revealed that H19 is increased in EC (12). However, the potential mechanisms of H19 in the development of EC are yet to be elucidated. The present study used a Transwell assay in order to determine whether H19 had an effect on the invasion of EC cells. Based on the expression of H19 in the EC cell lines, Eca-109 cells were selected for analysis. The Eca-109 cells were transfected with pCDNA, pCDNA-H19, siRNA/control or siRNA/H19, and the transfection efficiency was then validated using RT-qPCR (Fig. 2A and B). The assay revealed that upregulated H19 expression promoted Eca-109 cell invasion, whereas a downregulation of H19 inhibited the invasion ability of EC cell lines (Fig. 2C and D). The results indicated that H19 may have an important role in regulating the metastasis of EC.
Figure 2.
H19 regulates the invasive ability of cells. The expression of H19 in Eca-109 cells transfected with (A) pCDNA or pCDNA-H19 and (B) siRNA/control or siRNA/H19. The transfection efficiency was validated by RT-qPCR. A Transwell assay was performed in order to determine to invasive ability of cells treated with (C) pCDNA or pCDNA-H19 (mgnification, ×20) and (D) siRNA/control or siRNA/H19 (magnification, ×20) for 24 h. The representative images reveal invasive cells on the lower side of the membrane stained with crystal violet. The quantifications of cell invasion are presented as a percentage of the control cell number. All experiments were performed in triplicate and are presented as the mean ± standard error of the mean. *P<0.05. Each independent experiment was performed three times. siRNA, small interfering RNA.
Aberrant expression of H19 regulates cell proliferation in vitro
An MTT assay was performed in order to investigate whether H19 had an effect upon the proliferation of the EC cell lines. The survival rate of the cells transfected with pCDNA-H19 was markedly higher than that of the controls, whereas the survival rate of the cells transfected with siRNA/H19 was lower than that of the controls (Fig. 3A and B). The data indicated that aberrant expression of H19 was able to regulate cell proliferation in vitro.
Figure 3.
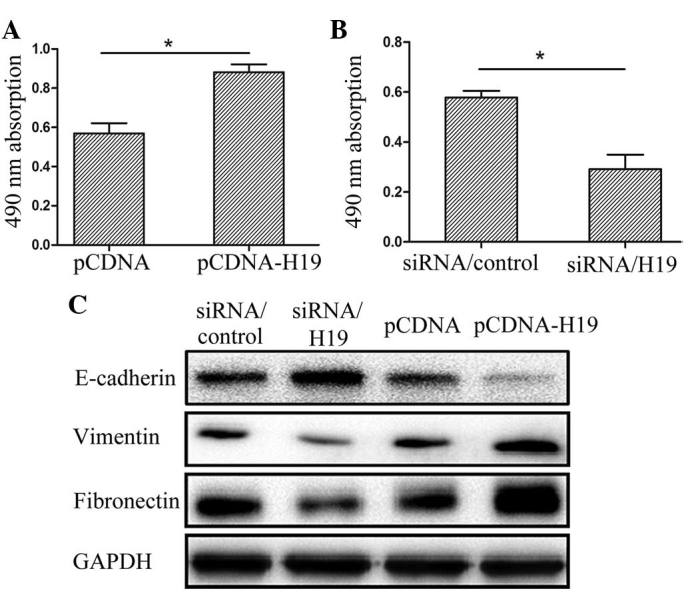
H19 regulates proliferation and epithelial-to-mesenchymal transition. The cells were treated with (A) pCDNA or pCDNA-H19 and (B) siRNA/control or siRNA/H19. (C) E-cadherin, Vimentin and fibronectin protein expression levels in Eca-109 cells transfected with pCDNA, pCDNA-H19, siRNA/control and siRNA/H19 were analyzed by western blotting. GAPDH was used as a loading control. The average values of the integrated optical densities were determined by analyzing five fields per slide. The data are presented as the mean ± standard error of the mean. *P<0.05. siRNA, small interfering RNA.
H19 regulates EMT
The Eca-109 cells were transfected with pCDNA, pCDNA-H19, siRNA/control or siRNA/H19 in order to determine whether H19 was involved in the EMT. The expression of the epithelial marker, E-cadherin, and the mesenchymal markers, fibronectin and Vimentin, was investigated using western blot analysis. At the protein level, the upregulation of H19 expression by pCDNA-H19 resulted in decreased E-cadherin expression and increased Vimentin and fibronectin expression. By contrast, the suppression of expression by siRNA/H19 resulted in increased E-cadherin expression and decreased Vimentin and fibronectin expression (Fig. 3C). Taken together, these findings indicated that H19 may be involved in the regulation of EMT marker expression in EC cell lines.
Discussion
EC is one of the most common causes of cancer-associated mortalities worldwide (13). The standard treatment for patients with early-stage disease, who have been diagnosed in accordance with the tumor, node and metastasis classification, is surgical resection. However, the majority of these patients subsequently develop metastasis, even following successful surgery (14). Identifying the mechanisms that underlie metastasis is therefore required, in order to improve treatment outcomes.
Previous data has identified that lncRNAs have regulatory roles in cancer proliferation, invasion and prognosis. In a previous study of EC, HNF1A-AS1 knockdown significantly inhibited cell proliferation and anchorage-independent growth, suppressed S-phase entry, and inhibited cell migration and invasion in multiple in vitro models of esophageal adenocarcinoma (15). In a further study, HOTAIR directly decreased the expression of WIF-1 by inducing promoter region histone H3K27 methylation and activation of the Wnt/β-catenin signaling pathway (16). The results of the present study indicated that the expression of H19 was higher in EC tissues (n=133) compared with that of the corresponding adjacent tissues. Furthermore, it was revealed that the aberrant expression of H19 affected the invasion potential of EC cell lines in vitro.
Decreased E-cadherin and increased Vimentin and Snail expression are characteristic of EMT; a process known to be significant in cancer invasion (17). Previous studies have established that EMT is associated with tumor invasiveness, metastasis and prognosis (18,19). Furthermore, a number of studies have identified functional associations between lncRNAs and key effectors of EMT during carcinogenesis and embryonic development, including LincRNA-ROR (20), MALAT-1 (21) and BANCR (22). In addition to its role in cancer progression, EMT contributes to chronic epithelial injury (23), which leads to tissue fibrosis and organ failure (24,25). The present study also revealed that an overexpression of H19 led to a decreased expression of the epithelial marker, E-cadherin, and increased expression of mesenchymal markers, Vimentin and Snail. The downregulation of H19 had the opposite effect. These results suggested that H19 may promote EC invasion by inducing EMT.
In conclusion, higher H19 expression levels were detected in EC tumor tissues than in corresponding adjacent tissues. The H19 expression levels were associated with tumor depth, stage and metastasis. Furthermore, H19 was able to regulate the invasion and proliferation of EC cells, and induce EMT in vitro.
Acknowledgements
The authors would like to thank Dr Linjie Si (The First Affiliated Hospital of Nanjing Medical University, Nanjing, China) for providing the EC samples.
References
- 1.Zhang F, Yang Z, Cao M, et al. MiR-203 suppresses tumor growth and invasion and down-regulates MiR-21 expression through repressing Ran in esophageal cancer. Cancer Lett. 2014;342:121–129. doi: 10.1016/j.canlet.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 2.Wang F, Li X, Xie X, Zhao L, Chen W. UCA1, a non-protein-coding RNA up-regulated in bladder carcinoma and embryo, influencing cell growth and promoting invasion. FEBS Lett. 2008;582:1919–1927. doi: 10.1016/j.febslet.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Wu ZH, Wang XL, Tang HM, et al. Long non-coding RNA HOTAIR is a powerful predictor of metastasis and poor prognosis and is associated with epithelial-mesenchymal transition in colon cancer. Oncol Rep. 2014;32:395–402. doi: 10.3892/or.2014.3186. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Yu B, Li J, et al. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5:2318–2329. doi: 10.18632/oncotarget.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutschner T, Hämmerle M, Eissmann M, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73:1180–1189. doi: 10.1158/0008-5472.CAN-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Wen M, Kwon Y, et al. CUL4A induces epithelial-mesenchymal transition and promotes cancer metastasis by regulating ZEB1 expression. Cancer Res. 2014;74:520–531. doi: 10.1158/0008-5472.CAN-13-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Ruan B, You N, et al. Downregulation of miR-200a induces EMT phenotypes and CSC-like signatures through targeting the β-catenin pathway in hepatic oval cells. PLoS One. 2013;8:e79409. doi: 10.1371/journal.pone.0079409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong H, Xie L, Tang C, et al. Snail1 correlates with patient outcomes in E-cadherin-preserved gastroesophageal junction adenocarcinoma. Clin Transl Oncol. 2014;16:783–791. doi: 10.1007/s12094-013-1149-3. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Li H, Feng J, et al. Lin28 induces epithelial-to-mesenchymal transition and stemness via downregulation of let-7a in breast cancer cells. PLoS One. 2013;8:e83083. doi: 10.1371/journal.pone.0083083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao YX, Cao Q, Yang Y, et al. Expression and prognostic significance of golgiglycoprotein73 (GP73) with epithelial-mesenchymal transition (EMT) related molecules in Hepatocellular Carcinoma (HCC) Diagn Pathol. 2013;8:197. doi: 10.1186/1746-1596-8-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Jiang G, Zhou J, et al. Down-regulation of miR-140 induces EMT and promotes invasion by targeting Slug in esophageal cancer. Cell Physiol Biochem. 2014;34:1466–1476. doi: 10.1159/000366351. [DOI] [PubMed] [Google Scholar]
- 12.Hibi K, Nakamura H, Hirai A, et al. Loss of H19 imprinting in esophageal cancer. Cancer Res. 1996;56:480–482. [PubMed] [Google Scholar]
- 13.Shigaki H, Baba Y, Watanabe M, Murata A, Ishimoto T, Iwatsuki M, Iwagami S, Nosho K, Baba H. PIK3CA mutation is associated with a favorable prognosis among patients with curatively resected esophageal squamous cell carcinoma. Clin Cancer Res. 2013;19:2451–2459. doi: 10.1158/1078-0432.CCR-12-3559. [DOI] [PubMed] [Google Scholar]
- 14.Koshy M, Esiashvilli N, Landry JC, Thomas CR, Jr, Matthews RH. Multiple management modalities in esophageal cancer: combined modality management approaches. Oncologist. 2004;9:147–159. doi: 10.1634/theoncologist.9-2-147. [DOI] [PubMed] [Google Scholar]
- 15.Yang X, Song JH, Cheng Y, et al. Long non-coding RNA HNF1A-AS1 regulates proliferation and migration in oesophageal adenocarcinoma cells. Gut. 2014;63:881–890. doi: 10.1136/gutjnl-2013-305266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge XS, Ma HJ, Zheng XH, et al. HOTAIR, a prognostic factor in esophageal squamous cell carcinoma, inhibits WIF-1 expression and activates Wnt pathway. Cancer Sci. 2013;104:1675–1682. doi: 10.1111/cas.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitamura K, Seike M, Okano T, et al. MiR-134/487b/655 cluster regulates TGF-β-induced epithelial-mesenchymal transition and drug resistance to gefitinib by targeting MAGI2 in lung adenocarcinoma cells. Mol Cancer Ther. 2014;13:444–453. doi: 10.1158/1535-7163.MCT-13-0448. [DOI] [PubMed] [Google Scholar]
- 18.Guo S, Xu X, Tang Y, et al. miR-15a inhibits cell proliferation and epithelial to mesenchymal transition in pancreatic ductal adenocarcinoma by down-regulating Bmi-1 expression. Cancer Lett. 2014;344:40–46. doi: 10.1016/j.canlet.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Yamada S, Fuchs BC, Fujii T, et al. Epithelial-to-mesenchymal transition predicts prognosis of pancreatic cancer. Surgery. 2013;154:946–954. doi: 10.1016/j.surg.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Hou P, Zhao Y, Li Z, et al. LincRNA-ROR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death Dis. 2014;5:e1287. doi: 10.1038/cddis.2014.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ying L, Chen Q, Wang Y, Zhou Z, Huang Y, Qiu F. Upregulated MALAT-1 contributes to bladder cancer cell migration by inducing epithelial-to-mesenchymal transition. Mol Biosyst. 2012;8:2289–2294. doi: 10.1039/c2mb25070e. [DOI] [PubMed] [Google Scholar]
- 22.Sun M, Liu XH, Wang KM, et al. Downregulation of BRAF activated non-coding RNA is associated with poor prognosis for non-small cell lung cancer and promotes metastasis by affecting epithelial-mesenchymal transition. Mol Cancer. 2014;13:68. doi: 10.1186/1476-4598-13-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vitalone MJ, Naesens M, Sigdel T, Li L, Hseih S, Sarwal MM. The dual role of epithelial-to-mesenchymal transition in chronic allograft injury in pediatric renal transplantation. Transplantation. 2011;92:787–795. doi: 10.1097/TP.0b013e31822d092c. [DOI] [PubMed] [Google Scholar]
- 24.López-Novoa JM, Nieto MA. Inflammation and EMT: An alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mucsi I, Rosivall L. Epithelial-mesenchymal transition in renal tubular cells in the pathogenesis of progressive tubulo-interstitial fibrosis. Acta Physiol Hung. 2007;94:117–131. doi: 10.1556/APhysiol.94.2007.1-2.11. [DOI] [PubMed] [Google Scholar]